ABSTRACT
Phloem tissue is essential for the translocation of nutrients, water, energy, and signals in plants. In order to study the chemical composition of phloem sap, several methods have been used for its collection including the dipotassium ethylenediamine tetraacetic acid (K2-EDTA) exudation, incision, and the stylectomy by aphids. Each method has advantages and disadvantages. Unfortunately, there is no ideal method that can be used for all plants or to collect ultrapure phloem sap with no cellular contamination. However, K2-EDTA exudation is the most used method because it is easy, fast, and results in a high quantity of phloem sap. In woody plants, it is easy to separate the bark. Using the bark which contains the phloem tissue would avoid the contamination with xylem sap when phloem sap is collected. Lately, we developed a simple and a quick method for the collection of the citrus phloem sap depending on the centrifugation of the detached bark tissue. Here, I report the advantages of the centrifugation method over the K2-EDTA exudation in collecting phloem sap from citrus. To emphasize the purity of collected saps, phloem sap (from bark tissue) and xylem sap (from inner part of stem) were collected using both methods. Using gas chromatography mass spectrometry, the centrifugation method showed less artifact peaks than the K2-EDTA exudation indicating more pure saps were collected. For instant, less hexoses were detected in phloem sap and the absence of sucrose in xylem sap in centrifugation method than in K2-EDTA exudation. More importantly, centrifugation method allowed accurate estimation of the concentrations of metabolites. This method could be successfully used for the collection of saps of citrus and other trees until the invention of a more specific method to collect ultra-pure phloem sap.
KEYWORDS: Phloem sap, xylem sap, K2EDTA exudation, GC-MS, Citrus
Text
The phloem plays a major role in the translocation of nutrients, water, energy, and signal molecules from source to sink in plants.1 In addition, the sieve elements transport a wide range of proteins and RNAs, which act as a long-distance signal in response to developmental cues and stresses.1 The phloem sap contains high quantities of sugars, amino acids, vitamins, and organic and inorganic acids.2,3 Sugars and amino acids are the major components of the phloem sap.2 Sucrose is the predominant sugar in phloem sap. Because the phloem sap is rich in nutrients and free of feeding deterrents and toxins, it is exclusively consumed by many phloem sap-feeding insects such as aphids.4 The composition of the phloem sap has gained a great attention in plant science and entomology.2 Some of the previous studies focused on nutrient intake and allocation in plants, whereas others investigated the effects of changes in the phloem sap composition on insect symbiont and honeydew composition.2 In addition, the phloem sap composition was studied as a tool to evaluate plant health.2
Several methods have been developed for the collection of the phloem sap including the dipotassium ethylenediamine tetraacetic acid (K2-EDTA), incision, and the stylectomy method. Each of these methods has its own advantages and drawbacks. In the K2-EDTA method, the tip of the petioles or a piece of bark tissue is incubated in K2-EDTA solution for a few hours at room temperature.5 The K2-EDTA prevents accumulation of callose and P-protein in the phloem sieve.1,6 This method is easy and works with most plants, and it can be used to collect large quantities of the phloem sap. Unfortunately, the phloem exudates obtained by the K2-EDTA method could be contaminated with substances from the xylem and other tissues. The source of this contamination is most likely due to the action of EDTA as a chelating agent which may release metal ions such as Ca2+ from the cell walls that enter the exudate. In addition, the K2-EDTA method cannot be used to estimate the concentration of phloem sap metabolites. The incision method can be used in plants which bleed spontaneously after cutting of the phloem tissues such as cucurbits and castor bean.7 The incision method is easy and fast. However, it cannot be used with most plants because the accumulation of callose and P-protein at the wound site stops the flow of phloem.6
To collect the phloem sap using the insect stylet, the stylet is cut during the insect feeding on the phloem sap and the exudate is collected using a microcapillary.1 This method is laborious, time-consuming, and can collect very small amounts of the phloem sap insufficient for exhaustive chemical analyses. The phloem sap collected using the stylectomy method was believed to be pure. However, the insect saliva proteins and other components could affect the chemical composition of the phloem sap collected using this method. In addition, the phloem sap collected using this method could also be mixed with the xylem sap since many of the phloem sap-feeders occasionally feed on the xylem sap.8 Moreover, the stylectomy method cannot be used with all insect species and it is limited to young plants.1 Because there is no ideal method for the collection of the phloem sap, it is recommended to use a combinatory approach in order to identify the artifacts.9
In our previous study, we described an easy method for the collection of citrus phloem sap.2 The collection of the citrus phloem sap was achieved by centrifugation of the separated bark tissues.2 Briefly, the bark was stripped into two pieces (1-cm pieces) using a sterile razor blade and was separated from the xylem. The separated tissues were rinsed with distilled water and dried with Kimwipes. The bark tissues were vertically placed in a 0.5-ml microcentrifuge tube punctured with small holes. The small tube was nested into a 2-ml Eppendorf tube. The sample was centrifuged at 12,000 x g for 15 min. The phloem sap obtained using this method was derivatized using methylchloroformate or trimethylsilyl (TMS) and analyzed using gas chromatography mass spectrometry (GC-MS). In addition, the phloem sap was prepared using the EDTA method and compared with that obtained by centrifugation. The GC-MS results showed that all the metabolites detected in the K2-EDTA exudate were detected also in the phloem sap obtained by the centrifugation method.
Herein, in order to fairly compare the purity of phloem sap collected by the two methods, both the phloem and the xylem saps were collected using the centrifugation technique and K2-EDTA method, and then saps were derivatized using TMS. The metabolites detected in sap are shown in Figure 1. These metabolites included several sugars, amino acids, sugar alcohols (polyols), sugar acids, and organic acids (Figure 1). All metabolites detected in the K2-EDTA exudates were detected in the phloem sap obtained by the centrifugation method (Figure 1). Sucrose was the most abundant sugar in the saps while it was hardly detected in xylem sap collected by centrifugation (Figure 1). Contrarily, hexoses were more abundant in xylem saps than in phloem saps. Amino acids, organic acids, and polyols were found at higher levels in the phloem saps.
Figure 1.
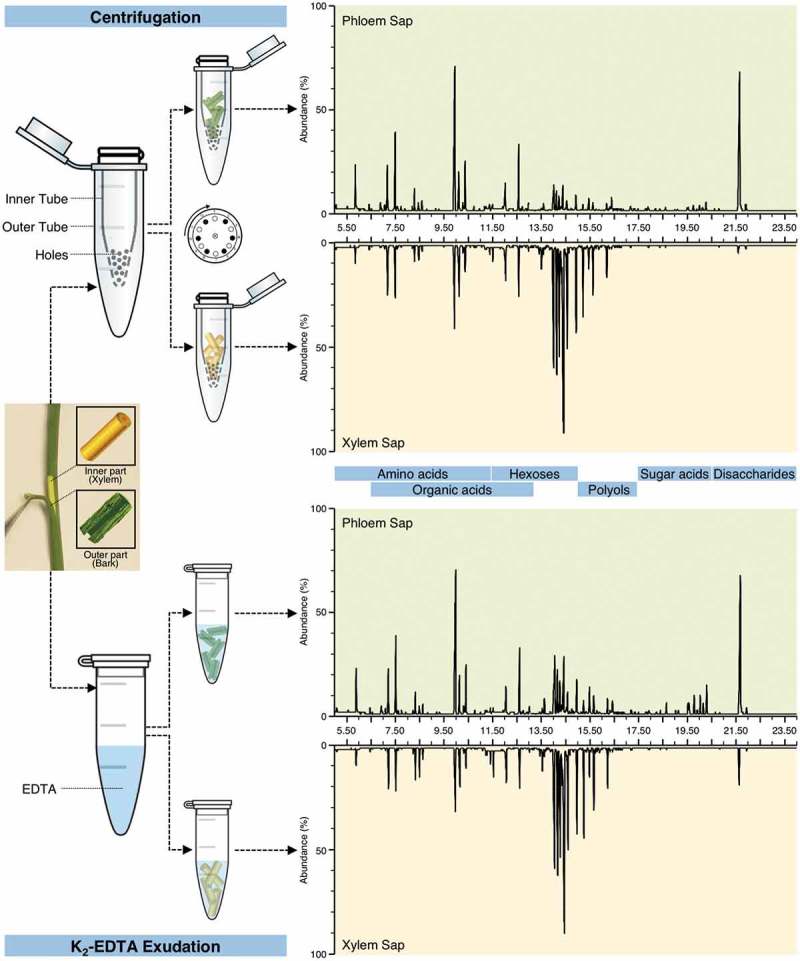
Chemical compositions of citrus phloem and xylem saps collected with K2-EDTA exudation and centrifugation techniques. Phloem sap collected by the centrifugation method shows a higher concentration of sucrose, amino acids, and organic acids and lower amount of hexoses and sugar acids than that found using the K2-EDTA method, indicating higher purity and less cellular contamination. Xylem saps with absent or little sucrose and high amounts of hexoses indicate that both methods of collection are good for xylem sap collection; however, centrifugation results in more pure saps and enable the quantification of compounds.
The current and the previous results2 showed that the centrifugation method was an excellent method for the collection of the citrus phloem and xylem saps. The centrifugation method has several advantages over the EDTA exudation method; it is easy, fast, and can be used to collect larger quantities of the phloem sap. In addition, the centrifugation method allowed accurate estimation of the phloem sap components because it is not diluted by the EDTA solution. Therefore, the volume of phloem sap collected can be directly measured. It is noteworthy to mention that contamination of the phloem sap by the cellular components during centrifugation is possible but is much less likely than when K2-EDTA is used. Overall, the advantages of centrifugation method overweigh this drawback. Consequently, this method could be successfully used for the collection of the phloem sap of citrus and other trees until the invention of more precise methods to collect ultra-pure phloem sap.
Funding Statement
This project is funded by the grant [no. 18-024] from Citrus Research and Development Foundation.
Acknowledgments
I thank Faraj Hijaz and Shelley E. Jones for the technical assistance and Lorraine Jones and Floyd Butz for maintaining the trees in greenhouses.
References
- 1.Dinant S, Bonnemain JL, Girousse C, Kehr J.. Phloem sap intricacy and interplay with aphid feeding. Comptes Rendus Biologies. 2010;333:504–515. doi: 10.1016/j.crvi.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Hijaz F, Killiny N.. Collection and chemical composition of phloem sap from Citrus sinensis L. osbeck (sweet orange). PLoS One. 2014:e101830. doi: 10.1371/journal.pone.0101830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hijaz F, Manthey J, Dv DM, Killiny N. Nucleotides, micro- and macro-nutrients, limonoids, flavonoids, and hydroxycinnamates composition in the phloem sap of sweet orange. Plant Signaling & Behavior. 2016;11:e1183084. doi: 10.1080/15592324.2016.1183084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ae D. Phloem-sap feeding by animals: problems and solutions. Journal of Experimental Botany. 2006;57:747–754. doi: 10.1093/jxb/erj067. [DOI] [PubMed] [Google Scholar]
- 5.King RW, Zeevaart JA. Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiology. 1974;53:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rennenberg H, Schneider S, Weber P. Analysis of uptake and allocation of nitrogen and sulphur compounds by trees in the field. J Exp Bot. 1996;47:1491–1498. doi: 10.1093/jxb/47.10.1491. [DOI] [Google Scholar]
- 7.Hall SM, Baker DA, Milburn JA. Phloem transport of 14C-labelled assimilates in Ricinus. Planta. 1971;100:200–207. doi: 10.1007/BF00387036. [DOI] [PubMed] [Google Scholar]
- 8.Pompon J, Quiring D, Goyer C, Giordanengo P, Pelletier Y. A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. Journal of Insect Physiology. 2011;57:1317–1322. doi: 10.1016/j.jinsphys.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Gaupels F, Knauer T, van Bel AJ. A combinatory approach for analysis of protein sets in barley sieve-tube samples using EDTA-facilitated exudation and aphid stylectomy. J Plant Physiol. 2008;165:95–103. doi: 10.1016/j.jplph.2007.07.023. [DOI] [PubMed] [Google Scholar]


