Abstract
Impairment of airway tight junctions induced by elevated levels of proinflammatory cytokines is implicated in the pathogenesis of inflammatory airway diseases. Pharmacological stimulation of G–protein coupled receptor (GPR) 40, a receptor of polyunsaturated fatty acids, have recently been shown to promote tight junction assembly in airway epithelial cells under non-inflammatory conditions. However, roles of GPR40 in regulating airway epithelial integrity in response to inflammatory insults are unknown. This study was aimed to investigate the effect of GPR40 stimulation on proinflammatory cytokine (TNFα and IL–1β)-induced tight junction disruption in human airway epithelial Calu–3 cells using GW9508, a GPR40 agonist. We found that stimulation of GPR40 by GW9508 attenuated the cytokine–induced airway epithelial barrier leakage as analyzed by measurements of transepithelial electrical resistance and transepithelial flux of fluorescently labeled dextran (molecular weight of 4 kDa). Furthermore, GW9508 prevented the cytokine-induced dislocalization of zonula occludens (ZO)–1, occludin and claudin–1. The barrier-protective effect of GW9508 was abolished by a GPR40 antagonist, but not a GPR120 antagonist. Immunofluorescence staining of NF–ĸB indicated that GW9508 had no effect on cytokine-induced NF–ĸB activation. Intriguingly, GW9508 inhibited cytokine-induced airway epithelial barrier disruption through suppression of extracellular signal–regulated kinase (ERK) phosphorylation in a phospholipase C (PLC) and calcium/calmodulin–dependent protein kinase kinase beta (CaMKKβ)-dependent manner. Collectively, this study uncovered the novel role of GPR40 in preventing cytokine–induced tight junction disruption in airway epithelial cells through mechanisms involving PLC–CaMKKβ-mediated suppression of ERK signaling. Pharmacological stimulation of GPR40 may be beneficial in the treatment of airway diseases.
Keywords: GPR40, airway epithelial cells, tight junctions, TNFα, IL–1β, ERK, COPD
INTRODUCTION
Tight junctions play an important role in limiting an influx of pathogens and noxious substances into lung tissues [1]. Defective airway epithelial barrier is involved in the pathogenesis of inflammatory lung diseases including pulmonary infection, asthma and chronic obstructive pulmonary disease (COPD) [2]. In COPD, cigarette smoke injures airway epithelial cells resulting in dislocalization of tight junction proteins (zonula occludens (ZO)–1 and occludin) and a transient reduction in airway barrier integrity [3]. Similarly, inhaled allergens promote transient airway barrier disruption in asthma [4]. Interestingly, airway epithelial tissues of both COPD and asthma patients are found to be leaky compared to healthy subjects [5–7]. It has been postulated that macrophage–derived proinflammatory cytokines including TNFα and IL–1β may contribute to the prolonged tight junction disruption in these patients [8, 9].
A number of studies have reported that TNFα and IL–1β induce tight junction disruption in airway epithelial cells [10–13]. These cytokines increase epithelial permeability in association with dislocalization of tight junction proteins including ZO–1 and occludin [11–13]. Long–term treatment with TNFα or IL–1β reduces expression of ZO–1 and occludin [10, 14]. These effects were mediated via either nuclear factor kappa B (NF-ĸB) or extracellular signal–regulated kinases (ERK) 1/2 [14–18]. Suppression of either NF-ĸB and ERK 1/2 signaling provides beneficial effects in alleviating airway epithelial disruption and severity of inflammatory lung diseases including lung injury and COPD [15, 19–21]. These lines of evidence suggest that inhibition of TNFα and IL–1β signaling in airway epithelial cells represent a promising strategy for the treatment of inflammatory lung diseases.
Recently, G–protein coupled receptor (GPR) 40 has been discovered as a receptor of polyunsaturated fatty acids (PUFA) including ω–3 fatty acids [22, 23]. GPR40 is expressed in various types of tissues including airway epithelial cells [24, 25]. Stimulation of GPR40 suppresses TNFα-induced downregulation of ZO–1 expression in intestinal epithelial cells [26]. Likewise, GPR40 stimulation inhibited TNFα–and IL–1β–induced inflammation and apoptosis in pancreatic β–cells by suppressing NF–ĸB signaling [27]. We recently reported that pharmacological stimulation of GPR40 promoted tight junction assembly in airway epithelial cells via a phospholipase C (PLC)– calcium/calmodulin–dependent protein kinase kinase beta (CaMKKβ)-AMP–activated protein kinase (AMPK)-dependent pathway [25]. Interestingly, plasma levels of ω –3, which is an endogenous agonist of GPR40, in COPD and asthmatic patients were found to be lower than that in healthy individuals [28, 29]. These data led us to hypothesize that pharmacological stimulation of GPR40 may suppress the airway epithelial barrier disruption induced by a mixture of proinflammatory cytokines involved in the pathogenesis of airway diseases i.e. TNFα–and IL–1β via inhibition of proinflammatory signaling cascades. Therefore, this study was performed to investigate the effect of pharmacological stimulation of GPR40 using GW9508, a GPR40 agonist, on suppressing the cytokine (TNFα–and IL–1β)–induced tight junction disruption and its underlying mechanisms in human airway epithelial Calu–3 cells.
MATERIAL AND METHODS
Chemicals and Materials
Calu–3 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). GW9508, DC260126, U0126, U73122, STO–609, compound C and fluorescein isothiocyanate (FITC)-dextran (MW 4 kD) were purchased from Sigma–Aldrich (Saint Louis, MO, USA). AH7614 was form Tocris Bioscience (Bristol, UK).
Cell culture
Calu–3 cells were cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F–12 medium supplemented with 1% non–essential amino acid, 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin (Life Technologies, Carlsbad, CA, USA). Cells were maintained in a humidified 95% O2/5% CO2 atmosphere at 37 °C.
Measurement of tight junction integrity
The integrity of epithelial tight junction was analyzed from transepithelial electrical resistance (TER) measurement and FITC–dextran flux assay. Calu–3 cells from passage 25–35 were seeded on Transwell permeable supports (2.5 × 105 cells/well) (Corning Life Sciences, Tewksbury, MA, USA) and cultured for 14 days (TER ≥ 900 Ω.cm2). After measuring TER before treatment, medium was replaced with FBS–free medium supplemented with vehicle or indicated treatments. TER was measured after treatment (at 1 h, 4 h, 8 h, and 24 h) using an EVOM2 volt/ohm meter (World Precision Instruments, Inc., Sarasota, FL, USA).
For FITC–dextran flux assay, cell monolayers were treated with vehicle, TNFα/IL–1β, GW9508, or TNFα/IL–1β plus GW9508. FITC–dextran 4 kDa (Sigma) was added into the apical media (1 mg/mL). Greater than 90% of this batch of FITC-dextran had molecular weight larger than 3 kDa as confirmed by dialysis (data not shown). Basolateral media were sampled after treatment (at 4 h, 8 h, and 24 h) and measured for FITC–dextran intensity (485/535 nm) using an EnVision microplate reader (PerkinElmer, Waltham, MA, USA).
Real–time RT PCR
Cells were grown onto 6–well plates at a density of 106 cells/well. After treatments, total RNA was extracted using the TRiZol reagent (Life Technologies, Carlsbad, CA). RNA was converted to cDNA using SuperScript II RNase H– Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions and was subjected to PCR. The reaction was performed using primers tagged with fluorescent dye (SYBR green) to monitor amplification of cDNA for CLDN1 (5’–CACCCTTGGCATGAAGTGTA–3’ and 5’–AGCCAATGAAGAGAGCCTGA–3’), ZO1 (5’–CGGTCCTCTGAGCCTGTAAG–3’ and 5’–GGATCTACATGCGACGACAA–3’), OCLN (5’–TCAGGGAATATCCACCTATCACTTCAG–3’ and 5’–CATCAGCAGCAGCCATGTACTCTTCAC–3’) and GAPDH (5’– GTCAGTGGTGGACCTGACCT –3’ and 5’– AGGGGTCTACATGGCAACTG –3’). cDNA amplification reactions were performed using an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA). The amount of target cDNA was compared to the amount of housekeeping sequence, i.e. GAPDH. Data were analyzed using ABI Prism software (Applied Biosystems, Foster City, CA).
Immunocytochemistry
For detection of ZO–1 occludin and claudin–1, cells were seeded on Transwell permeable supports (2.5 × 105 cells/well) and cultured for 14 days. After treatments, cells were washed with phosphate−buffered saline (PBS). Fixation was performed by 20–min incubation with 1:1 methanol/acetone at –20 °C followed by incubation with 3% bovine serum albumin (BSA). Then, cells were stained overnight with a mixture of 1:200 anti–ZO–1 antibodies (Invitrogen, California, USA; catalog number 339100; lot 1100420A) and 1:200 claudin–1 antibodies (Invitrogen, California, United States; catalog number 51–9000) or 1:200 occludin antibodies (Invitrogen, California, United States; catalog number 33–1500) and then conjugated with either Cy2- or Cy3-tagged anti-IgG antibodies (Jackson ImmunoResearch, PA, USA). Cells were counter-stained with DAPI for nuclei. Images were captured using either an Olympus fluorescence microscope or a Leica TCS SP8 MP Multiphoton Microscope. Densitometry of ZO–1/occludin along line scans crossing junctions (15 lines per experiment) was measured using Fiji image J software (version 1.52n). The junction to cytoplasm ratio of ZO-1 intensity was calculated, and correlation coefficient between ZO-1 and occludin densities was analyzed from line scanning plot profiles using GraphPad Prism5 software. Intensities of ZO–1 occludin and claudin-1 in whole images were also analyzed.
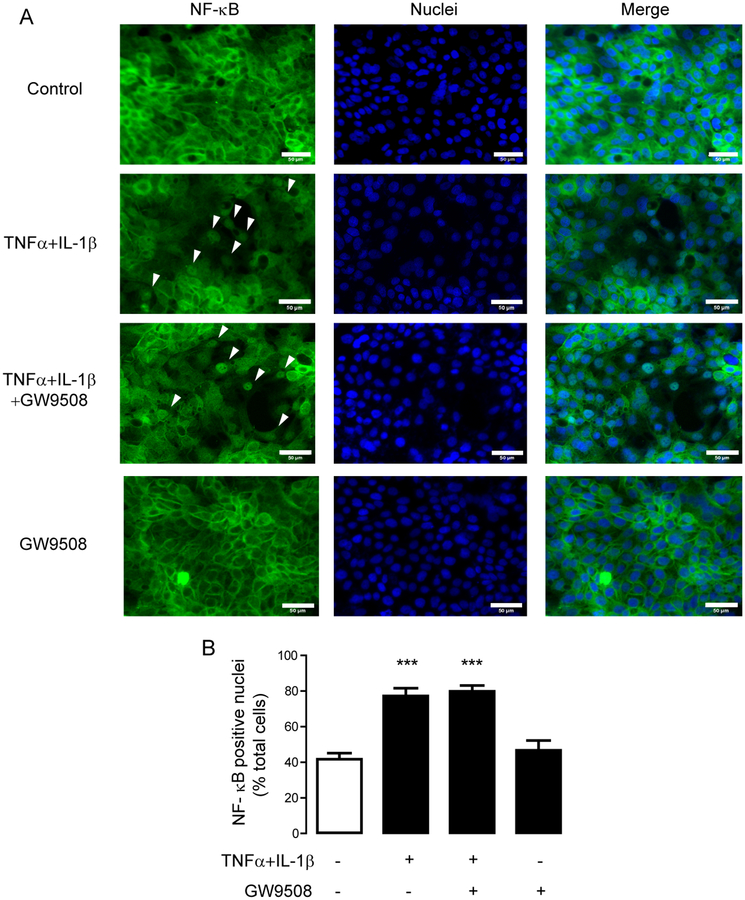
For detection of NF–ĸB, cells were fixation was performed by 15–min incubation with 3.7% paraformaldehyde followed by 10–min incubation with 0.1% triton–X. Cells were washed with PBS and incubated for 30 min in PBS containing 1%BSA, 22.52 mg/mL glycine and 1% Tween20. Then, cells were stained overnight with rabbit anti–p65 antibodies (Cell Signaling Technology, Boston MA, USA, catalog number 8242; lot 9) and conjugated with anti–rabbit IgG Alexa fluor 488 (Thermo Fisher Scientific Inc , MA, USA; catalog number A11034; lot 1670152). Nuclear region (DAPI) and cytosolic region (software algorithm) in each cell were defined by the Columbus Image Data Storage and Analysis System (PerkinElmer, Waltham, MA, USA). Intensity of Alexa fluor 488 in nuclear region and in cytosolic region was measured, and cells having a maximum intensity of Alexa fluor 488 in nuclear region greater than that in cytosolic region were counted as a NF-ĸB positive nuclei indicating nuclear translocation of NF– ĸB.
Western blot analysis
Cells were plated onto 6–well plates at a density of 106 cells/well. After treatments, cell lysates were harvested using a RIPA lysis buffer. Proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) before transferring to a nitrocellulose membrane. The membrane was blocked for an hour with 5% non–fat dried milk (Bio–Rad, Hercules, CA, USA) and incubated overnight with rabbit antibodies to phospho–p44/42 ERK (catalog number 9101; lot 30), p44/42 ERK (catalog number 9102; lot 27), or β–actin (catalog number 4970S; lot 14) (Cell Signaling Technology, Boston, MA, USA). The membrane was then washed for 4 times with Tris–Buffered Saline Tween–20 (TBST) and incubated for an hour at room temperature with 2.5% non–fat dried milk plus horseradish peroxidase conjugated goat antibodies to rabbit immunoglobulin G (Abcam, Cambridge, MA, USA; catalog number ab6721; lot GR194011–1). The signal was detected using Luminata Forte’ Western HRP Substrate (Merck Millipore, Billerica, MA, USA) captured by Omega Lum G Imaging System (Aplegen, San Francisco, CA). Densitometry analysis was performed using Image J software (version 1.51).
Statistical analysis
Results are presented as means ±S.E.M. One–way ANOVA with tukey’s post test was performed for multiple comparison. Two–way ANOVA followed by Bonferroni’s post hoc test was used for multiple comparison data in time series. Spearman correlation test was used for analyzing correlation coefficients. p value <0.05 was considered statistically significant.
3. RESULTS
3.1. Effect of GW9508 on the cytokine–induced tight junction disruption
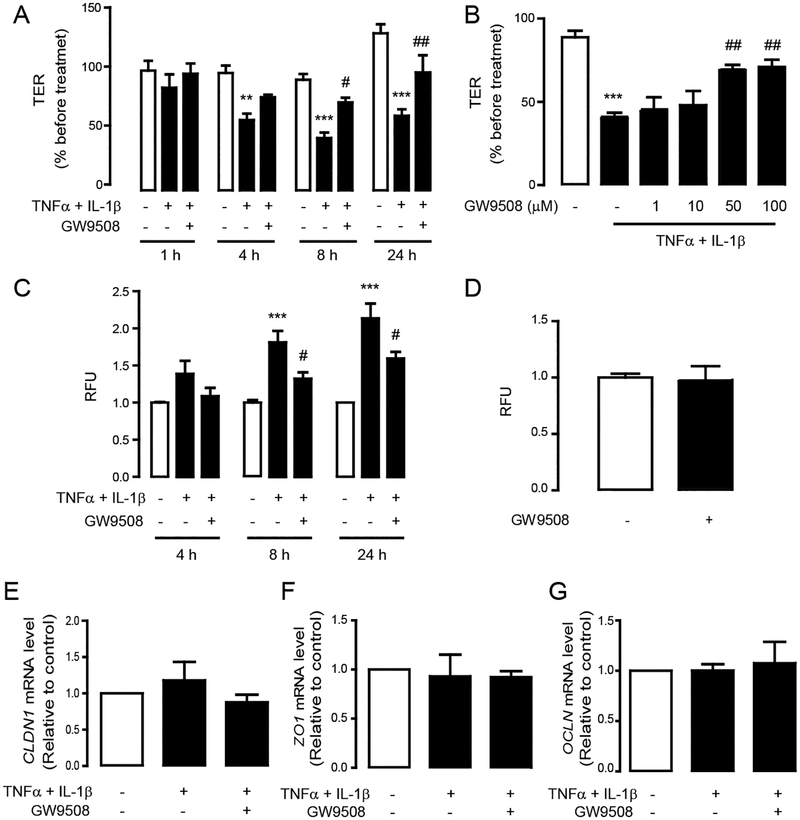
We have previously shown that GW9508 selectively activates GPR40 in Calu-3 cells [25]. To investigate the effect of GPR40 stimulation by GW9508 on the cytokine–induced barrier disruption, barrier integrity was analyzed using both TER measurement and FITC–dextran flux assay following the induction of barrier disruption by TNFα and IL–1β (each at 10 ng/mL) with or without co-treatment with GW9508 (50 μM). We found that TER of Calu–3 cells was markedly reduced by TNFα and IL–1β, indicating an induction of barrier disruption (Fig. 1A). Interestingly, GW9508 significantly suppressed the effect of cytokine on reducing TER in a time–dependent (Fig. 1A) and a dose–dependent (Fig 1B) manner. Paracellular flux was also examined using FITC-dextran, which was validated using dialysis to have molecular weight > 3 kDa by 90% (data not shown). Consistent with results obtained by TER, GW9508 (50 μM) significantly inhibited the cytokine–induced FITC–dextran flux at 8 h and 24 h post-treatment (Fig. 1C), whereas it had no effect on FITC–dextran flux in intact non-inflammatory Calu-3 cell monolayers (Fig. 1D). Taken together, these results suggest that GW9508 suppressed airway epithelial barrier disruption induced by TNFα and IL–1β.
Figure 1. Effect of GW9508 on cytokine–induced barrier disruption and expression of tight junction mRNAs.
TER measurement of Calu–3 cells after treatment with TNFα and IL–1β (10 ng/ml) plus vehicle or GW9508 at various times (A) and doses (B). Data are expressed as means of % of baseline TER±S.E.M (n=5–8). (C) Effect of GW9508 on preventing cytokine–induced barrier disruption. Cell monolayers were exposed to the indicated treatments and incubated with FITC–dextran. (D) Effect of GW9508 on barrier integrity of intact monolayers. Data are expressed as means of relative fluorescent unit (RFU) of control ±S.E.M (n=4–8). Effect of cytokines and GW9508 on mRNA expression of CLDN1 (E), ZO1 (F) and OCLN (G). Data are expressed as means of ratio of control (vehicle–treated group) ±S.E.M (n=4–8). ** p<0.01; *** p<0.001 compared with vehicle–treated group. # p<0.05; ## p<0.01 compared with TNFα–and IL–1β–treated group (one–way ANOVA).
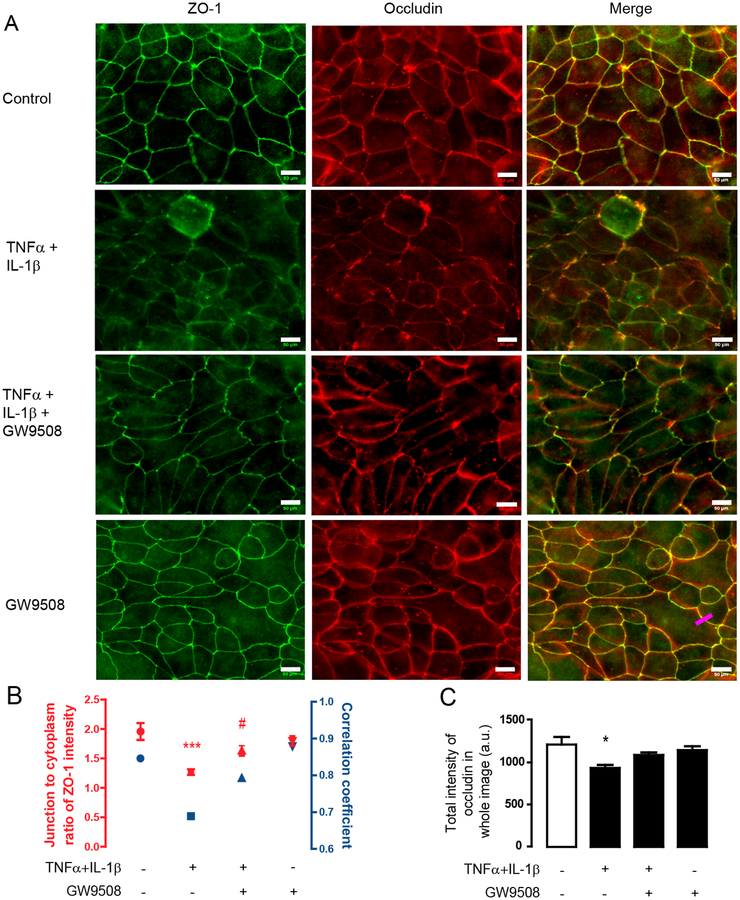
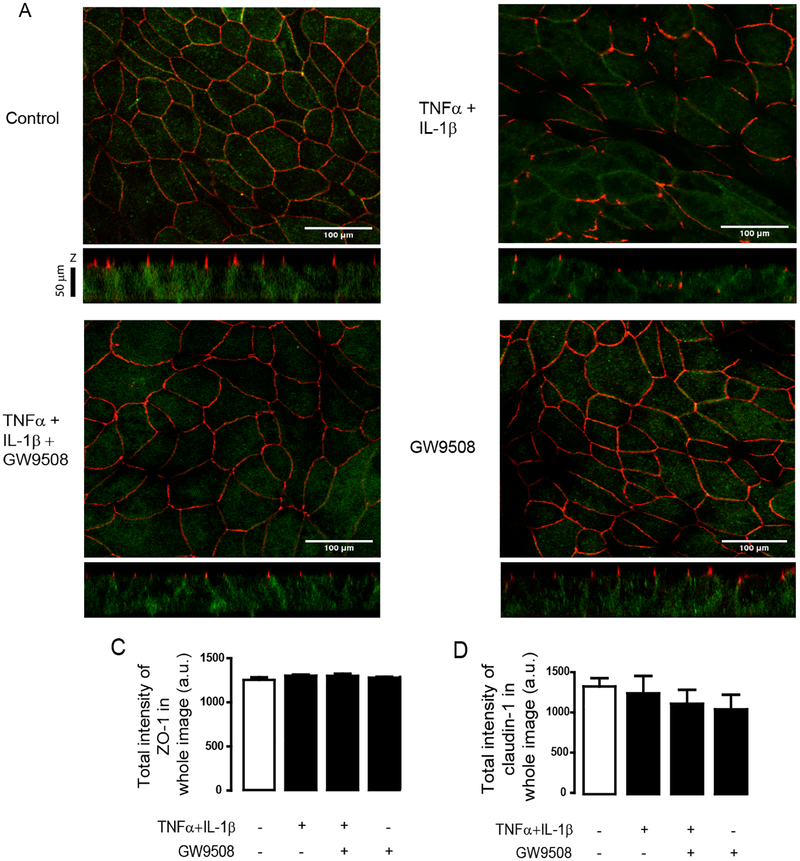
To investigate whether the barrier-protective effect of GW9508 involves changes in gene expression of tight junction components, we investigated mRNA expression of genes encoding tight junction proteins including CLDN1, ZO1 and OCLN, all of which play important roles in regulating airway epithelial permeability [30, 31]. We found that treatments with either a mixture of cytokines or cytokines plus GW9508 (50 μM) did not significantly alter the mRNA levels of CLDN1 (Fig. 1E), ZO1 (Fig. 1F) and OCLN (Fig 1. G) as compared to non-inflammatory healthy monolayers. These findings suggest that the effect of GPR40 agonist on protecting airway epithelial barrier integrity might not involve a change in mRNA expression of tight junction components and instead occurred at the protein level. Thus, we investigated whether GW9508 exerted its barrier-protective effect by changing localization pattern of tight junction proteins i.e. ZO–1, occludin and claudin–1 using immunofluorescence analyses. We found that TNFα and IL–1β reduced the ZO–1/occludin colocalization (Fig. 2) and altered the localization of ZO–1 (Fig. 2) and claudin–1 in apical junction area (Fig. 3A). These effects were suppressed by GW9508. Of note, GW9508 had no effect on total amounts of these tight junction components (Fig. 2C, 3B and 3C). Taken together, these results indicate that GW9508 preserved airway epithelial integrity by counteracting the effect of cytokines on inducing tight junction dislocalization.
Figure 2. Effect of GW9508 on cytokines-induced the disruption of occludin and ZO-1 localization.
Cells were treated with TNFα and IL–1β plus vehicle or GW9508 (50 μM). After 8 h of incubation, cells were fixed and immunostained for ZO-1 and occludin1. (A) Effect of GW9508 on regulating occludin/ZO-1 colocalization. Scale bars represent 50 μM. (B) Junction to cytoplasm ratio of ZO-1 intensity and correlation coefficient between ZO-1 and occludin. ZO-1 and occludin intensities along the labeling lines crossing the junctional area and nearby cytoplasmic regions (e.g.: Pink line in bottom right panel of Fig. 2A) was analyzed (15 lines per experiment; red). For correlation analysis, data of ZO-1 and occludin intensities in both junctional and cytoplasmic area were collected to calculate correlation coefficient between ZO-1 and occludin by Spearman correlation (blue). (C) Summarized data of total occludin in whole images *p<0.05 compared with vehicle–treated group (one–way ANOVA).
Figure 3. Effect of GW9508 on cytokine–induced tight junction dislocalization.
Cells were treated with TNFα and IL–1β plus vehicle or GW9508 (50 μM). After 8 h of incubation, cells were fixed and immunostained for ZO-1 and claudin-1. (A) Effect of GW9508 on regulating claudin-1 and ZO-1 localization. Scale bars represent 100 μM. (B,C) Summarized data of densitometry of ZO–1 (C) and claudin–1 (D) in whole images. Data are expressed as means of total intensity±S.E.M. (n=4).
3.2. Involvement of GPR40 in the barrier-protective effect of GW9508
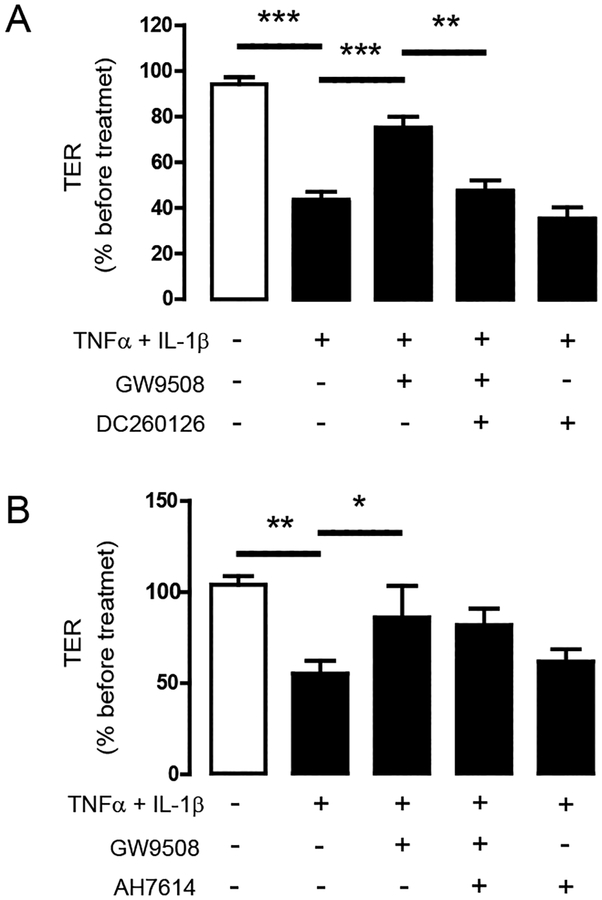
GW9508 has been reported to stimulate both GPR40 and GPR120 [32]. To investigate whether the effect of GW9508 on inhibiting cytokine-induced barrier disruption was via GPR40 or GPR120, Calu–3 cells were pretreated for an hour with a GPR40 antagonist DC260126 (3 μM) or GPR120 antagonist AH7614 (100 μM) before exposure to cytokines and GW9508 (50 μM). As shown in Fig. 4A, pretreatment with GPR40 agonist DC260126 abolished the effect of GW9508 on inhibiting cytokine–induced barrier disruption. In contrast, pretreatment with GPR120 antagonist AH7614 did not change the barrier-protective effect of GW9508 (Fig. 4B). These data confirm that GW9508 exerted its barrier-protective effect via GPR40.
Figure 4. Roles of GPR40 in mediating the barrier-protective effect of GW9508.
Cells were treated for 8 h with TNFα and IL–1β plus vehicle or GW9508. TER of Calu–3 cell monolayers was then measured. (A) Roles of GPR40. Cells were pretreated with GPR40 antagonist DC260126 (3 μM). (B) No involvement of GPR120. Cells were pretreated with GPR120 antagonist AH7614 (3 μM). Data are expressed as means of % of TER before treatment ±S.E.M (n=5–8). * p<0.05; ** p<0.01; *** p<0.001 (one–way ANOVA).
3.3. Barrier-protective effect of GPR40 stimulation is independent of NF–ĸB
Two major mediators of the effect of TNFα and IL–1β on inducing tight junction disruption are NF–ĸB and ERK1/2 [11, 16]. Accumulated lines of evidence have revealed that activation of NF–ĸB alters expression of tight junction components [14–16]. Since we found that both cytokines and GW9508 had no effect on expression of selected tight junction mRNAs, we hypothesized that the effect of GPR40 stimulation on attenuating cytokine–induced barrier disruption might not involve NF–ĸB signaling. To prove this hypothesis, we assessed NF–ĸB activation status by analyzing immunofluorescence staining of p65 subunit of NF–ĸB in the nuclear regions. As shown in Fig. 5, 2-h treatment with TNFα and IL–1β caused nuclear translocation of p65 NF–ĸB, which was unaffected by co-treatment with GW9508 (50 μM). These results indicate that the effect of GPR40 stimulation on preventing cytokine–induced tight junction disruption is independent of NF–ĸB.
Figure 5. No involvement of NF–ĸB in the GW9508 inhibition of cytokine–induced tight junction disruption.
(A) Effect of GPR40 stimulation on NF–ĸB activation. Cells were treated with TNFα and IL–1β plus vehicle or GW9508. After 2 h of incubation, cells were fixed and immunostained for NF–ĸB (green) and nuclear content was stained with Hoechst (blue). Scale bars represent 50 μM. (B) Summarized percentage of NF-ĸB positive nuclei. Intensity of Alexa fluor 488 in the nuclear and cytosolic regions were measured, and the cells having maximum intensity of Alexa fluor 488 in nuclear region greater than that in cytosolic region were counted as a NF-ĸB positive nuclei. Data are expressed as means amount±S.E.M. (n=4). *** p<0.001 compared with vehicle–treated group (one–way ANOVA).
3.4. Involvement of ERK1/2 in the barrier-protective effect of GPR40 stimulation
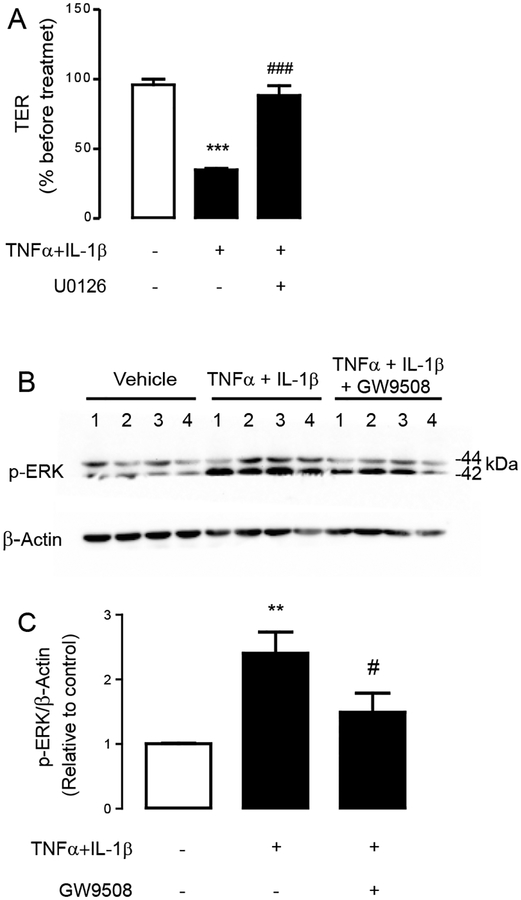
To test an involvement of ERK1/2 in mediating the tight junction disruption induced by TNFα and IL–1β, Calu-3 cells were pretreated for an hour with ERK1/2 inhibitor U0126 (10 μM) before incubation for 8 h with TNFα and IL–1β. We found that U0126 abolished the effect of TNFα and IL–1β on inducing tight junction impairment (Fig. 6A), suggesting that ERK1/2 mediated the cytokine–induced tight junction disruption. We next investigated whether stimulation of GPR40 by GW9508 suppressed ERK1/2 activation by performing western blot analyses of ERK1/2 phosphorylation (p44/ERK1 in the upper band and p42/ERK2 in the lower band). As shown in Fig. 6B and Fig. 6C, TNFα and IL–1β treatment significantly enhanced ERK1/2 phosphorylation, and this effect was significantly suppressed by co-treatment with GW9508 (50 μM). These findings indicate that GPR40 stimulation protects against cytokine-induced airway epithelial disruption by suppressing ERK1/2 signaling.
Figure 6. Involvement of ERK1/2 in the GW9508 supression of cytokine-induced tight junction disruption.
(A) Role of ERK1/2 junction disruption. Cells were treated with TNFα and IL–1β with or without pretreatment with ERK1/2 inhibitor U0126 (10 μM). After 8 h of incubation, TER of Calu–3 cell monolayers was measured. Data are expressed as means of % of TER before treatment±S.E.M (n=6). (B) Inhibitory effect of GW9508 on ERK1/2. Cells were treated for 2 h with TNFα and IL–1β plus vehicle or GW9508 before sample collection for western blot analysis of p–ERK1/2 and β–actin. Four sets of experiments were performed (C) Summarized data of western blot analysis. Data are expressed as mean of ratio of control (vehicle–treated group) ±S.E.M (n=13–17). ** p<0.01; *** p<0.001 compared with vehicle–treated group. # p<0.05; ### p<0.001 compared with TNFα–and IL–1β–treated group (one–way ANOVA).
3.5. Involvement of PLC and CaMKKβ in the barrier-protecting effect of GPR40 stimulation
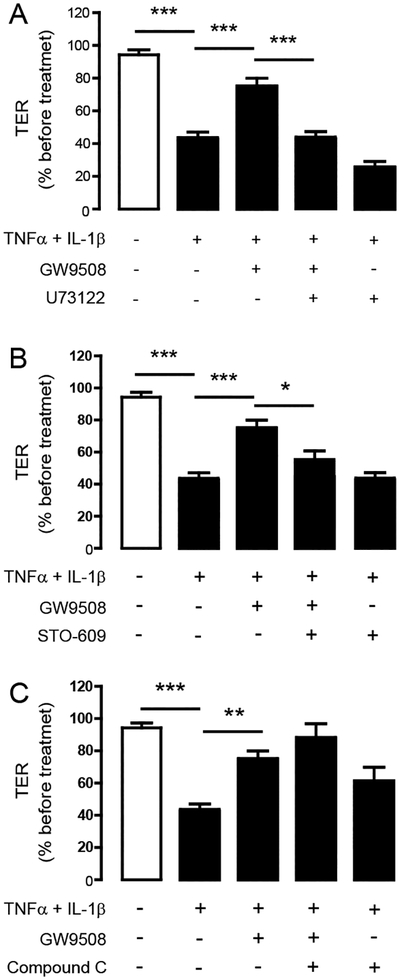
GPR40 is known to be coupled with Gq/11 [33, 34]. In addition, our previous study has found that GPR40 stimulation by GW9508 promoted tight junction assembly through a PLC-CaMKKβ–AMPK pathway [25]. Therefore, we investigated the involvement of PLC, CaMKKβ and AMPK in mediating the barrier-protective effect of GPR40 stimulation by GW9508. Pretreatment with PLC inhibitor U73122 (20 μM) or CaMKKβ inhibitor STO–609 (5 μM) completely abolished the protective effect of GW9508 against the cytokine-induced barrier disruption (Fig. 7A and Fig. 7B). To our surprise, AMPK inhibitor compound C did not affect the barrier-protective effect of GW9508 (Fig. 7C). These results suggest that the mechanism by which GPR40 stimulation protects against cytokine-induced airway epithelial disruption involves PLC-CaMKKβ-dependent pathway.
Figure 7. Involvement of PLC and CaMKKβ in the barrier-protective effect of GPR40 stimulation.
Cells were treated for 8 h with TNFα and IL–1β plus vehicle or GW9508. TER of Calu–3 cell monolayers was then measured. (A) Role of PLC. Cells were pretreated with PLC inhibitor U73122 (20 μM). (B) Role of CaMKKβ. Cells were pretreated with CaMKKβ inhibitor STO–609 (5 μM). (C) Role of AMPK. Cells were pretreated with AMPK inhibitor compound C (40 μM). Data are expressed as means of % of TER before treatment±S.E.M (n=6–7). * p<0.05; ** p<0.01; *** p<0.001 (one–way ANOVA).
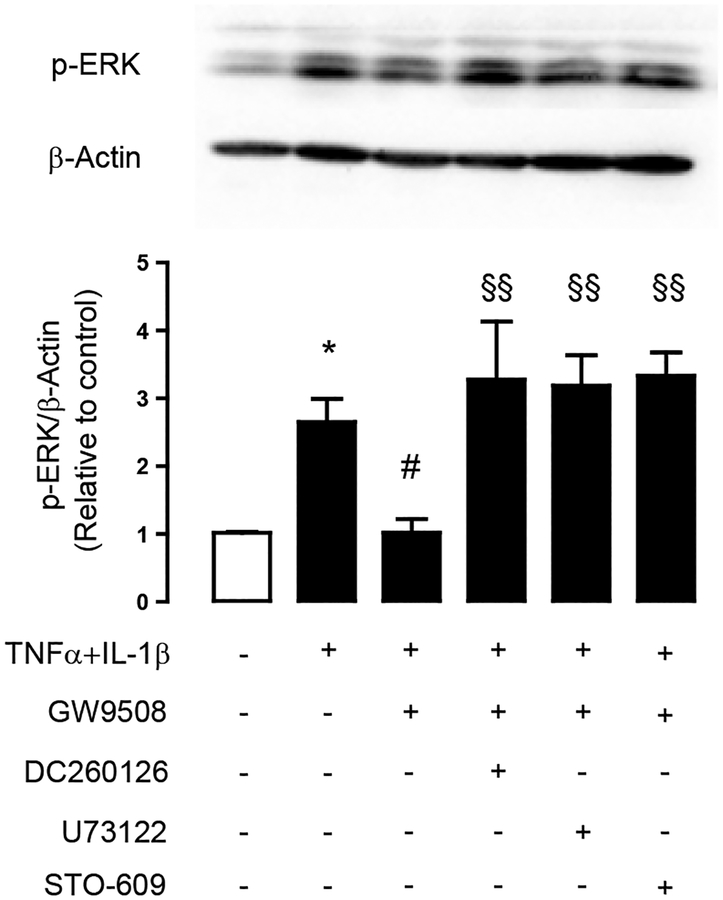
Furthermore, we investigated whether GW9508 suppressed ERK1/2 signaling via a GPR40–PLC–CaMKKβ pathway by performing western blot analysis of ERK1/2 phosphorylation. As shown in Fig. 8, pretreatment with GPR40 antagonist DC260126, PLC inhibitor U73122 or CaMKKβ inhibitor STO–609 completely abolished the inhibitory effect of GW9508 on cytokine-induced ERK1/2 phosphorylation. Altogether, these results indicate that GPR40 stimulation by GW9508 attenuated the cytokine–induced airway epithelial tight junction disruption via PLC–CaMKKβ–mediated suppression of ERK1/2 signaling.
Figure 8. Roles of PLC and CaMKKβ in the inhibition of ERK1/2 following pharmacological stimulation of GPR40.
Calu–3 cells were pretreated for an hour with indicated inhibitors, before incubation for 2 h with TNFα and IL–1β plus vehicle or GW9508. Samples were collected for western blot analysis. Data are expressed as mean of ratio of control (vehicle–treated group) ±S.E.M (n=5–6). * p<0.05 compared with vehicle–treated group. # p<0.05 compared with TNFα–and IL–1β–treated group. §§ p<0.001 compared with group of cytokines plus GW9508 (one way ANOVA).
DISCUSSION
Tight junctions impede inhaled noxious substances and pathogen in airway epithelial cells by regulating paracellular permeability [1]. In addition to a physiological role of GPR40 in promoting tight junction assembly [25], in this study, we demonstrated the protective effect of GPR40 stimulation against impairment of tight junctions induced by proinflammatory cytokines via mechanisms involving PLC–CaMKKβ-mediated suppression of ERK 1/2 signaling.
Perturbation of tight junctions is involved in the pathogenesis of inflammatory lung diseases. The dysregulation of tight junctions includes changes in expression of tight junction components or alteration in localization of tight junction proteins [35]. The present study showed that proinflammatory cytokines including TNFα and IL–1β impaired airway epithelial integrity by disrupting localization of ZO–1 and claudin–1 in the intercellular areas. However, it is also possible that these cytokines may have effects on other tight junction components such as claudin–2 and claudin–4.
In this study, we found that Calu-3 cells exhibit non-junctional expression of claudin-1. Unlike occludin and accessory proteins, such as junction ZO-1 and junctional adhesion molecules that are not usually presented in the non-junctional areas, several lines of evidence demonstrates that transmembrane claudins not only reside in the apical junctional complex, but also are observed in basolateral membranes [36], in intracellular cytoplasmic vesicles [37], or in the nucleus [38]. Apart from controlling the permeability of solutes through the tight junctions, non-canonical functions of claudin-1 on the basal membrane include regulating cell adhesion to the extracellular matrix by forming the complex with α2-integrin [36]. Claudin-1 has been found to be in the nucleus serving the transcriptional role in regulating the gene expression of E-cadherin in colon cancer cell lines [38]. A previous study also reported the extra-junctional expression of claudin-1 in airway epithelial cells with an unknown function [30]. That claudin-1 may have a non-junctional role in regulating airway inflammation is suggested by studies demonstrating that claudin-1 expression in airway smooth muscle cells, which do not form tight junctions, and that expression was augmented by treatment with TNFα and IL-1β [39, 40]. Further work is needed to define roles for non-junctional claudin-1 in airway epithelial cells.
Previous studies have revealed that activation of ERK1/2 caused an increase in tight junction permeability in epithelial cells. For example, ERK1/2 upregulates myosin like chain activity, which influences cytoskeleton dynamic resulting in tight junction disassembly [41]. Stimulation of ERK1/2 also leads to dislocalization of ZO–1 and epithelial to mesenchymal transition [42]. Indeed, our data showed that GPR40 stimulation reversed the effect of cytokines on tight junctions by resolving ZO–1, occludin and claudin–1 dislocalization. These results are consistent with the finding that GPR40 activation downregulated ERK1/2 activation. Similar to our findings, Sun et al., reported that ω–3 fatty acid treatment inhibits ERK1/2 phosphorylation in breast cancer cells [43]. Since ω–3 fatty acids are the endogenous agonist of GPR40, this effect might be partly explained by GPR40 activation. Of note, the cytokine tended to activate ERK2 more than ERK1. This finding might be explained by the higher expression of ERK2 than ERK1 in airway epithelial cells [44, 45]. Taken together, these findings indicate that GPR40 stimulation leads to ERK1/2 inhibition and consequently suppression of tight junction disruption under inflammatory conditions.
Previous study reported that stimulation of GPR40 inhibits the effect of TNFα on inducing barrier impairment, reducing expression of ZO–1 and increasing NF–ĸB expression in intestinal epithelial cells [26]. Using both real–time RT PCR and immunofluorescence analyses, we demonstrated that TNFα did not change expression of tight junction components at both mRNA and protein levels. TNFα induced nuclear translocation of NF–ĸB, but GPR40 stimulation did not affect NF–ĸB activation. These findings suggest the NF–ĸB–independent effect of GPR40 stimulation on preventing the cytokine-induced tight junction disruption in Calu–3 cells. The contradiction may be explained by the difference in exposure time, cytokine concentration and cell type.
Even though previous studies have reported that GW9508 stimulates both GPR40 and GPR120 [32], using a GPR40 antagonist and a GPR120 antagonist we showed that GW9508 exerted its inhibitory effect against cytokine-induced barrier disruption via GPR40 but not GPR120 in Calu–3 cells, which express both types of receptors. This finding is similar to our previous result demonstrating that GW9508 specifically stimulates GPR40 leading to an increase in [Ca2+]i and AMPK activation in Calu–3 cells. This functional bias of GW9508 toward the agonism of GPR40 has previously been reported in other studies [46, 47]. However, roles of GPR120 stimulation in regulating tight junction integrity in airway epithelial cells remain unknown and require further studies.
Several studies revealed that GPR40 is a Gq/11–coupled receptor that transduces signal through PLC activation with subsequent elevation in [Ca2+]i [22, 23, 33, 34]. In airway epithelial cells, GPR40–PLC–CaMKKβ–AMPK signaling plays an important role in regulating tight junction assembly [25]. In this study, a mechanism by which a GPR40 agonist suppresses ERK activation and tight junction disruption was explored using both TER measurements and immunoblotting analyses. Using inhibitors of GPR40, PLC, CaMKKβ which have no effect on TER [25], we found that GW9508 suppressed ERK1/2 activation via a GPR40–PLC–CaMKKβ-independent mechanism. We also investigated the involvement of AMPK in the GW9508 suppression of the cytokine-induced barrier disruption because AMPK is a well–known downstream target of CaMKKβ and it activation leads to attenuation of MEK–ERK signaling [48]. Surprisingly, our results indicate that AMPK is not involved in the protective effect of GPR40 stimulation against cytokine-induced barrier disruption. Further studies are required to identify the mechanisms by which CaMKKβ interferes with ERK1/2 signaling.
In summary, we identified the novel role of GPR40 in preventing cytokines–induced tight junction disruption in airway epithelial cells. Our data indicate that GPR40 stimulation attenuates the cytokine-induced tight junction disruption via suppression of ERK1/2 signaling through a PLC–CaMKKβ–dependent mechanism. GPR40 represents an important regulator of airway barrier integrity and may be a promising therapeutic target for the treatment of airway diseases.
ACKNOWLEDGEMENTS
This work was supported by the Thailand Research Fund (TRF), Mahidol University (grant BRG5980008 to CM) and NIH grant R01-AA025854 (MK). CM is a TRF Advanced Research Scholar. Financial support from the Thailand Research Fund and the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0113/2557) to AM and CM is acknowledged.
Footnotes
DISCLOSURE OF INTEREST
None
REFERENCE
- [1].Ganesan S, Comstock AT, Sajjan US, Barrier function of airway tract epithelium, Tissue barriers 1(4) (2013) e24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wittekindt OH, Tight junctions in pulmonary epithelia during lung inflammation, Pflugers Archiv : European journal of physiology 469(1) (2017) 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ, Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery, The European respiratory journal 39(2) (2012) 419–28. [DOI] [PubMed] [Google Scholar]
- [4].Georas SN, Rezaee F, Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation, The Journal of allergy and clinical immunology 134(3) (2014) 509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heijink IH, Noordhoek JA, Timens W, van Oosterhout AJ, Postma DS, Abnormalities in airway epithelial junction formation in chronic obstructive pulmonary disease, American journal of respiratory and critical care medicine 189(11) (2014) 1439–42. [DOI] [PubMed] [Google Scholar]
- [6].Hogg JC, Timens W, The pathology of chronic obstructive pulmonary disease, Annual review of pathology 4 (2009) 435–59. [DOI] [PubMed] [Google Scholar]
- [7].Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, Cremin C, Sones J, Djukanovic R, Howarth PH, Collins JE, Holgate ST, Monk P, Davies DE, Defective epithelial barrier function in asthma, The Journal of allergy and clinical immunology 128(3) (2011) 549–56 e1–12. [DOI] [PubMed] [Google Scholar]
- [8].Chung KF, Cytokines in chronic obstructive pulmonary disease, The European respiratory journal. Supplement 34 (2001) 50s–59s. [PubMed] [Google Scholar]
- [9].Hallsworth MP, Soh CP, Lane SJ, Arm JP, Lee TH, Selective enhancement of GM-CSF, TNF-alpha, IL-1 beta and IL-8 production by monocytes and macrophages of asthmatic subjects, The European respiratory journal 7(6) (1994) 1096–102. [PubMed] [Google Scholar]
- [10].Shang VC, Kendall DA, Roberts RE, Delta(9)-Tetrahydrocannabinol reverses TNFalpha-induced increase in airway epithelial cell permeability through CB2 receptors, Biochemical pharmacology 120 (2016) 63–71. [DOI] [PubMed] [Google Scholar]
- [11].Petecchia L, Sabatini F, Usai C, Caci E, Varesio L, Rossi GA, Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway, Laboratory investigation; a journal of technical methods and pathology 92(8) (2012) 1140–8. [DOI] [PubMed] [Google Scholar]
- [12].Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG, Regulation of airway tight junctions by proinflammatory cytokines, Molecular biology of the cell 13(9) (2002) 3218–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hardyman MA, Wilkinson E, Martin E, Jayasekera NP, Blume C, Swindle EJ, Gozzard N, Holgate ST, Howarth PH, Davies DE, Collins JE, TNF-alpha-mediated bronchial barrier disruption and regulation by src-family kinase activation, The Journal of allergy and clinical immunology 132(3) (2013) 665–675 e8. [DOI] [PubMed] [Google Scholar]
- [14].Al-Sadi RM, Ma TY, IL-1beta causes an increase in intestinal epithelial tight junction permeability, Journal of immunology 178(7) (2007) 4641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD, TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling, J Cell Sci 123(Pt 23) (2010) 4145–55. [DOI] [PubMed] [Google Scholar]
- [16].Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM, TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation, American journal of physiology. Gastrointestinal and liver physiology 286(3) (2004) G367–76. [DOI] [PubMed] [Google Scholar]
- [17].Aggarwal S, Suzuki T, Taylor WL, Bhargava A, Rao RK, Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers, The Biochemical journal 433(1) (2011) 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petecchia L, Sabatini F, Varesio L, Camoirano A, Usai C, Pezzolo A, Rossi GA, Bronchial airway epithelial cell damage following exposure to cigarette smoke includes disassembly of tight junction components mediated by the extracellular signal-regulated kinase 1/2 pathway, Chest 135(6) (2009) 1502–1512. [DOI] [PubMed] [Google Scholar]
- [19].Gu W, Song L, Li XM, Wang D, Guo XJ, Xu WG, Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways, Scientific reports 5 (2015) 8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schuh K, Pahl A, Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury, Biochemical pharmacology 77(12) (2009) 1827–34. [DOI] [PubMed] [Google Scholar]
- [21].Li D, Hu J, Wang T, Zhang X, Liu L, Wang H, Wu Y, Xu D, Wen F, Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells, Scientific reports 6 (2016) 37751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr., Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI, The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids, The Journal of biological chemistry 278(13) (2003) 11303–11. [DOI] [PubMed] [Google Scholar]
- [23].Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M, Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40, Nature 422(6928) (2003) 173–6. [DOI] [PubMed] [Google Scholar]
- [24].Gras D, Chanez P, Urbach V, Vachier I, Godard P, Bonnans C, Thiazolidinediones induce proliferation of human bronchial epithelial cells through the GPR40 receptor, American journal of physiology. Lung cellular and molecular physiology 296(6) (2009) L970–8. [DOI] [PubMed] [Google Scholar]
- [25].Moonwiriyakit A, Wattanaphichet P, Chatsudthipong V, Muanprasat C, GPR40 receptor activation promotes tight junction assembly in airway epithelial cells via AMPK-dependent mechanisms, Tissue barriers (2018) 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miyamoto J, Mizukure T, Park SB, Kishino S, Kimura I, Hirano K, Bergamo P, Rossi M, Suzuki T, Arita M, Ogawa J, Tanabe S, A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway, The Journal of biological chemistry 290(5) (2015) 2902–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Verma MK, Sadasivuni MK, Yateesh AN, Neelima K, Mrudula S, Reddy M, Smitha R, Biswas S, Chandravanshi B, Pallavi PM, Oommen AM, Jagannath MR, Somesh BB, Activation of GPR40 attenuates chronic inflammation induced impact on pancreatic beta-cells health and function, BMC cell biology 15 (2014) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shahar E, Boland LL, Folsom AR, Tockman MS, McGovern PG, Eckfeldt JH, Docosahexaenoic acid and smoking-related chronic obstructive pulmonary disease. The Atherosclerosis Risk in Communities Study Investigators, American journal of respiratory and critical care medicine 159(6) (1999) 1780–5. [DOI] [PubMed] [Google Scholar]
- [29].Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K, Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma, Eur Respir J 16(5) (2000) 861–5. [DOI] [PubMed] [Google Scholar]
- [30].Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG, Role of claudin interactions in airway tight junctional permeability, American journal of physiology. Lung cellular and molecular physiology 285(5) (2003) L1166–78. [DOI] [PubMed] [Google Scholar]
- [31].Gunzel D, Yu AS, Claudins and the modulation of tight junction permeability, Physiological reviews 93(2) (2013) 525–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S, Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules, British journal of pharmacology 148(5) (2006) 619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Suh HN, Huong HT, Song CH, Lee JH, Han HJ, Linoleic acid stimulates gluconeogenesis via Ca2+/PLC, cPLA2, and PPAR pathways through GPR40 in primary cultured chicken hepatocytes, Am J Physiol Cell Physiol 295(6) (2008) C1518–27. [DOI] [PubMed] [Google Scholar]
- [34].Fujiwara K, Maekawa F, Yada T, Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release, Am J Physiol Endocrinol Metab 289(4) (2005) E670–7. [DOI] [PubMed] [Google Scholar]
- [35].Capaldo CT, Nusrat A, Cytokine regulation of tight junctions, Biochim Biophys Acta 1788(4) (2009) 864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hagen SJ, Non-canonical functions of claudin proteins: Beyond the regulation of cell-cell adhesions, Tissue barriers 5(2) (2017) e1327839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR, Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation, Laboratory investigation; a journal of technical methods and pathology 88(10) (2008) 1110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, Neff J, Washington MK, Beauchamp RD, Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer, The Journal of clinical investigation 115(7) (2005) 1765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schlingmann B, Molina SA, Koval M, Claudins: Gatekeepers of lung epithelial function, Seminars in cell & developmental biology 42 (2015) 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fujita H, Chalubinski M, Rhyner C, Indermitte P, Meyer N, Ferstl R, Treis A, Gomez E, Akkaya A, O’Mahony L, Akdis M, Akdis CA, Claudin-1 expression in airway smooth muscle exacerbates airway remodeling in asthmatic subjects, The Journal of allergy and clinical immunology 127(6) (2011) 1612–21 e8. [DOI] [PubMed] [Google Scholar]
- [41].Gonzalez-Mariscal L, Tapia R, Chamorro D, Crosstalk of tight junction components with signaling pathways, Biochim Biophys Acta 1778(3) (2008) 729–56. [DOI] [PubMed] [Google Scholar]
- [42].Elsum IA, Martin C, Humbert PO, Scribble regulates an EMT polarity pathway through modulation of MAPK-ERK signaling to mediate junction formation, J Cell Sci 126(Pt 17) (2013) 3990–9. [DOI] [PubMed] [Google Scholar]
- [43].Sun H, Hu Y, Gu Z, Owens RT, Chen YQ, Edwards IJ, Omega-3 fatty acids induce apoptosis in human breast cancer cells and mouse mammary tissue through syndecan-1 inhibition of the MEK-Erk pathway, Carcinogenesis 32(10) (2011) 1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hammoud MK, Yosef HK, Lechtonen T, Aljakouch K, Schuler M, Alsaidi W, Daho I, Maghnouj A, Hahn S, El-Mashtoly SF, Gerwert K, Raman micro-spectroscopy monitors acquired resistance to targeted cancer therapy at the cellular level, Sci Rep 8(1) (2018) 15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Agarwal V, Asmat TM, Dierdorf NI, Hauck CR, Hammerschmidt S, Polymeric immunoglobulin receptor-mediated invasion of Streptococcus pneumoniae into host cells requires a coordinate signaling of SRC family of protein-tyrosine kinases, ERK, and c-Jun N-terminal kinase, The Journal of biological chemistry 285(46) (2010) 35615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mizuta K, Zhang Y, Mizuta F, Hoshijima H, Shiga T, Masaki E, Emala CW Sr., Novel identification of the free fatty acid receptor FFAR1 that promotes contraction in airway smooth muscle, American journal of physiology. Lung cellular and molecular physiology 309(9) (2015) L970–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Puebla C, Cisterna BA, Salas DP, Delgado-Lopez F, Lampe PD, Saez JC, Linoleic acid permeabilizes gastric epithelial cells by increasing connexin 43 levels in the cell membrane via a GPR40- and Akt-dependent mechanism, Biochim Biophys Acta 1861(5) (2016) 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y, Lee SX, Ou Y, Asara JM, Cantley LC, Zheng B, Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation, Molecular cell 52(2) (2013) 161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]