Abstract
Slack (Slo 2.2), a member of the Slo potassium channel family, is activated by both voltage and cytosolic factors, such as Na+ ([Na+]i) and Cl− ([Cl−]i). Since Slo family is known to play a role in hypoxia, and since hypoxia/ischemia is associated with an increase in H+ and CO2 intracellularly, we hypothesized that the Slack channel may be affected by changes in intracellular concentrations of CO2 and H+. To examine this, we expressed the Slack channel in Xenopus oocytes and the Slo 2.2 protein was allowed to be inserted into the plasma membrane. Inside-out patch recordings were performed to examine the response of Slack to different CO2 concentrations (0.038%, 5%, 12%) and to different pH levels (6.3, 6.8, 7.3, 7.8, 8.3). In the presence of low ([Na+]i (5 mM), the Slack channel open probability decreased when exposed to decreased pH or increased CO2 in a dose-dependent fashion (from 0.28±0.03, n=3, at pH 7.3 to 0.006±0.005, n=3, p=0.0004, at pH 6.8; and from 0.65±0.17, n=3, at 0.038% CO2 to 0.22±0.07, n=3, p=0.04 at 12% CO2). In the presence of high [Na+]i (45 mM), Slack open probability increased (from 0.03±0.01 at 5 mM [Na+]i, n=3, to 0.11±0.01, n=3, p=0.01) even in the presence of decreased pH (6.3). Since Slack activity increases significantly when exposed to increased [Na+]i, even in presence of increased H+, we propose that Slack may play an important role in pathological conditions during which there is an increase in the intracellular concentrations of both acid and Na+, such as in ischemia/hypoxia.
Keywords: Na+-activated K+ channel, brain, hypoxia/ischemia
Introduction
Potassium (K+) channels belong to many families of channels and are important regulators of cell excitability in normal and abnormal conditions (Hille, 2001). More recently, for example, Na+-activated K+ channels (KNa) have been identified in the heart (Kameyama et al., 1984; Niu and Meech, 2000) and central nervous system (CNS) of avians (Dryer, 1991) and mammals (Bhattacharjee et al., 2002; Franceschetti et al., 2003). KNa channels are thought to play a role in terminating neuronal excitability when activated by an increase in [Na+]i (Kameyama et al., 1984; Dryer, 2003; Franceschetti et al., 2003). The Slack (sequence like a Ca2+- activated K+ channel) channel is a newly recognized KNa channel in the Slo family (though Slack and its ortholog in C. elegans are only 7% (Joiner et al. 1998) identical to the voltage- and Calcium-dependent Slo and the voltage-dependent but calcium-insensitive Slo3 (see reviews by Gribkoff et al., 2001; Bhattacharjee and Kaczmarek, 2005). In mammals, Slack has been identified in the CNS (Bhattacharjee et al., 2002) and hypothesized to be important in regulating neuronal excitation in hypoxia/ischemia due particularly to its sensitivity to both Na+ and Cl− (Bhattacharjee and Kaczmarek, 2005). The Slack channel cloned first by Joiner et al. (1998) and then by Yuan et al. (2003) shares most of the characteristics as those of the native Slack channels, with the exception of cooperative activation by Na+ and Cl− (Yuan et al. 2003 and Dryer 2003).
During brain or heart hypoxia/ischemia, there is decreased tissue perfusion which decreases O2 delivery and CO2 removal. As a result of the low tissue pO2, a shift to anaerobic metabolism occurs leading to lactic acidosis (Nedergaard and Goldman, 1993). In addition, as a result of CO2 accumulation, respiratory acidosis develops to promote a further drop in pH. The switch in metabolism decreases the amount of ATP available to support ATP-dependent exchangers (e. g. the Na+-K+ ATPase), which usually help maintain ionic gradients (Mitani and Shattock, 1992; Dobrota et al., 1999; Lipton, 1999). In order to examine the properties of these channels during pathophysiological conditions, we studied the Slack channel in an isolated environment where each variable can be manipulated independently. Hence, we studied Slack in an oocyte system to focus on its properties and the responses to changes in CO2 and pH, both of which change in a major way in brain or heart hypoxia/ischemia.
Methods
Preparation of cRNA
The Slack α (Slo 2.2) cDNA plasmid was inserted into a Bluescript vector (kindly provided by W. Joiner and L. Kaczmarek, Yale University School of Medicine, New Haven, CT) and transformed using ampicillin selection in Top Ten Competent Cells (Invitrogen, Carlsbad, CA). The Slack cDNA was amplified, purified using Qiaprep Spin Miniprep Kit (Qiagen, Valencia, CA), and then linearized by the Not I restriction enzyme (Invitrogen, Carlsbad, CA). The Slack cDNA (0.24 μg/μl) was in-vitro transcribed using the mMessage mMachine T3 (Ambion, Austin, TX), polymerase to generate a cRNA transcript (1.0–2.0 μg/μl) that when translated in vivo. The cRNA was purified using the RNeasy Mini kit (Qiagen, Valencia, CA).
Oocyte injection
The African claw frog (Xenopus laevis, Nasco, Fort Atkinson, WI) was anesthetized using 3-benzoic acid and the oocytes were removed surgically. Seventy nl of cRNA of Slack α (mSlo-2.2) subunit was injected into isolated oocytes and allowed to be translated into the protein product, and inserted into the oocyte plasma membrane. Oocytes were recorded from three to ten days after the injection.
Solutions
A symmetrical potassium solution was applied to both sides of the oocyte membrane when in the inside-out patch configuration consisting of (mM): 130 KCl, 5 Na- d-gluconate, 10 HEPES, 5 EGTA, and 29 Glucose, and pH was adjusted to 7.3 with KOH and glacial acetic acid. Chemicals were purchased from Sigma-Aldrich (Saint Louis, MO). Osmolarity of the solution was adjusted to around 300 mOsm.
To test the effect of Cl− on Slack channel activity, a solution made up of the following (in mM) 3 KCl, 127 K-d-gluconate, 5 Na-d-gluconate, 10 HEPES, 5 EGTA and 29 glucose, was applied to both sides of the membrane. The bath solution (cytosolic side) was later replaced with a solution (in mM) composed of 130 KCl, 5 Na-d-gluconate, 10 HEPES, 5 EGTA and 29 glucose to examine the response of Slack to increased [Cl−]i. Both solutions were adjusted to 293–299 mOsm and a pH of 7.3.
To test the effect of Na+ on Slack channel activity, a solution made up of the following (in mM) 30 KCl, 10 K-d-gluconate, 5 Na-d-gluconate, 85 n-methyl-d-glucamine, 10 HEPES, 5 EGTA and 10 glucose, was applied to both sides of the membrane. The bath solution (cytosolic side) was later replaced with a solution (in mM) composed of 30 KCl, 10 K-d-gluconate, 90 Na-d-gluconate, 10 HEPES, 5 EGTA and 10 glucose to examine the response of Slack to increased intracellular [Na+]i. Both solutions were adjusted to 275–290 mOsm and a pH of 7.3.
To determine the effect of varying pH on Slack activity, the bath solution was first adjusted to pH 8.3 with KOH to maintain equal concentrations of K+ and then glacial acetic acid was added to achieve more acidic pH values. The inside-out patches of oocytes were perfused with the bath solution bubbled with either 5% or 12% CO2 to test the effect of higher CO2 on Slack channel properties.. The perfusion solution was bubbled with either 5% or 12% CO2 for >2 hours before use to allow for adequate equilibration time.
To ensure quick and efficient solution exchange, some experiments were performed with a “microchamber” perfusion system. In this system, the tip of the recording pipette was deeply inserted into the microchamber made by a glass ball from the tip of a thin wall capillary glass tubing (1.5 mm OD, Warner Instruments, Hamden, CT). The solution exchange was almost instantaneous without any delay. The flow rate was around 0.5–1.0 ml/min, controlled by gravity. To make sure that there are no technical errors related to the order by which we bathed the patches, the same solution exchange was performed in both directions: from a low to a high concentration and from a high to a low concentration of a particular ion, with some experiments repeatedly performed several times with different concentrations of one particular ion (e.g. Na+ or Cl−).
Electrophysiology
Patch-clamp experiments were performed using the PC-501 amplifier (Warner Instruments, Hamden, CT). Recordings were made in inside-out configuration with borosilicate glass pipettes (World Precision Instruments, Serasota, FL) pulled with a Sutter puller (P-87, Sutter Instruments, Navato, FL), with a resistance of 5–10 MΩ. The signal was low-pass filtered at 2–5 kHz, sampled at 5–10 kHz and stored in a PC-DOS based computer using pClamp 5 and 6 software (Axon Instruments, Foster City, CA). Data were then analyzed using pClamp 9 software. Voltages in the text are all given comparing the internal side of the oocyte membrane to the external side. The open probability was defined as the time a particular channel (Po) or channels (NPo) in the patch spend in the open versus the total recording time.
Results
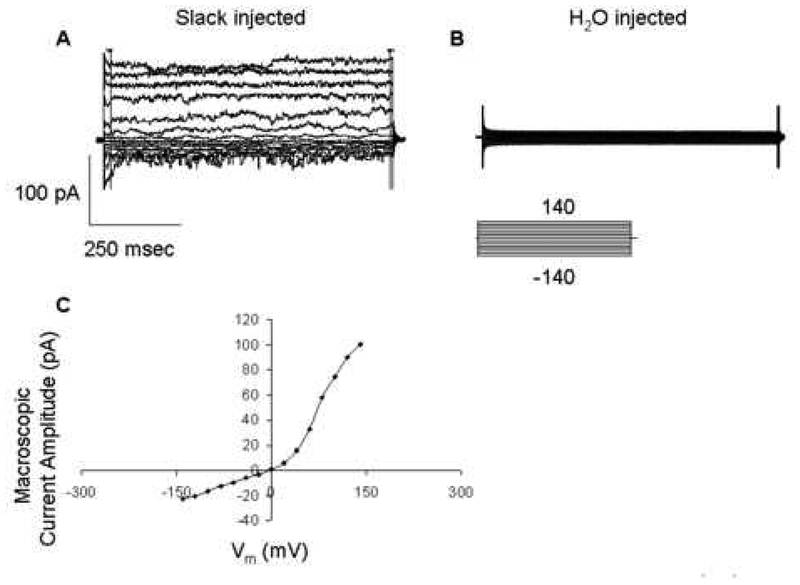
Very few endogenous channels were present in the oocyte and the Slack channel was not present. Three to ten days after the injection of the Slack α subunit cRNA into Xenopus oocytes, recordings were done. A current could be detected using the inside-out configuration of the patch clamp technique (Figure 1A).
Figure 1.
Slack channel activity is present in the Slack cRNA-transfected oocyte (A) and not in the H2O-injected oocyte (B). IV relationship in expressed Slack channel (C). Recordings were performed with a protocol under (B). Symmetrical 130 mM KCl and 5 mM Na-gluconate solutions were used in both pipette and bath for the macro-patch recordings (inside-out).
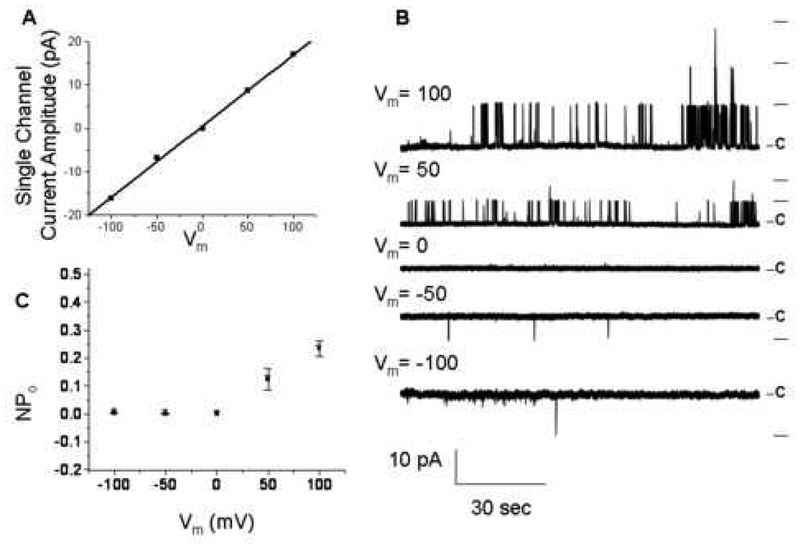
The transfection of Slack (Slo 2.2) induced a detectable macroscopic K+ current that was not present in the water injected oocyte (Figure 1B). In response to the voltage protocol shown in Figure 1B, an outwardly rectifying current was observed (Figure 1C). The Slack channel was voltage-dependent, increasing single channel amplitude in response to increasing changes in Vm (Figure 2A, B). Furthermore, an increase in NPo was obtained at positive Vm (Figure 2C). The single channel I-V relationship could be fitted with a linear regression, yielding a single channel slope conductance of 120 ± 5 pS (n=3) (Figure 2A). In addition, this single channel conductance is within the range of published conductances for the Slack α subunit in symmetrical K+ solutions (from 88 to 160 pS) (Yuan et al., 2003).
Figure 2.
Voltage dependency of Slack single channel opening. Slack single channel activities were recorded using symmetrical solutions (see Fig. 1) in both pipette and bath at different Vm (B) and single Slack channel amplitudes were plotted against different Vm (A). Open probability (NPo) of Slack single channel increases with increased Vm (C) (n=3).
In addition to being voltage-dependent, Slack, being a member of the Slo family, is also modulated by intracellular factors. Single channel (inside-out patch) experiments were performed to examine the sensitivity of the Slack channel to the [Cl−]i. In low [Cl−]i solutions (3 mM), the open probability of single Slack channels was very low (Fig. 3A, D) and there seems to be no voltage dependency of NPo (Fig. 3D). Single Slack channel activity increased remarkably when the intracellular side of the membrane was exposed to 130 mM Cl− at all voltages from −140 to 140 mV. In high Cl− solutions, there is a voltage dependency of NPo with a low open probability in negative voltage ranges. NPo increased with an increase in Vm from 40 to 100 mV and the NPo reached a plateau at around 100 mV. Shifting the internal solution from 3 mM Cl− to 130 mM Cl− did not shift the single channel reversal potential (Fig. 3C, and A, B traces at 0 mV), which confirms that the currents obtained from these experiments are indeed K+ currents (symmetrical K+ in both intracellular and extracellular sides of the membrane in both low and high Cl− solutions) and not Cl− currents. Shifting internal solutions from 3 mM Cl− to 130 mM Cl− did not change single Slack channel amplitude (Fig. 3C). There is no difference of single channel amplitudes in the two Cl− solutions with the exception of currents at +140 mV. This Cl− sensitivity has been also found in a previous publication on Slack (Bhattacharjee and Kaczmarek, 2005).
Figure 3.
Single Slack channel activity is sensitive to intracellular chloride. Slack channel activity increases when the [Cl−]i increased from 3 (Fig. 3A) to 130 mM (Fig. 3B). Single channel currents were evoked at Vm −140 to 140 mV with a 20 mV increment from Vm 0 mV. Only currents from selected voltages listed on the right side of the Fig. 3B are shown for simplicity. The experiment started with symmetrical solutions (3 mM KCl and 127 mM K-d-gluconate) in both pipette and bath and then bath solutions (intracellular side) were replaced with solutions containing130 mM KCl. Traces in A and B are from two different patches. C. Current-voltage relationship of single Slack channel. Each current in is an average of 2 to 39 (n=11.6±2.2 for 3 mM Cl− experiments; n=22.6±2.0 for 130 mM Cl− experiments) measurements from 6 patches of 3 mM Cl− experiments and 7 patches of 130 mM Cl− experiments. D. Relationship between Vm and NPo. Each point in Fig. 3D is from 1 to 5 (n=2.9±0.5 for 3 mM Cl− experiments; n=3.5±0.3 for 130 mM) measurements.
* shows statistical difference between 3 and 130 mM Cl− experiments (p<0.05).
We also examined the sensitivity of the Slack channel to [Na+]i. Single channel (inside-out patch) experiments were performed to examine the sensitivity of the Slack channel to the [Na+]i. In low [Na+]i solutions (5 mM), the open probability of single Slack channels was low (Fig. 3A, D). Single Slack channel activity significantly increased when the intracellular side of the membrane was exposed to 90 mM Na+ at all voltages from −140 to 140 mV (with the exception at +60 mV). The normalized relationship between NPo and Vm for both low and high Na+ experiments indicates that the Na+ concentration does not change the voltage dependency of the Slack single channel open probability. Shifting internal solutions from 5 mM to 90 mM Na+ did not alter the single channel reversal potential (Fig. 4C, and A, B traces at 0 mV), which confirms that the currents are carried by K+ ions. Changing the internal solutions from 5 mM to 90 mM Na+ significantly increased single Slack channel amplitude (Fig. 4C).
Figure 4.
Single Slack channel activity is sensitive to intracellular sodium. Slack channel activity increases when the [Na+]i increased from 5 (Fig. 4A) to 90 mM (Fig. 4B). Single channel currents were evoked at Vm −140 to 140 mV with a 20 mV increment from Vm 0 mV. Only currents from selected voltages listed on the right side of the Fig. 4B are shown for simplicity. The experiment started with symmetrical solutions (30 mM KCl, 10 mM K-d-gluconate and 5 mM Na-d- gluconate and 85 n-methyl-d-glucamine) in both pipette and bath and then bath solutions (intracellular side) were replaced with solutions containing 30 mM KCl, 10 K-d-gluconate and 90 mM Na-d-gluconate. Traces in A and B are from the same patch and the order of the experiment was 90 mM Na+ experiment first and 5 mM Na+ experiment second. C. Current-voltage relationship of single Slack channel. Each current in Fig. 4C is an average of 2 to 18 (n=12.1±1.0 for 5 mM Na+ experiments; n=13.5±1.1 for 90 mM Na+ experiments) measurements from 4 patches of 5 mM Na+ experiments and 4 patches of 90 mM Na+ experiments. D. Relationship between Vm and NPo. Each point in Fig. 3D is from 2 to 7 (n=5.4±0.4 for 5 mM Na+ experiments; n=4.3±0.4 for 90 mM Na+ experiments) measurements.
* shows statistical difference between 5 and 90 mM Na+ experiments (p<0.05).
We then tested the effect of a decrease in intracellular pH (pHi) and an increase in CO2 on the activity of the Slack channel. Inside-out patches were held at Vm equal to −100 mV and perfused with different solutions of varying pH to examine the effects of pHi changes on channel activity. There was a dose-dependent inhibition of channel activity when the Slack channel was exposed to acidic pH, reflected by a decrease in NPo (p<0.05, n=3, for pH 6.8 vs. pH 7.3 and p<0.05, n=3, for pH6.3 vs. pH 7.3) (Figure 5A). There was an increase in channel NPo when Slack was exposed to basic pH values (p<0.05, n=3, for pH 8.3 vs. pH 7.3) (Figure 5B), though at pH 7.8, NPo was not significantly different from that at pH 7.3 (p>0.05, n=3). Insets in this figure show channel opening in an expanded time frame at the indicated points. The effect of pH on the channel was partially reversible. The NPo was restored close to its original level in most cases when pH was returned to 7.3. The relationship of NPo versus pH could be fitted by the Boltzmann equation and yielded a middle point 0.17 of NPo at pH 7.2.
Figure 5.
Slack single channel activity is modulated by intracellular pH. Slack single channel activities were initially recorded using symmetrical solutions (see Fig. 1) in both pipette and bath at Vm = −100 mV. Intracellular pH was then increased or decreased by replacing the original bath solution with a solution having the same chemical composition but for pH which was adjusted by acetic acid or KOH. When exposed to acidic [pH]i, Slack channel activity decreases (A). When exposed to basic [pH]i, Slack channel activity increases (B). Insets show channel opening in higher resolution. Slack NPo increases with increased pH (C) (n=3, Vm = −100mV).
To examine the effect of [CO2]i, inside-out patches (cytosolic side facing the bath solution) recordings were performed at Vm = −100 mV and the bath was perfused with solutions of varying CO2 concentrations. pH of the bath solution was decreased with increased CO2. In room air, pH of the bath solution was 7.29±0.01 (n=3), With 5% CO2, pH was 6.42±0.06 (n=3) and with 12% CO2, pH was 6.14±0.06 (n=4). There was a dose- dependent inhibition of channel activity when the Slack channel was exposed to 5% as reflected by a decreased NPo (p>0.05, n=3, for 0.038% vs. 5% and p<0.05, n=3, for 0.038% vs. 12%) (Figure 6A, B). Insets show channel opening in an expanded time frame at the indicated points. Based on our data, we estimated that the NPo was decreased by about 41%, when 5% CO2 was in bath solution. With increased CO2 (12%), NPo was decreased further to about 66%.
Figure 6.
Slack single channel activity is modulated by hypercapnia. Slack single channel activities were initially recorded using symmetrical solutions (see Fig. 1) in both pipette and bath at Vm = −100 mV. Bath (intracellular side) solution was replaced with a solution bubbled with either 5% (A) or 12% (B) CO2. Insets show channel opening in higher resolution. Slack NPo decreases with increased CO2 concentration (C) (n=3, Vm = −100mV).
The Slack channel pH sensitivity was examined when the [Na+]i was increased to 45 mM. Insets show clear channel opening at different pH values when exposed to 45 mM Na+. The Slack channel increased its activity significantly (p<0.05), especially at the higher pHs, when exposed to increased [Na+]i even in the presence of decreased pHi (Figure 7, n=3).
Figure 7.
Slack single channel activity is increased when exposed to increased [Na+]i even in the presence of decreased [pH]i. Slack channel activity was initially recorded using symmetrical solutions (130 mM KCl, 5 mM Na-d-gluconate, 85 mM n-methy-d-glucamine) in both pipette and bath at Vm = −100 mV and later the bath (intracellular side) was replaced with a solution containing 130 mM KCl, 45 mM Na-d-gluconate and 45 n-methy-d-glucamine. Slack single channel activity decreased when the bath was changed from 7.3 to 6.3 even when the bath contained high [Na+]i (45 mM) (A). Slack single channel activity increased dramatically when exposed to [pH]i 8.3 (A). Insets show channel opening at different [pH]i in higher resolution. Slack NPo is significantly increased with increased [Na+]i at all pH values (p< 0.05) (B) (n=3, Vm= −100mV).
Discussion
Based on our experimental data, the channel we expressed in oocytes is the Slack (Slo 2.2) channel. The current is a voltage-dependent, outwardly rectifying current with a slope conductance in the range reported in the literature for this channel (Yuan et al., 2003). Our work has demonstrated that 1) the Slack current from this study is a K+ current since single Slack currents reversed at 0 mV with symmetrical K+ solutions (Fig. 3C and 4C); 2) the Slack current from this study is voltage-dependent and outwardly rectifying (Fig. 1C); 3) the Slack current is sensitive to intracellular Cl− with physiological intracellular Cl− concentrations (130 mM) increasing open probability (Fig. 3D) of single Slack channel but does not modify slope conductance (Fig. 3C); 4) the Slack current is sensitive to intracellular Na+, with physiological concentrations (e.g. 5 mM) keeping this channel in moderate open status. Higher concentrations of Na+ increase not only single channel open probability (Fig. 4D) but also single channel slope conductance, keeping the driving force the same (Fig. 4C); 5) Slack channel has a decreased open probability when exposed to decreased pH or increased CO2 and 6) Slack channel activity is modulated by [Na+]i in the presence of decreased pH.
The Slack channel is widely expressed throughout the CNS (Bhattacharjee et al., 2002) and has been hypothesized to be important in neuronal function, especially because of its known [Na+]i sensitivity (Bhattacharjee et al., 2003; Yuan et al., 2003; Bhattacharjee and Kaczmarek, 2005; Santi et al., 2006). Since we have shown in this study that the Slack channel is active in 5 mM [Na+]i, which is within the range of the resting neuronal [Na+]i (Rose, 2002), we believe that Slack is most likely active in neurons under basal conditions and contributes to setting the resting membrane potential. Our data show that the Slack channel increases its activity when [Na+]i is increased, resulting in increased K+ efflux and promoting membrane repolarization. Therefore, the increased neuronal [Na+]i can be considered to be a “second messenger” feedback ion that terminates cell excitation by activating Slack.
We and others have also shown that hypoxia and ischemia are associated with a substantial increase in [Na+]i, primarily as a result of the inhibition of the Na+-K+ ATPase (as suggested by Friedman and Haddad, 1994), Na+ influx through voltage-gated Na+ channels (Hammarstrom and Gage, 2002; see a review by Banasiak et al., 2004), glutamate receptors (Muller and Somjen, 2000), Na+/H+, Na+/Ca2+ exchanger (as suggested by Friedman and Haddad, 1994), or mainly NMDA channel minorly through AMPA or voltage-gated Na+ channels (Rose and Konnerth, 2001; see a review by Rose 2002). As expected from our current results, this increase in [Na+]i enhances the open probability of Slack. However, clearly there are a variety of intracellular factors and other ion channels which would determine ionic gradients and shape neuronal excitability and the relative role of Slack depends on its expression and temporal activation in specific neurons in the CNS.
In ischemia or hypoxia there is usually a build-up of CO2 and metabolic by- products, which would decrease pHi (Yao and Haddad, 2004). We have shown in this work that Slack activity is dose-dependently decreased by acidic pHi (see Figure 5) or by increased CO2 (see Figure 6). Either pHi or CO2 or both could contribute to the inhibitory effect on Slack activity. Acid sensitive channels have been reported (Kir: Wu et al., 2004; ASIC (acid sensitive ion channel-1, Na channel): Zhang et al., 2006). One characteristic of acid-sensitive K channels is in its regulation by hyper- or hypocapnia (Jiang et al., 2004). However, what was particularly interesting to us was that high [Na+]i attenuated the acid- induced inhibition of Slack activity (see Figure 7). We propose that, during pathological conditions such as hypoxia or ischemia, Slack channel activity is under the influence of at least resultant opposing factors: increased [Na+]i and decreased pHi, which produce a net modulatory signal to regulate Slack channel activity.
We and others have shown that hypoxia decreases the open probability of BK channels in either acutely dissociated (Liu et al., 1999) or membrane delimited preparations (Lewis et al., 2002). The mechanism behind this inhibition of K+ channels has not been fully resolved. It is thought that CO2 exerts its inhibitory effect in the form of H+ ions intracellularly ([H+]i). The increased [H+]i may alter charges on the residues that line the mouth of the pore through which the K+ ions must pass, thereby affecting the sensitivity of the voltage sensor of the channel. Ruppersberg et al. (1991) reported changes in pH dependency of inactivation of KA channel mutation of cysteine with serine residue in the ball domain of RCK4 in the N-terminus suggesting that a change in structure would affect channel gating kinetics. Furthermore, the increased [H+]i may cause protons to line the inner membrane surface altering the voltage difference across the membrane which may affect the threshold (for example, Ca threshold, Haddad and Jiang, 1997) for K+ channel activation.
In summary, we have shown that the Slack channel is active even when [Na+]i is low and that its opening probability decreases when exposed to decreased pH or hypercapnia. In the presence of increased [Na+]i, the Slack channel opening probability increases, even when exposed to decreased pHi. The Slack channel may play an important role in pathological conditions such as in ischemia/hypoxia.
ACKNOWLEDGEMENTS
Supported by NIH Grant# 5 PO1 HD 32573-11 (GGH). We also thank Dr. Amjad Kanaan for his thoughtful criticisms and Nuny Morgan and Orit Gavrialov for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Banasiak KJ, Burenkova O, and Haddad GG (2004) Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience, 126: 31–44. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Gan L, and Kaczmarek LK (2002) Localization of the Slack potassium channel in the rat central nervous system. J. Comp. Neurol, 454: 241–254, 2002. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, and Kaczmarek LK (2003) Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J. Neurosci, 23: 11681–11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, and Kaczmarek LK (2005) For K+ channels, Na+ is the new Ca2+. Trends Neurosci., 28: 422–428. [DOI] [PubMed] [Google Scholar]
- Dobrota D, Matejovicova M, Kurella EG, and Boldyrev AA (1999) Na/K-ATPase under oxidative stress: molecular mechanisms of injury. Cell. Mol. Neurobiol, 19: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer SE (2003) Molecular identification of the Na+-activated K+ channel. Neuron, 37: 727–728. [DOI] [PubMed] [Google Scholar]
- Dryer SE (1991) Na(+)-activated K+ channels and voltage-evoked ionic currents in brain stem and parasympathetic neurones of the chick. J. Physiol, 435: 513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti S, Lavazza T, Curia G, Aracri P, Panzica F, Sancini G, Avanzini G, and Magistretti J (2003) Na+-activated K+ current contributes to postexcitatory hyperpolarization in neocortical intrinsically bursting neurons. J. Neurophysiol, 89: 2101–2111. [DOI] [PubMed] [Google Scholar]
- Friedman JE, and Haddad GG (1994) Anoxia induces an increase in intracellular sodium in rat central neurons in vitro. Brain Res, 663: 329–334. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Starrett JE Jr., and Dworetzky SI (2001) Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist, 7: 166–177. [DOI] [PubMed] [Google Scholar]
- Haddad GG, and Jiang C (1997) O2-sensing mechanisms in excitable cells: role of plasma membrane K+ channels. Annu. Rev. Physiol, 59: 23–42. [DOI] [PubMed] [Google Scholar]
- Hammarstrom AK, and Gage PW (2002) Hypoxia and persistent sodium current. Eur Biophys J., 31: 323–330. [DOI] [PubMed] [Google Scholar]
- Hille B (2001) Ion channels of excitable membrane. Third edition, Sinauer; Sunderland, MA. [Google Scholar]
- Jiang C, Rojas A, Wang R, and Wang X (2004) CO2 central chemosensitivity: why are there so many sensing molecules? Resp. Physiol. Neurobiol, 45:115–126. [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Tang MD, Wang LY, Dworetzky SI, Boissard CG, Gan L, Gribkoff VK, and Kaczmarek LK (1998) Formation of intermediate-conductance calcium- activated potassium channels by interaction of Slack and Slo subunits. Nat. Neurosci, 1: 462–469. [DOI] [PubMed] [Google Scholar]
- Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, and Irisawa H (1984) Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature, 309: 354–356. [DOI] [PubMed] [Google Scholar]
- Lewis A, Peers C, Ashford ML, and Kemp PJ (2002) Hypoxia inhibits human recombinant large conductance, Ca(2+)-activated K(+) (maxi-K) channels by a mechanism which is membrane delimited and Ca(2+) sensitive. J. Physiol, 540: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P (1999) Ischemic cell death in brain neurons. Physiol. Rev, 79: 1431–1568. [DOI] [PubMed] [Google Scholar]
- Liu H, Moczydlowski E, and Haddad GG (1999) O(2) deprivation inhibits Ca(2+)- activated K(+) channels via cytosolic factors in mice neocortical neurons. J. Clin. Invest, 104: 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani A, and Shattock MJ (1992) Role of Na-activated K channel, Na-K-Cl cotransport, and Na-K pump in [K]e changes during ischemia in rat heart. Am. J. Physiol, 263: H333–340. [DOI] [PubMed] [Google Scholar]
- Muller M, and Somjen GG (2000) Na(+) dependence and the role of glutamate receptors and Na(+) channels in ion fluxes during hypoxia of rat hippocampal slices. J. Neurophysiol, 84: 1869–1880. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, and Goldman SA (1993) Carrier-mediated transport of lactic acid in cultured neurons and astrocytes. Am. J. Physiol, 265: R282–289. [DOI] [PubMed] [Google Scholar]
- Niu XW, and Meech RW (2000) Potassium inhibition of sodium-activated potassium (K(Na)) channels in guinea-pig ventricular myocytes. J. Physiol, 526 Pt 1: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR (2002) Na+ signals at central synapses. Neuroscientist, 8: 532–539. [DOI] [PubMed] [Google Scholar]
- Rose CR, and Konnerth A (2001) NMDA receptor-mediated Na+ signals in spines and dendrites. J. Neurosci, 21: 4207–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg JP, Stocker M, Pongs O, Heinemann SH, Frank R, and Koenen M (1991) Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature, 352: 711–714. [DOI] [PubMed] [Google Scholar]
- Santi CM, Ferreira G, Yang B, Gazula VR, Butler A, Wei A, Kaczmarek LK, and Salkoff L (2006) Opposite regulation of Slick and Slack K+ channels by neuromodulators. J. Neurosci, 26: 5059–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xu H, Shen W, and Jiang C (2004) Expression and coexpression of CO2- sensitive Kir channels in brainstem neurons of rats. J. Membr. Biol, 197:179–191. [DOI] [PubMed] [Google Scholar]
- Yao H, and Haddad GG (2004) Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium, 36: 247–255. [DOI] [PubMed] [Google Scholar]
- Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, and Salkoff L (2003) The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron, 37: 765–773. [DOI] [PubMed] [Google Scholar]
- Zhang P, Sigworth FJ and Canessa CM (2006) Gating of acid-sensitive ion channel-1: release of Ca2+ block vs. allosteric mechanism. J. Gen. Physiol 127:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]