ABSTRACT
Directional and non-directional environmental cues are able to induce polar behaviors of plants, which are termed tropic and nastic movements, respectively. While molecular mechanisms underlying the directionality of tropic movements are relatively well studied, it is poorly understood how the polarity of nastic movements is determined in response to non-directional stimuli, such as ambient temperatures. It has recently been shown that thermal induction of leaf hyponasty is stimulated by developmentally programmed polar auxin transport in Arabidopsis. Under warm environments, the PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) transcription factor binds to the promoter of PINOID (PID) gene, whose gene product modulates the polar trafficking of the auxin transporter PIN-FORMED 3 (PIN3). Notably, PIF4 binding to the PID promoter occurs predominantly in the abaxial petiole cells than the adaxial petiole cells, leading to differential PID expression and thus asymmetric auxin accumulation in the petiole cells. In addition, ASYMMETRIC LEAVES 1 (AS1), the well-characterized leaf polarity-determining epigenetic regulator, promotes the PID expression by modulating the patterns of histone 4 acetylation (H4Ac) in the PID chromatin. These observations demonstrate that developmental programming of the thermonastic leaf movement through polar auxin distribution enables plants to bend their leaves upward in response to non-directional thermal stimuli, contributing to cooling plant body temperatures under warm temperature conditions. We propose that a developmentally predetermined polarity plays a major role in governing the directionality of various nastic movements in plants.
KEYWORDS: Leaf thermonasty, AS1, polar auxin transport, thermomorphogenesis
Text
Environmental stimuli under natural conditions can be categorized into two major groups depending on whether they harbor directional information or not: directional or non-directional ones. Both two types of stimuli are capable of generating directional behaviors of specific plant organs, termed tropic and nastic movements, respectively. The tropic and nastic movements require asymmetric growth in plant tissues. The best characterized is the polar auxin transport, which directs asymmetric cell elongation during tropic movements.1,2 The polar auxin flow also plays an important role in regulating the directionality of nastic movements.3 A well-known example of nastic plant behaviors is the upward bending of leaves under warm environments, frequently termed leaf thermonasty.4 It has been reported that thermonastic leaf movements are promoted by the asymmetric growth between the adaxial and abaxial petiole cells.3 However, it is largely unknown whether and how polar auxin transport elicits the directional movements of plant organs under warm temperature conditions.
The protein kinase PINOID (PID) has been proven to shape the asymmetric growth of plant organs by regulating the polar localization of the auxin transporter PIN-FORMED 3 (PIN3).5,6 It has been observed that PIN3 is preferentially localized to the outer membranes of the abaxial petiole cells at warm temperatures. Recently, it has been observed that the thermal induction of PID expression is higher in the abaxial petiole cells than in the adaxial petiole cells.3 Notably, the temperature-dependent differential expression of PID gene is diminished in the pif4-101 mutant that lacks the PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) transcription factor, suggesting that PIF4 is required for the polar auxin transport during leaf thermonasty. Accordingly, the pif4-101 mutant did not exhibit leaf thermonasty. These observations indicate that PIF4 promotes leaf thermonasty by modulating the differential transcription of PID gene in the adaxial and abaxial petiole cells.3
A critical question is how PIF4 is associated with the PID function in directing polarized auxin flow during leaf thermonastic movements. Chromatin immunoprecipitation (ChIP) assays revealed that PIF4 binds directly to the PID promoters under warm temperature conditions. Furthermore, the DNA-binding affinity of PIF4 to the PID promoters was more prominent in the abaxial petiole cells, indicating that the thermo-responsive action of the PIF4 transcription factor is differentiated along the adaxial-abaxial polarity of the leaf petioles.
It is particularly interesting that the PIF4-mediated polar auxin transport occurs predominantly in the abaxial petiole cells in response to non-directional warm temperature stimuli, raising a central question as to molecular mechanisms that determine the polarity of leaf thermonasty. Light and gravity, which are two major determinants of polarized plant growth,1,2 do not affect the polarity of leaf thermonasty,3 suggesting that certain intrinsic signals would be involved in specifying the polarity of leaf thermonasty.
It has been well characterized that the epigenetic regulator ASYMMETRIC LEAVES 1 (AS1) modulates the expression of leaf polarity-specifying genes in plants,7–9 raising a possibility that the leaf polarity specifier is functionally linked with leaf thermonasty. In support of this notion, it was found that leaf thermonasty and differential PID expression patterns were severely impaired in the AS1-deficient as1-1 mutant. In addition, the thermally induced DNA binding of PIF4 to the PID promoters was also disrupted in the as1-1 mutant. On the basis of the biochemical nature of AS1 function in mediating histone modifications,10 the patterns of H4Ac were examined in the genomic DNA sequence harboring the PID locus. The results have shown that while the thermal-induction of H4Ac in the PID locus is prominent in the abaxial petiole cells in wild-type plants, it is compromised in the as1-1 mutant. It is thus apparent that the AS1-mediated developmental signals determine the patterns of asymmetric growth of the adaxial and abaxial petiole cells by modulating PIF4 binding to the PID promoters under warm environments.
It is evident that the directional leaf hyponastic movements occur under warm environments. A next question is how temperature sensing mechanisms are functionally associated with the thermonastic leaf movement. It is known that the conversion of the PHYTOCHROME B (phyB) photoreceptor from the physiologically active Pfr form to the inactive Pr form is accelerated at warm temperatures,11 supporting that the phytochrome photoreceptors act as a temperature sensor.
A challenging question is whether the phytochrome-mediated temperature sensing is related with leaf thermonastic movement. We found that overexpression of a constitutively active form of phyB, YHB, markedly diminished leaf hyponasty at warm temperatures (Figure 1a), suggesting that the thermal reversion of phyB is required for the leaf thermonastic leaf movement. It has been previously reported that phyB negatively regulates the protein stability of the downstream PIF4 transcription factor.12 Consistent with the thermal inactivation of phyB, we observed that PIF4 protein accumulates to a higher level at warm temperatures (Figure 1b), further supporting that the phyB-mediated temperature sensing mechanism is functionally associated with leaf thermonasty.
Figure 1.
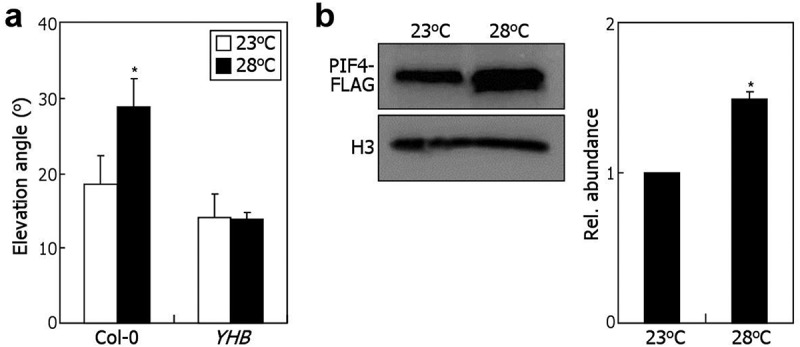
The phytochrome photoreceptors are functionally linked with leaf thermonasty.
Three measurements, each consisting of 16 individual plants grown under identical conditions, were averaged and statistically analyzed (t-test, *P < .01). Bars indicate standard error of the mean. (a) Leaf thermonasty in YHB transgenic plants. The YHB transgenic plants express a constitutively active form of phytochrome B. Elevation angles of the 5th and 6th rosette leaves relative to the horizontal plane were measured using three-week-old plants grown either at 23°C or 28°C for 6 h. (b) Thermal induction of PIF4 protein accumulation. Transgenic plants expressing the pPIF4:PIF4-FLAG construct were grown at 23°C for three weeks and then exposed to either 23°C or 28°C for 6 h. Leaf petioles were harvested for total protein extraction. The PIF4-FLAG proteins were immunologically detected using an anti-FLAG antibody. Immunological detection of histone 3 (H3) was performed in parallel as loading control.
Plants always bend their leaves upward in response to non-directional temperature stimuli.3,4 It is well known that plants exhibit distinct morphological alterations, such as hypocotyl overgrowth and increased leaf hyponasty, at warm temperatures,13–15 collectively termed thermomorphogenesis. The thermomorphogenic responses are known to facilitate leaf cooling and protect the heat-labile shoot apical meristem from the warm or hot soil surface;4 suggesting a possibility that leaf thermonasty contributes to cooling body temperatures. In agreement with the hypothesis, the temperature of the leaves arrested close to the soil surface was higher than the leaves exhibiting leaf hyponasty under warm environments.3 Together, these results indicate that the directionality of thermonastic leaf movement enables plants to promote the cooling capacity of plant organs.
Our findings provide an excellent research system for the developmental control of environmental adaptation in plants. Polarity of thermonastic leaf movement is shaped by the developmentally programmed polar auxin transport. Why is it advantageous for plants to utilize intrinsic signals rather than environmental cues in directing thermonastic leaf movement? It is speculated that directional information given by developmental signals would be more advantageous than directional external stimuli under certain circumstances, in which plants are exposed to distorted direction of light or gravity in darkness or on a slope. It will be interesting to explore whether our leaf thermonastic model is also applicable to other nastic movements, such as flooding-induced hyponastic growth in Arabidopsis and nastic behaviors in mimosa and Venus flytrap:16–18
Funding Statement
This work was supported by the Leaping Research (NRF-2018R1A2A1A19020840) and Global Research Lab (NRF-2012K1A1A2055546) Programs provided by the National Research Foundation of Korea (NRF) and the Next-Generation BioGreen 21 Program (PJ013134) provided by the Rural Development Administration of Korea. Y.-J.P. was partially supported by Global Ph.D. Fellowship Program through NRF (NRF-2016H1A2A1906534).
References
- 1.Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski Ł, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol. 2011;13:447–452. doi: 10.1038/ncb2208. [DOI] [PubMed] [Google Scholar]
- 2.Rakusová H, Gallego-Bartolomé J, Vanstraelen M, Robert HS, Alabadí D, Blázquez MA, Benková E, Friml J.. Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J. 2011;67:817–826. doi: 10.1111/j.1365-313X.2011.04636.x. [DOI] [PubMed] [Google Scholar]
- 3.Park YJ, Lee HJ, Gil KE, Kim JY, Lee JH, Lee H, Cho HT, Vu LD, De Smet I, Park CM.. Developmental programming of thermonastic leaf movement. Plant Physiol. 2019. doi: 10.1104/pp.19.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA. High temperature exposure increases plant cooling capacity. Curr Biol. 2012;22:R396–R397. doi: 10.1016/j.cub.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 5.Kleine-Vehn J, Huang F, Naramoto S, Zhang J, Michniewicz M, Offringa R, Friml J. PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell. 2009;21:3839–3849. doi: 10.1105/tpc.109.071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zourelidou M, Absmanner B, Weller B, Barbosa IC, Willige BC, Fastner A, Streit V, Port SA, Colcombet J, de la Fuente van Bentem S, et al. Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. Elife. 2014;3:e02860. doi: 10.7554/eLife.02860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–709. doi: 10.1038/35079629. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki M, Takahashi H, Iwakawa H, Nakagawa A, Ishikawa T, Tanaka H, Matsumura Y, Pekker I, Eshed Y, Vial-Pradel S, et al. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development. 2013;140:1958–1969. doi: 10.1242/dev.085365. [DOI] [PubMed] [Google Scholar]
- 9.Merelo P, Ram H, Pia Caggiano M, Ohno C, Ott F, Straub D, Graeff M, Cho SK, Yang SW, Wenkel S, et al. Regulation of MIR165/166 by class II and class III homeodomain leucine zipper proteins establishes leaf polarity. Proc Natl Acad Sci U S A. 2016;113:11973–11978. doi: 10.1073/pnas.1516110113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo M, Yu CW, Chen FF, Zhao L, Tian G, Liu X, Cui Y, Yang JY, Wu K. Histone deacetylase HDA6 is functionally associated with AS1 in repression of KNOX genes in Arabidopsis. PLoS Genet. 2012;8:e1003114. doi: 10.1371/journal.pgen.1003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, et al. Phytochromes function as thermosensors in Arabidopsis. Science. 2016;354:886–889. doi: 10.1126/science.aaf6005. [DOI] [PubMed] [Google Scholar]
- 12.Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. Phytochrome‐mediated inhibition of shade avoidance involves degradation of growth‐promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 13.Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol. 2009;19:408–413. doi: 10.1016/j.cub.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Ye S, Yu P, Breen G, Cohen JD, et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci U S A. 2011;108:20231–20235. doi: 10.1073/pnas.1110682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park YJ, Lee HJ, Ha JH, Kim JY, Park CM. COP1 conveys warm temperature information to hypocotyl thermomorphogenesis. New Phytol. 2017;215:169–280. doi: 10.1111/nph.14581. [DOI] [PubMed] [Google Scholar]
- 16.Sasidharan R, Voesenek LA. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015;169:3–12. doi: 10.1104/pp.15.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Q, Dai E, Han X, Xie S, Chao E, Chen Z. Fast nastic motion of plants and bioinspired structures. J R Soc Interface. 2015;12:0598. doi: 10.1098/rsif.2015.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433:421–425. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]


