ABSTRACT
The objective of our study was to provide a comparative assessment of previously reported magnetic resonance imaging (MRI) parameters in primary and secondary pseudotumor cerebri (PTC) patients, to examine their diagnostic contribution, and to evaluate their association with symptoms, neuro-ophthalmological findings, laboratory results, and cerebrospinal fluid characteristics.
Twenty-eight consecutive patients with PTC were included in the study. Age- and sex-matched 20 individuals with normal neurologic examination served as the control group. Modified Dandy Criteria were used for the diagnosis of PTC. Orbital and cranial MRI and MR venography of all patients and controls were assessed by three radiologists.
According to our study, posterior flattening of the globe (64% sensitive, 100% specific), optic nerve sheath distention (46% sensitive, 100% specific), vertical tortuosity of the optic nerve (30% sensitive, 95% specific), and partial empty sella (43% sensitive, 100% specific) emerged as particularly valuable markers for a diagnosis of PTC.
KEYWORDS: Intracranial hypertension, MRI, partial empty sella, optic nerve
Introduction
Pseudotumor cerebri (PTC) is distinguished by headache and papilledema due to high cerebrospinal fluid (CSF) pressure in the absence of intracranial lesion or hydrocephalus and does not cause any localized neurological deficits other than abducens nerve palsy.1,2 There is no identified cause of intracranial hypertension in patients with primary PTC, while the term “secondary PTC” is preferred for the patients with an underlying pathology causing increased CSF pressure.
Modified Dandy criteria, which need brain imaging, have been used for the diagnosis of this condition since 1985.1,2 Previously, imaging had played a diagnostic part in eliminating other causes of intracranial hypertension and papilledema only. However, lately, imaging findings such as vertical tortuosity of the optic nerves, posterior flattening of the globes, optic nerve protrusion, distention of the optic nerve sheath, and inferior tonsillar displacement have been described in PTC patients. 3 Therefore, it is an interesting question, whether MRI findings alone might yield sufficient clues for the diagnosis and follow-up of idiopathic PTC, without any interventional investigation such as a lumbar puncture (LP).
The aim of our study was to provide a comparative assessment of previously reported MRI parameters in primary and secondary PTC patients, to examine their diagnostic contribution, and to evaluate their association with symptoms, neuro-ophthalmological findings, laboratory results, and CSF characteristics.
Methods
Patients over 18 years of age presenting to the neurology and neuro-ophthalmology outpatient clinics, between 2011 and 2014 with bilateral papilledema and no localized neurologic findings other than abducens palsy, if a diagnosis of intracranial mass lesion and hydrocephalus was excluded by imaging and an initial CSF pressure of ≥250 cm H2O was measured were included in the study. Also, patients with cerebral venous thrombosis as documented by magnetic resonance venography (MRV) were included, provided that there was no venous infarction or hematoma. Modified Dandy criteria were used for the diagnosis of primary PTC, and the former group of patients with normal MRV was classified as idiopathic PTC, and the latter group of patients with venous thrombosis in MRV was classified as secondary PTC. Written informed consent was obtained from all patients and this study was approved by institutional review board.
Age- and gender-matched subjects attending to the neurology outpatient clinic with symptoms other than those suggesting an increased intracranial pressure and with normal neurological examination findings served as the control group.
Exclusion criteria included age over 60 years, presence of localized neurological findings in addition to abducens palsy, unilateral papilledema, presence of intracranial mass as documented by MRI, presence of additional pathological findings in addition to elevated CSF pressure, a diagnosis of or treatment for PTC within the past year, use of medications that may affect CSF pressure, a contraindication for MRI, patient’s refusal of LP, and unwillingness to participate.
Headache characteristics, accompanying symptoms of headache, neck pain, tinnitus, visual symptoms, systemic diseases, weight gain within the past 6 months, current medicines as well as the duration from initial symptoms to diagnosis were recorded. Body mass index (BMI) was calculated for each patient.
A neuro-ophthalmological examination and visual field testing (VFT) with a Humphrey Field Analyzer at the ophthalmology unit were performed. The loss of visual field was categorized in three groups including enlargement in the blind spot, lower nasal defect, concentric narrowing, and others. Blood chemistry, complete blood counts, sedimentation, iron parameters, and thyroid function tests were checked in all patients.
Cranial and orbital MRI, in addition to MRV were performed in a single session in both controls and patients using a 3.0 Tesla Siemens MRI (Siemens HealthCare, Erlangen, Germany) device. The images were assessed separately by a neuroradiology specialist and two radiology residents who were blinded to diagnoses. A concordance between at least two of the radiologists was considered to indicate a positive reading, which was recorded.
Cranial, orbital MRI and MRV studies were evaluated in terms of partial empty sella, slit like ventricles, tight subarachnoid spaces, posterior flattening of the globes, optic nerve protrusion, vertical tortuosity of the optic nerves, distention of the optic nerve sheaths, inferior tonsillar displacement, and thrombotic or hypoplastic cerebral sinuses.
Partial empty sella was defined as presence of CSF in >50% the pituitary fossa (Figure 1).3–6 Slit-like ventricles were defined as decrease in the dimensions of lateral ventricles as compared to average values in the general population.4,5,7,8 Similarly, tight subarachnoid spaces were defined as the decreased dimensions of sulci and cisternae in cranial MR images. Posterior flattening of the globe was diagnosed when the normal convexity at the origin of the optic nerve was flattened in the orbital MRI (Figure 2).8,9 Optic nerve protrusion was defined as the loss of its convexity at the point of entry into globe and attainment of a concave form, as shown by orbital MRI (Figure 3).10,11 Vertical tortuosity of the optic nerve is defined as the presence of an “S” shaped optic nerve in sagittal orbital MR images (Figure 4).3 Distention of the optic nerve sheath is diagnosed in the presence of a calculated CSF distance of >2 mm around the optic nerve in coronal cross-sections (Figure 5).3 Inferior tonsillar displacement is defined as a >5 mm downward displacement of cerebellar tonsils as compared to normal anatomical location. Thrombotic and hypoplastic cerebral sinuses were assessed using MRV.
Figure 1.
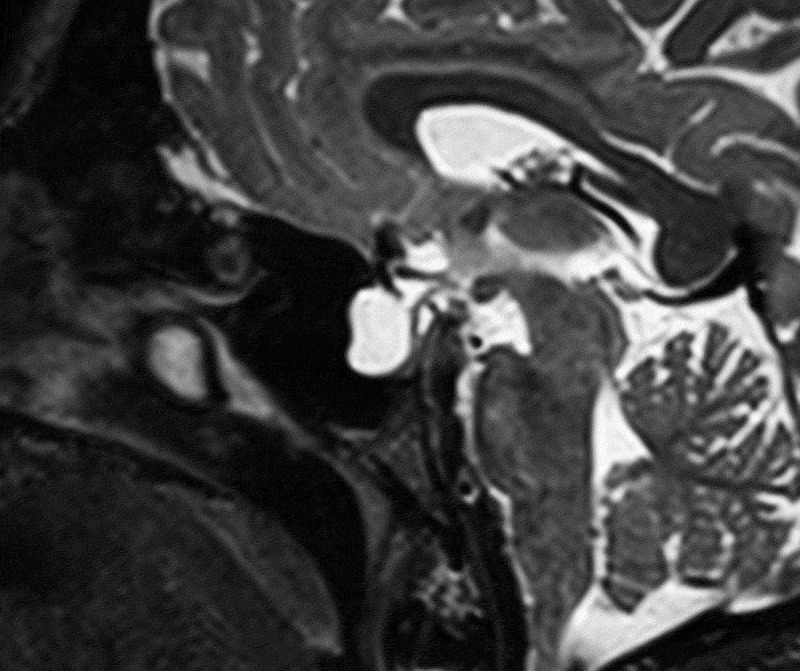
Partial empty sella in sagittal T1 weighted image.
Figure 2.
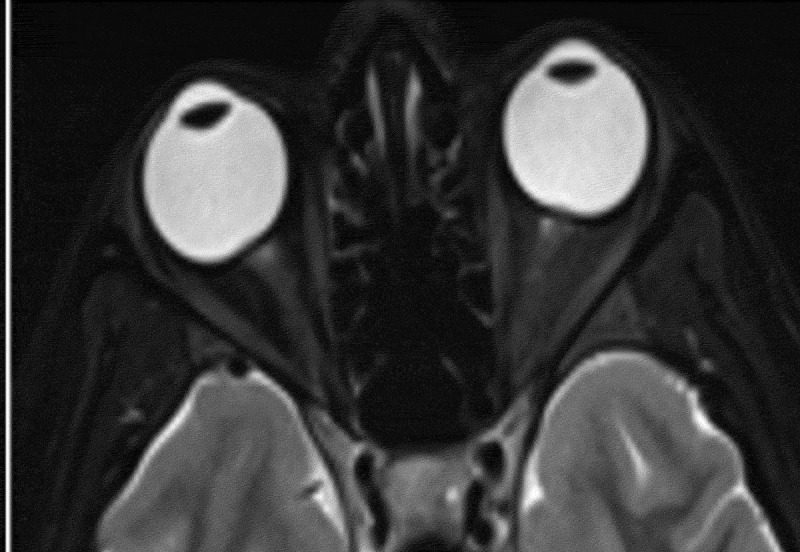
Posterior flattening of the globe in axial T2 weighted image.
Figure 3.
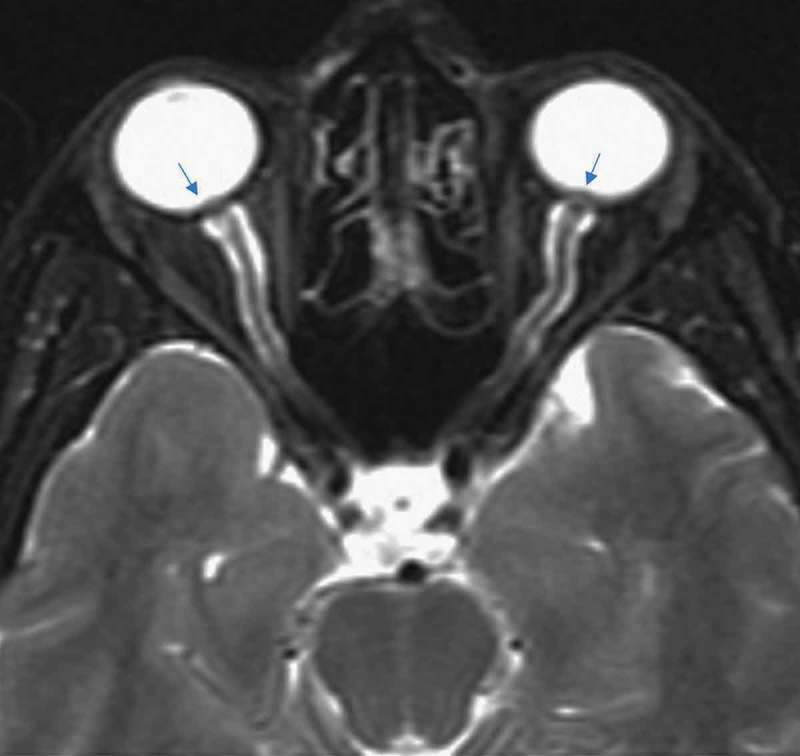
Optic nerve protrusion (arrows) in axial T2 image.
Figure 4.
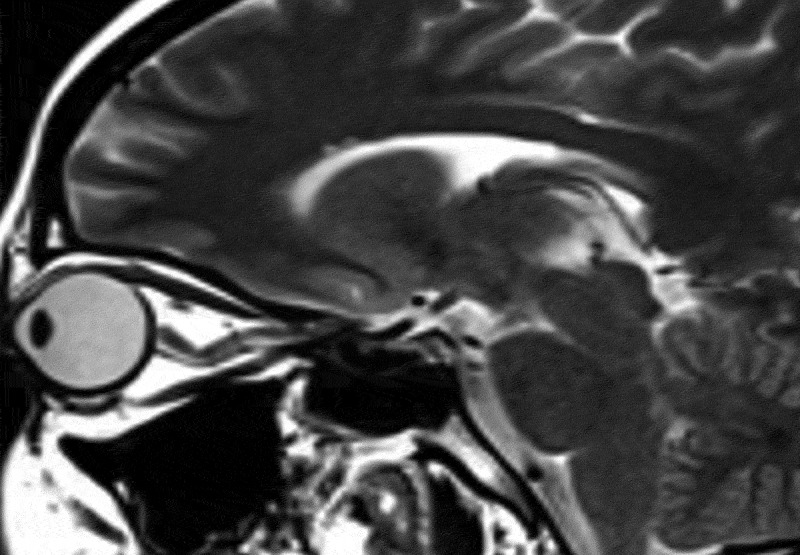
Vertical tortuosity of the optic nerve in sagittal T2 weighted image.
Figure 5.
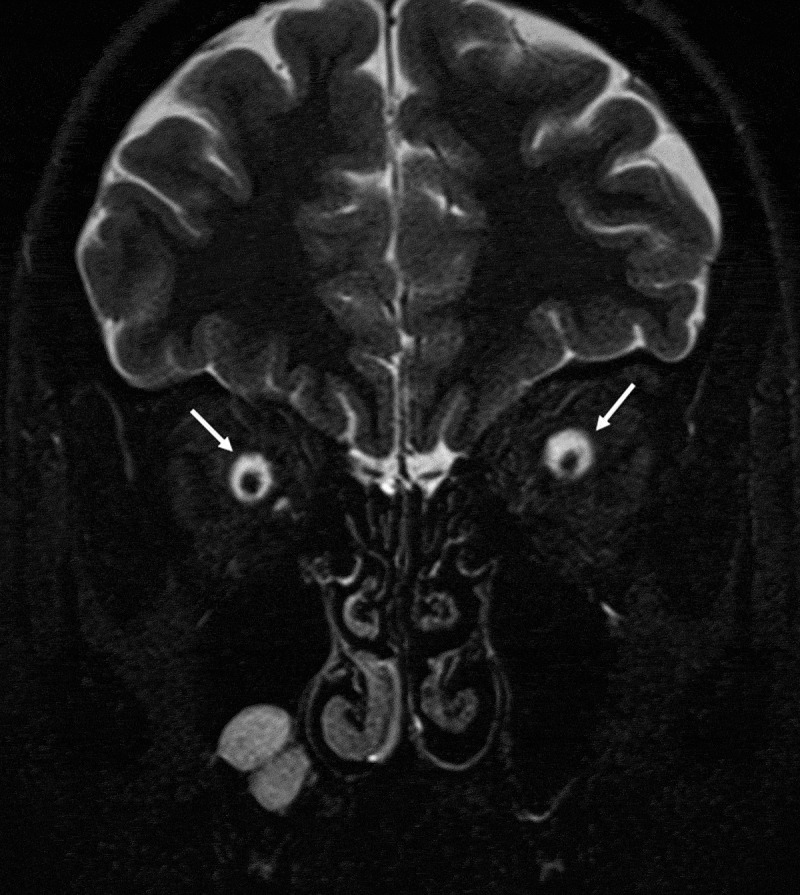
Distention of the optic nerve sheath in coronal T2 weighted image.
Two patients did not undergo an orbital MRI, as cranial MRI and MRV provided adequate information. Following radiological examination, LP was performed in all patients, and CSF opening pressure, cell content, cytology, and biochemistry were evaluated.
Statistical analyses were performed to compare the findings of MRI in patient and control groups in order to detect any differences that can be meaningful in the diagnosis and follow-up of PTC. Also inter-observer reliability between the three radiologists was assessed. The association between MR findings and clinical, neuro-ophthalmological or CSF characteristics was examined. Continuous variables were assessed using mean ± standard deviation and median (minimum–maximum), while categorical variables were summarized with percentages. The between-group comparisons for continuous variables were performed with Mann–Whitney U and Wilcoxon tests, while chi-square and Fisher exact tests were used for the categorical data. The correlations between two continuous variables were evaluated with Pearson correlation, Kolmogorof–Smirnov, and Shapiro–Wilk non-parametric tests. All statistical analyses were performed with SPSS Statistics for Windows (version 20, IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
Results
Of the 28 patients included in the study, 24 were female and 4 were male, with a mean age of 28.5 ± 9.8 years (18–60). Control group consisted of 18 female and 2 male subjects, with a mean age of 33 ± 8.73 years (24–49). The two groups in terms of gender distribution and mean age were similar. A VFT was performed in 26 patients, and the remaining two patients were uncooperative for a VFT. Medical history and neurological findings of the PTC patients were shown on Table 1. All patients had a CSF pressure ≥ 25 cm H2O, and they had normal CSF cell content, biochemistry, and cytology.
Table 1.
Medical history and neurological findings of the patients.
| Medical history | Patient (n) | % | Neurologic examination | Patient (n) | % |
|---|---|---|---|---|---|
| Headache | 27 | 94.4 | Decreased visual acuity | 7 | 25 |
| Persistent | 25 | 92.6 | |||
| Paroxysmal | 2 | 7.40 | |||
| Neck pain | 5 | 17.9 | Computerized visual field defect | 14 | 50 |
| Nausea | 2 | 7.14 | Colour vision defect | 2 | 7.14 |
| Tinnitus | 5 | 17.9 | Abducens palsy | 5 | 17.9 |
| Diplopia | 6 | 21.4 | |||
| Unilateral visual loss | 11 | 39.3 | |||
| Bilateral visual loss | 6 | 21.4 | |||
| Systemic disease | 11 | 39.3 | |||
| Weight gain in last 6 months | 7 | 25 | |||
| 12 | 42.9 |
A diagnosis of primary PTC was established in 24 patients, while 4 had secondary PTC. In the latter group of patients, three had venous thrombosis, and one had type 1 Chiari malformation, which was corrected surgically.
Fourteen patients had a BMI of ≥25 kg/m2, and 14 had a BMI of <25 kg/m2. Four male patients had a BMI <25 kg/m2. The average BMI in female and male patients were 26.37 ± 4.8 (min: 19, max: 35, median: 24.5) and 22 ± 2.9 kg/m2 (min: 18, max: 25, median: 22.5), respectively, with no significant difference between two genders (p > 0.05). No correlation between the CSF opening pressure and BMI was observed in the patient group.
Thus, partial empty sella, posterior flattening of the globe, optic nerve protrusion, optic nerve sheath distention, and vertical tortuosity of the optic nerve occurred at a significantly higher frequency among the patients than controls (Table 2).The diagnostic performance of all MRI criteria were calculated (Table 2). Four criteria (partial empty sella, optic nerve vertical tortuosity, distention of the optic nerve sheath, posterior globe flattening) were discriminative between patient and control groups (area under the curve (AUC): 0.714, p = 0.012; AUC: 0.779, p = 0.001; AUC: 0.732, p = 0.007; AUC: 0.821, p = 0.000, consequtively). Optic nerve protrusion was not discriminative between two groups (AUC: 0.661, p = 0.60). Posterior flattening of the globe yielded both highest sensitivity and specificity (64%, 100%, consequtively) as well as highest AUC (0.821), followed by optic nerve vertical tortuosity (sensitivity: 60%, specificity: 95%), optic nerve sheath distention (sensitivity: 46%, specificity: 100%), and partial empty sella (sensitivity: 43%, specificity: 100%) (Table 2).
Table 2.
Diagnostic value of single and combined MRI parameters.
| True positive | True negative | False positive | False negative | p | AUC (p) (95% CI) |
Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Single parameters | ||||||||
| Partial empty sella | 12/28 | 20/20 | 0/20 | 16/28 | 0.001 | 0.714 (0.012) (57–85) |
43% (26–60) |
100% (83–100) |
| Posterior flattening of the globe | 18/28 | 20/20 | 0/20 | 10/28 | < 0.001 | 0.821 (0.000) (70–94) | 64% (45–79) |
100% (83–100) |
| Optic nerve vertical tortuosity | 17/28 | 19/20 | 1/20 | 11/28 | < 0.001 | 0.779 (0.001) (64–91) | 60% (42–76) |
95% (76–99) |
| Optic nerve sheath distention | 13/28 | 20/20 | 0/20 | 15/28 | 0.001 | 0.732 (0.007) (59–87) | 46% (29–64) |
100% (83–100) |
| Optic nerve protrusion | 9/28 | 20/20 | 0/20 | 19/28 | 0.006 | 0.661 (0,060) (50–81) | 32% (17–50) |
100% (83–100) |
| Slit-like ventricules | 1/28 | 20/20 | 0/20 | 27/28 | 1.000 | 0.518 (0.834) (35–68) | 3% (6–17) |
100% (83–100) |
| Tight subarachnoid spaces | 1/28 | 20/20 | 0/20 | 27/28 | 1.000 | 0.518 (0.834) (35–68) | 3% (6–17) |
100% (83–100) |
| Inferior tonsillar displacement | 2/28 | 20/20 | 0/20 | 26/28 | 0.504 | 0.536 (0.676) (37–70) | 7% (2–22) |
100% (83–100) |
| Combined parameters | ||||||||
| One of them is positive | 23/28 | 19/20 | 1/20 | 5/28 | 0.000 | 0.886 (0.000) (78–99) |
82% (64–92) |
95% (76–99) |
| Optic nerve tortuosity-posterior globe flattening | 22/28 | 19/20 | 1/20 | 6/28 | 0.000 | 0.868 (0.000) (76–97) | 78% (59–91) |
95% (75–99) |
| Partial empty sella- Posterior flattening of the globe | 21/28 | 20/20 | 0/20 | 7/28 | 0.005 | 0.875 (0.000) (77–98) | 75% (55–89) |
100% (83–100) |
| Partial empty sella-optic nerve vertical tortuosity | 21/28 | 19/20 | 1/20 | 7/28 | 0.000 | 0.850 (0.000) (73–96) | 75% (55–89) |
95% (75–99) |
| Optic nerve tortuosity-optic nerve distention | 20/28 | 19/20 | 1/20 | 7/28 | 0.000 | 0.832 (0.000) (71–95) | 71% (52–84) |
95% (75–99) |
| Partial empty sella-optic nerve distention | 18/28 | 20/20 | 0/20 | 10/28 | 0.000 | 0.821 (0.000) (70–94) | 64% (45–79) |
100% (83–100) |
| Posterior globe flattening-optic nerve distention | 20/28 | 20/20 | 0/20 | 8/28 | 0.000 | 0.857 (0.000) (74–96) | 71% (52–84) |
100% (83–100) |
True positive: proportion of positive results among the patient group; true negative: proportion of negative results among the control group; false positive: proportion of positive results among the control group; false negative: proportion of negative results among the patient group.
Pairwise combinations of MRI findings occurring at a significantly higher frequency among patients than in controls (partial empty sella, optic nerve vertical tortuosity, distention of the optic nerve sheath, posterior globe flattening) were also assessed (Table 2). Twenty-one patients (75%) had either partial empty sella or flattening of posterior aspect of the globe (sensitivity: 75%; specificity: 100%); 18 (64%) patients had either partial empty sella or distention of the optic nerve sheath (sensitivity: 64%; specificity: 100%); 21 patients (75%) had partial empty sella or vertical tortuosity of the optic nerve (sensitivity: 75%; specificity: 95%); 20 patients (71%) had posterior flattening of the globe and optic nerve sheath distention (sensitivity: 71%; specificity:100 %); 22 patients (78%) had posterior flattening of the globe with vertical tortuosity of the optic nerve (sensitivity: 78%; specificity: 95%); 20 patients (71%) had optic nerve tortuosity or optic nerve distention. Twenty-three patients (82%) had at least one of these four criteria (partial empty sella, optic nerve vertical tortuosity, distention of the optic nerve sheath, posterior globe flattening) (sensitivity: 82%, specificity: 95%).
MRV did not show any significant differences between the two groups in terms of narrowed transverse sinuses (p > 0.05).
Inter-observer reliability was highest for inferior tonsillar displacement (k = 0.850), followed by partial empty sella (k = 0.811), posterior flattening of the globe (k = 0.639), vertical tortuosity of the optic nerve (k = 0.639), distention of the optic nerve sheath (k = 0.579), protrusion of the optic nerve (k = 0.478), and slit like ventricules (k = 0.130).
MRI findings were not significantly different between two genders within the patient group (p > 0.05). In two patients with episodic headache, inferior tonsillar displacement was detected, and this association was statistically significant (p = 0.003). Otherwise, there was no association between the MRI findings and other clinical features (p > 0.05). MRI findings were not associated with impaired VFT, impaired colour vision, diplopia, and gaze palsy in the patient group (p > 0.05).
High CSF pressure was associated with impairment of the VFT (p = 0.002), with a correlation between increased CSF pressure and VFT (p = 0.04). Eleven patients with a CSF pressure greater than 33.5 cm H2O had impaired VFT. However, there was no significant association between CSF pressure and MRI findings.
Discussion
This study was aimed to assess the role of MRI findings in the diagnosis of PTC and their association with clinical and laboratory findigs, as well as the inter-observer reliability between radiologists.
Majority of participants were female, and number of obese women was similar to the subjects with a normal BMI, at odds with most of the previous published reports. On the other hand, in a study from Turkey, obesity was present in 30% of these subjects which was less frequent than reported in studies from Western countries. Male and female participants in our study were comparable in terms of BMI. Previous studies found similar BMI in males diagnosed with idiopathic intracranial hypertension (IIH) and in control subjects, which is consistent with our observations.12–14
The most common symptom at presentation was headache (96.7%), similar to a previous comprehensive review (92%).15 Except for one patient, all had headache. This patient without headache presented with transient visual obscurations and bilateral papilledema, had a CSF pressure of 26 cm H2O and normal VFT, and was diagnosed with primary PTC. Neuroradiological imaging did not reveal any pathological findings but he benefited from lumbar drainage, suggesting that he was diagnosed at an early stage of the condition. A cranial imaging should be performed and PTC should be considered in the differential diagnosis of all patients presenting with papilledema, irrespective of the presence of headache.
There was a significant association between episodic headache and inferior tonsillar displacement. Headache may be induced or worsened by some physical activities and Valsalva maneuver.16,17 Although our results were consistent with these data, since triggering physical activities were not questioned in the initial history taking of our patients, this association has not been clearly elucidated.
Previous studies failed to find associations between orbital MRI and neuro-ophthalmological examination findings such as stage of the papilledema, visual field, and visual acuity.18,19 Consistent with these data, no such associations between MRI findings and neuro-ophthalmological findings (impaired visual acuity, impaired VFT result, diplopia, gaze palsy) were observed in our study.
Significant associations between IIH and posterior flattening of the globe, distention of the optic nerve sheath, vertical tortuosity of the optic nerve, and empty sella were verified in different studies.20,21 Posterior flattening of the globe was reported to be the most sensitive pathological finding for PTC, which was also consistent with our study.3,20 Except for the optic nerve protrusion, all other MRI findings (posterior flattening of the globe, distention of the optic nerve sheath, vertical tortuosity of the optic nerve, empty sella, and preliminary optic nerve contrast uptake) occurred more frequently among patients.3
Increased dimensions of sella and pituitary deformity as a result of chronically elevated intracranial pressure in PTC patients have been shown in previous studies.22,23 In a prospective study, presence of empty sella had a sensitivity of 2.5%, while partially empty sella was 80% sensitive and 75% specific for PTC.24 Specificity rates were similar to our study; however, we have found lower sensitivity rates.
Association of Chiari type 1 malformation (CM1) with papilledema and benefit of CM1 patients from decompression surgery have been reported.25–29 Both CM1 and PTC patients may suffer from tonsillar descent because of different pathophysiological mechanisms. CM1 is considered as a congenital condition, while PTC results in tonsillar descent due to intracranial hypertension. 27 Intracranial hypertension due to tonsillar descent was accepted to represent a secondary PTC and it has been proposed that correction of tonsillar descent could normalize the intracranial pressure.28,30 However, there are patients who have improved with decompression surgery, as well as others in whom the condition recurred or did not result in an improvement, necessitating a shunt surgery.31 In our patient group, one had CMI. The patient did not improve with medical treatment and worsened after repeated LPs. She was operated by neurosurgeons for posterior fossa decompression. Postoperatively, papilledema and diplopia resolved completely. This patient represents one of the secondary PTC cases in our study. The patient’s response to decompression surgery suggests that PTC was due to CM1 rather than tonsillar descent was caused by intracranial hypertension.
There have been several reports of transverse sinus stenosis due to elevated CSF, as documented by MRV. However, some authors suggest that intracranial hypertension conversely may arise from hypoplastic or narrowed transverse sinuses, which represent anatomic variations. Such patients may also benefit from stenting.32–34 The cause-and-effect relationship has not been clearly understood. Transverse sinus hypoplasia is a rather common finding in the general population, with some studies reporting unilateral transverse sinus flow impairment in up to 30% of individuals in the general population. However, bilateral transvers sinus stenosis is not common and should be considered as an underlying factor of PTC, and patients with this finding should be evaluated as possible candidates for this syndrome. In our study, patients and controls did not differ significantly with regard to the frequency of transverse sinus hypoplasia or stenosis. In patients with transverse sinus stenosis, no control MRV was performed after the treatment. Therefore, it was not possible to determine whether stenosis was an etiological factor or the result of the condition. Three patients had thrombosis in the transverse sinuses. One of these secondary PTC patients had a history of hormone treatment for pregnancy. Another patient had a past history of orbital myositis. During the follow-up, she had positive laboratory results for lupus anticoagulant and control MRI showed dural contrast enhancement. No underlying infection could be detected; thus a diagnosis of autoimmune pachymeningitis was made. In the third case with superior sagittal sinus thrombosis, no etiological factors could be identified.
In our study, frequency of partial empty sella, posterior flattening of the globe, protrusion of the optic nerve, optic nerve sheath distention, and vertical tortuosity of the optic nerve were significantly different between patient and control groups, consistent with previous reports. Partial empty sella, posterior flattening of the globe, optic nerve sheath distention, and vertical tortuosity of the optic nerve were discriminative between patient and control group with sensitivity and specificity of 43% and 100%; 64% and 100%; 46% and 100%, and 60% and 95%, respectively. However, the AUC calculated for optic nerve protrusion was not statistically significant (p = 0.060).
Partial empty sella and cerebellar tonsillar herniation, which are based on actual measurements, showed the highest kappa values in this study. On the other hand, low kappa values for the distention of the optic nerve sheath, which is another parameter obtained from the measurement, indicate that it is difficult to measure this finding. Due to higher kappa values and sensitivities as compared to other MRI findings, posterior flattening of the globe, vertical tortuosity of the optic nerve, optic nerve distention, and partial empty sella emerged as particularly valuable markers for a diagnosis and follow-up of PTC.
Pairwise combinations of meaningful MRI findings showed a higher sensitivity, although still modest, for the combination of the posterior flattening of the globe and vertical tortuosity of the optic nerve. If at least one of the following four MRI parameters (optic nerve tortuosity, partial emtpy sella, posterior flattening of the globe, distention of the optic nerve sheath) is present, sensitivity and true positive rates reached the highest values.
Significantly higher occurrence of impaired VFT was detected in patients with a CSF pressure exceeding 33.5 cm H2O. Elevated CSF pressure correlates with the impairment of VFT, demonstrating the fact that above a certain CSF pressure, there is more marked compression on the optic nerves and that the optic nerve injury becomes more apparent in CSF pressure values greater than the one used as a criteria for the diagnosis of PTC (i.e. ≥ 25 cm H2O).
The major limitation of our study is its small sample size. Also, greater expertise of the neuro-radiologists performing the readings could yield higher sensitivity and specificity. Furthermore, improvement in MRI findings could be checked through control MRI studies and the association between pathological findings and CSF pressure could be more clearly delineated. Recent studies assessing PTC patients reported narrowing in Meckel’s cavity and cavernous sinuses with MRI and dilatation in foramen ovale with cranial CT imaging.1 These changes were not assessed in our study, although a retrospective assessment of these findings is planned.
According to our study, partial empty sella, optic nerve sheath distention, posterior flattening of the globe, and vertical tortuosity of the optic nerve were found to show a significant correlation with PTC. These parameters are supportive findings in the diagnosis of PTC. However, a sizeable number of PTC cases may be missed by focusing on these MRI parameters only. MRI may be also useful in the follow-up of patients or in situations where LP cannot be performed.
Declaration of interest
There is no conflict of interest of any of the authors.
References
- 1.Friedman DI, Liu GT, Digre KB.. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. [DOI] [PubMed] [Google Scholar]
- 3.Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105:1686–1693. doi: 10.1016/S0161-6420(98)99039-X. [DOI] [PubMed] [Google Scholar]
- 4.George AE. Idiopathic intracranial hypertension: pathogenesis and the role of MR imaging. Radiology. 1989;170:21–22. doi: 10.1148/radiology.170.1.2909099. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson HG, Shapiro JH. Pseudotumor Cerebri. Radiology. 1964;82:202–210. doi: 10.1148/82.2.202. [DOI] [PubMed] [Google Scholar]
- 6.Silbergleit R, Junck L, Gebarski SS, Hatfield MK. Idiopathic intracranial hypertension (pseudotumor cerebri): MR imaging. Radiology. 1989;170:207–209. doi: 10.1148/radiology.170.1.2909098. [DOI] [PubMed] [Google Scholar]
- 7.Lightfoote WE, Pressman BD. Increased intracranial pressure: evaluation by computerized tomography. Am J Roentgenol Radium Ther Nucl Med. 1975;124:195–198. [DOI] [PubMed] [Google Scholar]
- 8.Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48:521–527. doi: 10.1007/s00234-006-0095-y. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky MC. Flattening of the posterior sclera: hypotony or elevated intracranial pressure? Am J Ophthalmol. 2004;138:511–512. doi: 10.1016/j.ajo.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Gass A, Barker GJ, Riordan-Eva P, et al. MRI of the optic nerve in benign intracranial hypertension. Neuroradiology. 1996;38:769–773. [DOI] [PubMed] [Google Scholar]
- 11.Jinkins JR, Athale S, Xiong L, Yuh WT, Rothman MI, Nquyen PT. MR of optic papilla protrusion in patients with high intracranial pressure. AJNR Am J Neuroradiol. 1996;17:665–668. [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–309. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz R, Kliper E, Stern N, Dotan G, Berliner S, Kesler A. The obesity pattern of idiopathic intracranial hypertension in men. Graefes Arch Clin Exp Ophthalmol. 2013;251:2643–2646. doi: 10.1007/s00417-013-2420-6. [DOI] [PubMed] [Google Scholar]
- 14.Pollak L, Zohar E, Glovinsky Y, Huna-Baron R. Reevaluation of presentation and course of idiopathic intracranial hypertension a large cohort comprehensive study. Acta Neurol Scand. 2013;127:406–412. doi: 10.1111/ane.12060. [DOI] [PubMed] [Google Scholar]
- 15.Ljubisavljevic S, Zidverc TJ, Covickovic SN, Spasic M, Kostic V. Idiopathic intracranial hypertension from the perspective of headache center. Acta Neurol Belg. 2013;113:487–492. doi: 10.1007/s13760-013-0228-0. [DOI] [PubMed] [Google Scholar]
- 16.Riveira C, Pascual C. Is Chiari type I malformation a reason for chronic daily headache. Curr Pain Headache Rep. 2007;11:53–55. [DOI] [PubMed] [Google Scholar]
- 17.Watkins JS. Paroxysmal headache due to the Chiari malformation. Dis Nerv Syst. 1969;30:693–695. [PubMed] [Google Scholar]
- 18.Padhye LV, Van Stavern GP, Sharma A, Viets R, Huecker JB, Gordon MO. Association between visual parameters and neuroimaging features of idiopathic intracranial hypertension. J Neurol Sci. 2013;332:80–85. doi: 10.1016/j.jns.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saindane AM, Bruce BB, Riggeal BD, Newman NJ, Biousse V. Association of MRI findings and visual outcome in idiopathic intracranial hypertension. AJR Am J Roentgenol. 2013;201:412–418. doi: 10.2214/AJR.12.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agid R, Farb RI. Neuroimaging in the diagnosis of idiopathic intracranial hypertension. Minerva Med. 2006;97:365–370. [PubMed] [Google Scholar]
- 21.Maralani PJ, Hassanlou M, Torres C, et al. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin Radiol. 2012;67:656–663. doi: 10.1016/j.crad.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Ranganathan S, Lee SH, Checkver A, et al. Magnetic resonance imaging finding of empty sella in obesity related idiopathic intracranial hypertension is associated with enlarged sella turcica. Neuroradiology. 2013;55:955–961. doi: 10.1007/s00234-013-1207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyung SE, Botelho JV, Horton JC. Enlargement of the sella turcica in pseudotumor cerebri. J Neurosurg. 2014;120:538–542. doi: 10.3171/2013.10.JNS131265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuh WT, Zhu M, Taoka T, et al. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging. 2000;12:808–813. [DOI] [PubMed] [Google Scholar]
- 25.Choudhari KA, Cooke C, Tan MH, Gray WJ. Papilloedema as the sole presenting feature of Chiari I malformation. Br J Neurosurg. 2002;16:398–400. [DOI] [PubMed] [Google Scholar]
- 26.Vaphiades MS. Resolution of papilledema after neurosurgical decompression for primary Chiari I malformation. Am J Ophthalmol. 2002;133:673–678. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JC, Bakir B, Lee A, Yalamanchili SS. Papilloedema due to Chiari I malformation. BMJ Case Rep. 2011. doi: 10.1136/bcr.08.2011.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bejjani GK. Association of the adult chiari malformation and idiopathic intracranial hypertension: more than a coincidence. Med Hypotheses. 2003;60:859–863. [DOI] [PubMed] [Google Scholar]
- 29.Istek S. Chiari type 1 malformation in a pseudotumour cerebri patient: is it an acquired or congenital Chiari malformation? BMJ Case Rep. 2014. doi: 10.1136/bcr-2013-201845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banik R, Lin D, Miller NR. Prevalence of Chiari I malformation and cerebellar ectopia in patients with pseudotumor cerebri. J Neurol Sci. 2006;247:71–75. doi: 10.1016/j.jns.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Aiken AH, Hoots JA, Saindane AM, Hudgins PA. Incidence of cerebellar tonsillar ectopia in idiopathic intracranial hypertension: a mimic of the Chiari I malformation. AJNR Am J Neuroradiol. 2012;33:1901–1906. doi: 10.3174/ajnr.A3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bussière M, Falero R, Nicolle D, Proulx A, Patel V, Pelz D. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2010;31:645–650. doi: 10.3174/ajnr.A1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol. 2011;32:1408–1414. doi: 10.3174/ajnr.A2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnet A. Idiopathic intracranial hypertension: stent or not. Rev Neurol. 2012;168:685–690. doi: 10.1016/j.neurol.2012.07.014. [DOI] [PubMed] [Google Scholar]


