ABSTRACT
To investigate the clinical characteristics and the effectiveness of maintenance therapy of anti-AQP4 antibody positive optic neuritis in Japanese patients, medical records from 69 patients (103 eyes) were retrospective reviewed. The status of relapse in patients who received maintenance therapy following acute therapy was compared with that before maintenance therapy in patients who started maintenance therapy ≥6 months after acute therapy. In Japan, anti-AQP4 antibody positive optic neuritis was characterized by older onset age and poor visual outcome. The yearly rate and total number of relapses were lower when maintenance therapy was followed immediately after acute therapy.
KEYWORDS: Maintenance therapy, anti-aquaporin 4 antibody, Japanese, optic neuritis, neuromyeritis optica
Introduction
Neuromyelitis optica (NMO) is characterized by severe optic neuritis and transverse myelitis. Since the discovery of anti-aquaporin 4 antibody (anti-AQP4 antibody), which is a specific autoantibody in NMO,1,2 the immunological mechanism of the disease was elucidated. Unlike multiple sclerosis (MS), which is a demyelinating disease, NMO is an astrocytopathic disorder in which complement- or cell-mediated activation of anti-AQP4 antibody causes destruction of astrocytes. Consequently, the clinical features of NMO and MS are also different, which have led to marked changes in the view of optic neuritis associated with NMO.3,4
In Japan, Takagi et al.5 first reported a case series focused on anti-AQP4 antibody positive optic neuritis in 2009. Thereafter, Nakao et al.6 described the clinical characteristics of anti-AQP4 antibody positive optic neuritis. They reported that 37.8% of the MS-associated and idiopathic optic neuritis cases were positive for anti-AQP4 antibody, and that anti-AQP4 antibody positive optic neuritis tended to occur bilaterally with serious impairment of residual visual function.
For acute-phase treatment of anti-AQP4 antibody positive optic neuritis, high-dose intravenous corticosteroid infusion is generally conducted.7,8 In patients who do not respond to mega-dose corticosteroids, plasma exchange has been reported to be effective.9–13 Cases that are refractory to both therapies have poor visual outcome. Moreover, after acute-phase therapy, implementation of maintenance therapy for the purpose of preserving the residual visual function and prevention of relapse is now recognized as the standard treatment.
In the present study, we tried to clarify the clinical characteristics of anti-AQP4 antibody positive optic neuritis in Japan by retrospectively reviewing the medical records of a relatively large series of these cases. In patients with anti-AQP4 antibody positive optic neuritis who were observed long-term for 2 years or longer, we examined the situation of relapse and maintenance therapy, as well as the visual function at the last follow-up. On the other hand, there are some patients who developed optic neuritis before anti-AQP4 antibody was discovered, and hence they did not receive maintenance therapy after acute treatment. Thereafter, they were found to be anti-AQP4 antibody positive and started receiving maintenance therapy, and have been observed long-term until now. Therefore, there was an interval between acute treatment for onset attack and start of maintenance therapy in these cases.
Currently, since acute treatment continuing on to maintenance therapy has become the standard treatment for optic neuritis, there are few cases that do not receive maintenance therapy after acute treatment. Hence, it is difficult to analyze the effect of maintenance therapy on relapse. To investigate the beneficial effect of continuous maintenance therapy from acute treatment, we adopted the following strategy. We analyzed the relapse rate in the above-mentioned cases with an interval between acute therapy for onset attack and initiation of maintenance therapy and compared with more recent cases that received maintenance therapy continuous from acute therapy for onset attack.
Subjects and methods
The present retrospective study was performed by reviewing patients’ medical records. Among patients diagnosed with and/or treated for optic neuritis at the Neuro-ophthalmology Outpatient Clinic, Inoue Eye Hospital or at the Department of Ophthalmology, Kitasato University School of Medicine between 2000 and 2014, those who had anti-AQP4 antibody positive optic neuritis proven by detection of anti-AQP4 antibody in blood collected during follow-up were included in the present study.
Optic neuritis was diagnosed based on interview, past history, and ophthalmologic examinations (visual acuity, intraocular pressure, flicker fusion threshold, status of relative afferent pupillary defect, slit lamp examination, fundus examination, visual field test, fundus fluorescein angiography, and head MRI). Measurements of anti-AQP4 antibody were performed after obtaining informed consent, and blood was collected before treatment for optic neuritis or during follow-up observation. After collection, the blood sample was kept cold on ice and transported to the Department of Neurology, Niigata University School of Medicine or the Department of Neurology, Tohoku University School of Medicine for anti-AQP4 antibody testing. Anti-AQP4 antibody positivity was determined qualitatively using a cell-based assay (CBA).14,15
In all the patients, residual visual function was evaluated from the final visual acuity at the last follow-up. In patients who were followed long-term for 2 years or longer, we also examined the treatment at onset of optic neuritis, status of relapse, and maintenance therapy.
The grouping of subjects and data are shown in Figure 1. The subjects consisted a group diagnosed with anti-AQP4 antibody positive optic neuritis shortly after onset of optic neuritis and received maintenance therapy continuous from acute treatment for onset attack of optic neuritis (group A), and a group diagnosed with optic neuritis before anti-AQP4 antibody was discovered and subsequently received maintenance therapy upon detection of anti-AQP4 antibody after a time lapse from acute treatment of onset attack (group B). For patients in group B, the data from onset attack to before start of maintenance therapy [group B(pre)] and the data after initiation of maintenance therapy [group B (post)] were analyzed separately. The status of relapse was compared between group A and group B(pre), and also between group B(pre) and group B(post).
Figure 1.
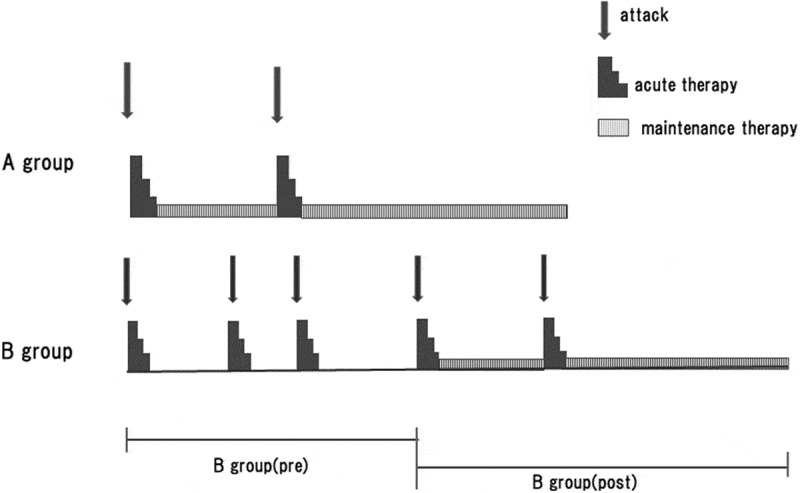
The grouping of subjects and data are shown in Figure 1. The subjects consisted of a group diagnosed with anti-AQP4 antibody positive optic neuritis shortly after onset of optic neuritis and received maintenance therapy continuous from acute treatment for onset attack of optic neuritis (group A), and a group diagnosed with optic neuritis before anti-AQP4 antibody was discovered and subsequently received maintenance therapy upon detection of anti-AQP4 antibody after a time lapse from acute treatment of onset attack (group B). For patients in group B, the data from onset attack to before start of maintenance therapy [group B (pre)] and the data after initiation of maintenance therapy [group B (post)] were analyzed separately.
After acute treatment, the corticosteroids were tapered, and the start of maintenance therapy was defined as the time when corticosteroids were used at a maintenance dose of prednisolone 20 mg/day or methylprednisolone 12 mg/day, or when low dose corticosteroids were used in combination with immunosuppressants.
This study was approved by the Ethics committee at Inouye Eye Hospital (201806–2).
Statistical analysis
Statistical analysis was performed using SPSS software (IBM SPSS Statistics Ver.22.0).
Data was reported using mean and standard deviation. Fisher test was performed to
determine difference in sex, the type of onset attack (optic neuritis or transverse myelitis) and disease course between Group A and Group B(pre). Mann-Whitney U test was used for assessment of non-parametric data [age at onset, follow-up period, annual relapse rate and total no. of attack between the group A and group B(pre)]. The significance level was set at p < 0.05.
Results
Clinical characteristics of all subjects
Review of medical records identified 69 patients (103 eyes) who satisfied the inclusion criteria, which Table 1 shows the demographic and clinical characteristics of optic neuritis in all subjects. The patients comprised 7 males and 62 females. The mean observation duration was 100.0 ± 74.0 months (range 3.0–400 months). The mean age at onset of optic neuritis was 49.9 years (range 8–72 years, median 51.5 years). During the follow-up period, 35 patients (50.7%) manifested unilateral optic neuritis, and 34 patients (49.3%) had bilateral optic neuritis, 12 (17.4%) of the latter developed optic neuritis almost simultaneously in both eyes (within one month). The onset symptom was optic neuritis in 65 of 69 patients (94.2%), and 51 patients (73.9%) did not show symptoms of myelitis during the follow-up period.
Table 1.
Demographic, clinical and outcome data of all patients with aquaporin-4 antibody-positive optic neuritis analyzed.
| No. of patients (eyes) | 69 patients (103 eyes) |
| Female/male | 62/7 (89.9% female) |
| Mean age at onset (range: median) years | 49.9 (8–72: median 51.5) |
| Mean disease duration (range) months | 100.0 ± 74.0 (3.0–400) |
| Onset attack: | |
| Optic neuritis | 65/69 patients (94.2%) |
| Transverse myelitis | 4/69 patients (5.8%) |
| Course of disease: | |
| Optic neuritis only | 51/69 patients (73.9%) |
| Bilateral episodes | 34/69 patients (49.3%) |
| Simultaneous bilateral episodes | 12/69 patients (17.4%) |
| Visual outcome: | |
| ≥1.0 | 23/103 eyes (22.3%) |
| ≤0.1 | 58/103 eyes (56.3%) |
| Total blindness | 14/103 eyes (13.6%) |
| Bilateral visual acuity ≤0.1 | 14/69 patients (20.3%) |
| Unilateral visual acuity ≤0.1 | 25/69 patients (36.2%) |
| Acute treatment | |
| High-dose intravenous methylprednisolone | 55/69 patients(79.7%) |
| High-dose intravenous methylprednisolone + plasma exchange | 14/69 patients (20.3%) |
The final visual acuity during follow-up recovered to 1.0 (decimal visual acuity) or above in 23 of 103 eyes (22.3%), while the final visual acuity was 0.1 or below in 58 of 103 eyes (56.3%), including no light perception in 14 of 103 eyes (13.6%). When analyzed by patient, 14 of 69 patients (20.2%) had visual acuity of 0.1 or below in both eyes, and 25 of 69 patients (36.2%) had 0.1 or below in one eye. Among 12 eyes with simultaneous bilateral episodes, 4 patients (33.3%) had visual acuity of 0.1 or below in both eyes, and 6 patients (50.0%) had 0.1 or below in either eye. In 84 eyes with documented visual acuity at onset attack of optic neuritis, visual acuity after treatment of the onset attack was 0.1 or below in 47 eyes (56.0%), including 14 eyes (16.7%) with no light reception.
Regarding acute-phase treatment for onset attack of optic neuritis, intravenous infusion of high-dose corticosteroids (methylprednisolone 1000 mg/ day) for 3 to 5 days was conducted. Fourteen patients (20.3%) did not respond to corticosteroid treatment and underwent plasma exchange. The results of anti-AQP4 antibody testing were available within approximately two weeks. During the acute phase of optic neuritis, high-dose intravenous corticosteroids were administered without waiting for the antibody result.
All 14 patients who underwent plasma exchange had bilateral episodes, including 3 patients with simultaneous bilateral episodes. Of the 14 patients, 12 (85.7%) had seriously impaired residual visual function (visual acuity 0.1 or below), including 7 patients (50%) with 0.1 or below in both eyes. One patient in whom plasma exchange was selected during onset attack of optic neuritis showed poor visual recovery, and the other patients underwent plasma exchange because of poor visual recovery after corticosteroid pulse therapy at relapse of optic neuritis, and only two patients showed good visual outcome with final visual acuity of 1.2.
Follow-up for 2 years or longer was possible in 55 patients, 38 (69.1%) of whom experienced relapse during the follow-up period. The maintenance therapies at the final follow-up of the observation period in these 55 patients were as follows: low-dose corticosteroids (prednisolone 15 mg/day or methylprednisolone 8 mg/day or lower) in 36 patients (65.5%), low-dose corticosteroids combined with immunosuppressant in 11 patients (20.0%), immunosuppressant only in 3 patients (5.5%), and no medication in 5 patients (9.1%) (1 patient refused medication because of wishing to get pregnant, 1 patient refused medication because of old age and blindness in both eyes, 3 patients refused treatment itself). The immunosuppressants used were methotrexate in three patients, azathioprine in seven patients, tacrolimus in two patients, and tocilizumab in two patients.
Comparison of relapse rate between, with and without maintenance therapy
The status of relapse was analyzed by comparing group A with group B(pre) (Figure 1). Table 2 shows the demographic and clinical data of 15 patients in group A and 27 patients in group B (pre) who were followed for over 6 months and did not start maintenance therapy for at least 6 months after acute therapy for onset attack.
Table 2.
Demographic, clinical, and outcome data of patients with aquaporin-4 antibody positive optic neuritis analyzed for relapse.
| Maintenance therapy continued after acute therapy Group A |
Maintenance therapy not started for more than 6M after acute therapy Group B (pre) |
P value | |
|---|---|---|---|
| No. of patients | 15 patients | 27 patients | |
| Female/Male | 15/0 (100% female) | 25/2 (92.6% female) | NC(F) |
| Median age at onset (range) | 47.4 (18–71 years: median 53.0) years | 45.2 (8–72 years: median 48.0) years | NC(M) |
| Onset attack: | |||
| Optic neuritis | 14/15 patients (93.3%) | 25/27 patients (92.6%) | NC(F) |
| Transverse myelitis | 1/15 patients (6.7%) | 2/27 patients (7.4%) | NC(F) |
| Mean follow-up period (range) | 79.5 ± 45.7 (21–189) months | 78.1 ± 85.4 (6–381) months | NC(M) |
| Disease course: | |||
| Optic neuritis only (%) | 11/15 patients (73.3%) | 25/27 patients (92.6%) | NC(F) |
| Simultaneous bilateral episode | 4/15 patients (26.7%) | 6/27 patients (22.2%) | NC(F) |
Mann-Whitney U-test (M), Fisher test (F), NC: not significant.
There was no significant difference in mean follow-up duration between the two groups. In both groups, optic neuritis was the onset attack in the majority of patients and optic neuritis was the only symptom during the clinical course in most of the patients. The relapse rate was 0.08 ± 0.10 per year in group A and 0.39 ± 0.51 per year in group B(pre), and was significantly lower (p = 0.0020, Mann-Whitney U test) in group A that received maintenance therapy immediately after onset (Figure 2). Moreover, the total number of attacks during follow-up was 1.93 ± 1.58 in group A and 2.79 ± 1.60 in group B (pre), and was significantly smaller (p = 0.0221, Mann-Whitney U test) in group A (Figure 3).
Figure 2.
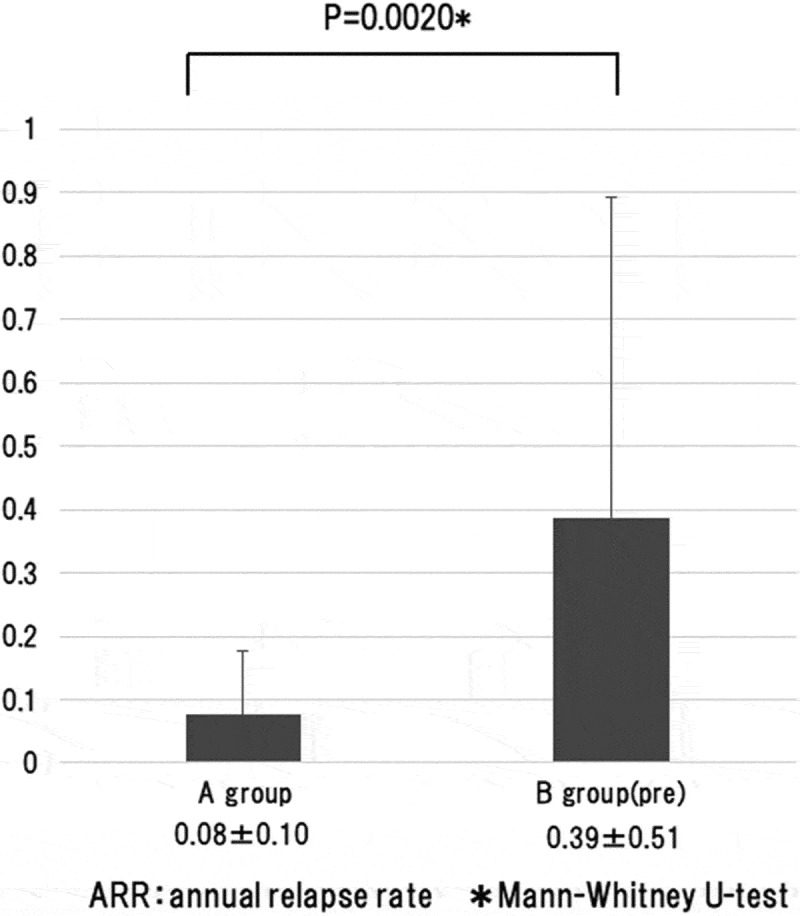
The annual relapse rate was analyzed by comparing group A with group B (pre). That was 0.08 ± 0.10 per year in group A and 0.39 ± 0.51 per year in group B (pre), and was significantly lower (p = 0.0020, Mann-Whitney U test) in group A that received maintenance therapy immediately after onset.
Figure 3.
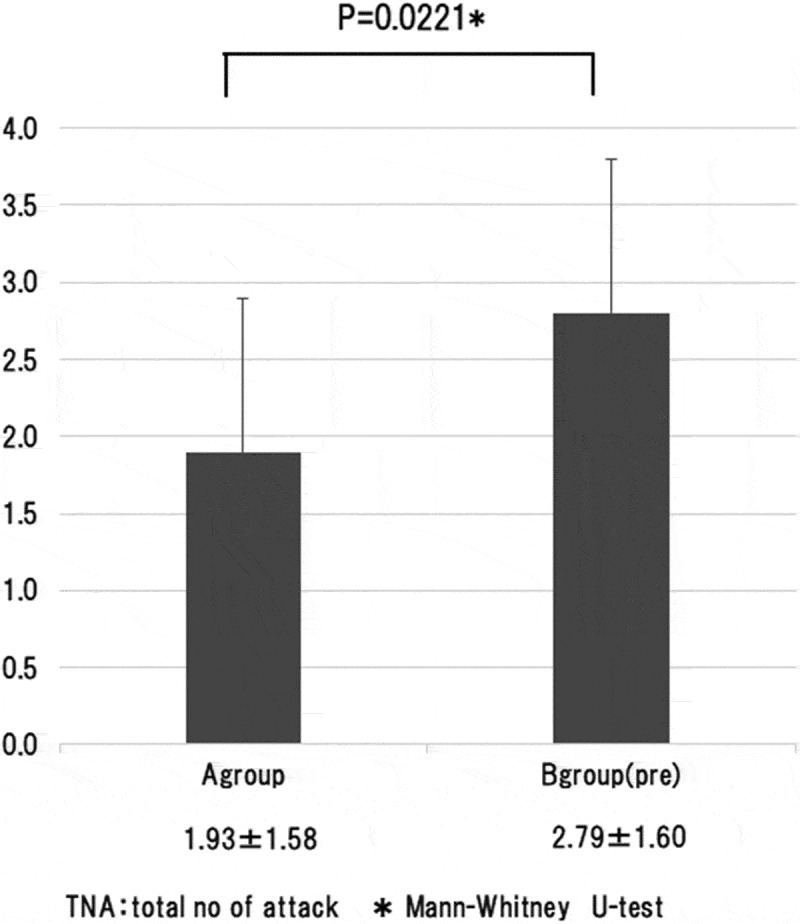
The total number of attacks during follow-up was analyzed by comparing group A with group B (pre). That was 1.93 ± 1.58 in group A and 2.79 ± 1.60 in group B (pre), and was significantly smaller (p = 0.0221, Mann-Whitney U test) in group A.
In group B, the status of relapse was compared before the start of maintenance therapy [group B(pre)] and after maintenance therapy was initiated [group B(post)]. The relapse rate was 0.39 ± 0.51 per year in group B(pre) and 0.05 ± 0.15 per year in group B (post), showing a significant decrease (P < 0.001, Wilcoxon test) in relapse rate after initiation of maintenance therapy even in the same patients.
Discussion
Clinical characteristics of all subjects
Anti-AQP4 antibody positive optic neuritis and NMO are considered not demyelinating diseases, but severe astrocytopathic disorders.3,4 Moreover, their clinical features include frequently bilateral disease, diverse age of onset including elderly onset, frequent relapses, and often refractory to mega-dose corticosteroids treatment, which are different from other types of optic neuritis.
In 2015, Wingerchuk et al.16 proposed new diagnostic criteria for neuromyelitis optica spectrum disorders (NMOSD). According to these diagnostic criteria, anti-AQP4 antibody positive optic neuritis is diagnosed as NMOSD even in the absence of myelitis or recurrent optic neuritis. On the other hand, in the case of anti-AQP4 antibody negative optic neuritis, cases with recurrent optic neuritis are included in NMOSD, but this group likely includes anti-myelin-oligodendrocyte glycoprotein (MOG) antibody positive cases. Therefore, our present results are restricted only to the cases of anti-AQP4 antibody positive optic neuritis.
Figure 4 summarizes the clinical feature and visual outcome of anti-AQP4 antibody positive optic neuritis reported in the literature.6,17–21 In the present study that examined a relatively large series of anti-AQP4 antibody positive optic neuritis in Japanese, the clinical picture also showed female predominance (90%), which is consistent with previous reports.5,6 Kitley et al.17 reported that Asian has older onset age compared to Caucasian and Afro-Caribbean, and that young-onset patients commonly present with optic neuritis while older patients are more likely to develop myelitis. In our series that contained a large proportion of onset attack of optic neuritis, the age of onset was the oldest compared to other reports.6,17–21 In anti-AQP4 antibody positive optic neuritis, altitudinal hemianopsia is observed at a relatively high frequency.22 When this occurs in the elderly, the condition may be clinically diagnosed as non-arteritic ischemic optic neuropathy, and blood collection for detailed investigations may not be done, consequently anti-AQP4 antibody may be overlooked.
Figure 4.
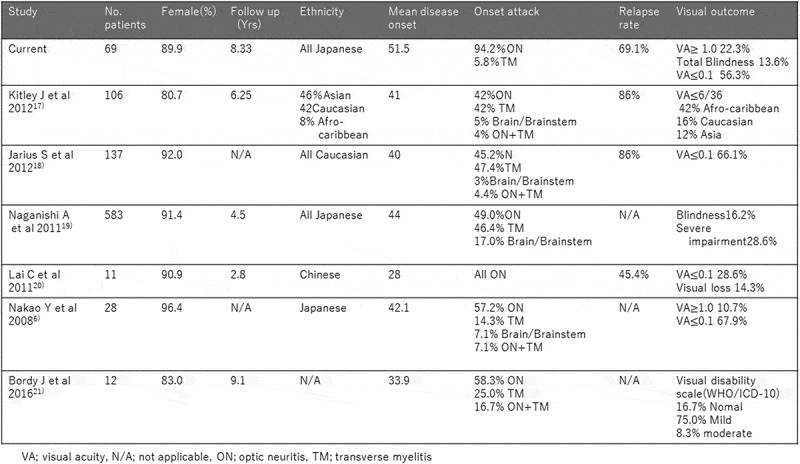
Studies detailing clinical features of aquaporin-4 antibody positive optic neuropathy.
On the other hand, in our cohort study, the vast majority (94.2%) of the patients had onset attack of optic neuritis, and as many as 73.9% of the patients manifested optic neuritis alone without other symptoms during the clinical course, and these characteristics may indicate a subcategory of optic neuritis. Since these patients had onset attack of optic neuritis and did not develop other neurological symptoms thereafter, many patients are probably followed only at the department of ophthalmology. From the viewpoint that the subjects in the present study did not represent the entire NMOSD, caution has to be exercised in interpreting the results. Nevertheless, this study indicates that neuro-ophthalmologists should be alerted for the existence of anti-AQP4 positive optic neuritis without myelitis.
In the analysis of visual outcome, 22.3% of 103 eyes regained normal vision (visual acuity of 1.0 or above) at the final follow-up. Brody et al.21 reported that 16.7% recovered normal vision, and Nakao et al.6 reported 10.7%. Compared to their results, the rate of recovering normal vision was similar to or slightly better than previous reports. However, in a study that examined the visual field defect in eyes with anti-AQP4 antibody positive optic neuritis, only 10% of the patients recovered normal visual field.22 Therefore, even though visual acuity may recover to normal level, visual field defect may remain. In addition, OCT studies have shown that the papillary retinal nerve fiber layer and the ganglion cell complex in the macular region are thinner compared to other types of optic neuritis.23 Since we did not evaluate visual field or OCT finding in the present study, the outcome of good visual acuity cannot be interpreted as full recovery of visual function. In this study, we did not investigate the status of optic disc edema, length of inflammation on MRI. However, studies of anti-AQP4 antibody positive optic neuritis have shown a high frequency of retrobulbar optic neuritis6 and long inflammation lesion on MRI, suggesting an association with the visual function outcome.16,24
In the present study, 56.3% of eyes that developed optic neuritis had final visual acuity of 0.1 or below, including total blindness in 13.6% and 0.1 or below in both eyes in 20.2%. Poor residual visual function was especially common in patients with simultaneous bilateral episodes, with 33.3% of the patients having 0.1 or below in both eyes. Visual outcome in previous report varies depending on race. Apart from Brody et al.21 who reported recovery of visual function (with only slight impairment of residual visual function) in 92% of the eyes, other reports indicated severe visual function impairment. In the study of Kitley et al.,17 which included Japanese patients, 16.2% were totally blind, and 28.6% had seriously impaired residual visual function. From our present findings and those of Kitley et al.,17 we can conclude that NMO has poor visual outcome also in Japanese patients.
In addition, when examining 84 eyes with documented visual acuity at recovery from the onset attack, 56.0% of the patients had visual acuity of 0.1 or below at recovery, including total blindness in 16.7%. The proportions of visual acuity of 0.1 or below and total blindness did not differ greatly at the last follow-up and at onset attack. These findings suggest that the onset attack with the patient in an untreated state tends to cause the most severe optic neuritis and is highly refractory to treatment. However, the subsequent maintenance therapy not only reduces the number of relapsing attacks, but may also reduce the severity of optic neuritis during relapse.
For NMO, corticosteroid pulse therapy (methylprednisolone 1000 mg for 3–5 days) is currently considered to be the first-line treatment in the acute phase.7,8 Following the pulse therapy, corticosteroids are commonly tapered (methylprednisolone, prednisone, or dexamethasone). Unlike MS and idiopathic optic neuritis, some cases of NMO do not respond or respond inadequately to corticosteroid pulse therapy. In these cases, plasma exchange has been reported to be effective. 9–13 In addition, a combination of corticosteroid pulse therapy and plasma exchange has been reported to be more effective than corticosteroid pulse or plasma exchange alone.25 Furthermore, high-dose γ globulin therapy26,27 has been shown to be effective in patients who respond poorly to corticosteroid pulse therapy.
In our study, plasma exchange was conducted in only 20.2% of the patients, mostly cases refractory to treatment, such as those with bilateral episodes or resistant to corticosteroids. Although there was a case that underwent plasma exchange at onset attack and achieved recovery of visual acuity to normal level, the number of cases in which plasma exchange was chosen as first-line treatment was limited, as described above. Further study is needed to examine which cases should be treated with steroid pulse therapy alone and which cases should be treated in combination with plasma exchange. The visual outcome was very poor in anti-AQP4 antibody positive cases in this study. Therefore, in cases suspected of anti-AQP4 antibody positive, such as those with bilateral episode showing poor visual function and severe cases with long inflammation of the optic nerve on MRI, active treatment with massive steroid pulse therapy (methylprednisolone 1000 mg for 5 days) and plasma exchange should be considered before the antibody result is known.
Comparison of relapse rate between, with and without maintenance therapy
Anti-AQP4 antibody positive optic neuritis has high relapse rates, which may cause severe impairment of visual function. Therefore, prevention of relapse is important. Various therapies have been used to prevent relapse of NMO, including low dose corticosteroids 28 and immunosuppressants (azathioprine,29,30 methotrexate,31,32 mitoxantrone,33,34 rituximab,35,36 eculizumab,37 tacrolimus,38 and mycophenolate mofetil39), all of which achieved reduction in relapse rate. In the present series of 55 patients who were followed for a relatively long period of 2 years or longer, the relapse rate was 69.1%. In this study, the most frequently used maintenance therapy was low-dose corticosteroids. This result indicates that the relapse prevention effect of maintenance therapy can be expected even with low-dose corticosteroids, as reported by Watanabe et al.28 On the other hand, the number of patients using immunosuppressants was small. Further long-term study with a large number of patients is required to compare corticosteroid + immunosuppressant combined therapy with steroid monotherapy, and to examine the efficacy of various immunosuppressants.
Our patients consisted of those who were diagnosed with anti-AQP4 antibody positive optic neuritis immediately after onset of optic neuritis and received maintenance therapy continuous from acute therapy (group A), and those who were diagnosed with optic neuritis before anti-AQP4 antibody was discovered and subsequently received maintenance therapy upon detection of anti-AQP4 antibody after a time lapse from onset and acute treatment (group B). To investigate the beneficial effect of continuous maintenance therapy from acute treatment, we compared the relapse rate between group A and the data before starting maintenance therapy in group B [group B (pre)].
The current treatment for anti-AQP4 antibody positive optic neuritis is generally acute therapy continuing on to maintenance therapy. Few patients do not receive maintenance therapy after acute treatment for optic neuritis. In addition, comparative study using controls with risk of relapse is difficult. Therefore, our research approach of comparing relapse rate during maintenance therapy in patients who received maintenance therapy continuous from acute treatment and the relapse rate before starting maintenance therapy in patients who did not start maintenance therapy for at least 6 months after acute treatment for onset attack may be acceptable.
Both the relapse rate per year and the total number of relapses during follow-up were significantly lower in group A than in group B. Moreover, in group B, the relapse rate was significantly lower after initiation of maintenance therapy [group B (post)] compared to before maintenance therapy [group B (pre)] even in the same patients. These results together indicate that maintenance therapy is essential for prevention of relapse. Kitley et al.17 also reported that relapse occurs at a rate of 49% within one year, and 70% within two years after disease onset, but maintenance therapy after the onset attack can delay the relapse.
Conclusion
Anti-AQP4 antibody positive optic neuritis is a severe optic neuritis with poor visual outcome. Anti-AQP4 antibody testing should be conducted from the time of onset attack of optic neuritis. Conducting appropriate acute-phase treatment and continuous transition from acute therapy to maintenance therapy are important.
Funding Statement
This work was not funded.
Disclosure Statement
The authors report no conflicts of interest. The authors are solely responsible for the contents and writing of the article.
References
- 1.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 2.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR.. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucchinetti CF, Guo Y, Popescu BF, Fujihara K, Itoyama Y, Misu T. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol. 2014;24:83–97. doi: 10.1111/bpa.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujihara K. Neuromyelitis optica and astrocytic damage in its pathogenesis. J Neurol Sci. 2011;306:183–187. doi: 10.1016/j.jns.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Takagi M, Tanaka K, Suzuki T, Miki A, Nishizawa M, Abe H. Anti-aquaporin-4-antibody-positive optic neuritis. Acta Ophthalmol. 2009;87:562–566. doi: 10.1111/j.1755-3768.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakao Y, Yamamoto H, Arimura E, et al. Clinical features of anti-aquaporin 4 antibody-positive optic neuritis in Japanese. Shinkei Ganka. 2008;25:327–342. [Google Scholar]
- 7.Trebst C, Jarius S, Berthele A, et al. Neuromyelitis Optica Study Group (NEMOS). Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol. 2014;261:1–16. doi: 10.1007/s00415-013-7161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowarik MC, Soltys J, Bennett JL. The treatment of neuromyelitis optica. J Neuroophthalmol. 2014;34:70–82. doi: 10.1097/WNO.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Kim W, Huh SY, Lee KY, Jung IJ, Kim HJ. Clinical efficacy of plasmapheresis in patients with neuromyelitis optica spectrum disorder and effects on circulating anti-aquaporin-4 antibody levels. J Clin Neurol. 2013;9:36–42. doi: 10.3988/jcn.2013.9.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe S, Nakashima I, Misu T, Miyazawa I, Shiga Y, Fujihara K, Itoyama Y. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler. 2007;13:128–132. doi: 10.1177/1352458506071174. [DOI] [PubMed] [Google Scholar]
- 11.Bonnan M, Cabre P. Plasma exchange in severe attacks of neuromyelitis optica. Mult Scler Int. 2012;2012:787630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnan M, Valentino R, Olindo S, Mehdaoui H, Smadja D, Cabre P. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult Scler. 2009;15:487–492. doi: 10.1177/1352458508100837. [DOI] [PubMed] [Google Scholar]
- 13.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–146. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, Watanabe S, Shiga Y, Kanaoka C, Fujimori J, Sato S, Itoyama Y. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Tani T, Tanaka M, et al. Anti-aquaporin 4 antibody in selected Japanese multiple sclerosis patients with long spinal cord lesions. Mult Scler. 2007;13:850–855. doi: 10.1177/1352458507076976. [DOI] [PubMed] [Google Scholar]
- 16.Wingerchuk DM, Banwell B, Bennett JL, et al. International panel for NMO diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitley J, Leite MI, Nakashima I, Waters P, McNeillis B, Brown R, Takai Y, Takahashi T, Misu T, Elsone L, Woodhall M, George J, Boggild M, Vincent A, Jacob A, Fujihara K, Palace J. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain. 2012;135:1834–1849. doi: 10.1093/brain/aws109. [DOI] [PubMed] [Google Scholar]
- 18.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaishi A, Takagi M, Umemura A, Tanaka M, Kitagawa Y, Matsui M, Nishizawa M, Sakimura K, Tanaka K. Clinical features of neuromyelitis optica in a large Japanese cohort: comparison between phenotypes. J Neurol Neurosurg Psychiatry. 2011;82:1360–1364. doi: 10.1136/jnnp-2011-300403. [DOI] [PubMed] [Google Scholar]
- 20.Lai C, Tian G, Takahashi T, Liu W, Yang L, Zhang X. Neuromyelitis optica antibodies in patients with severe optic neuritis in China. J Neuroophthalmol. 2011;31:16–19. doi: 10.1097/WNO.0b013e3181f8a693. [DOI] [PubMed] [Google Scholar]
- 21.Brody J, Hellmann MA, Marignier R, Lotan I, Stiebel-Kalish H. Neuromyelitis optica spectrum disorder: disease course and long-term visual outcome. J Neuroophthalmol. 2016;36:356–362. doi: 10.1097/WNO.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 22.Yamagami A. Investigation of the visual field defects pattern of anti-aquaporin 4 antibody optic neuriti. Shinkei Ganka. 2015;32:135–141. [Google Scholar]
- 23.Bennett JL, de Seze J, Lana-Peixoto M, Palace J, Waldman A, Schippling S. Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler. 2015;21:678–688. doi: 10.1177/1352458514567216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna S, Sharma A, Huecker J, Gordon M, Naismith RT, Van Stavern GP. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica multiple sclerosis. J Neuroophthalmol. 2012;32:216–220. doi: 10.1097/WNO.0b013e318254c62d. [DOI] [PubMed] [Google Scholar]
- 25.Merle H, Olindo S, Jeannin S, Valentino R, Mehdaoui H, Cabot F, Donnio A, Hage R, Richer R, Smadja D, Cabre P. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol. 2012;130:858–862. doi: 10.1001/archophthalmol.2012.1126. [DOI] [PubMed] [Google Scholar]
- 26.Feasby T, Banwell B, Benstead T, Bril V, Brouwers M, Freedman M, Hahn A, Hume H, Freedman J, Pi D, Wadsworth L. Guidelines on the use of intravenous immune globulin for neurologic conditions. Transfus Med Rev. 2007;21:S57–107. doi: 10.1016/j.tmrv.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Nakao Y, Nakamura Y, Aomatsu K, Hirano M, Sakaoto H. Intravenous immunoglobulin treatment in corticosteroid refractory anti-aquaporin 4 antibody-seropositive optic neuritis. Shinkei Ganka. 2012;29:424–433. [Google Scholar]
- 28.Watanabe S, Misu T, Miyazawa I, Nakashima I, Shiga Y, Fujihara K, Itoyama Y. Low-dose corticosteroids reduce relapses in neuromyelitis optica: a retrospective analysis. Mult Scler. 2007;13:968–974. doi: 10.1177/1352458507077189. [DOI] [PubMed] [Google Scholar]
- 29.Costanzi C, Matiello M, Lucchinetti CF, Weinshenker BG, Pittock SJ, Mandrekar J, Thapa P, McKeon A. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77:659–666. doi: 10.1212/WNL.0b013e31822a2780. [DOI] [PubMed] [Google Scholar]
- 30.Elsone L, Kitley J, Luppe S, et al. Long-term efficacy, tolerability and retention rate of azathioprine in 103 aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder patients: a multicentre retrospective observational study from the UK. Mult Scle. 2014;20:1533–1540. doi: 10.1177/1352458514525870. [DOI] [PubMed] [Google Scholar]
- 31.Kitley J, Elsone L, George J, Waters P, Woodhall M, Vincent A, Jacob A, Leite MI, Palace J. Methotrexate is an alternative to azathioprine in neuromyelitis optica spectrum disorders with aquaporin-4 antibodies. J Neurol Neurosurg Psychiatry. 2013;84:918–921. doi: 10.1136/jnnp-2012-304774. [DOI] [PubMed] [Google Scholar]
- 32.Ramanathan RS, Malhotra K, Scott T. Treatment of neuromyelitis optica/neuromyelitis optica spectrum disorders with methotrexate. BMC Neurol. 2014;14:51. doi: 10.1186/s12883-014-0196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Kim W, Park MS, Sohn EH, Li XF, Kim HJ. Efficacy and safety of mitoxantrone in patients with highly relapsing neuromyelitis optica. Arch Neurol. 2011;68:473–479. doi: 10.1001/archneurol.2010.322. [DOI] [PubMed] [Google Scholar]
- 34.Weinstock-Guttman B, Ramanathan M, Lincoff N, Napoli SQ, Sharma J, Feichter J, Bakshi R. Study of mitoxantrone for the treatment of recurrent neuromyelitis optica (Devic disease). Arch Neurol. 2006;63:957–963. doi: 10.1001/archneur.63.7.957. [DOI] [PubMed] [Google Scholar]
- 35.Kim SH, Huh SY, Lee SJ, Joung A, Kim HJ. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110–1117. doi: 10.1001/jamaneurol.2013.3071. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg BM, Graves D, Remington G, Hardeman P, Mann M, Karandikar N, Stuve O, Monson N, Frohman E. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler. 2012;18:1022–1026. doi: 10.1177/1352458511432896. [DOI] [PubMed] [Google Scholar]
- 37.Pittock SJ, Lennon VA, McKeon A, Mandrekar J, Weinshenker BG, Lucchinetti CF, O'Toole O, Wingerchuk DM. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 2013;12:554–562. doi: 10.1016/S1474-4422(13)70076-0. [DOI] [PubMed] [Google Scholar]
- 38.Zheng X, Zhang X, Liu X, Mu W, Yang W, Liu Y, Ge P, Li H. Patient with neuromyelitis optica spectrum disorder combined with Sjögren’s syndrome relapse free following tacrolimus treatment. Intern Med. 2014;53:2377–2380. [DOI] [PubMed] [Google Scholar]
- 39.Jacob A, Matiello M, Weinshenker BG, Wingerchuk DM, Lucchinetti C, Shuster E, Carter J, Keegan BM, Kantarci OH, Pittock SJ. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch Neurol. 2009;66:1128–1133. doi: 10.1001/archneurol.2009.175. [DOI] [PubMed] [Google Scholar]


