Abstract
Background
Infection in acute pancreatitis (AP) is associated with nutritional therapies including naso-gastric (NG), naso-jejunal (NJ), and total parenteral nutrition (TPN). To examine infections among NG, NJ, TPN, and no nutritional support (NNS) in treating patients with AP.
Methods
The investigators completed comprehensive search in the Cochrane library, EMBASE, PubMed, Web of Science, and ClinicalTrials.gov without restriction on language and publication date before January 21, 2019. They also searched the reference lists of relevant studies for randomized controlled trials (RCTs) comparing NG, NJ, TPN, and NNS among patients with AP. Quantitative synthesis was conducted in a contrast-based network meta-analysis. To clarify effects, a network meta-analysis was conducted to calculate the surface under the cumulative ranking curve (SUCRA). Beside of overall infections, the event rates of infected pancreatic necrosis, bacteremia, line infection, pneumonia, urinary tract infection, and other types of infections were measured.
Results
The network meta-analysis of 16 RCTs showed that NJ had significantly lower overall infection rates compared with TPN (risk ratio: 0.59; 95% confidence interval: 0.38, 0.90); and NG had a larger effect size and higher rank probability compared with NJ, TPN, and NNS (mean rank = 1.7; SUCRA = 75.8). TPN was the least preferred (mean rank = 3.2; SUCRA = 26.6).
Conclusions
NG and NJ may be preferred therapies for treating patients with AP. Clinicians may consider NG as a first-line treatment for patients with AP (including severe AP) and even in patients receiving prophylactic antibiotics. In addition, we found that NNS should be avoided when treating patients with severe AP.
Introduction
Nutritional therapy is an important topic for patients with acute pancreatitis, and it covers standard therapy and nutritional therapy.[1] Standard therapy refers to when patients can tolerate it. Nutritional therapy involves three routes including nasogastric tube (NG) feeding, nasojejunal tube (NJ) feeding enteral and parenteral nutrition. NG feeding and NJ feeding are enteral nutrition. Parenteral nutrition can be provided independently (total parenteral nutrition, TPN), or be combined with enteral nutrition. Numerous randomized controlled trials (RCTs) and systematic review have attempted to determine the most effective nutrition therapy among NG, NJ, TPN, and NNS in past decades.[2, 3] Although the guideline suggested that enteral nutrition is superior to TPN in terms of reducing the infectious complication of predicted severe acute pancreatitis (pSAP),[4] further discussion is still required.
Firstly, recommendations about nutritional routes for acute pancreatitis usually are based on relevant systematic reviews.[2, 3, 5] They included important RCTs.[6–13] Nevertheless, the previous systematic reviews of enteral nutrition typically refer to the nasojejunal route. As we know, NG is located differently from NJ and may directly stimulate pancreatic exocrine secretion or even exacerbate pancreatic inflammation.[14] Thus, the definitions of enteral routes require clarification, and NJ and NG feeding should be discussed separately. Secondly, two systematic reviews cited by the guideline performed several errors.[4, 15] Data in the two systematic reviews differed from the original reports. For instance, we found that the data for local septic complications (infected necrosis and pancreatic abscess formation) in the TPN differs from the original report by Petrov et al.,[2, 5, 12] and data for infectious complication also differ from the article by Eckerwall et al. [3, 8] The errors should be corrected. Thirdly, there is a need for understanding the effects of prophylactic antibiotics for reducing infections in patients with pSAP,[16, 17] but previous systematic reviews did not considered prophylactic antibiotics usage in their analyses. Moreover, no evidence synthesized NG, NJ, TPN, and NNS in consistency model.
Therefore, we aimed to determine the safety of various nutritional therapies for acute pancreatitis through network meta-analysis of infectious complications. In addition, we also explored the influence from severity and prophylactic antibiotics usage. Our results may provide a clear and practical guidance to nutritional therapies for treating patients with acute pancreatitis.
Methods
This systematic review with network meta-analysis was conducted by a multi-disciplinary research team involving gastroenterologist, dietitian, and an experienced researcher in systematic review and network meta-analysis.[18, 19] The experienced researcher also participated in gastroenterological studies.[20, 21] Our team followed the PRISMA guidelines, and the study protocol was registered on PROSPERO (CRD42017084125): https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=84125.
Data sources and searches
We selected RCTs of nutritional therapies for acute pancreatitis from the EMBASE, PubMed, Web of Science, and Cochrane library databases. The primary systematic search strategy with relevant terms was completed in PubMed and was adapted to other databases before January 21st, 2019 (S1 Table). The search strategy consisted of free-text, medical subject headings (MeSH in PubMed and Emtree in EMBASE), abbreviations, appropriate Boolean algebras, and no restrictions on language and publication date.
Study selection
Two authors (P.H.H and Y.N.K) screened citations identified by systematic searches. Disagreement during the study selection was resolved through discussion. Inclusion criteria for the selection were defined beforehand as follows: (1) patients with pancreatitis; (2) treated by NNS, TPN, or enteral therapies; and (3) RCT. This study used kappa coefficient for thee inter-rater reliability for study selection. The discussion for disagreement involved four steps including (1) reading methods together, (2) explaining why the author would like to include, (3) explaining why the other author would like to exclude, and (4) making decision together.
Data extraction and quality assessment
Two authors (P.H.H and C.Y.L) individually reviewed the included RCTs for quality evaluation and data extraction. The quality of the RCTs was evaluated using the Cochrane risk of bias tool. Disagreements on the risk of bias evaluations were resolved through discussion. The authors identified relevant information and extracted outcome data. The relevant information included age, prophylactic antibiotics, and severity of acute pancreatitis. Data on infectious events from previous meta-analyses did not consider differences between the numbers of infectious patients and infectious events. Unclear definitions may lead to unclear or mistaken outcomes; therefore, we used the clear definition of the number of infectious patients as a standard counting unit in all analyses. The outcomes of this systematic review were overall infections (total number of infectious patients), infected pancreatic necrosis, bacteremia, line infection, pneumonia, urinary tract infections, and other types of infection (OTIs). The OTIs included bile culture, sepsis, unspecified drain, and wound infection.
Data synthesis and analysis
We performed a quantitative synthesis through a meta-analysis, which used the risk ratio (RR) for binary data and was conducted in a random-effects model. The effect size was calculated using a 95% confidence interval (CI) and P value. A P value of <0.05 was considered statistically significant in all analyses. A small study bias in the meta-analysis was examined using a funnel plot with Egger’s regression intercept. Inconsistency in the network meta-analysis was examined using the Lu–Ades loop inconsistency test. Network meta-analysis was conducted in STATA version 14.
To examine effects, we calculated the surface under the cumulative ranking curve (SUCRA) and further analyzed the overall infection rate based on the severity of acute pancreatitis and prophylactic antibiotics usage. The SUCRA is a statistical technique with advantage as more than two comparators in a meta-analysis model by demonstrating a hierarchy of intervention rankings. It provides the probability of an intervention being among the most effective interventions.[22] For further analysis of the severity of acute pancreatitis, the network meta-analysis synthesized RCTs that included only patients with pSAP. For further analysis of prophylactic antibiotics, the network meta-analysis synthesized only RCTs that administered prophylactic antibiotics to every patient.
Results
A total of 628 potential references were identified in databases through systematic searches (n = 617) and hand search (n = 11), of which 177 were duplicated. Of the remaining 451 references, we excluded 409 after title and abstract screening. Subsequently, we retrieved the full text of 42 references for further review. Finally, we excluded 17 of the 42 references because they met the exclusion criteria. The excluded 17 references were conference abstract without complete information (n = 9),[23–31] systematic review (n = 3),[32–34] relevant document (n = 2),[35–37] and no comparison for nutritional routes (n = 2).[38, 39] Among the 42 references, two authors had agreements on 40 references (25 were included and 15 were excluded), and there were two references were disagreed in the individual review.[38, 39] After the discussion, the two references were excluded because of no comparison between nutritional routes. The kappa coefficient (0.899) reflected that the authors had similar judgement. The 25 eligible references after the full-text review were from 22 RCTs, and they were included in this study for qualitative and quantitative synthesis.[6–13, 40–56] Fig 1 presents a flow diagram of study selection according to PRISMA guidelines.
Fig 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of study identification process.
Characteristics and quality of included studies
The 22 RCTs included in the study consisted of 1379 patients and were conducted in the United States,[40, 45, 51] Canada,[11] China,[13, 41, 54–56] Croatia,[53] Greece,[10] Hungary,[47] India,[7, 43, 52] New Zealand,[44, 46, 48, 49] Russia,[12] Scotland,[42, 50] Spain,[6] Sweden,[8] and the United Kingdom[9] from 1984 to 2012. These RCTs included 4 therapies: NNS (n = 190), TPN (n = 420), NG (n = 163), and NJ (n = 562). The overall quality of the RCTs is shown in S2 Table. The available information showed that the age of patients in each RCT ranged from 36–72 years. In total, 780 men (58.43%) were included in the RCTs. Eleven RCTs provided all patients with prophylactic antibiotics,[7, 9, 10, 12, 13, 41, 50, 53–56] and 17 RCTs only included patients with SAP (Table 1).[6–13, 41–43, 50, 52–56] Among the 22 trials, two authors had agreements on 264 main extractions (196 information were extracted and 68 items were no information), and there were 22 disagreements in the individual review. The kappa coefficient (0.808) indicated that the agreement between authors was acceptable. Further information is shown in S3 Table.
Table 1. Characteristics of the included randomized controlled trials.
| Inclusion | All | All | Sample size | Age | Implementing TPN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Region | period | SAP1 | PAB | TPN | NNS | NJ | NG | TPN | NNS | NJ | NG | timing |
| Abou-Assi [40] | USA | 2000/1-2000/12 | 27 | 26 | 50 2 | 48 | About 48 hours | ||||||
| Casas [6] | Spain | N/A | ✓ | 11 | 11 | 55.6 | 61.2 | Immediate | |||||
| Doley [7] | India | 2006/7-2007/12 | ✓ | ✓ | 25 | 25 | 41.1 | 38.4 | Within 72 hours | ||||
| Du [41] | China | 2009/3-2013/12 | ✓ | ✓ | 40 | 40 | 43 3 | 41 | N/A | ||||
| Eckerwall [8] | Sweden | 2002/1-2004/12 | ✓ | 26 | 24 | 68 3 | 71 3 | Within 24 hours | |||||
| Entock [42] | Scotland | 1997/10-2000/7 | ✓ | 22 | 27 | 58 | 63 3 | N/A | |||||
| Gupta [9] | UK | 1996/11-1998/4 | ✓ | ✓ | 10 | 11 | 57 | 65 | About 24 hours | ||||
| He [55] | China | N/A | ✓ | ✓ | 22 | 25 | 40.2 | 39.6 | Within 48 hours | ||||
| Kalfarentzos [10] | Greece | 1990/7-1995/12 | ✓ | ✓ | 20 | 18 | 67.2 | 63 | Within 48 hours | ||||
| Kumar [43] | India | 2002/9-2003/12 | ✓ | 14 | 16 | 35.57 | 43.25 | N/A | |||||
| McClave [45] | USA | N/A | 16 | 16 | 45.1 | 47.64 | Within 48 hours | ||||||
| Louie [11] | Canada | 1999/7-2001/12 | ✓ | 18 | 10 | 59 | 65.3 | Within 24 hours | |||||
| MIMOSA trial [44, 46, 48, 49] | New Zealand | 2010/5-2011/4 | 18 | 17 | 55 | 41 | N/A | ||||||
| Olah [47] | Hungary | 1995/1-1996/5 | 48 | 41 | 43.8 | 47.2 | Within 24 hours | ||||||
| Petrov [12] | Russia | 2002/3-2004/12 | ✓ | ✓ | 35 | 35 | 52 3 | 51 3 | Within 72 hours | ||||
| Powell [50] | Scotland | 1996/12-1998/6 | ✓ | ✓ | 14 | 13 | 52 | 64 | N/A | ||||
| Sax [51] | America | 1984/7-1985/12 | 28 | 26 | 39.8 | 39.8 | Within 24 hours | ||||||
| Singh [52] | India | 2005/1-2007/12 | ✓ | 39 | 39 | 39.7 | 39.1 | N/A | |||||
| Stimac [53] | Croatia | 2007/5-2012/2 | ✓ | ✓ | 107 | 107 | 72 | 69 3 | N/A | ||||
| Wang [54] | China | 2006/1–2011/12 | ✓ | ✓ | 60 | 61 | 41.7 | 43.15 | Within 48 hours | ||||
| Wu [13] | China | 2003/11-2007/12 | ✓ | ✓ | 54 | 53 | 54 | 52 | About 48 hours | ||||
| Zhang [56] | China | 2006/1-2009/10 | ✓ | ✓ | 42 | 42 | 48.6 | 47.4 | About 72 hours | ||||
1 N/A, not applicable; NG, naso-gastric; NJ, naso-jejeunal; NNS, no nutrition support; PAB, prophylactic anti-biotics; SAP, severe acute pancreatitis; TPN, total parenteral nutrition.
2 Mean (all such values unless otherwise indicated).
3 Median.
Primary outcome: Total number of infectious patients
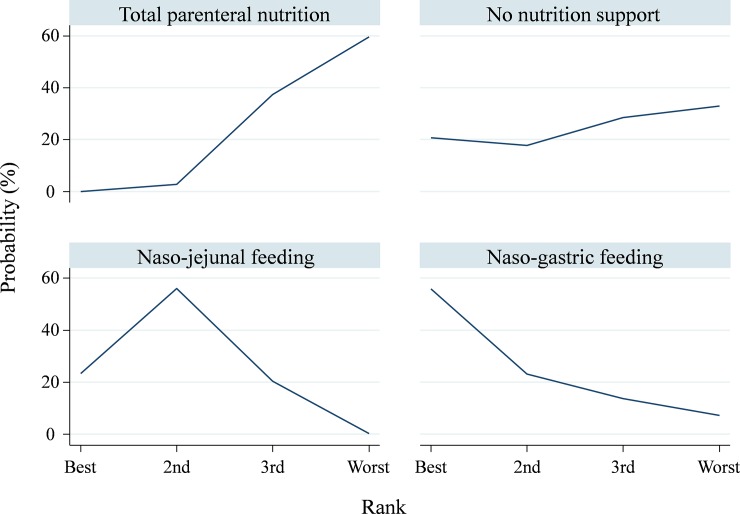
Of the 22 RCTs, 16 showed data on the total number of infectious patients.[6–12, 41, 45, 47, 51–56] In overall, per infected patient proximately had one to two kind of infections. In the consistency model, the results showed that NJ led to a significantly lower total infection rate compared with TPN (RR, 0.59; 95% CI, 0.38, 0.90), but NG had the largest effect size compared with other therapies (Fig 2 and Appendix A1 in S1 File). The SUCRA value based on cumulative ranking probabilities showed that NG may be the optimal therapy (mean rank = 1.7; SUCRA = 75.8), and TPN should be avoided when treating patients with acute pancreatitis (mean rank = 3.6; SUCRA = 14.5) (Fig 3). To assess influence of inclusion year and TPN implementing timing on the pooled result, meta-regression in network meta-analysis model did not find significant influence (S4 Table). No inconsistency (loop inconsistency χ2 = 5.40; P = 0.07) (Appendix A2 in S1 File) or small study bias (Egger’s test t = −1.68; P = 0.12) was detected in this consistency model (Appendix A3 in S1 File)
Fig 2. Network geometry of total number of infectious patients among nutritional therapies and no nutritional support.
Fig 3. Surface under the cumulative ranking curve of total number of infectious patients among nutritional therapies and no nutritional support.
Table 2 presents a summary of further network meta-analysis for total infection (pSAP and prophylactic antibiotics). The 12 RCTs that recruited patients with pSAP showed that the result in the consistency model was similar to the overall pooling.[6–12, 41, 52, 54–56] The NJ led to a significantly lower total infection rate compared with TPN (RR, 0.56; 95% CI, 0.34, 0.92), but NG had the largest effect size compared with other therapies (Table 2 and Appendix B1 in S1 File). Moreover, the SUCRA value showed that NG may be the optimal nutritional route (mean rank = 1.6; SUCRA = 80.9), and TPN should be avoided when treating patients with acute pancreatitis (mean rank = 3.2; SUCRA = 26.6) (Appendix B2 in S1 File). No evidence indicated inconsistency (loop inconsistency χ2 = 3.68; P = 0.06) (Appendix B3 in S1 File) or small study bias (Egger’s test t = -1.75; P = 0.11) in this network meta-analysis (Appendix B4 in S1 File).
Table 2. Summary of further network meta-analysis for total number of infectious patients.
| Therapy | Effect size | Inconsistency | Small study bias | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | studies | RR1 | 95% CI | I2 | χ2 | P | t | P |
| Total number of infectious patients (pSAP) | 3.68 | 0.06 | -1.75 | 0.11 | |||||
| NNS | TPN | 1 | 1.41 | 0.38, 5.16 | N/A | ||||
| NJ | TPN | 8 | 0.562 | 0.34, 0.92 | 45.8% | ||||
| NG | TPN | 1 | 0.47 | 0.16, 1.43 | N/A | ||||
| NJ | NNS | AIC | 0.40 | 0.10, 1.60 | N/A | ||||
| NG | NNS | AIC | 0.34 | 0.06, 1.85 | N/A | ||||
| NG | NJ | 2 | 0.84 | 0.30, 2.36 | 0% | ||||
| Total number of infectious patients (PAB) | 2.07 | 0.15 | -1.02 | 0.34 | |||||
| NNS | TPN | 1 | 0.97 | 0.28, 3.40 | N/A | ||||
| NJ | TPN | 6 | 0.59 | 0.35, 1.00 | 56.6% | ||||
| NG | TPN | AIC | 0.19 | 0.01, 5.30 | N/A | ||||
| NJ | NNS | 1 | 0.61 | 0.16, 2.29 | N/A | ||||
| NG | NNS | AIC | 0.19 | 0.01, 6.16 | N/A | ||||
| NG | NJ | 1 | 0.32 | 0.01, 8.61 | N/A | ||||
| Infected pancreatic necrosis | 5.70 | 0.06 | 0.35 | 0.73 | |||||
| NNS | TPN | 1 | 1.08 | 0.43, 2.70 | N/A | ||||
| NJ | TPN | 6 | 0.392 | 0.26, 0.58 | 0% | ||||
| NG | TPN | 1 | 0.312 | 0.11, 0.91 | N/A | ||||
| NJ | NNS | 1 | 0.362 | 0.14, 0.97 | N/A | ||||
| NG | NNS | AIC | 0.29 | 0.07, 1.17 | N/A | ||||
| NG | NJ | 2 | 0.80 | 0.29, 2.19 | 0% | ||||
| Bacteremia | No | loop | 0.51 | 0.631 | |||||
| NNS | TPN | 1 | 0.15 | 0.01, 2.83 | N/A | ||||
| NJ | TPN | 4 | 0.54 | 0.24, 1.21 | 0% | ||||
| NG | TPN | AIC | 0.52 | 0.17, 1.56 | N/A | ||||
| NJ | NNS | AIC | 3.51 | 0.17, 72.44 | N/A | ||||
| NG | NNS | AIC | 3.39 | 0.15, 76.54 | N/A | ||||
| NG | NJ | 2 | 0.96 | 0.46, 2.04 | 0% | ||||
| Line infection | No | loop | 1.15 | 0.29 | |||||
| NNS | TPN | 2 | 0.79 | 0.10, 6.38 | 59.9% | ||||
| NJ | TPN | 8 | 0.192 | 0.07, 0.50 | 0% | ||||
| NJ | NNS | AIC | 0.24 | 0.02, 2.38 | N/A | ||||
| Pneumonia | No | loop | -1.49 | 0.20 | |||||
| NJ | TPN | 4 | 0.72 | 0.27, 1.87 | 0% | ||||
| NG | TPN | AIC | 0.32 | 0.07, 1.40 | N/A | ||||
| NG | NJ | 3 | 0.44 | 0.14, 1.37 | 0% | ||||
| Other type infection | 1.83 | 0.18 | -1.52 | 0.17 | |||||
| NJ | TPN | 6 | 0.87 | 0.56, 1.34 | 36.5% | ||||
| NG | TPN | 1 | 0.98 | 0.16, 5.87 | N/A | ||||
| NG | NJ | 2 | 1.14 | 0.19, 6.66 | 53.1% | ||||
1 AIC, adjusted indirect comparison; N/A, not applicable; NG, naso-gastric; NJ, naso-jejeunal; NNS, no nutrition support; PAB, prophylactic anti-biotics; pSAP, predicted severe acute pancreatitis; RR, risk ratio; CI, confidence interval; TPN, total parenteral nutrition.
2 P < 0.05.
In the 9 RCTs that provided all patients prophylactic antibiotics,[7, 9, 10, 12, 41, 53–56] the results of the network meta-analysis showed no significant differences in total infection rates among different therapies (Table 2 and Appendix C1 in S1 File). However, the SUCRA value showed that NG may be the best therapy (mean rank = 1.6; SUCRA = 80.2), and TPN should be avoided when treating patients with acute pancreatitis (mean rank = 3.3; SUCRA = 22.3) (Appendix C2 in S1 File). No inconsistency (loop inconsistency χ2 = 2.07; P = 0.15) (Appendix C3 in S1 File) or small study bias (Egger’s test t = -1.02; P = 0.34) was observed in the present network meta-analysis (Appendix C4 in S1 File).
Secondary outcomes
Table 2 summarizes the network meta-analyses for infected pancreatic necrosis, bacteremia, line infection, pneumonia, and OTIs, and their ranking probabilities were summarized in S5 Table. infected pancreatic necrosis was reported in 11 RCTs.[6, 8, 10–13, 43, 52, 53, 55, 56] The network meta-analysis consistently showed that NJ led to a significantly lower infected pancreatic necrosis rate when compared with NNS (RR, 0.36; 95% CI, 0.14, 0.97) and with TPN (RR, 0.39; 95% CI, 0.26, 0.58). Furthermore, NG led to a significantly lower infected pancreatic necrosis rate when compared with TPN (RR, 0.31, 95% CI, 0.11, 0.9). The SUCRA showed that NG may be the optimal therapy (mean rank = 1.4; SUCRA = 86.8) for avoiding infected pancreatic necrosis, and NNS may be the least effective treatment, very likely resulting in infected pancreatic necrosis (mean rank = 3.5; SUCRA = 16.8) among patients with acute pancreatitis (Appendix D1-D4 in S1–File).
Bacteremia was reported in 7 RCTs.[6, 7, 10, 43, 45, 51, 52] The network meta-analysis showed no significant difference in bacteremia rates among the various therapies. However, the SUCRA showed that NNS may have the lowest probability of bacteremia (mean rank = 1.5; SUCRA = 82.2), whereas TPN may have the highest probability of leading to bacteremia (mean rank = 3.7; SUCRA = 9.8) among the 4 therapies (Appendix E1-E3 in S1 File).
Ten RCTs reported data on line infection.[6, 9–13, 40, 45, 51, 55] Because no RCT that compared NG with other therapies reported line infection, the consistency model was only conducted for comparisons among NNS, TPN, and NJ. The network meta-analysis showed that NJ had significantly lower line infection rates when compared with TPN (RR, 0.19; 95% CI, 0.07, 0.50). The SUCRA showed that NJ may have the lowest probability of leading to line infection (mean rank = 1.1; SUCRA = 94.3), and TPN may have the highest probability of leading to line infection (mean rank = 2.6; SUCRA = 20.9) among the 3 therapies (Appendix F1-F3 in S1 File).
Seven RCTs reported pneumonia.[9, 10, 12, 41, 43, 45, 52] The consistency model could only be conducted for comparisons among TPN, NJ, and NG, because no RCT that compared NNS with other therapies reported pneumonia. The network meta-analysis showed no significant difference in pneumonia rates among the 3 therapies. However, the SUCRA implied that NG may be the optimal therapy (mean rank = 1.1; SUCRA = 92.9) for avoiding pneumonia, and TPN may have the highest probability of leading to pneumonia (mean rank = 2.7; SUCRA = 15.8) among the 3 therapies (Appendix G1-G3 in S1 File).
Five RCTs reported data on urinary tract infections.[6, 9, 10, 12, 45] Because these 5 RCTs only compared NJ with TPN, no consistency model was required. We conducted a head-to-head meta-analysis for urinary tract infections, and results showed no significant difference in urinary tract infection rates between NJ and TPN (RR, 0.76; 95% CI, 0.27, 2.09) with very low heterogeneity (I-square = 0%) (Appendix H1 and H2 in S1 File).
Nine RCTs reported data on OTIs.[6–8, 13, 40, 43, 45, 52, 54] Because no RCT that compared NNS with other therapies reported OTIs, the consistency model was only conducted for comparisons among TPN, NJ, and NG. The results showed no significant difference in OTI rates among the 3 therapies. However, the SUCRA showed that NJ may have the lowest probability of leading to OTIs (mean rank = 1.7; SUCRA = 64.8), and TPN may have the highest probability of leading to OTIs (mean rank = 2.2; SUCRA = 37.8) among the 3 therapies (Appendix I1-I4 in S1 File).
Discussion
This is the first network meta-analysis comparing infections among three nutritional support routes and NNS among acute pancreatitis. We summarized available data on overall infectious complications from 16 RCTs, and our analyses also detailed for six infectious events including infected pancreatic necrosis, bacteremia, line infection, pneumonia, urinary tract infections, and OTIs. Then, we found that NG may be the most preferred therapy and TPN may be the least preferred.
An important finding is about NG showing a favorable result in preventing infected pancreatic necrosis and pneumonia. To our knowledge, infected pancreatic necrosis was categorized as severe acute pancreatitis, and it may lead to morbidity and mortality.[57, 58] The superior ranking of enteral nutrition in our study may reflected that enteral feeding reduced bacterial translocation and further reducing colonization of the necrotic pancreatic tissue through keeping gut structure and function.[59] In contrast, NNS was the least effective therapy for preventing infected pancreatic necrosis because the hypercatabolic status and lack of nutritional support during long periods of illness lead to poor outcomes among pSAP patients.
Besides, we found another crucial piece of evidence for verifying the safety of using NG feeding in leading pneumonia. Aspiration is known to be an etiology of pneumonia, and in enteral nutrition, NG has long been believed to be more likely to cause aspiration than NJ.[60] However, our evidence did not support the assumption. The aforementioned evidence resonates with recent guidelines that support the efficacy and safety of NG and NJ in pSAP.[4, 15] The potential mechanism may be that pancreatic exocrine function was diminished significantly in patients with acute pancreatitis, and it has a negative correlation with the severity of acute pancreatitis.[61] As a result, NG is less likely to cause pancreatic stimulation and inflammation through an enzyme attack during a severe course. In the present study, although the result of NG being superior to NJ was nonsignificant, NG has numerous advantages. Specifically, it is much easier to insert, has a low dislodge rate, can achieve the same target caloric intake as NJ, and possesses the same risk of changing to TPN as NJ.[62]
Regarding the event of bacteremia, NNS is the most recommended therapy, NG is second, and TPN is also the least preferred. Two methodological problems may help to understand why NNS is the most recommended therapy. First, only one RCT was involved in the analysis.[51] Second, most participants in the RCT had mild acute pancreatitis. This result echoed current guidelines in terms of when treating patients with mild acute pancreatitis. NNS may be superior, because it may relieve patients’ symptoms rapidly.[63] However, the bacteremia rate in enteral nutrition is still lower than in TPN. Most studies that featured the event of bacteremia were on patients with pSAP,[6, 7, 10, 51, 52] and this implied the importance of enteral nutrition in such patients. This result supports the previous research indicating enteral nutrition prevents bacterial translocation.[64]
Our study further considered potential factors: severity and prophylactic antibiotics usage. Firstly, patients with pSAP are more likely to possess morbidities including pancreatic necrosis and infectious events.[58] We defined pSAP according to RCTs that mentioned the severity of acute pancreatitis,[6–13, 41–43, 50, 52–56] and NG seemed to be the preferred nutritional therapy among patients with pSAP. Secondly, physicians usually prescribed prophylactic antibiotics to patients with pSAP to prevent further infectious complications. In the further analysis of prophylactic antibiotics usage, the 9 included RCTs only recruited patients with pSAP. Most of them used imipenem and fluoroquinolone. Then, NG still seemed to be the most recommended treatment, and TPN was not recommended. We observed some interesting changes in effect size when comparing the results from 16 RCTs with the results from those providing all patients prophylactic antibiotics. The effect size of NG and TPN in the further analysis of prophylactic antibiotics (RR, 0.19) was lower than that in all RCT analyses (RR, 0.48). Moreover, the effect size of NG and NJ in the further analysis of prophylactic antibiotics (RR, 0.32) was also lower than that in all RCT analyses (RR, 0.82). These interesting findings implies that NG may be a superior choice, especially for those pSAP with prophylactic antibiotics.
Limitations
This study has some limitations. Firstly, we could not control the etiology of acute pancreatitis. Etiology usually causes different infectious complication and require different treatments in acute pancreatitis. Regrettably, the RCTs included in this study did not differentiate etiologies. Thus, etiology might have influenced the results of this study. Secondly, the inclusion criteria about severity in the included RCTs used various scales, and the pSAP in this study was based the declaration in original study. We cannot exclude this potential bias from severity measurement. Thirdly, we did not consider the total caloric intake. Recent large trials,[65, 66] CALORIES and NUTRIREA-2, indicated that different caloric intakes may influence the outcome of infectious complications. The 22 included RCTs set different caloric goals and caloric achievements. Caloric intake was another difficult-to-control confounding factor. Fourthly, we did not get any response from authors for missing data. That is to say, our results still cannot completely reflect all evidence because of those missing data though our evidence is the first network meta-analysis of the effects nutritional routes on acute pancreatitis. Lastly, SUCRA was regarded to have a substantial degree of imprecision in ranking.[67] Nevertheless, in our study, the effect size and probability ranking showed obvious trends in the outcomes, except for in OTIs. These clear ranks provide clear and practical information. Notably, these limitations were not well-controlled in previous systematic reviews either.
Conclusions
In conclusion, acute pancreatitis is an inflammatory disease with unregulated activation of trypsin in pancreatic acinar cells. Our evidence echoed that the era of “gut rousing” replaced “pancreatic rest”.[68] To prevent further infectious complications, selecting an adequate nutritional support is crucial. Overall, NG was shown to be the most preferred therapy for acute pancreatitis and TPN was shown to be the least preferred. Moreover, NNS must be avoided in treating patients with severe acute pancreatitis. More evidence is required to further analyze the etiology, feeding time, and caloric intake in acute pancreatitis.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Ms. Yun-Ya Hsu for her helpful comments and English editing on earlier version of this article.
Abbreviations
- AP
acute pancreatitis
- CI
confidence interval
- NG
nasogastric
- NJ
nasojejunal
- NNS
no nutritional support
- OTI
other type of infection
- pSAP
predicted severe acute pancreatitis
- RCT
randomized control trial
- RR
risk ratio
- SUCRA
surface under the cumulative ranking curve
- TPN
total parenteral nutrition
Data Availability
All relevant data are included in the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN Journal of parenteral and enteral nutrition. 2016;40(2):159–211. Epub 2016/01/17. 10.1177/0148607115621863 . [DOI] [PubMed] [Google Scholar]
- 2.Petrov MS, van Santvoort HC, Besselink MG, van der Heijden GJ, Windsor JA, Gooszen HG. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acute pancreatitis: a meta-analysis of randomized trials. Archives of surgery (Chicago, Ill: 1960). 2008;143(11):1111–7. Epub 2008/11/19. 10.1001/archsurg.143.11.1111 . [DOI] [PubMed] [Google Scholar]
- 3.Yi F, Ge L, Zhao J, Lei Y, Zhou F, Chen Z, et al. Meta-analysis: total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Internal medicine (Tokyo, Japan). 2012;51(6):523–30. Epub 2012/03/28. 10.2169/internalmedicine.51.6685 . [DOI] [PubMed] [Google Scholar]
- 4.Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. The American journal of gastroenterology. 2013;108(9):1400–15; 16. Epub 2013/07/31. 10.1038/ajg.2013.218 . [DOI] [PubMed] [Google Scholar]
- 5.Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;(1):Cd002837 Epub 2010/01/22. 10.1002/14651858.CD002837.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casas M, Mora J, Fort E, Aracil C, Busquets D, Galter S, et al. [Total enteral nutrition vs. total parenteral nutrition in patients with severe acute pancreatitis]. Revista espanola de enfermedades digestivas: organo oficial de la Sociedad Espanola de Patologia Digestiva. 2007;99(5):264–9. Epub 2007/07/27. . [DOI] [PubMed] [Google Scholar]
- 7.Doley RP, Yadav TD, Wig JD, Kochhar R, Singh G, Bharathy KG, et al. Enteral nutrition in severe acute pancreatitis. JOP: Journal of the pancreas. 2009;10(2):157–62. Epub 2009/03/17. . [PubMed] [Google Scholar]
- 8.Eckerwall GE, Axelsson JB, Andersson RG. Early Nasogastric feeding in predicted severe acute pancreatitis—A clinical, randomized study. Annals of Surgery. 2006;244(6):959–67. 10.1097/01.sla.0000246866.01930.58 WOS:000242474700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Patel K, Calder PC, Yaqoob P, Primrose JN, Johnson CD. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II > or = 6). Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al. ]. 2003;3(5):406–13. Epub 2003/10/04. 10.1159/000073657 . [DOI] [PubMed] [Google Scholar]
- 10.Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. The British journal of surgery. 1997;84(12):1665–9. Epub 1998/02/04. . [PubMed] [Google Scholar]
- 11.Louie BE, Noseworthy T, Hailey D, Gramlich LM, Jacobs P, Warnock GL. 2004 MacLean-Mueller prize enteral or parenteral nutrition for severe pancreatitis: a randomized controlled trial and health technology assessment. Can J Surg. 2005;48(4):298–306. Epub 2005/09/10. [PMC free article] [PubMed] [Google Scholar]
- 12.Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23(5–6):336–44; discussion 44–5. Epub 2006/12/14. 10.1159/000097949 . [DOI] [PubMed] [Google Scholar]
- 13.Wu XM, Ji KQ, Wang HY, Li GF, Zang B, Chen WM. Total enteral nutrition in prevention of pancreatic necrotic infection in severe acute pancreatitis. Pancreas. 2010;39(2):248–51. Epub 2009/11/17. 10.1097/MPA.0b013e3181bd6370 . [DOI] [PubMed] [Google Scholar]
- 14.Cassim MM, Allardyce DB. Pancreatic secretion in response to jejunal feeding of elemental diet. Ann Surg. 1974;180(2):228–31. Epub 1974/08/01. 10.1097/00000658-197408000-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IAP/APA WG. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al. ]. 2013;13(4 Suppl 2):e1–15. Epub 2013/09/27. 10.1016/j.pan.2013.07.063 . [DOI] [PubMed] [Google Scholar]
- 16.van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, et al. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66(11):2024–32. Epub 2017/08/26. 10.1136/gutjnl-2016-313595 . [DOI] [PubMed] [Google Scholar]
- 17.Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;(5):Cd002941 Epub 2010/05/14. 10.1002/14651858.CD002941.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao CC, Lin YS, Chu HC, Fang TC, Wu MS, Kang YN. Association of Renal Function and Direct-Acting Antiviral Agents for HCV: A Network Meta-Analysis. Journal of clinical medicine. 2018;7(10). Epub 2018/10/03. 10.3390/jcm7100314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin EY, Kuo YK, Kang YN. Effects of three common lumbar interbody fusion procedures for degenerative disc disease: A network meta-analysis of prospective studies. International journal of surgery (London, England). 2018;60:224–30. Epub 2018/11/25. 10.1016/j.ijsu.2018.11.009 . [DOI] [PubMed] [Google Scholar]
- 20.Huang YJ, Chen CY, Chen RJ, Kang YN, Wei PL. Topical diltiazem ointment in post-hemorrhoidectomy pain relief: A meta-analysis of randomized controlled trials. Asian journal of surgery. 2018;41(5):431–7. Epub 2017/07/13. 10.1016/j.asjsur.2017.06.002 . [DOI] [PubMed] [Google Scholar]
- 21.Huang YJ, Kang YN, Huang YM, Wu AT, Wang W, Wei PL. Effects of laparoscopic vs robotic-assisted mesorectal excision for rectal cancer: An update systematic review and meta-analysis of randomized controlled trials. Asian journal of surgery. 2019. Epub 2019/01/06. 10.1016/j.asjsur.2018.11.007 . [DOI] [PubMed] [Google Scholar]
- 22.Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Systematic reviews. 2017;6(1):79–. 10.1186/s13643-017-0473-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhary A, Puli SR, Ibdah JA, Bechtold ML. Nasogastric tube feeding in acute pancreatitis: A meta-analysis of randomized controlled trials. Gastrointest Endosc. 2009;69(5):AB315 10.1016/j.gie.2009.03.893 [DOI] [Google Scholar]
- 24.Kumar S, Bhandar V, Vyas HG. Clinical outcome in low socioeconomic patients with severe acute pancreatitis treated either with early nasogastric feeding or total parenteral nutrition-a comparative study. Gastroenterology. 2010;138(5):S888. [Google Scholar]
- 25.Lou J, Zhao H, Yu J, Luo Y, Chen F, Chen J. Nasogastric or nasojejunal tube feeding in pediatric acute pancreatitis: A clinical, randomized pilot study. Journal of Pediatric Gastroenterology and Nutrition. 2016;62:48 10.1097/01.mpg.0000484500.48517.e7 [DOI] [Google Scholar]
- 26.Moparty E, Kumar PS, Umadevi M, Ramanna M. A comparison of nasogastric and nasojejunal feeding in the enteral nutrition of acute pancreatitis. Indian Journal of Gastroenterology. 2015;34(1):A79 10.1007/s12664-015-0600-5 [DOI] [Google Scholar]
- 27.Olivier N. Nutritional management of acute pancreatitis in a human immunodeficiency virus-infected patient. South African Journal of Clinical Nutrition. 2013;26(4):212–5. [Google Scholar]
- 28.Rolniak S, Raina A, Hegazi R, Cerita-Wagner PK, Kandil HM, Hughes SJ, et al. Simultaneous Nasogastric Decompression with Mid-Jejunal Feeding Avoids Total Parenteral Nutrition (TPN) and Early Surgery in the Management of Complicated Acute Pancreatitis and Gastric Outlet Obstruction. Gastroenterology. 2009;136(5):A76–A. WOS:000275277200337. [Google Scholar]
- 29.Wang B, Dong L, Kang Y. The application of early enteral nutrition via naso-jejunal catheter in the treatment of severe acute pancreatitis. Intensive Care Medicine. 2009;35:S148. [Google Scholar]
- 30.Wiesen A, Sideridis K, Jalal PK, Bank S. Effect of early oral or nasogastric feeding on the outcome of cases with acute pancreatitis. American Journal of Gastroenterology. 2006;101(9):S128–S9. WOS:000240656100258. [Google Scholar]
- 31.Windsor J, McIlroy K, Grayson L, Phillips A, Petrov M. Early nasogastric tube feeding versus nil-by-mouth in patients with mild and moderate acute pancreatitis: A randomised controlled trial. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al. ]. 2014;14(3):S98. [Google Scholar]
- 32.Nally D, Kelly E, Byrne M, Ridgway PF. Nasogastric nutrition in severe acute pancreatitis: A systematic review and meta-analysis. Irish Journal of Medical Science. 2014;183(1):S53 10.1007/s11845-013-1062-3 [DOI] [PubMed] [Google Scholar]
- 33.Petrov MS, Correia MITD, Windsor JA. Nasogastric tube feeding in predicted severe acute pancreatitis. A systematic review of the literature to determine safety and tolerance. Journal of the Pancreas. 2008;9(4):440–8. [PubMed] [Google Scholar]
- 34.Samaraee AA, McCallum IJD, Coyne PE, Seymour K. Nutritional strategies in severe acute pancreatitis: A systematic review of the evidence. The surgeon: journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2010;8(2):105–10. 10.1016/j.surge.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 35.Buchman A. Nasogastric versus nasojejunal feeding in severe acute pancreatitis. Current gastroenterology reports. 2005;7(4):300–1. Epub 2005/07/27. . [PubMed] [Google Scholar]
- 36.Petrov MS. Moving beyond the 'pancreatic rest' in severe and critical acute pancreatitis. Crit Care. 2013;17(4). 10.1186/cc12770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López A. Pancreas and biliary duct: Parenteral, nasogastric enteral or nasojejunal enteral nutrition in severe acute pancreatitis? Gastroenterologia y Hepatologia. 2008;31(10):702–3. [DOI] [PubMed] [Google Scholar]
- 38.Wang YZ, Ding YB, Wu J, Deng B, Xiao WM. Treatment of 64 cases severe acute pancreatitis with early enteral nutrition and intestinal barrier protective agents. World Chinese Journal of Digestology. 2007;15(33):3545–8. [Google Scholar]
- 39.Bakker OJ, van Brunschot S, van Santvoort HC, Besselink MG, Bollen TL, Boermeester MA, et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. The New England journal of medicine. 2014;371(21):1983–93. Epub 2014/11/20. 10.1056/NEJMoa1404393 . [DOI] [PubMed] [Google Scholar]
- 40.Abou-Assi S, Craig K, O'Keefe SJD. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: Results of a randomized comparative study. American Journal of Gastroenterology. 2002;97(9):2255–62. 10.1111/j.1572-0241.2002.05979.x WOS:000178229700018. [DOI] [PubMed] [Google Scholar]
- 41.Du Z, Wang W, Chen L, Luo J, Zhou L, Zhou X. The application of enteral nutrition by nasogastric tube in severe acute pancreatitis. Parenteral & Enteral Nutrition. 2015;22(3):168–70. [Google Scholar]
- 42.Eatock FC, Chong P, Menezes N, Murray L, McKay J, Carter CR, et al. A Randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. American Journal of Gastroenterology. 2005;100(2):432–9. 10.1111/j.1572-0241.2005.40587.x WOS:000226726300027. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A, Singh N, Prakash S, Saraya A, Joshi YK. Early enteral nutrition in severe acute pancreatitis: A prospective randomized controlled trial comparing nasojejunal and nasogastric routes. J Clin Gastroenterol. 2006;40(5):431–4. 10.1097/00004836-200605000-00013 WOS:000237742900013. [DOI] [PubMed] [Google Scholar]
- 44.Ma JM, Pendharkar SA, O'Grady G, Windsor JA, Petrov MS. Effect of Nasogastric Tube Feeding vs Nil per Os on Dysmotility in Acute Pancreatitis: Results of a Randomized Controlled Trial. Nutr Clin Pract. 2016;31(1):99–104. 10.1177/0884533615603967 WOS:000369048300013. [DOI] [PubMed] [Google Scholar]
- 45.McClave SA, Greene LM, Snider HL, Makk LJK, Cheadle WG, Owens NA, et al. Comparison of the safety of early enteral vs parenteral nutrition in mild acute pancreatitis. J Parenter Enter Nutr. 1997;21(1):14–20. 10.1177/014860719702100114 WOS:A1997WB86100003. [DOI] [PubMed] [Google Scholar]
- 46.McKenzie SJ, Premkumar R, Askelund KJ, Pendharkar SA, Phillips AR, Windsor JA, et al. The effect of enteral nutrition on adipokines in patients with acute pancreatitis. Journal of nutritional science. 2015;4:e33 Epub 2015/10/27. 10.1017/jns.2015.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olah A, Pardavi G, Belagyi T, Nagy A, Issekutz A, Mohamed GE. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition (Burbank, Los Angeles County, Calif). 2002;18(3):259–62. Epub 2002/03/08. . [DOI] [PubMed] [Google Scholar]
- 48.Pendharkar SA, Plank LD, Windsor JA, Petrov MS. Quality of Life in a Randomized Trial of Nasogastric Tube Feeding in Acute Pancreatitis. J Parenter Enter Nutr. 2016;40(5):693–8. 10.1177/0148607115574290 WOS:000379609300011. [DOI] [PubMed] [Google Scholar]
- 49.Petrov MS, McIlroy K, Grayson L, Phillips ARJ, Windsor JA. Early nasogastric tube feeding versus nil per os in mild to moderate acute pancreatitis: A randomized controlled trial. Clin Nutr. 2013;32(5):697–703. 10.1016/j.clnu.2012.12.011 WOS:000325600900004. [DOI] [PubMed] [Google Scholar]
- 50.Powell JJ, Murchison JT, Fearon KC, Ross JA, Siriwardena AK. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. The British journal of surgery. 2000;87(10):1375–81. Epub 2000/10/24. 10.1046/j.1365-2168.2000.01558.x . [DOI] [PubMed] [Google Scholar]
- 51.Sax HC, Warner BW, Talamini MA. Early total parenteral nutrition in acute pancreatitis: Lack of beneficial effects. American journal of surgery. 1987;153(1):117–24. 10.1016/0002-9610(87)90211-x [DOI] [PubMed] [Google Scholar]
- 52.Singh N, Sharma B, Sharma M, Sachdev V, Bhardwaj P, Mani K, et al. Evaluation of Early Enteral Feeding Through Nasogastric and Nasojejunal Tube in Severe Acute Pancreatitis A Noninferiority Randomized Controlled Trial. Pancreas. 2012;41(1):153–9. 10.1097/MPA.0b013e318221c4a8 WOS:000298375900021. [DOI] [PubMed] [Google Scholar]
- 53.Stimac D, Poropat G, Hauser G, Licul V, Franjic N, Zujic PV, et al. Early nasojejunal tube feeding versus nil-by-mouth in acute pancreatitis: A randomized clinical trial. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al. ]. 2016;16(4):523–8. 10.1016/j.pan.2016.04.003 WOS:000390288700007. [DOI] [PubMed] [Google Scholar]
- 54.Wang G, Wen J, Xu L, Zhou S, Gong M, Wen P, et al. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. The Journal of surgical research. 2013;183(2):592–7. Epub 2013/06/04. 10.1016/j.jss.2012.12.010 . [DOI] [PubMed] [Google Scholar]
- 55.He X-l, Ma Q-j, Lu J-g, Chu Y-k, Du X-l. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplementation on outcome in severe acute pancreatitis (SAP) 2004. 43–7 p. [Google Scholar]
- 56.Zhang Y-S, Shu X-L, Zhong J-X, Sheng Y, Meng B-L. Total parenteral nutrition combined with enteral nutrition in treatment of severe acute pancreatitis. Academic Journal of Second Military Medical University. 2011;32(7):737–40. 10.3724/SP.J.1008.2011.00737 [DOI] [Google Scholar]
- 57.Dellinger EP, Forsmark CE, Layer P, Levy P, Maravi-Poma E, Petrov MS, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256(6):875–80. Epub 2012/06/28. 10.1097/SLA.0b013e318256f778 . [DOI] [PubMed] [Google Scholar]
- 58.Forsmark CE, Yadav D. Predicting the Prognosis of Acute Pancreatitis. Ann Intern Med. 2016;165(7):523–4. Epub 2016/07/28. 10.7326/M16-1581 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deitch EA. Bacterial translocation: the influence of dietary variables. Gut. 1994;35(1 Suppl):S23–7. 10.1136/gut.35.1_suppl.s23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomes GF, Pisani JC, Macedo ED, Campos AC. The nasogastric feeding tube as a risk factor for aspiration and aspiration pneumonia. Curr Opin Clin Nutr Metab Care. 2003;6(3):327–33. Epub 2003/04/12. 10.1097/01.mco.0000068970.34812.8b . [DOI] [PubMed] [Google Scholar]
- 61.Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al. ]. 2003;3(4):303–8. Epub 2003/08/02. 10.1159/000071768 . [DOI] [PubMed] [Google Scholar]
- 62.Nally DM, Kelly EG, Clarke M, Ridgway P. Nasogastric nutrition is efficacious in severe acute pancreatitis: a systematic review and meta-analysis. The British journal of nutrition. 2014;112(11):1769–78. Epub 2014/10/22. 10.1017/S0007114514002566 . [DOI] [PubMed] [Google Scholar]
- 63.Gupta K, Wu B. In the clinic. Acute pancreatitis. Ann Intern Med. 2010;153(9):ITC51–5; quiz ITC516. Epub 2010/11/03. 10.7326/0003-4819-153-9-201011020-01005 . [DOI] [PubMed] [Google Scholar]
- 64.Kotani J, Usami M, Nomura H, Iso A, Kasahara H, Kuroda Y, et al. Enteral nutrition prevents bacterial translocation but does not improve survival during acute pancreatitis. Archives of surgery (Chicago, Ill: 1960). 1999;134(3):287–92. Epub 1999/03/24. . [DOI] [PubMed] [Google Scholar]
- 65.Reignier J, Boisrame-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet (London, England). 2018;391(10116):133–43. Epub 2017/11/13. 10.1016/s0140-6736(17)32146-3 . [DOI] [PubMed] [Google Scholar]
- 66.Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. Trial of the route of early nutritional support in critically ill adults. The New England journal of medicine. 2014;371(18):1673–84. Epub 2014/10/02. 10.1056/NEJMoa1409860 . [DOI] [PubMed] [Google Scholar]
- 67.Trinquart L, Attiche N, Bafeta A, Porcher R, Ravaud P. Uncertainty in Treatment Rankings: Reanalysis of Network Meta-analyses of Randomized Trials. Ann Intern Med. 2016;164(10):666–73. Epub 2016/04/19. 10.7326/M15-2521 . [DOI] [PubMed] [Google Scholar]
- 68.Petrov MS, Windsor JA. Nutritional management of acute pancreatitis: the concept of 'gut rousing'. Curr Opin Clin Nutr Metab Care. 2013;16(5):557–63. Epub 2013/06/27. 10.1097/MCO.0b013e3283638ed1 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are included in the paper and its Supporting Information files.