ABSTRACT
Plants face various stresses during the growth and development processes. The specific transcription factors bind to the cis-acting elements upstream of the stress resistance genes, specifically regulating the expression of the gene in plants and increasing the adaptability of plants to environmental stress. The transcription factor-mediated gene expression regulatory networks play an important role in plant stress response pathways. MYB (v-myb avian myeloblastosis viral oncogene homolog) transcription factor is one of the largest members of the transcription factor family in plants. It participates and has a great influence on all aspects of plant growth and development. It plays an important role in plant secondary metabolic regulation, hormone and environmental factor responses, cell differentiation, organ morphogenesis, and cell cycle regulation. This review mainly introduces the characteristics, structure, and classification of MYB transcription factors, as well as the abiotic stress resistance to drought, salt, temperature, and other functions in breeding, and provides a reference for the research and utilization of transcription factors in the future.
KEYWORDS: MYB transcription factor, drought stress, salt stress, temperature stress, breeding
Introduction
Transcription factors, also known as trans-acting factors, usually refer to a class of proteins encoded by genes that specifically bind to relevant cis-acting elements in the promoter region of a gene to activate gene expression.1 The regulation of target gene expression at the transcriptional level is an important way for plants to grow and develop physiologically. The specific expression of genes is regulated by a single regulatory factor and is also controlled by multiple regulatory factors. In the plant stress response system, the regulation of functional gene expression by transcription factors is a key link in plant stress response.2,3
From the perspective of protein structure, the transcription factor consists of four parts: DNA binding region, transcriptional regulatory domain, oligomerization site, and nuclear localization signal. These functional regions determine the structure and characteristics of transcription factors.4,5 Transcription factors can be divided into a number of different families according to the specificity of the DNA binding region, and there are mainly four types related to plant stress resistance: bZIP, WRKY, AP2/ERF, and MYB.6 MYB transcription factors are widely distributed in higher plants, and they are the most abundant and most functional in the family of transcription factors, and play the most important role in plant response to stress resistance. Functional studies have shown that MYB is involved in plant secondary metabolism,7 hormone, and environmental factor responses, and plays an important regulatory role in cell differentiation, cell cycle, and leaf morphogenesis.8-14
The MYB transcription factor was first discovered in the avian leukosis virus and was named “v-MYB” virus.15 In higher plants, the first cloned MYB transcription factor from gramineous plants was ZmMYBC1 gene. This gene mainly regulates the biosynthesis of corn anthocyanins.16 The complete MYB consists of a three-part structure: the DNA structure binding region (DBD), the transcriptional activation domain, and the negative regulatory region.17,18
DBD is the most conservative and generally contains 1–3 incomplete repeats (R), represented by R1, R2, and R3. Each repeat region R consists of 51–52 conserved amino acid residues and spacer sequences, each containing about one tryptophan residue or other hydrophobic groups in about 18 amino acids, which form a helix-turn-helix (HTH) structure. A three-dimensional core structure is formed centering on the tryptophan residue, and tryptophan acts as a hydrophobic core, which is important for maintaining the configuration of HTH.19 Based on the number of MYB domains, the MYB family can be divided into four classes, 1R-, R2R3-, 3R-, and 4R-MYB proteins (Figure 1).20 The R2R3-MYB protein is specific for plants and is the most abundant species in plants, with more than 100 R2R3 MYB members in the genomes of dicots and monocots.
Figure 1.
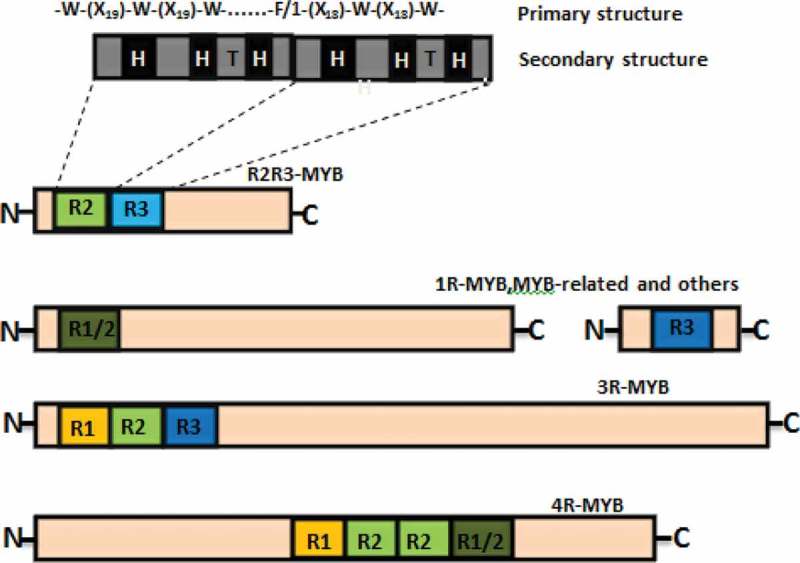
Classification of plant MYB transcription factors.
Abiotic stresses such as drought, high salt, low temperature, and high-temperature limit plant growth and development. The ability of plants to resist abiotic stress is inseparable from the expression of transcription factor-regulated stress-related functional genes. MYB transcription factors are widely found in plants and are involved in almost all aspects of plant development and metabolism. The MYB transcription factor can control the morphology and pattern of the cells. When plants are stressed by the external environment, they can effectively regulate the expression of related functional genes in the body through their signaling pathways, and then stimulate a series of physiology. Biochemical reactions form an efficient and orderly signal conditioning network to reduce or eliminate the damage to plants. This response of plants is a complex process involving multiple genes, multiple signaling pathways, and multiple gene products. Transcriptional control plays a leading role in the response of plants to environmental stress.
MYBs in plant resistance to drought stress
Drought stress is the main factor affecting biological development, which severely limits plant growth and reduces crop yield and quality.21 Under drought stress, the roots of the plants lose water and cannot provide sufficient water supply for the growth of the plants. The stomas on the surface of the leaves are closed, the absorption of CO2 is reduced, resulting in a decrease in photosynthesis rate. The production of reactive oxygen species (ROS) in plants and the homeostasis between their own antioxidant systems is disrupted, and cells are subjected to oxidative stress, which damages the membrane system. The permeability of the membrane is increased, the ions are leaked, the selection of the cell membrane is lost, and the cell structure is destroyed. At the same time, the increase of active oxygen also increases the activity of hydrolase, the activity of synthetase decreases, the synthesis of protein is blocked, the imbalance of material and energy metabolism in plants, which adversely affects the growth and development of plants.22-26
The study found that most of the MYB transcription factors are involved in the response of plants to drought stress. There are more than 198 MYB genes in Arabidopsis thaliana and more than 183 genes in rice.27 A large number of MYB genes involved in abiotic stress belong to the R2R3 type group and the R1R2R3-MYB group.28 Studies have shown that under stress, MYB transcription factors bind to MYB binding elements in the promoter regions of many functional genes to activate the expression of stress-responsive genes. For example, AtMYB2 and AtMYB60 of Arabidopsis thaliana have been shown to be involved in drought tolerance stress in plants.9,29 Overexpression of Arabidopsis thaliana AtMYB2 increased expression in ABA-mediated drought stress response and caused high-efficiency expression of various stress-inducing genes such as drought response genes RD22 and ADH1 in transgenic plants (Figure 2).30,31 AtMYB60 and AtMYB96 regulate stomatal movement, drought stress, and disease resistance through ABA signaling cascades. The expression of AtMYB60 is rapidly down-regulated by ABA and dehydration stress, and the transcriptional activation of epidermal wax biosynthesis by AtMYB96 contributes to the drought resistance of Arabidopsis thaliana.32
Figure 2.

A model for the induction of the rd22 gene under water stress conditions.
Ectopic expression of the MdSIMYB1 gene in apples enhances plant tolerance to drought and other abiotic stresses, mainly by activating the up-regulated stress response genes NtDREB1A, NtERD10B and NtERD10C.33 Overexpression of the stress-responsive PtsrMYB gene isolated from Poncirus trifoliata enhances plant tolerance to drought. Overexpression of PtsrMYB can interact with the promoter of PtADC, improve polyamine biosynthesis, less water loss, lower levels of MDA and reactive oxygen species, and increased plant drought tolerance.34 The GmMYBJ1 protein isolated from soybean is targeted to the nucleus, and the expression of GmMYBJ1 induced by abiotic stress reflects enhanced tolerance to abiotic stress.35 A rice-associated gene OsMYB48-1 can be induced by various abiotic stresses. Many ABA-related genes are up-regulated in OsMYB48-1 overexpressing plants, and transgenic plants show resistance to drought and high salt stress.36 Studies have shown that BcMYB1 in the Sphagnum moss is strongly induced by drought, and can produce a certain degree of response to stress such as PEG, high salt and low temperature, but exogenous abscisic acid (ABA) and MDA. Under the treatment of salicylic acid (SA), the expression level is very low, indicating that it may participate in the regulation of gene expression through an ABA-independent pathway to respond to drought.37 In potato, a single MYB domain TF has been shown to be involved in drought tolerance by activating drought-related genes in StMYB1R-1.38 Overexpression of single-repeat MYB TF AmMYB1 from gray mangroves confers tolerance to transgenic tobacco.39
MYBs in plant resistance to salt stress
When plants are exposed to high salt environments, excess soluble salts in the soil are toxic to most plants. Na and Ca salts are more harmful to plants, especially Na salts. Ions from the external environment accumulate in the cytoplasm and have many negative effects on metabolism. Numerous studies have shown that salt stress can affect plant photosynthesis,40-47 related enzyme denaturation inactivation, increase respiratory consumption, accumulate a large number of toxic metabolites, affect the synthesis of assimilation products, and ultimately leading to plant death. Under salt stress, the roots of the plant absorb water difficulty, and the water infiltration in the body reduces the supply of water in the plant, causing cell dehydration and threatening the survival of the plant,48,49 which seriously affects normal growth.50 When plants are subjected to salt stress, the ion dynamic balance of the plants is disturbed,51-57 and high salt seriously affects the membrane permeability,58-61 and the absorbed salt ions are toxic to plants62. Excessive salinity leads to osmotic stress in cells, destroying normal metabolism and seed germination.63-70 In China, about 100 million hectares of land have become salted land. Halophytes can grow in high-salt environments, mainly through dilute salts, salt secretion, and salt rejection to avoid salt damage, so halophytes can be used to improve saline-alkali soils.71-79 Certain genes,80-81 enzymes,82-85 hormones, and ions86-88 are involved in the salt stress response of plants.
The salt-tolerant and drought-tolerant regulation of MYB transcription factors involved in the ABA signaling pathway has different degrees of intersection with other signal transduction pathways in plants. When plants are subjected to salt stress, the ABA content is significantly increased, and the salt tolerance of plants is enhanced by signal transduction and induction of transcriptional levels and up-regulation of related genes. The specific effects are to alleviate osmotic stress and ionic stress caused by excessive salt, maintain water balance, and maintain cell membrane structure integrity.89 At the same time, under salt stress, ABA can induce the accumulation of proline in the plant, and enhance the activity of related protective enzymes.90 Studies on the abscisic acid receptor in Arabidopsis thaliana indicate that it is rapidly regulated by PYR/PYL/RCARs, protein phosphate 2C (PP2Cs) and SNF1-related protein kinase 2s (nRK2s).91 Studies on the abscisic acid receptor in Arabidopsis thaliana indicate that it is induced by PYR/PYL/RCARs, protein phosphate 2C (PP2Cs), and SNF1-related protein kinase 2s (nRK2s).92 When plants are subjected to salt stress, ABA rapidly synthesizes and binds to PYR/PYL and PP2C, then enhances the activity of SnRK2, phosphorylates the bZIP transcription factor, and binds bZIP to the response element AREB. The final ABA response gene is activated and expressed.93,94 For example, the R2R2-MYB transcription factor AtMYB41 can not only respond to ABA, drought, and salt stress, but also affect cell wall development.95 The R2R3-MYB salt stress-responsive transcription factor OsMPS plays a role in the regulation of plant hormones and cell wall synthesis in rice.96 The involvement of the MYB transcription factor in the hormone synthesis pathway plays an important role in enhancing plant salt tolerance.
AtMYB44 and AtMYB2 in Arabidopsis thaliana responded to salt stress at mRNA level, and overexpression of AtMYB44 and AtMYB2 in transgenic Arabidopsis thaliana could increase the resistance of transgenic plants to salt stress.97,98 AtMYB41 and AtMYB96 genes are not expressed under normal growth conditions but can be induced by high levels drought, ABA, and salt stress.99,100 The expression of four R2R3-MYB genes, AhMYBl, AhMYB2, AhMYB6, and AhMYB7 in peanuts increased significantly under salt stress; the mRNA abundance of four MYB-related genes, AhMYBl2, AhMYBl8, AhMYB28, and AhMYB30 increased,101 indicating that at least eight MYB genes in peanut are induced by salt stress. The R2R3-MYB genes TaMYB32, TaMYB33, TaMYB56-B, and TaMYB73 are all induced by salt stress in wheat, and the salt tolerance of the transgenic Arabidopsis thaliana plants overexpressing these genes is enhanced.102-105 Some R2R3-MYB genes in other plants, such as rice OsMYB2, apple MdSIMYB1, sugarcane PScMYBAS1, and Phyllostachys pubescens PeMYB2, can increase salt tolerance of transgenic plants.106,107 In addition, a 3R-MYB gene OsMYB3R-2 in rice is also involved in salt stress regulation.108 OsMYB48-1 (Figure 3) is an MYB-related gene and is slightly induced by salt stress, but overexpression of this gene in rice can significantly increase salt tolerance of the transgenic plants.36
Figure 3.

Proposed model revealing the function of OsMYB48-1 in the regulation of drought and salt stress.
MYBs in plant resistance to temperature stress
Temperature is the key environmental factors and can affect plant yield, growth and geographical distribution. Temperatures that are too high or too low can adversely affect plant growth and development. The response of plants to temperature is mainly manifested in physiological, molecular and biochemical levels.109 Continued cold stress reduces plant water uptake and affects cell membrane systems as well as chlorophyll synthesis, photosynthesis, and other processes. Under low-temperature stress, the oxygen absorption rate is reduced, and excess oxygen is converted into active oxygen, causing a series of physiological and biochemical changes in plants. At low temperatures, regulatory factors involved in related signal transduction and expression patterns of functional genes are adversely altered.110-112 High-temperature stress can also cause damage to the physiological metabolism and growth of plants. The stability of biofilms in plants under high-temperature stress, the balance between oxides and antioxidants, the metabolism of amines, photosynthesis, changes in heat shock proteins, and the mechanism of their heat resistance are destroyed. The transpiration and the loss of water affects the activity of key enzymes and affects photosynthesis and growth and metabolism of plants.113
Under low-temperature stress, the expression of plant MYB transcription factors also has outstanding research results. After 50 days of low-temperature treatment of transgenic soybean and wild-type soybean, the survival rate of GmMYB76 and GmMYB177 transgenic plants is much higher than that of wild type, and the proline of transgenic plants is significantly increased, indicating GmMYB76 and GmMYB1 genes in soybean participated in low-temperature response.114 In rice, OsMYB4 is previously reported to have a positive effect on cold and drought tolerance in transgenic Arabidopsis thaliana, tomato, and apple.115-117 In a recent study, the molecular characteristics of OsMYB2 clearly demonstrated its regulation in low-temperature tolerance in rice.106 OsMYB3R-2 is involved in the cold signaling pathway by targeting the cell cycle and putative DREB/CBF.118 In addition, a recent study showed that OsMYBS3 is critical for conferring tolerance to cold stress in rice.108 In Arabidopsis thaliana, MYB15 interacts with ICE1 and binds to the MYB binding site of the CBF1, 2, 3 promoters. At low temperatures, the transcription levels of CBF3, CBF2, and CBF1 in overexpressing MYB15 plants decrease. The low-temperature tolerance of MYB15 overexpressing plants is weakened, indicating that MYB15 negatively regulates the expression of the CBF gene and thus controls the low-temperature tolerance of plants.119
The MYB68 gene in Arabidopsis thaliana was specifically expressed in the root sheath cells. Under high-temperature stress, the activity of MYB68 in roots was significantly enhanced, and the growth of MYB68 mutant was lower than that of wild type, indicating that MYB68 is involved in Arabidopsis thaliana response to high-temperature stress.120 OsMYB55 exhibits tolerance to high temperatures through enhanced amino acid metabolism.121
MYBs in plant breeding
Drought, soil salinization, low temperature, and high temperature are the limiting factors that restrict plant growth and development. MYB transcription factors are widely found in plants, involved in cell differentiation, cell cycle regulation, hormone and environmental factor responses, plant development, and metabolism. In all aspects, it plays an important role in regulating plant secondary metabolism and morphogenesis of leaves and other organs. Therefore, it is more effective to improve or enhance the stress resistance of a key transcription factor. Methods and approaches have important guiding significance for breeding.
Recent studies have shown that MYBs are involved in the regulation of plant anthocyanidogenesis and play an important role in the coloration of peels, flesh, and even leaves.122 MYB transcription factors can control the morphology and pattern of cells. A series of genes related to root hair development, such as TTG, CPC, WER, and GL2, were isolated from Arabidopsis thaliana. Two of the MYB transcription factors, CPC and WER, are key genes in the development of root epidermal cells.123 Overexpression of AtMYB24 gene in Arabidopsis thaliana causes the plant to be short and the flower organs are poorly developed, the anthers are not cracked, and the pollen is inactive.124 MYB transcription factors are also involved in the metabolism of plant sub-products. In recent years, a series of MYB transcription factors have been found to be involved in the regulation of plant flavonoid synthesis pathways, which in turn affect the coloration of plant flower organs and fruits.125 MYB transcription factors play an important role in the secondary transcriptional regulation of secondary wall synthesis. At present, many major secondary wall synthesis-related MYB transcription factors have been isolated and identified. AC cis-acting elements are important elements for the binding of MYB transcription factors to lignin-synthesizing genes. Therefore, the secondary growth regulation network is elucidated by genetic engineering. Realize the genetic improvement of a specific synthetic pathway of the secondary wall, and improve the application efficiency of the forest in the paper industry and renewable bioenergy.126 During the process of plant microsporogenesis and the development of male gametophyte, MYB transcription factor promotes the development of microspore mother cells into normal fertile pollen by regulating the expression of related functional genes.127
Some genes of the MYB gene family in grape are involved in regulating the differentiation of floral organs. It is a candidate gene regulating the sex differentiation of flower organs. VlMYBA1-3 transcription factor was found to be closely related to flower organ or skin coloration.128 Two MYB genes, LHMYB6 and LHMYB12, were isolated from wild lily, and they have homologous genes with petunia. Yeast two-hybrid showed that they interacted with LhbHLH2 protein, LHMYB12 coordinated well with anthocyanin staining in petals, filaments, and styles, whereas LHMYB6 cooperated with petal spots and pigment accumulation in leaves. The discovery of these functions has brought new references and help to the breeding of plant coloring such as fruit trees, vegetables, and flowers.129
Outlook
Abiotic stresses such as drought, high salt, low temperature, and high temperature limit the growth of plants and affect agricultural production. The ability of plants to resist abiotic stress is inseparable from the expression of stress-regulated genes related to transcription factors. MYB transcription factors are widely distributed in plants and are involved in the regulation of various stages of plant growth and development. The function of the MYB transcription factor also appears to be diversified during evolution. When a plant suffers from adverse damage, the plant can effectively regulate the expression of related genes through signal transduction, which in turn triggers a series of physiological and biochemical reactions and reduces damage to plants. The response process of plants is a complex process involving multiple genes, multiple signaling pathways, and multiple gene products. The tolerance to drought, high salt, and temperature stress often does not depend on a single factor but is controlled by multiple factors. The internal members of the MYB transcription factor family have a certain correlation in structure and function, and the biological functions of unknown MYB transcription factors are predicted by analyzing their protein structure and characterization patterns. However, studying individual genes in isolation and their expression does not fully reflect the underlying laws. So far, the study of MYB transcription factors has focused on the regulation of single stress conditions, and the mechanism of its regulation in the interaction of different signaling pathways has not been studied in depth. The function of most MYB transcription factors is redundant, and traditional molecular biology methods are difficult to reveal its function and regulation mechanism. With the development of CRISPR/Cas9 and other technologies, the research of MYB transcription factor regulatory network will become a hot issue.
The MYB transcription factor not only clarifies the molecular mechanism of action but also shows a good application prospect. The use of transcription factors to improve and enhance the comprehensive resistance of plants has become a promising approach. By enhancing the role of some key MYB transcription factors to promote these anti-reverse gene resources, the comprehensive and fundamental improvement of plant stress resistance is of great significance to improve the plant‘s ability to resist environmental stress. Further research on the regulation mechanism of MYB transcription factors located downstream of the signaling pathway in the interaction of multiple signaling pathways in plants is of great value for understanding the abiotic stress response process of plants.
Funding Statement
This work was support from Major Program of Shandong Provincial Natural Science Foundation (2017C03), Shandong Provincial Natural Science Foundation (ZR2016JL028). The Opening Foundation of Shandong Provincial Key Laboratory of Crop Genetic Improvement, Ecology and Physiology (SDKL2018008-3)
Acknowledgments
We are grateful for financial support from Major Program of Shandong Provincial Natural Science Foundation (2017C03), Shandong Provincial Natural Science Foundation (ZR2016JL028). The Opening Foundation of Shandong Provincial Key Laboratory of Crop Genetic Improvement, Ecology and Physiology (SDKL2018008-3).
Author Contributions
Jinlu Li and Guoliang Han wrote the manuscript. Na Sui and Cuifeng Sun modified the article. All authors read and approved the final manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zhang C, Long Y, Ji F, Meng J.. Transcriptional regulation of plant genes and its significance in biology. J Hereditas. 2007;29:793–799. doi: 10.1360/yc-007-0793. [DOI] [PubMed] [Google Scholar]
- 2.Honma T, Goto K.. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature. 2001;409:525–529. doi: 10.1038/35054083. [DOI] [PubMed] [Google Scholar]
- 3.Krizek B, Meyerowitz E. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development. 1996;122:11–22. [DOI] [PubMed] [Google Scholar]
- 4.Meshi T, Iwabuchi M. Plant transcription factors. J Plant Cell Physiol. 1995;36:1405–1420. [PubMed] [Google Scholar]
- 5.Chen Q, Tang H, Dong X, Hong Y, Hou Y, Lou Y, Jiang Y, Huang Q. Progress in the study of plant Myb transcription factors. J Genom Appl Biol. 2009;28:365–372. [Google Scholar]
- 6.Liu L, Du H, Tang X, et al. The roles of MYB transcription factors on plant defense responses and its molecular mechanism. J Hereditas (Beijing). 2008;30:1265–1271. doi: 10.3724/SP.J.1005.2008.01265. [DOI] [PubMed] [Google Scholar]
- 7.Uimari A, Strommer J. Myb26: a MYB‐like protein of pea flowers with affinity for promoters of phenylpropanoid genes. Plant J. 1997;12:1273–1284. doi: 10.1046/j.1365-313x.1997.12061273.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Peng S, Huang G, Wu K, Fu X, Chen Z.. Association of decreased expression of a Myb transcription factor with the TPD (tapping panel dryness) syndrome in Hevea brasiliensis. J Plant Mol Biol. 2003;51:51–58. doi: 10.1023/A:1020719420867. [DOI] [PubMed] [Google Scholar]
- 9.Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennisl ES. Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. J Genet. 1998;149:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lea US, Slimestad R, Lillo SC. Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. J Planta. 2007;225:1245–1253. doi: 10.1007/s00425-006-0414-x. [DOI] [PubMed] [Google Scholar]
- 11.Suo J, Liang X, Pu L, Zhang Y, Xue Y. Identification of GhMYB109 encoding a R2R3 MYB transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L.). J Biochimica Et Biophysica Acta. 2003;1630:25–34. doi: 10.1016/j.bbaexp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee MM, Schiefelbcin J. Cell pattern in the Anbidopsis root epidermis determined by lateral inhibition with feedback. Plant Cell. 2002;14(61):l–618. doi: 10.1105/tpc.010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang XY, Li JG, Pei M, Gu H, Chen ZL, Qu LJ. Over-expression of a flower-specific transcription factor gene causes aberrant anther development. J Plant Cell Rep. 2007;26:219–228. doi: 10.1007/s00299-006-0229-z. [DOI] [PubMed] [Google Scholar]
- 14.Klempnauer KH, Gonda TJ, Michael Bishop J. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. J Cell. 1982;31:453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- 15.Paz-Ares J. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. Embo J. 1987;6:3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frampton J. Myb transcription factors: their role in growth, differentiation and disease. J Proteins Cell Regul. 2004;2:1–2. [Google Scholar]
- 17.Thompson MA, Ramsay RG. MYB: an old oncoprotein with new roles. J Bio Essays. 1995;17:341–350. doi: 10.1002/(ISSN)1521-1878. [DOI] [PubMed] [Google Scholar]
- 18.Ogata K, KANEI ISHII C, Sasaki M, Hatanaka H, Nagadoi A, Enari M, Nakamura H, Nishimura Y, Ishii S, Sarai A.. The cavity in the hydrophobie core of Myb DNA binding domain is reserved for DNA recognition and trans—activation. J Nat Struct Biol. 1996;3:178–187. doi: 10.1038/nsb0296-178. [DOI] [PubMed] [Google Scholar]
- 19.Ralf Stracke MW, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. J Curr Opin Plant Biol. 2001;4:447–456. doi: 10.1016/S1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- 20.Martin C. MYB transcription factors in plants. Trends Genet. 1997;13:67–73. doi: 10.1016/S0168-9525(96)10049-4. [DOI] [PubMed] [Google Scholar]
- 21.Lu HD, Xue JQ, Guo DW. Efficacy of planting date adjustment as a cultivation strategy to cope with drought stress and increase rainfed maize yield and water-use efficiency. J Agric Water Manag. 2016;179: 227–235. [Google Scholar]
- 22.Zheng Y, Liao C, Zhao S, Wang C, Guo Y. The glycosyl transferase QUA1 regulates chloroplast-associated calcium signaling during salt and drought stress in Arabidopsis. Plant Cell Physiol. 2017;58:329–341. doi: 10.1093/pcp/pcw192. [DOI] [PubMed] [Google Scholar]
- 23.Guo YY, Tian SS, Liu SS, Wang WQ, Sui N.. Energy dissipation and antioxidant enzyme system protect photosystem II of sweet sorghum under drought stress. Photosynthetica. 2017;56:861–872. doi: 10.1007/s11099-017-0741-0. [DOI] [Google Scholar]
- 24.Hou L, Liu W, Li Z, Huang C, Fang X, Wang Q, Liu X. Identification and expression analysis of genes responsive to drought stress in peanut. Russ J Plant Physiol. 2014;61:842–852. doi: 10.1134/S1021443714060089. [DOI] [Google Scholar]
- 25.Liu J, Zhang F, Zhou J, Chen F, Wang B, Xie X. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol Biol. 2012;78:289–300. doi: 10.1007/s11103-011-9853-2 [DOI] [PubMed] [Google Scholar]
- 26.Tang G, Shao F, Xu P, Shan L, Liu Z. Overexpression of a peanut NAC gene, AhNAC4, confers enhanced drought tolerance in tobacco. Russ J Plant Physiol. 2017;64:525–535. doi: 10.1134/S1021443717040161. [DOI] [Google Scholar]
- 27.Chen Y, Yang X, He K, et al. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. J Plant Mol Biol. 2006;60:107–124. doi: 10.1007/s11103-005-2910-y. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L. REVIEW: biochemical and molecular characterization of plant MYB transcription factor family. J Biochem Biokhimiia. 2009;74:1–11. doi: 10.1134/S0006297909010015. [DOI] [PubMed] [Google Scholar]
- 29.Cominelli E, Galbiati M, Vavasseur A, Conti L, Sala T, Vuylsteke M, Leonhardt N, Dellaporta SL, Tonelli C.. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. J Curr Biol. 2005;15:1196–1200. doi: 10.1016/j.cub.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Urao T, Ito T, et al. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in Abscisic acid signaling. J Plant Cell. 2003;15:63–78. doi: 10.1105/tpc.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. Role of arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. J Plant Cell. 1997;10:1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo P, Lee S, Suh M, Park MJ, Go YS, Park CM.. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. J Plant Cell. 2011;23:1138–1152. doi: 10.1105/tpc.111.083485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, Cao Z, Hao Y. Overexpression of a R2R3 MYB gene MdSIMYB1 increases tolerance to multiple stresses in transgenic tobacco and apples. J Physiol Plant. 2013;150:76–87. doi: 10.1111/ppl.12069. [DOI] [PubMed] [Google Scholar]
- 34.Sun P, Zhu X, Huang X, Liu J. Overexpression of a stress-responsive MYB transcription factor of Poncirus trifoliata confers enhanced dehydration tolerance and increases polyamine biosynthesis. J Plant Biochem Physiol. 2014;78:71–79. doi: 10.1016/j.plaphy.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Su L, Li J, Liu D, Zhai Y, Zhang HJ, Li XW, Zhang QL, Wang Y, Wang QY.. A novel MYB transcription factor, GmMYBJ1, from soybean confers drought and cold tolerance in Arabidopsis thaliana. J Gene. 2014;538:46–55. doi: 10.1016/j.gene.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Xiong H, Li J, Liu P, Duan J, Zhao Y, Guo X, Li Y, Zhang H, Ali J, Li Z, et al. Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. J Plos One. 2014;9: e92913. doi: 10.1371/journal.pone.0092913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen B, Wang Y, Hu Y, Wu Q. Cloning and characterization of a drought-inducible MYB gene from Boea crassifolia. J Plant Sci. 2005;168:493–500. doi: 10.1016/j.plantsci.2004.09.013. [DOI] [Google Scholar]
- 38.Shin D, Moon S, Han S, Kim BG, Park SR, Lee SK, Yoon HJ, Lee HE, Kwon HB, Baek D, et al. Expression of StMYB1R-1, a novel potato single MYB-like domain transcription factor, increases drought tolerance. J Plant Physiol. 2011;155:421–432. doi: 10.1104/pp.110.163634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganesan G, Sankararamasubramanian HM, Harikrishnan M, Ashwin G, Parida A.. A MYB transcription factor from the grey mangrove is induced by stress and confers NaCl tolerance in tobacco. J Exp Bot. 2012;63:4549–4561. doi: 10.1093/jxb/ERS135. [DOI] [PubMed] [Google Scholar]
- 40.Sui N, Han G. Salt-induced photoinhibition of PSII is alleviated in halophyte Thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol Plant. 2014;36:983–992. doi: 10.1007/s11738-013-1477-5. [DOI] [Google Scholar]
- 41.Liu S, Wang W, Li M, Wang S, Sui N. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. J Acta Physiol Plant. 2017;39:207. doi: 10.1007/s11738-017-2501-y. [DOI] [Google Scholar]
- 42.Feng Z, Deng Y, Fan H, Sun Q, Sui N, Wang B. Effects of NaCl stress on the growth and photosynthetic characteristics of Ulmus pumila, L. seedlings in sand culture. Photosynthetica. 2014;52:313–320. doi: 10.1007/s11099-014-0032-y. [DOI] [Google Scholar]
- 43.Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ.. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant. 2016;38:102. doi: 10.1007/s11738-016-2113-y. [DOI] [Google Scholar]
- 44.Sui N, Yang Z, Liu M, Wang B. Identification and transcriptomic profiling of genes involved in increasing sugar content during salt stress in sweet sorghum leaves. BMC Genomics. 2015;16:534. doi: 10.1186/s12864-015-1760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng T, Chen J, Zhang J, Shi S, Zhou Y., Lu Lu, Wang P, Jiang Z, Yang J, Zhang S, et al. Physiological and proteomic analyses of leaves from the halophyte Tangut Nitraria reveals diverse response pathways critical for high salinity tolerance. Front Plant Sci. 2015;6:30. doi: 10.3389/fpls.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Liu Y, Chen M, Song Y, Song J, Wang B. Relationships between ion and chlorophyll accumulation in seeds and adaptation to saline environments in Suaeda salsa populations. Plant Biosyst. 2012;146:142–149. doi: 10.1080/11263504.2012.727880. [DOI] [Google Scholar]
- 47.Zhang S, Song J, Wang H, Feng G. Effect of salinity on seed germination, ion content and photosynthesis of cotyledons in halophytes or xerophyte growing in Central Asia. J Plant Ecol. 2010;3:259–267. doi: 10.1093/jpe/rtq005. [DOI] [Google Scholar]
- 48.Nxele X, Klein A, Ndimba B. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S Afr J Bot. 2017;108:261–266. doi: 10.1016/j.sajb.2016.11.003. [DOI] [Google Scholar]
- 49.Song J, Wang B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: suaeda salsa as a promising model. Ann Bot. 2014;115:541–553. doi: 10.1093/aob/mcu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan F, Chen M, Yang J, Ling B, Wang B. A system for the transformation and regeneration of the recreto halophyte Limonium bicolor. In Vitro Cell Dev. 2014;50:610–617. doi: 10.1007/s11627-014-9611-7. [DOI] [Google Scholar]
- 51.Yuan F, Leng B, Wang B. Progress in studying salt secretion from the salt glands in recretohalophytes: how do plants secrete salt? Front Plant Sci. 2016;7:977. doi: 10.3389/fpls.2016.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, Cai H, Liu Y, Zhu Y, Ji W, Bai X. A study on physiological characteristics and cmparison of salt resistance of two medicago sativa at the seedling stage. Acta Pratacult Sin. 2015;22:250–256. [Google Scholar]
- 53.Han N, Shao Q, Bao H, Wang B. Cloning and characterization of a Ca2+/H+, antiporter from halophyte Suaeda salsa L. Plant Mol Biol Report. 2011;29:449–457. doi: 10.1007/s11105-010-0244-7. [DOI] [Google Scholar]
- 54.Feng Z, Deng Y, Zhang S, Liang X, Yuan F, Hao J, Zhang J, Sun S, Wang B. K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using nanoSIMS. Plant Sci. 2015;238:286–296. doi: 10.1016/j.plantsci.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 55.Song J, Shi W, Liu R, Xu Y, Sui N, Zhou J, Feng G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biol. 2017;32:107–114. doi: 10.1111/psbi.2017.32.issue-2. [DOI] [Google Scholar]
- 56.Guo P, Wei H, Zhang W, Bao Y. Physiological responses of alfalfa to high-level salt stress: root ion flux and stomatal characteristics. Int J AgricBiol. 2016;18:125–133. [Google Scholar]
- 57.Zhao K, Song J, Fan H, Zhou S, Zhao M. Growth response to ionic and osmotic stress of NaCl in salt-tolerant and salt-sensitive maize. J Integr Plant Biol. 2010;52:468–475. doi: 10.1111/j.1744-7909.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- 58.Cui F, Sui N, Duan G, Liu Y, Han Y, Liu S, Wan S, Li G. Identification of metabolites and transcripts involved in salt stress and recovery in peanut. Front Plant Sci. 2018;9:217. doi: 10.3389/fpls.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, Cai H, Liu Y, Zhu Y, Ji W, Bai X. A study on physiological characteristics and comparison of salt resistance of two Medicago sativa at the seedling stage. Acta Pratacult Sin. 2013;22:250–256. [Google Scholar]
- 60.Tao L, Liu R, He X, Wang B. Enhancement of superoxide dismutase and catalase activities and salt tolerance of euhalophyte Suaeda salsa L. by mycorrhizal fungus glomus mosseae. Pedosphere. 2012;22:217–224. doi: 10.1016/S1002-0160(12)60008-3. [DOI] [Google Scholar]
- 61.Song J, Zhou J, Zhao W, Xu H, Wang F, Xu Y, Wang L, Tian C. Effects of salinity and nitrate on production and germination of dimorphic seeds applied both through the mother plant and exogenously during germination in Suaeda salsa. Plant Spec Biol. 2016;31:19–28. doi: 10.1111/1442-1984.12071. [DOI] [Google Scholar]
- 62.Zhou J, Fu T, Sui N, Gou J, Feng G, Fan J, Song J. The role of salinity in seed maturation of the euhalophyte Suaeda salsa. Plant Biosyst. 2016;150:83–90. doi: 10.1080/11263504.2014.976294. [DOI] [Google Scholar]
- 63.Guo Y, Jia W, Song J, Wang D, Chen M, Wang B. Thellungilla halophila is more adaptive to salinity than Arabidopsis thaliana at stages of seed germination and seedling establishment. Acta Physiol Plant. 2012;34:1287–1294. [Google Scholar]
- 64.Yao S, Chen S, Zhao J, Xu D, Lan H, Zhang F. Effect of three salts on germination and seedling survival of dimorphic seeds of Chenopodium album. Botany. 2010;88:821–828. doi: 10.1139/B10-052. [DOI] [Google Scholar]
- 65.Wang F, Xu Y, Wang S, Shi W, Liu R, Feng G, Song J. Salinity affects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol Biochem. 2015;95:41–48. doi: 10.1016/j.plaphy.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Xu Y, Liu R, Sui N, Shi W, Wang L, Tian C, Song J. Changes in endogenous hormones and seed-coat phenolics during seed storage of two Suaeda salsa populations. Aust J Bot. 2016;64:325–332. doi: 10.1071/BT16014. [DOI] [Google Scholar]
- 67.Guo J, Suo S, Wang B. Sodium chloride improves seed vigour of the euhalophyte Suaeda salsa. Seed Sci Res. 2015;25:335–344. doi: 10.1017/S0960258515000239. [DOI] [Google Scholar]
- 68.Guo J, Li Y, Han G, Song J, Wang B. NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Funct Plant Biol. 2018;45:350–361. doi: 10.1071/FP17181. [DOI] [PubMed] [Google Scholar]
- 69.Liu Q, Liu R, Ma Y, Song J. Physiological and molecular evidence for Na+ and Cl− exclusion in the roots of two Suaeda salsa populations. Aquat Bot. 2018;146:1–7. doi: 10.1016/j.aquabot.2018.01.001. [DOI] [Google Scholar]
- 70.Zhang T, Song J, Fan J, Feng G. Effects of saline-waterlogging and dryness/moist alternations on seed germination of halophyte and xerophyte. Plant Spec Biol. 2015;30:231–236. doi: 10.1111/1442-1984.12056. [DOI] [Google Scholar]
- 71.Feng Z, Sun Q, Deng Y, Sun S, Zhang J, Wang B. Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiol Plant. 2014;36:2729–2741. doi: 10.1007/s11738-014-1644-3. [DOI] [Google Scholar]
- 72.Leng B, Yuan F, Dong X, Wang J, Wang B. Distribution pattern and salt excretion rate of salt glands in two recretohalophyte species of Limonium, (Plumbaginaceae). S Afr J Bot. 2018;115:74–80. doi: 10.1016/j.sajb.2018.01.002. [DOI] [Google Scholar]
- 73.Yuan F, Lyu M, Leng B, Zheng G, Feng Z, Li P, Zhu X, Wang B. Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant Cell Environ. 2015;38:1637–1657. doi: 10.1111/pce.12514. [DOI] [PubMed] [Google Scholar]
- 74.Yuan F, Chen M, Yang J, Song J, Wang B. The optimal dosage of60co gamma irradiation for obtaining salt gland mutants of exo-recretohalophyte Limonium bicolor (Bunge) O. Kuntze. Pak J Bot. 2015;47:71–76. [Google Scholar]
- 75.Yuan F, Lyu M, Leng B, Zhu X, Wang B. The transcriptome of NaCl-treated Limonium bicolor, leaves reveals the genes controlling salt secretion of salt gland. Plant Mol Biol. 2016;91:241–256. doi: 10.1007/s11103-016-0460-0. [DOI] [PubMed] [Google Scholar]
- 76.Zhang H, Zhang L, Gao B, Fan H, Jin J, Botella M, Jiang L, Lin J. Golgi apparatus-localized synaptotagmin 2 is required for unconventional secretion in Arabidopsis. PLoS One. 2011;6:e26477. doi: 10.1371/journal.pone.0026477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han G, Wang M, Yuan F, Sui N, Song J, Wang B. The CCCH zinc finger protein gene AtZFP1, improves salt resistance in Arabidopsis thaliana. Plant Mol Biol. 2014;86:237–253. doi: 10.1007/s11103-014-0226-5. [DOI] [PubMed] [Google Scholar]
- 78.Qi Y, Liu W, Qiu L, Zhang S, Ma L, Zhang H. Overexpression of glutathione S-transferase gene increases salt tolerance of Arabidopsis. Russ J Plant Physiol. 2010;57:233–240. doi: 10.1134/S102144371002010X. [DOI] [Google Scholar]
- 79.Qi Y, Wang F, Zhang H, Liu W. Overexpression of Suadea salsa S-adenosylmethionine synthetase gene promotes salt tolerance in transgenic tobacco. Acta Physiol Plant. 2010;32:263–269. doi: 10.1007/s11738-009-0403-3. [DOI] [Google Scholar]
- 80.Sun Z, Qi X, Wang Z, Li P, Wu C, Zhang H, Zhao Y. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol Biochem. 2013;69:82–89. doi: 10.1016/j.plaphy.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 81.Chen M, Song J, Wang B. NaCl increases the activity of the plasma membrane H+-ATPase in C3 halophyte Suaeda salsa callus. Acta Physiol Plant. 2010;32:27–36. doi: 10.1007/s11738-009-0371-7. [DOI] [Google Scholar]
- 82.Li K, Pang C, Ding F, Sui N, Feng Z, Wang B. Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis chloroplasts enhances salt tolerance of plants. S Afr J Bot 2012;78:235–245. doi: 10.1016/j.sajb.2011.09.006. [DOI] [Google Scholar]
- 83.Shao Q, Han N, Ding T, Zhou F, Wang B. SsHKT1;1 is a potassium transporter of the C3 halophyte Suaeda salsa that is involved in salt tolerance. Funct Plant Biol. 2014;41:790–802. doi: 10.1071/FP13265. [DOI] [PubMed] [Google Scholar]
- 84.Sui N, Tian S, Wang W, Wang M, Fan H. Overexpression of glycerol-3-phosphate acyltransferase from Suaeda salsa improves salt tolerance in Arabidopsis. Front Plant Sci. 2017;8:1337. doi: 10.3389/fpls.2017.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Z, Wang Y, Wei X, Zhao X, Wang B. Transcription profiles of genes related to hormonal regulations under salt stress in sweet sorghum. Plant Mol Biol Rep. 2017;35:1–14. doi: 10.1007/s11105-017-1047-x. [DOI] [Google Scholar]
- 86.Shen X, Wang Z, Song X, Xu J, Jiang C, Zhao Y. Transcriptomic profiling revealed an important role of cell wall remodeling and ethylene signaling pathway during salt acclimation in Arabidopsis. Plant Mol Biol 2014;86:303–317. doi: 10.1007/s11103-014-0230-9. [DOI] [PubMed] [Google Scholar]
- 87.Ding F, Chen M, Sui N, Wang B. Ca2+ significantly enhanced development and salt-secretion rate of salt glands of Limonium bicolor under NaCl treatment. S AfrJ Bot. 2010;76:95–101. doi: 10.1016/j.sajb.2009.09.001. [DOI] [Google Scholar]
- 88.Han N, Lan W, He X, Shao Q, Wang B. Expression of a Suaeda salsa, vacuolar H +/Ca2+, transporter gene in Arabidopsis, contributes to physiological changes in salinity. Plant Mol Biol Rep. 2011;30:470–477. doi: 10.1007/s11105-011-0353-y. [DOI] [Google Scholar]
- 89.Jaschke WD, Peuke AD, Pate JS, Hartung W.. Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (\r, Ricinuscommunis\r, L.) under phosphate deficiency and moderate salinity J Exp Bot. 1997;48:1737–1747. doi: 10.1093/jxb/48.9.1737. [DOI] [Google Scholar]
- 90.Hao G, Sun Z, Zhang L, Du K. A research overview of the plant resistance to adverse environment by using abscisic acid. J Chin Agric Sci Bull. 2009;25:212–215. [Google Scholar]
- 91.Agarwal PK, Jha B. Transcription factors in plants and ABA dependent and independent abiotic stress signaling. J Biologia Plantarum Prague. 2010;54:201–212. doi: 10.1007/s10535-010-0038-7. [DOI] [Google Scholar]
- 92.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. J Science. 2009;324:1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phang TH, Shao G, Lam HM. Salt tolerance in soybean. J Integr Plant Biol. 2008;50:1196–1212. doi: 10.1111/j.1744-7909.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 94.Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI.. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. J Genes Dev. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cominelli E, Sala T, Calvi D, et al. Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability. Plant J. 2008;53:53–64. doi: 10.1111/j.1365-313X.2007.03310.x. [DOI] [PubMed] [Google Scholar]
- 96.Schmidt R, Schippers JH, Mieulet D, Obata T, Fernie AR, Guiderdoni E, Mueller-Roeber B. MULTIPASS, a rice R2R3-type MYB transcription factor, regulates adaptive growth by integrating multiple hormonal pathways. Plant J. 2013;76:258–273. doi: 10.1111/tpj.12286. [DOI] [PubMed] [Google Scholar]
- 97.Yoo J, Park C, Kim J, Do Heo W, Cheong MS, Park HC Kim MC, Moon BC, Choi MS, Kang YH, et al. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem. 2005;280:3697–3706. doi: 10.1074/jbc.M408237200 [DOI] [PubMed] [Google Scholar]
- 98.Jung C, Seo J, Han S, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ.. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. J Plant Physiol 2007;146:623–635. doi: 10.1104/pp.107.110981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cominelli E, Sala T, Calvi D, Gusmaroli G, Tonelli C. Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability. Plant J. 2008;53:12. [DOI] [PubMed] [Google Scholar]
- 100.Seo P, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM.. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. J Plant Physiol. 2009;151:275–289. doi: 10.1104/pp.109.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen N, Yang Q, Pan L, Chi X, Chen M, Hu D, Yang Z, Wang T, Wang M, Yu S.. Identification of 30 MYB transcription factor genes and analysis of their expression during abiotic stress in peanut (Arachishypogaea L.). J Gene. 2014;533:332–345. doi: 10.1016/j.gene.2013.08.092. [DOI] [PubMed] [Google Scholar]
- 102.Zhang L, Zhao G, Jia J, Liu X, Kong X. Molecular characterization of 60 isolated wheat MYB genes and analysis of their expression during abiotic stress. J Exp Bot. 2011a;63:203–214. doi: 10.1093/jxb/err264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L, Zhao G, Xia C, Jia J, Liu X, Kong X. Overexpression of a wheat MYB transcription factor gene, TaMYB56-B, enhances tolerances to freezing and salt stresses in transgenic Arabidopsis. Gene. 2012;505:100–107. doi: 10.1016/j.gene.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 104.He Y, Li W, Lv J, Jia Y, Wang M, Xia G. Ectopic expression of a wheat MYB transcription factor gene, TaMYB73, improves salinity stress tolerance in Arabidopsis thaliana. J Exp Bot. 2012;63:1511–1522. doi: 10.1093/jxb/err389. [DOI] [PubMed] [Google Scholar]
- 105.Qin Y, Wang M, Tian Y, He W, Han L, Xia G. Over-expression of TaMYB33 encoding a novel wheat MYB transcription factor increases salt and drought tolerance in Arabidopsis. MolBiol Rep. 2012;39:7183–7192. [DOI] [PubMed] [Google Scholar]
- 106.Yang A, Dai X, Zhang W. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Exp Bot. 2012;63:2541–2556. doi: 10.1093/jxb/err431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prabu G, Prasad D. Functional characterization of sugarcane MYB transcription factor gene promoter (PScMYBAS1) in response to abiotic stresses and hormones. Plant Cell Rep. 2012;31:661–669. doi: 10.1007/s00299-011-1183-y. [DOI] [PubMed] [Google Scholar]
- 108.Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K. Overexpression of an R1R2R3 MYB gene OsMYB3R-2, increases tolerance to freezing, drought, salt stress in transgenic Arabidopsis. Plant Physiol. 2007;143:1739–1751. doi: 10.1104/pp.106.094532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Agarwal M, Hao Y, Kapoor A, Dong C, Fujii H, Zheng X, Zhu J. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J BiolChem 2006;281: 37636–37645. [DOI] [PubMed] [Google Scholar]
- 110.Yang J, Li M, Xie X, Han G, Sui N, Wang B. Deficiency of phytochrome B alleviates chilling-induced photoinhibition in rice. Am J Bot. 2013;100:1860–1870. doi: 10.3732/ajb.1200574. [DOI] [PubMed] [Google Scholar]
- 111.Cheng S, Yang Z, Wang M, Song J, Sui N, Fan H. Salinity improves chilling resistance in Suaeda salsa. Acta Physiol Plant. 2014;36:1823–1830. [Google Scholar]
- 112.Sui N. Photoinhibition of Suaeda salsa to chilling stress is related to energy dissipation and water-water cycle. Photosynthetica. 2015;53:207–212. doi: 10.1007/s11099-015-0080-y. [DOI] [Google Scholar]
- 113.Peiqin C, Songlin Y, Yanni Z,Kang X. A review on plant heat stress physiology. J Chin Agric Sci Bull. 2006;22:223–227. [Google Scholar]
- 114.Liao Y, Zou H, Wang H, Zhang WK, Ma B, Zhang JS, Chen SY.. Soybean GmMYB76, GmMYB92, and GmMYB177 genes confer stress tolerance in transgenic Arabidopsis plants. J Cell Res. 2008;18:1047–1060. doi: 10.1038/cr.2008.280. [DOI] [PubMed] [Google Scholar]
- 115.Pasquali G, Biricolti S, Locatelli F, Baldoni E, Mattana M. Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Rep. 2008;27:1677–1686. doi: 10.1007/s00299-008-0587-9. [DOI] [PubMed] [Google Scholar]
- 116.Vannini C, Campa M, Iriti M, Genga A, Faoro F, Carravieri S, Rotino GL, Rossoni M, Spinardi A, Bracale M. Evaluation of transgenic tomato plants ectopically expressing the rice Osmyb4 gene. Plant Sci. 2007;173:231–239. doi: 10.1016/j.plantsci.2007.05.007. [DOI] [Google Scholar]
- 117.Vannini C, Locatelli F, Bracale M, Magnani E, Marsoni M, Osnato M, Mattana M, Baldoni E, Coraggio I. Overexpression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. Plant J. 2004;37:115–127. doi: 10.1046/j.1365-313X.2003.01938.x [DOI] [PubMed] [Google Scholar]
- 118.Ma Q, Dai X, Xu Y, Guo J, et al. Enhanced tolerance to chilling stress in OsMYB3R-2 transgenic rice is mediated by alteration in cell cycle and ectopic expression of stress genes. Plant Physiol. 2009;150:244–256. doi: 10.1104/pp.108.133454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Su C, Wang Y, Hsieh T, Lu C, Tseng T, Yu S. A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol. 2010;153:145–158. doi: 10.1104/pp.110.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feng C, Andreasson E, Mattson O, Mock HP, Mattsson O, Mundy J.. Arabidopsis MYB68 in development and responses to environmental cues. Plant Sci. 2004;167:1099–1107. doi: 10.1016/j.plantsci.2004.06.014 [DOI] [Google Scholar]
- 121.Ashraf EK, Yong-Mei B, Kosala R, Perrin H, Beatty, Good AG, Steven J, Rothstein S. The rice R2R3-MYB transcription factor OsMYB55 is involved in the tolerance to high temperature and modulates amino acid metabolism. PLoS One. 2017;7:e52030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deng Z, Jin L, Li J, Wang W, Yang L, Wang X. Pathways of MYB transcription factors regulating anther development and pollen formation. J Acta Botanica Boreali-Occidentalia Sinica. 2013;33:850–856. [Google Scholar]
- 123.Lee MM, Schiefelbein J. Cell pattern in the Arabidopsis root epidermis determined by lateral inhibition with feedback. J Plant Cell. 2002;14:611–618. doi: 10.1105/tpc.010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang X, Li J, Pei M, Gu H, Chen Z, Qu L. Over- expression of a flower- specific transcription factor gene AtMYB24 causes aberrant anther development. J Plant Cell. 2007;26:219–228. doi: 10.1007/s00299-006-0229-z. [DOI] [PubMed] [Google Scholar]
- 125.Noda KI, Glover BJ, Linstead P, Martin C.. Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. J Nat. 1994;369:661–664. doi: 10.1038/369661a0. [DOI] [PubMed] [Google Scholar]
- 126.Zhao K, Liu N, Yu Y, Zheng K, Chen Q, Ni Z. Identification and genetic analysis of transgenic GhMYB4 cotton. J Mol Plant Breed. 2018;16:7304–7308. [Google Scholar]
- 127.Xue Y. Research advances in the secondary growth-associated MYB transcription factors in plants. J Anhui Agric Sci. 2012;40:7650–7655. [Google Scholar]
- 128.Chiou C, Yeh K. Differential expression of MYB gene (OgMYB1) determines color patterning in floral tissue of oncidium gower ramsey. Plant Mol Biol 2008;66:379–388. doi: 10.1007/s11103-007-9275-3. [DOI] [PubMed] [Google Scholar]
- 129.Yamagishi M, Shimoyamada Y, Nakatsuk T, Masuda K.. Two R2R3-MYB genes, homologs of petunia AN2, regulate anthocyanin biosyntheses in flower tepals, tepal spots and leaves of asiatic hybrid Lily. J Plant Cell Physiol. 2010;51:463–474. doi: 10.1093/pcp/pcq011. [DOI] [PubMed] [Google Scholar]


