Abstract
Introduction
Rotavirus causes severe-diarrheal diseases in infants. An estimation of 138 million rotavirus-associated diarrheal cases and 215,000 deaths occur every year globally. In December 2016, West-Shewa zone in Ethiopia reported unidentified gastrointestinal diarrhea outbreak. We investigated to identify the causative agent of the outbreak to support response operations.
Methods
Medical records were reviewed, and the daily line list was collected from health facilities. Descriptive data analysis was done by time, person and place. Stool specimens were first tested by antigen capture enzyme immunoassay (EIA) technique and further confirmed by reverse-transcription polymerase chain reaction (RT-PCR) as a gold standard. The product of RT-PCR was genotyped for each gene using G1-G4, G8-G9 and G12 primers for VP7 gene and P(4), P(6), P(8) and P(14) primers for VP4 gene.
Results
A total of 1,987 diarrheal cases (5.7 per 1000) and five deaths (case-fatality rate 0.25%) were identified and epidemiologically-linked to confirmed rotavirus from December 2016 to February 2017. Among the cases, 1,946 (98%) were < 5 children. Fourteen (74%) of the 19 tested stool specimens were positive for rotavirus by EIA and RT-PCR. Majority of strains detected were G12P(6) (25%) and G-negative P(8) (25%) followed by G9P(8) (19%), G1P(8) (13%) and G3/G2 P(8), G12P(8), and G-negative P(6) (6% each).
Conclusion
Diarrheal outbreak which occurred in West-Shewa zone of Ethiopia was associated with rotavirus and relatively more affected districts with low vaccination coverage. Routine rotavirus vaccination quality and coverage should be evaluated and the surveillance system needs to be strengthened to detect, prevent and control a similar outbreak.
Keywords: Rotavirus, diarrhea, outbreak, genotype, vaccine, Ethiopia
Introduction
Rotavirus is a genus Rotavirus in the family Reoviridae and causes severe diarrhea and vomiting in infants [1]. Rotavirus has a genome consisting of 11 segments of double-stranded RNA [2, 3]. Most segments encode a single polypeptide, allowing the virus to express six structural viral proteins (VP1-VP7) and six nonstructural proteins (NSP1- NSP6) [3]. Rotavirus-infected persons shed high concentrations of rotavirus in the stool. The disease transmits from an infected person to another by fecal-oral route through close person-to-person contact and by fomites. Less commonly, the virus is transmitted by consuming contaminated water or food [4]. Rotavirus is stable and may persist viably in the environment for weeks or even for months if not disinfected [4-6]. Individuals infected by rotavirus disease manifest some watery diarrhea of limited duration to severe diarrhea with vomiting and fever that can result in dehydration with shock, electrolyte imbalance and even death [7]. Following an incubation period of 1-3 days, the illness often begins shortly and vomiting frequently precedes the onset of diarrhea. The gastrointestinal symptoms usually resolved in 3-7 days after the first onset of the illness [8]. Rotavirus is the most common cause of severe, dehydrating gastroenteritis in infants and young children worldwide [9]. An estimated 138 million annual cases and 215,000 deaths in under five children reported in both developing and developed countries, principally in Asia and sub-Saharan Africa [10-12]. Rotavirus infection accounts for 40% of childhood gastroenteritis hospitalizations and 37% of diarrhea-related deaths in children under five years old [13]. Diarrheal disease is a leading killer and causing approximately 16 percent of deaths in children less than five years of age in Ethiopia [14]. Rotavirus is one of the top diarrhea diseases and affect the lives of more than 28,000 Ethiopian children of under five years old every year [15] which makes Ethiopia one of the five developing countries accounted for more than half of all rotavirus deaths: The Democratic Republic of the Congo, Ethiopia, India, Nigeria and Pakistan [16, 17].
Diarrheal diseases caused by rotavirus cannot be clinically differentiated from that caused by other enteric pathogens; specific diagnosis needs testing of fecal specimens with commercially available assays [7]. ELISA, Test device and Latex Agglutination tests are a sensitive diagnostic test for rotavirus detection [18]. However, PCR provided the best overall sensitivity and specificity [19]. This diagnostic testing technique is not usually available for routine patient management. At the hospital level, routine laboratory confirmation is not usually performed as the clinical management mostly relies on appropriate rehydration therapy. Hospital-based rotavirus surveillance in under five children was initiated in 2007 in Addis Ababa to estimate the burden of rotavirus gastroenteritis in children less than five years of age. Studies have shown that rotavirus accounts for 18%-28% of diarrhea hospitalizations among children < 5 years of age in Ethiopia [20-23]. Ethiopia introduced the Rotarix vaccine into its routine immunization program in November 2013 with two doses in 6 and 10 weeks of age [15, 24]. The WHO required 90% and 80% vaccination coverage at the national and district level respectively for all vaccine-preventable diseases including rotavirus [25]. In Ethiopia, the national rotavirus vaccination coverage was 56.0% while it was 50.2% in Oromia region that included West Shewa in 2016 [26]. In pre-vaccine period (2007-2011), the most prevalently detected genotypes in Addis Ababa were G1P(8) (20%), G12P(8) (17%) and G3P(6) (15%) [27]. In the 2nd week of December 2016, West Shewa zone of Oromia regional state reported rotavirus suspected acute diarrhea outbreak. As the number of daily cases increasing the investigation was warranted and conducted to identify the underlying causative agent of the outbreak to support outbreak management and response operations.
Methods
West Shewa zone is one of the zones in Oromia regional state of Ethiopia to the west of Addis Ababa. Based on the 2007 Census conducted by the Central Statistical Agency of Ethiopia (CSA), this Zone has a total population of 2,058,676, of which 1,028,501 are men, and 1,030,175 are women. The investigation was warranted by the Ethiopian Public Health Institute as part of outbreak response and management. Subsequent permissions obtained from Oromia Regional Health Bureau, West Shewa Health Department, Nono, Danno and Jibat districts health offices to investigate the outbreak. The outbreak case definition for rotavirus was “any person presenting to a health facility with acute diarrhea and/ or vomiting or any person in which clinician suspected rotavirus in Nonno, Danno, Jibat districts after December 16, 2016.” Clinicians were reported cases met the case definition for the duration of the outbreak. Medical records were reviewed and all cases were line listed up on getting verbal permission from medical directors of health facilities. Patients' age, outbreak area setting, contact history, and date of symptoms of onset were used to epidemiologically link gastrointestinal diarrheal cases with laboratory-confirmed rotavirus cases. The descriptive cross-sectional study design was employed, and data analysis was done by time, person and place using Microsoft Excel. The incident rate was calculated by dividing the number of the case to the population (Source: 2007 Census) and expressed per 1,000. The age-specific incident rates were also calculated by dividing the number of cases within a specific age group or sex to respective age group population and multiplying by 1,000. Three years of routine rotavirus vaccination coverage data were received from West Shewa Health Department and compared with suspected rotavirus incidence rate. Incident rate and vaccination coverage were depicted on the map using the Arc Geographic Information System (Arc GIS) 10.4.1 version to illustrate the most affected districts.
Rotavirus infection was determined by using an antigen capture enzyme immunoassay (EIA; ProSpecTTMRotavirus kit, Oxoid Ltd, United Kingdom) on the collected 19 fecal specimens at the national laboratory of the Ethiopian Public Health Institute (EPHI). RT-PCR was used as a gold standard for further confirmation and genotyping. All positive samples were further characterized by molecular methods at the Rotavirus Regional Reference Laboratory (RRRL): South Africa Medical Research Council (SAMRC) Diarrheal Pathogens Research Unit, Department of Virology, SefakoMakgatho Health Sciences University, Pretoria, South Africa. As described before, the VP7 and VP4 genes were amplified by reverse transcription polymerase chain reaction (RT-PCR) using the outer primer sets sBeg/End9 and Con2/Con3 [28, 29]. RT-PCR products for each gene were genotyped using type-specific primers such as G1-G4, G8-G9, and G12 for VP7 gene and primers P(4), P(6), P(8) and P(14) for VP4 gene, respectively. The investigation was conducted to support the response operations or management of the outbreak by identifying the causative agent so as to pinpoint and specify the interventions. Ethiopian public health Institute is mandated by the Council of Ministers to conduct diseases surveillance, epidemiological and laboratory investigations and respond to the outbreaks [30].
Results
A total of 1,987 diarrheal cases (5.7 per 1,000) and five deaths (case fatality rate 0.25%) were identified and epidemiologically linked to confirmed rotavirus from 16 December 2016 to 21 February 2017. Among the cases, 1121 (56%) were male, and the rest of 866 (44%) cases were female (Table 1). Only 32 (1.6%) of the cases were hospitalized. The majority of the cases, 1946 (98%), were under the age of five years old with nine months median age and interquartile range 14 months (Q1 = 6 months and Q3 = 20 months). A total of 19 stool specimens were collected during the diarrhea outbreak in the affected districts. All children sampled were with unknown rotavirus vaccination history. Among the collected samples, 14 (74%) were positive for rotavirus by EIA. Male and female contribute 7 (50%) each of the positive cases (Table 1). With regard to genotyping results, majority of the strains found were G12P(6) (25%) and G-negative P(8) (25%) followed by G9P(8) (19%),G1P(8) (13%) and G3/G2 P(8), G12P(8) and G-negative P(6) (6% each), respectively. The crude incidence rate was 5.7 cases per 1,000 populations with some variation among different affected districts. Nonno is the most affected district with the incident rate of 8.5 cases per 1,000 populations. The age group specific incident rate showed that young children less than five years of age were the most affected age groups with the incident rate of 38.2 cases per 1,000 populations of the same age (Table 2). The outbreak started in the 2nd week of December 2016 in Nonno district. It was further spread to Danno and Jibat districts of West Shewa zone and increased to reach high pick on the 3rd week of January 2017 and the number of daily cases started dropping in the same week until the 3rd week of February 2017 (Figure 1). Among total cases, 1224 (62%) were not vaccinated for rotavirus; 693 (35%) were vaccinated while the vaccination statuses of 70 (4%) of the cases were not known (Table 3). Routine rotavirus vaccination coverage was relatively low in Nonno district followed in Danno District over years (Table 4). The 2016/2017 routine 2nd dose vaccination coverage and incidence rate showed in Figure 2.
Table 1.
Rotavirus cases, deaths and laboratory specimen results by district, West Shewa Zone, Oromia Region, Ethiopia, 2017
| District | Cases | Deaths | Laboratory test results | ||||
|---|---|---|---|---|---|---|---|
| Total | Female | Male | Sample | Positive | Positivity Rate | ||
| Danno | 914 | 395 | 519 | 0 | 11 | 7 | 63.6% |
| Nonno | 983 | 436 | 547 | 0 | - | - | - |
| Jibat | 90 | 35 | 55 | 5 | 8 | 7 | 87.5% |
| Total | 1987 | 866 | 1121 | 5 | 19 | 14 | 73.7% |
Table 2.
Rotavirus cases incident rate per 1,000 populations by age groups and districts, West Shewa Zone, Oromia Region, Ethiopia, 2017
| District | 0-4 Years | 5-14 Years | 15-44 Years | 45 and above Years | Total |
|---|---|---|---|---|---|
| Danno | 887 (44.6%) | 13 (0.3%) | 13 (0.2%) | 1 (0.1%) | 914 (6.7%) |
| Nonno | 971 (57.7%) | 7 (0.2%) | 5 (0.1%) | 0 (0.0%) | 983 (8.5%) |
| Jibat | 88 (6.2%) | 2 (0.1%) | 0 (0.0%) | 0 (0.0%) | 90 (0.9%) |
| Total | 1946 (38.2%) | 22 (0.2%) | 18 (0.1%) | 1 (0.0%) | 1987 (5.7%) |
Figure 1.
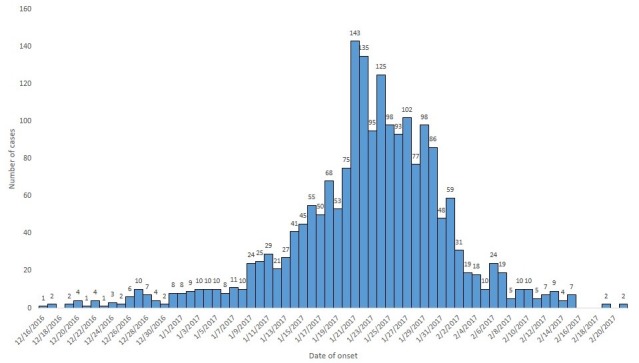
Trend of rotavirus-associated diarrheal cases by date of onset, West Shewa Zone, Oromia region, Ethiopia, 2017
Table 3.
Rotavirus vaccination status of the cases, West Shewa Zone, Oromia Region, Ethiopia, 2017
| District | Unknown | Unvaccinated | Vaccinated | Total |
|---|---|---|---|---|
| Danno | 4 (0.4%) | 755 (82.6%) | 155 (16.9%) | 914 (100%) |
| Nonno | 19 (1.9%) | 452 (46.0%) | 512 (52.1%) | 983 (100%) |
| Jibat | 47 (52.2 | 17 (18.9%) | 26 (28.9%) | 90 (100%) |
| Total | 70 (3.5%) | 1224 (61.6%) | 693 (34.9%) | 1987 (100%) |
Table 4.
Routine rotavirus vaccination administrative coverage by district, West Shewa Zone, Oromia Region, Ethiopia, 2014-2017
| District | 2014/2015 | 2015/2016 | 2016/2017 | |||
|---|---|---|---|---|---|---|
| 1st dose % | 2nd dose % | 1st dose % | 2nd dose % | 1st dose % | 2nd dose % | |
| Danno | 106 | 95 | 101 | 88 | 87 | 72 |
| Nonno | 97 | 92 | 99 | 81 | 76 | 67 |
| Jibat | 101 | 96 | 106 | 98 | 106 | 95 |
| Total | 101 | 94 | 101 | 88 | 87 | 76 |
Figure 2.
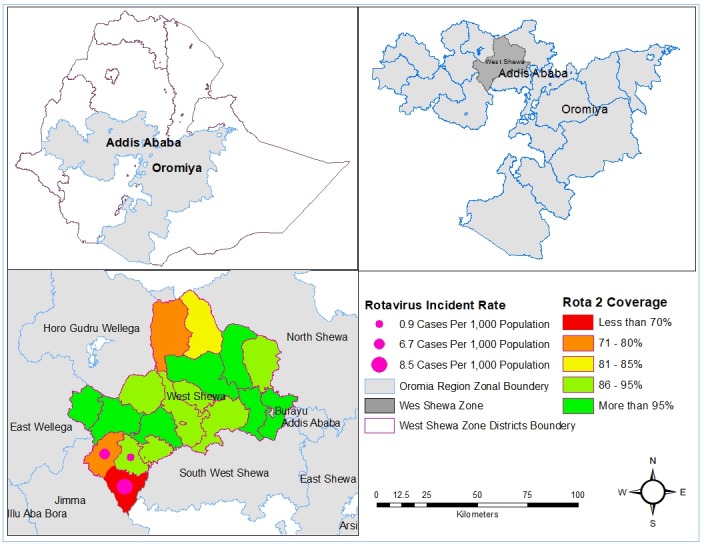
Map of Ethiopian showing rotavirus incidence rate and rotavirus vaccination coverage by districts, 2017
Discussion
This is first reported and investigated rotavirus-associated diarrheal outbreak occurred in three districts of West Shewa Zone of Oromia Region in Ethiopia. The diarrheal outbreak affected the health of many children is associated with rotavirus where 62% of the cases were not vaccinated. In sub-Saharan Africa 45.5% in 2000 and 38.9% in 2013 deaths of diarrheal diseases attributed to rotavirus [31]. Children aged less than five years old were most affected (38 cases per 1,000 populations) by the outbreak with a median age of nine months. Children under five years of age are most vulnerable to the disease [10]. In low-income countries, the median age at the primary rotavirus infection ranges from 6 to 9 months [32, 33]. Similar to our observation, in the Solomon Islands at which the highest attack rate during the outbreak occurred in the < 5 years age groups (32%), which was > 14 times higher than in the ≥ 5 years age groups (2%) [34]. During this outbreak children under five years account for 98% of the total cases which is supported by a study conducted in South Tarawa, Kiribati, that indicates 93.4% of the cases attributed to the rotavirus outbreak and all deaths were under five years old [10]. AS compared with the 2.5% rotavirus CFR estimated by WHO, the CFR we reported here is low [35]. Another study in Ethiopia also reported 2.4% CFR due to rotavirus which is higher as compared to our report [36]. The lower CFR in our investigation might be attributed to the early detection and treatment of cases at health facilities. In our investigation, we observed G12P(6) and G-negative P(8), G9P(8) were circulating dominantly followed by G1P(8), G3/G2 P(8), G12P(8), and G-negative P(6) in the outbreak areas. Whereas, the previous study showed that G3P (6), G1P (8) and G2P (4) are common strains of rotaviruses circulating in Ethiopia [10, 22, 31, 37]. In rural Southern Ethiopia, it was reported that 43.6% of children less than five years of age had diarrhea as a result of rotavirus [38].
G-P combinations such as G1P(8), G2P(4), G3P(8), G4P(8) and G9P(8) contribute about 90% of all human rotavirus infections in which G1P(8) is the most prevalent combination worldwide. Surveillance studies indicate that G1P(8) strain contribute 52.2% of rotavirus strains globally and 17.4% in Africa [39]. Several rotavirus types are circulating in Asia and Africa with greater strain diversity. Among the total rotavirus cases, 52.1%, 28.9% and 16.9% cases were reported with the history of at least one dose of rotavirus vaccination in Nonno, Jibat and Danno districts respectively. The proportion of infected vaccinated children is high in Nonno district which might be attributed to the quality of vaccination. The study conducted in the Republic of Moldova indicates that 25% moderate to severe cases received at least one dose of rotavirus vaccine [40]. The case-control studies conducted in Malawi and Botswana indicate that rotavirus vaccine efficacy for two doses were 64% and 54% respectively which is low compared with developed countries [41, 42]. The administrative vaccination coverage for the last three years varies from district to district. In contrast to the vaccination status of the patients, the vaccination coverage was low in Nonno district. Nonno was the most affected district with a high number of cases and incidence rate. In the district vaccination coverage for the 2nd dose of rotavirus vaccine was 81% in 2015/2016 and 67% in 2016/2017 which was less than the World Health Organization's minimum requirement [25, 43]. Similarly, the vaccination coverage in Danno district was less to prevent the outbreak. However, the rotavirus vaccination coverage was high in Jibat district. In Jibat district effectiveness of the vaccine needs to be further studied and evaluated. Even though rotavirus vaccine efficacy is actually high, the effectiveness of rotavirus vaccine is less in developing countries as compared with developed countries [44]. We also believe that, as the reliability of the administrative vaccination concerns, the true vaccination coverage even might be less than what was reported which might be contributing factors for the outbreak.
Conclusion
The diarrheal outbreak occurred in three districts of South West Shewa zone of Oromia region in Ethiopia was associated with different rotavirus strains and relatively more affected districts with low vaccination coverage. The existing routine rotavirus vaccination needs to be evaluated and the surveillance system needs to be strengthened to detect, prevent and control a similar future outbreak.
What is known about this topic
Rotavirus is the most common cause of severe gastroenteritis and responsible for more than one-third of diarrheal diseases related to hospitalizations and deaths in infants under five years old. Currently, there is a safe and effective vaccine to prevent rotavirus infections.
What this study adds
Rotavirus caused diarrheal disease outbreak and mostly affected under five years children in West Shewa Zone of Oromia Region of Ethiopia. The occurrence of the outbreak suggested there is an inadequate rotavirus vaccination coverage in the area.
Competing interests
All Authors declared no competing interests.
Acknowledgments
The authors wish to thank Oromia Regional Health Bureau, West Shewa Zonal health Department, Nono, Donno, and Jibat district health offices. We would also like to acknowledge Rotavirus Regional Reference Laboratory (RRRL): SAMRC Diarrheal Pathogens Research Unit, Department of Virology, SefakoMakgatho Health Sciences University, Pretoria, South Africa, for their support in laboratory analysis, WHO Ethiopia country office and Drs. Jason Mwenda and Goitom G/Medhin from African Regional WHO Office for their support.
Authors’ contributions
The work presented here was of a collaborative nature. Abyot Bekele Woyessa proposed and designed the analysis, carried out data cleaning, categorizing, analysis and write-up of the manuscript. Almaz Abebe leads the laboratory investigation, participate in the interpretation of the result and manuscript preparation. Berhane Beyene, Mesfin Tefera, Esete Assefa, Hiwot Ketema, Birke Teshome, and Ayenachew Bekele participated in laboratory analysis. Shambel Habebe, Diriba Sufa, Dagnachew Alemu, Habtamu Tilahun, Mengistu Biru and Gemechu Shume participated in the fieldwork. All authors have participated in the interpretation of findings and review of the manuscript. All authors have read and approved the final manuscript.
References
- 1.Mizukoshi F, Kuroda M, Tsukagoshi H, Sekizuka T. A food-borne outbreak of gastroenteritis due to genotype G1P rotavirus among adolescents in Japan. Microbiol Immunol. 2014;58(9):536–9. doi: 10.1111/1348-0421.12176. [DOI] [PubMed] [Google Scholar]
- 2.Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Estes MK, Gentsch JR, et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch Virol. 2008;153(8):1621–9. doi: 10.1007/s00705-008-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthijnssens J, Ciarlet M, Mcdonald SM, Attoui H. Uniformity of Rotavirus Strain Nomenclature Proposed by the Rotavirus Classification Working Group (RCWG) Jelle. Arch Virol. 2011;156(8):1397–413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4..Center for Disease Control and Prevention (CDC) Rotavirus. 2014. pp. 263–7. Accessed on 16 Jan 2019. [Google Scholar]
- 5.Keswick BH, Pickering LK, DuPont HL, Woodward WE. Survival and detection of rotaviruses on environmental surfaces in day care centers. Appl Environ Microbiol. 1983;46(4):813–6. doi: 10.1128/aem.46.4.813-816.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmér M, Paxéus N, Magnius L, Enache L, Arnholm B, Johansson A, et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl Environ Microbiol. 2014;80(21):6771–81. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parashar UD, Nelson EAS, Kang G. Diagnosis, management and prevention of rotavirus gastroenteritis in children. Bmj. 2013 Dec 30;347:f7204. doi: 10.1136/bmj.f7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . Rotavirus. Atlanta: Vaccine Preventable Disease Surveillance Manual; pp. 1–17. Accessed on 16 Jan 2019. [Google Scholar]
- 9.Kaufman HW, Chen Z. Trends in Laboratory Rotavirus Detection: 2003 to 2014. Pediatrics. 2016;138(4):e20161173–e20161173. doi: 10.1542/peds.2016-1173. [DOI] [PubMed] [Google Scholar]
- 10.Tate JE, Burton AH, Boschi-pinto C, Parashar UD, Health W, Coordinated O, et al. Global, Regional and National Estimates of Rotavirus Mortality in Children < 5 Years of Age, 2000-2013. Clin Infect Dis. 2016;62(Suppl 2):S96–105. doi: 10.1093/cid/civ1013. [DOI] [PubMed] [Google Scholar]
- 11.Freed GL, Turbitt E. The global imperative to address vaccine-preventable diseases. R Aust Coll Gen Pract. 2016;45(1):14–6. [PubMed] [Google Scholar]
- 12.Donato CM, Cowley D, Snelling TL, Akopov A, Kirkness EF, Kirkwood CD. Characterization of a G1P rotavirus causing an outbreak of gastroenteritis in the Northern Territory, Australia, in the vaccine era. Emerg Microbes Infect. 2014 Jul;3(7):e47. doi: 10.1038/emi.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez PP, King AA, Yunus M, Faruque ASG, Pascual M. Differential and enhanced response to climate forcing in diarrheal disease due to rotavirus across a megacity of the developing world. PNAS. 2016;113(15):4092–7. doi: 10.1073/pnas.1518977113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . World Health Statistics 2015: Global Health Observatory (GHO) data.; [Google Scholar]
- 15.PATH . Rotavirus disease and vaccines in Ethiopia. Seattle, USA: 2013. Accessed on 16 Jan 2019. [Google Scholar]
- 16.Verguet S, Murphy S, Anderson B, Johansson KA, Glass R, Rheingans R. Public finance of rotavirus vaccination in India and Ethiopia: an extended cost-effectiveness analysis. Vaccine. 2013;31(42):4902–10. doi: 10.1016/j.vaccine.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(2):136–41. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 18.Younis Abdul-Redha AL, Khafaji HJA-J. Detection of rotavirus in diarrhea stool samples of children with acute gastroenteritis in Babylon. Int Res J Microbiol. 2013;4(3):84–88. [Google Scholar]
- 19.Ye S, Lambert SB, Grimwood K, Nimmo GR, Sloots TP, Kirkwood CD, et al. Comparison of test specificities of commercial antigen-based assays and in-house PCR Methods for detection of rotavirus in Stool. J Clin Microbiol. 2015;53(1):295–7. doi: 10.1128/JCM.02251-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhe L, Fredrikzon B, Habte D. Clinical profile of rotavirus enteritis in Ethiopian children. Ethiop Med J. 1986;24(1):1–6. [PubMed] [Google Scholar]
- 21.Abebe A, Abebe S, Giday M, Taffesse B. Rotavirus infection in under-five children in Yekatit 12 hospital. Ethiop J Heal Dev. 1995;9(1):71–5. [Google Scholar]
- 22.Stintzing G, Back E, Tufvesson B, Johnsson T, Wadström T, Habte D. Seasonal fluctuations in the occurrence of enterotoxigenic bacteria and rotavirus in paediatric diarrhoea in Addis Ababa. Bull World Health Organ. 1981;59(1):67–73. [PMC free article] [PubMed] [Google Scholar]
- 23.Bizuneh T, Mariam ZS, Abebe A, Lema E. Rotavirus infection in under-five children in Jimma Hospital, Southwest Ethiopia. Ethiop J Heal Dev. 2004;18(1):19–24. [Google Scholar]
- 24.Almaz Abebe, Mekonen Getahun, Seheri Mapaseka L, Berhane Beyene, Essete Assefa, Birke Teshome, et al. Impact of rotavirus vaccine introduction and genotypic characteristics of rotavirus strains in children less than 5 years of age with gastroenteritis in Ethiopia: 2011-2016. Vaccine. 2018;36(46):7043–7. doi: 10.1016/j.vaccine.2018.09.048. [DOI] [PubMed] [Google Scholar]
- 25.World Human Organization Global vaccine action plan 2011-2020. Accessed on 16 Jan 2019. [Google Scholar]
- 26.Central Statistical Agency (CSA) Ethiopia and ICF . Demographic and Health Survey. Addis Ababa, Ethiopia and Rockville, Maryland, USA; 2016. [Google Scholar]
- 27.Abebe A, Teka T, Kassa T, Seheri M, Beyene B, Teshome B, et al. Hospital-based surveillance for rotavirus gastroenteritis in children younger than 5 years of age in Ethiopia: 2007-2012. Pediatr Infect Dis J. 2014;33(SUPPL 1):2007–12. doi: 10.1097/INF.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 28.Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30(6):1365–73. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28(2):276–82. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Federal Democratic Republic of Ethiopia parlament . Ethiopian Public Health Institute Establishment Council of Ministers Regulation No. 301/2013. Federal Negarit Gazette. 2014. Jan, p. 7175. Accessed on 16 Jan 2019. [Google Scholar]
- 31.Shahrabadi MS, Ahmadi E. Epidemiology of Rotavirus Infection in Certain Countries. Iran J Virol. 2014;8(4):34–42. [Google Scholar]
- 32.Cunliffe NA, Kilgore PE, Bresee JS, Steele AD, Luo N, Hart CA, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull World Health Organ. 1998;76(5):525–37. [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization Rotavirus vaccines WHO position paper-January 2013. Wkly Epidemiol Rec. 2013;88(5):49–64. [PubMed] [Google Scholar]
- 34.Precipitated O, Islands S, Jones FK, Ko AI, Becha C, Joshua C, et al. Increased rotavirus prevalence in diarrheal outbreak precipitated by localized flooding, Solomon Islands, 2014. Emerg Infect Dis. 2016;22(5):875–9. doi: 10.3201/eid2205.151743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization Rotavirus. Geneva: World Health Organization; 2018:1–11. [Google Scholar]
- 36.Malede Mequanent Sisay, Segni Mekonen Gedefa, Addisu Jember Zeleke GAS. Risk Factors of Rotavirus Outbreak Among Children in Kurmuk District, Benishangul Gumuz Regional State, Ethiopia. JOJ Case Stud. 2018;8(1):555730. [Google Scholar]
- 37.Stella SI, Ajayi A. Viral causes of diarrhea in children in Africa: a literature review. J Mol Biol Tech. 2017;1(1):104. [Google Scholar]
- 38.Yassin MA, Kirby A, Mengistu AA, Arbide I, Dove W, Beyer M, et al. Unusual norovirus and rotavirus genotypes in Ethiopia. Paediatr Int Child Health. 2012;32(1):51–5. doi: 10.1179/1465328111Y.0000000047. [DOI] [PubMed] [Google Scholar]
- 39.Todd S, Page NA, Duncan Steele A, Peenze I, Cunliffe NA. Rotavirus strain types circulating in Africa: review of studies published during 1997-2006. J Infect Dis. 2010;202(S1):S34–42. doi: 10.1086/653555. [DOI] [PubMed] [Google Scholar]
- 40.Gheorghita S, Birca L, Donos A, Wasley A, Birca I, Cojocaru R, et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clin Infect Dis. 2016;62(Suppl 2):S140–6. doi: 10.1093/cid/civ1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, Nakagomi O, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: An observational and case-control study. Lancet Infect Dis. 2015;15(4):422–8. doi: 10.1016/S1473-3099(14)71060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gastañaduy PA, Steenhoff AP, Mokomane M, Esona MD, Bowen MD, Jibril H, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clin Infect Dis. 2016;62(62 Suppl 2):S161–7. doi: 10.1093/cid/civ1207. [DOI] [PubMed] [Google Scholar]
- 43.Vakili R, Hashemi AG, Khademi G, Ajilian M. Immunization coverage in WHO regions: a review article. Indian J Paediatr. 2015;3(15):111–8. [Google Scholar]
- 44.Vesikari T. Success of rotavirus vaccination in Africa: good news and remaining questions. Lancet Glob Heal. 2016;4(2):e76–7. doi: 10.1016/S2214-109X(15)00318-6. [DOI] [PubMed] [Google Scholar]


