Abstract
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory disease in young children, elderly and immunocompromised adults. There is no licensed vaccine against RSV although development of an effective and safe RSV vaccine has been a high priority for several decades. Among the various vaccine platforms, the viral-vectored RSV vaccines based on licensed cold-adapted live attenuated influenza vaccine (LAIV) might offer an advantage of inducing adequate mucosal CD8 T cell immunity at the infection site of respiratory pathogens. We constructed two recombinant LAIV viruses expressing immunodominant T-cell epitopes of RSV M2–1 protein. The results in this study provide evidence that RSV CD8 T cell epitopes delivered by LAIV viral vector could confer protection against RSV infection without causing pulmonary eosinophilia and inflammatory RSV disease in mice. In addition, these chimeric LAIV-RSV vaccines retained their attenuated phenotype and ability to protect against virulent influenza virus, thus providing a unique approach to fight against two dangerous respiratory viral pathogens using a single vaccine preparation.
Keywords: Respiratory syncytial virus, vaccine enhanced disease, T cell immunity, live attenuated influenza vaccine, viral vector
1. Introduction
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory disease in infants and young children. Globally, each year RSV results in over 30 million episodes of acute lower respiratory tract infections among children younger than 5 years, with over 100,000 deaths (Shi et al., 2017). Besides this vulnerable young age group, RSV infection also presents substantial health burden for the elderly and high-risk adults (Falsey et al., 2005; Griffiths et al., 2017). The development of RSV vaccine has been challenging due to the concerns of enhanced respiratory disease (ERD) associated with alum-adjuvanted formalin inactivated RSV (FI-RSV) vaccine. FI-RSV vaccine administered to infants and toddlers in 1960s failed with catastrophic outcomes: 80% FI-RSV vaccinated children who were subsequently infected with natural RSV needed hospitalizations, resulting in two fatal cases (Kim et al., 1969).
FI-RSV vaccination was shown to induce T helper type 2 (Th2)-biased CD4 T cells with low levels of cytotoxic CD8 T cells and less protective antibodies, causing ERD in animal models after RSV challenge (Collins et al., 2013). Previous studies of FI-RSV vaccine-induced ERD demonstrated an abundance of Th2 (IL-4, IL-5) cytokines and high pulmonary eosinophilic infiltrates in FI-RSV-immunized mice after RSV challenge, compared with intranasal administration of live-attenuated RSV (Graham et al., 1993; Knudson et al., 2015). It was also demonstrated that mice immunized with recombinant vaccinia virus expressing RSV G protein (vvG) exhibited severe pulmonary eosinophilia similar to FI-RSV vaccine post-RSV challenge, whereas vv expressing F protein along with T cell epitope from RSV M2 protein (M282–90) did not (Srikiatkhachorn and Braciale, 1997). Further studies demonstrated that RSV M2-specific CD8 T cells inhibited pulmonary eosinophilia by reducing the number of Th2 CD4 T cells in the lungs after RSV challenge (Olson and Varga, 2007). These studies provide a rationale for designing safe and efficacious CD8 T cell-based RSV vaccines. However, effective generation of RSV-specific cytotoxic T lymphocyte (CTL) may require internalization and processing of viral proteins leading to class I MHC-driven antigen presentation. Live-attenuated vaccines and vectored RSV products would undergo cytoplasmic processing, leading to MHC I presentation and an immune response resembling natural infection (FDA, 2017).
Live attenuated influenza vaccine (LAIV) viruses might serve as an attractive vector delivery system for the immunogenic epitopes of respiratory pathogens, such as RSV (Isakova-Sivak et al., 2016a). LAIVs are an effective inducer of cross-reactive CTL responses (Korenkov et al., 2018; Mohn et al., 2017) by promoting MHC I-driven presentation of the inserted RSV-specific CD8 T-cell epitopes. In a previous proof-of-concept study, we generated two LAIV-RSV chimeric vaccines expressing H2-Kd-restricted T-cell epitopes of RSV M2–1 protein and demonstrated the establishment of strong, fully functional RSV M2-specific CD8 T-cell responses in BALB/c mice (Kotomina et al., 2018). Here we investigated whether the recombinant LAIV-RSV vectored vaccines would be safe and afford dual protection against virulent influenza virus and RSV without developing inflammatory disease post-RSV challenge in mice.
2. Materials and Methods
Cells, peptides and viruses
Cells:
Hep-2 cells were obtained from the American Type Culture Collection (ATCC CCL-23). The cells were maintainedin Dulbecco’s Modified Eagle Medium (DMED) supplemented with 5% fetal bovine serum (FBS) and penicillin-streptomycin solution.
Peptides:
RSV M282–90 (SYIGSINNI) peptide, an epitope for CD8 T cells was obtained from GenScript (Piscataway, NJ, USA).
Viruses:
RSV A2 strain virus was grown in Hep-2 cells cultured in DMEM supplemented with 1% FBS and penicillin-streptomycin solution at 37°C in 5% CO2 atmosphere. After 3 days of incubation the virus solution was harvested, sonicated and clarified at 2000 rpm at 4°C. The resulted RSV stock was aliquoted and stored at −70°C.
To obtain FI-RSV stocks, 37% formalin was added to RSV solution to a final dilution 1:4000 v/v. Inactivation was carried out for 3 days at 37°C with shaking, followed by ultracentrifugation at 30,000 rpm for 1 hour at 4°C to remove the remaining formalin from the supernatant. The pellet was resuspended in sterile Dulbecco’s phosphate-buffered saline (DPBS) and stored at −80°C in aliquots. The standard Bradford method was used to determine protein concentration in FI-RSV stocks.
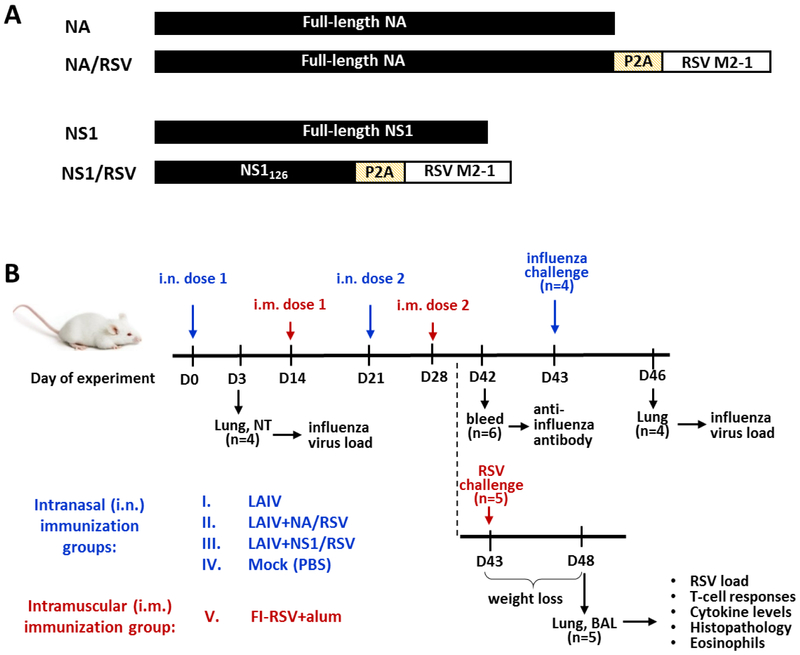
Recombinant LAIV-RSV viruses were previously generated by the means of reverse genetics and contained HA and NA genes of A/Anhui/1/2013 (H7N9) virus and the remaining six genes from A/Leningrad/134/17/57 master donor virus (Kotomina et al., 2018). These viruses expressed the RSV cassette containing human RSV A2 strain M2–1 fragment (amino acids 70 to 101 and 114 to 146) in the C-terminal NA [LAIV+NA/RSV] or NS1 truncated to 126 amino acids [LAIV+NS1/RSV] open reading frames (Figure 1A). The RSV cassette also contained porcine teschovirus-1 P2A auto cleavage site at the N-terminus (Kim et al., 2011) and stop-codon at the C-terminus (Figure 1A). The P2A peptide was inserted between influenza and RSV genes to facilitate the T-cell epitope-presenting pathway upon translation in infected cells, as well as to prevent possible negative effects of the RSV fragment on the influenza protein functions during the life cycle of recombinant LAIV. An H7N9 LAIV reassortant virus was also generated by the means of reverse genetics (Stepanova et al., 2019a) and was used as a vector control in this study.
Figure 1.
(A) Schematic design of native and recombinant viral proteins. Self-cleavage site P2A promotes the cleavage of foreign RSV epitopes upon translation in an infected cell. (B) Overview of study design and procedures.
Reassortant influenza virus A/Shanghai/2/2013(H7N9)-PR8-IDCDC-RG32A bearing HA and NA from A/Shanghai/2/2013 (H7N9), and the remaining six genes from egg-adapted donor virus A/PR/8/34 (H1N1) was obtained from the Centers for Disease control and Prevention (CDC, Atlanta, USA). Influenza viruses were propagated in 10–11 days old chicken embryos and stored at −70°C in aliquots. The infectious virus titer was determined in eggs by the method of Reed and Muench and expressed as log10EID50/ml.
In vitro RSV titer determination
An aliquot of RSV was 10-fold diluted in DMEM and 6-well plates seeded with Hep-2 cells were inoculated with each dilution and incubated for 2 hours at 37°C and 5% CO2. Four days after inoculation, the cells were fixed in 1% formaldehyde in phosphate-buffered saline (PBS) and plates were stored at 4°C overnight. The final titer of RSV was determined by immunostaining plaque assay using primary anti-RSV F monoclonal antibody (MAB 8599, EMD Millipore Corp., USA) and secondary horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Southern Biotech) as described (Hwang et al., 2017; Lee et al., 2017). The plaques were visualized using HRP-sensitive DAB colored substrate (Sigma-Aldrich). The RSV viral titer was expressed as plaque forming units (PFU) per ml.
Mouse immunization and challenge
All study procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Georgia State University. The vaccination, blood collection and virus challenge were conducted under isoflurane anesthesia.
Groups of thirteen 6–8 weeks old female BALB/c mice were intranasally (i.n.) inoculated with two doses of LAIV-RSV constructs and LAIV vector at a dose of 106 EID50 in a volume of 50 μl three weeks apart (Figure 1B). Control mice (Mock) received PBS. In a formalin-inactivated alum-precipitated group (FI-RSV+alum) five mice were intramuscularly injected with 100 μl of FI-RSV vaccine at a 2 μg dose emulsified in aluminum hydroxide adjuvant (2mg/ml, Sigma), twice with a 2-week interval. Three days after immunization, four mice from LAIV and LAIV-RSV groups were sacrificed and lung and nasal turbinate tissues were collected for virus titration.
Immunized mice were challenged i.n. either with RSV A2 strain at a dose of 2×105 PFU per mouse (n=5) or with virulent influenza virus H7N9-PR8 at a dose of 1×105 EID50 (n=4). Influenza-challenged mice were sacrificed on day 3 post infection to determine viral load in lungs and nasal turbinates. RSV-challenged mice were sacrificed on day five post-infection and bronchoalveolar lavage fluid (BAL) and lung tissues were collected for virological, immunological, and histopathology analyzes (Figure 1B).
Anti-influenza antibody responses
Blood samples were collected from six mice in each group on day 14 after the second dose. Antibody immune responses to influenza virus were assessed by hemagglutination inhibition assay (HAI) and enzyme-linked immunosorbent assay (ELISA) as previously described (Isakova-Sivak et al., 2014). For HAI, the sera were treated with receptor destroying enzyme (RDE) (Denka Seiken, Tokyo, Japan) to remove non-specific inhibitors and quantitated against four HA units of H7N9 LAIV virus. The ELISA was performed by using H7N9 LAIV (50 ng per well of sucrose purified virus) as a coating antigen on high-sorbent 96-well plates (Greiner, Germany). Ten-fold dilutions of the sera were prepared starting from 1:10 and added to the coated wells, followed by incubation with HRP-conjugated goat anti-mouse IgG antibody (Sigma). The detection of antibody binding was performed with 3,3’, 5,5’;-tetramethylbenzidine substrate (1-Step Ultra TMB–ELISA Substrate Solution, Thermo). The antibody levels were presented as the mean optical density (OD450) values for each serum dilution.
RSV load in mouse lungs
Lung sections from individual mice were weighed and tissue homogenates were prepared for virus load analyses. The Hep-2 cells seeded on 48 well plates a day earlier were infected with 2-fold dilutions of the homogenates in incomplete DMEM starting from 1:5. Plaque assay for RSV was performed as described above. RSV viral load was calculated and expressed as PFU per gram lung tissue.
Influenza virus load in mouse tissues
The influenza viral load in the upper and lower respiratory tract was determined on day 3 after inoculation or on day 3 post influenza challenge. Nasal turbinates and lungs were collected at indicated time points and stored at −70°C until used for homogenization. Tissue homogenates were prepared using a small TissueLyzer LT (QIAGEN, Germany) bead mill in 1 ml of sterile PBS containing antibiotic-antimycotic (Invitrogen, UK) and clarified supernatants were used to determine virus titers in eggs as described above. The limit of detection was 1.2 log10EID50/ml.
Cytokine levels after RSV challenge
The BAL fluid was collected from RSV-challenged mice by infusing 1ml of sterile PBS into lungs via trachea using an 18-gauge catheter (Exelint International Co., USA). After separation of BAL fluid and cells by centrifugation, BAL cells were used for cellular analysis by flow cytometry. The levels of cytokines in BAL fluid were determined by following the manufacturer protocol ELISA Ready-SET-Go® Kit (eBioscience, USA) as previously described (Lee et al., 2017).
Flow cytometry
On day 5 post-RSV challenge, lung tissues were homogenized completely through a 70 micron cell strainer. Lung lymphocytes separation from other population by the centrifugation through layered 44%/67% Percoll was performed at 2,800 rpm for 15 minutes. Layered cells were harvested, washed with PBS. BAL and lung cells were stained with phenotypic marker monoclonal antibodies (eBioscience or BD Pharmingen) specific for CD11b (clone M1/70), CD11c (clone N418), Siglec F (clone E50–2440) in the presence of anti-mouse CD16/32 (clone 93). For intracellular cytokine staining, BAL and lung cells were in vitro stimulated with M282–90 CD8+ T cell peptide (5 μg/ml) for 5h. Cytokine secreting lymphocytes were fixed/permeabilized using BD Cytofix/Cytoperm™ Plus kit after staining of surface markers with CD3 (clone 17A2) and CD8 (clone 53–6.7, eBioscience) and then stained with cytokine antibodies including IFNγ (clone XMG1.2) and TNFα (clone MP6-XT22). The results were obtained using Becton-Dickinson LSR-II/Fortessa flow cytometer (BD Pharmingen) and analyzed by Flowjo software (Tree Star Inc., USA).
Lung histopathology
For the histological analysis, mice were sacrificed under the isoflurane anesthesia at 5 days after RSV A2 infection. The superior lobe of right lung was harvested individually and fixed in 10% neutral buffered formalin for 48 hours, dehydrated in 70% ethanol for 2 hours before embedding in paraffin blocks. To assess the histopathology associated with RSV-infection 5μm longitudinal sections were stained in Hematoxylin and Eosin (H&E), hematoxylin-congo red (H&CR) and Periodic acid–Schiff (PAS) as previously described (Hwang et al., 2017; Lee et al., 2017). In brief, stained slides were digitally scanned with light microscope. For the histopathology blind scoring system, histology photographs with at least 10 sections per lung tissue from individual mouse were assessed for severity in inflammation and focal aggregates of infiltrating cells on a scale of 0–5 as similarly described in previous studies (Derscheid et al., 2013; Klopfleisch, 2013). The pathologist scored the pneumonia conditions in lung tissues in pre-bronchiolar, perivascular, interstitial, and alveolar spaces. Goblet cell hyperplasia expressing PAS+ mucin was examined in 50 randomly selected lung airways from the PAS stained slides and scored as percentages of positive from 5 individual lung sections of each mouse.
Statistical analysis
Data were analyzed with the GraphPad Prism 6.0 software (GraphPad Software Inc). Statistical significance of group measures was determined by nonparametric Mann-Whitney U test. The differences were considered significant with p<0.05.
3. Results
3.1. The recombinant LAIV-RSV constructs maintain attenuated and protect mice against influenza infection
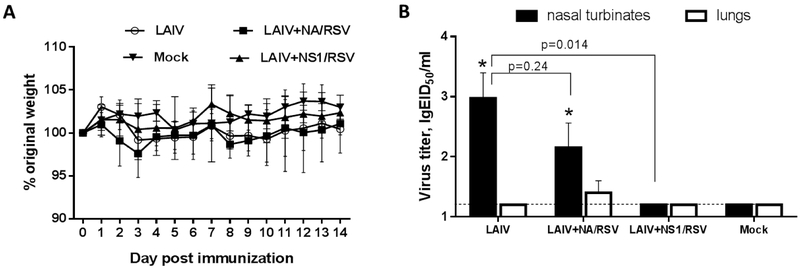
Mice immunized with two doses of recombinant LAIV-RSV viruses did not display signs of disease, such as weight loss during immunization (Figure 2A). In addition, recombinant vaccine viruses did not replicate in mouse lungs, suggesting attenuated phenotypes characteristic of a cold-adapted influenza virus (Figure 2B). Interestingly, the LAIV+NS1/RSV virus was not detected in mouse upper respiratory tract, whereas recombinant LAIV+NA/RSV virus with the insertion in NA gene was observed at comparable titers as the control LAIV vector virus in this regard (Figure 2B). These findings are consistent with previous studies reporting that truncation of NS1 protein to 126 amino acids significantly reduced viral titers of replication in mouse tissues (Ferko et al., 2001; Zhou et al., 2010).
Figure 2.
Characterization of LAIV-RSV constructs in vivo. (A) Weight loss during the immunization phase. (B) Replication of the vaccine viruses in the respiratory tract of BALB/c mice at day 3 after inoculation. (*) indicates a significant difference with Mock control group (p<0.05, Mann-Whitney U test).
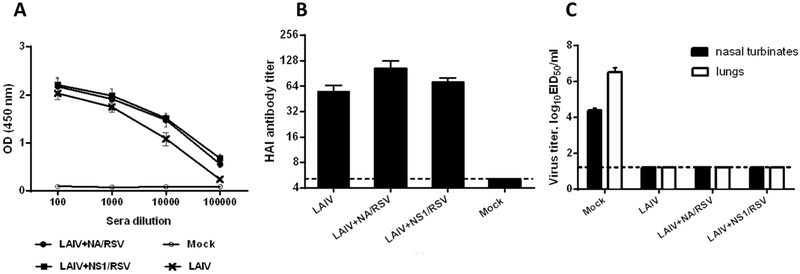
Despite the differences in the ability to replicate in mouse respiratory tracts, both engineered LAIV-RSV vaccines were similarly immunogenic in mice. Prime boost vaccination with recombinant LAIV-RSV induced high levels of HAI and serum IgG antibody which were comparable to the LAIV viral vector (Figure 3A,B). As a result, all immunized mice were fully protected from replication of homologous mouse-adapted H7N9-PR8 challenge virus, whereas the virus replicated to high titers in Mock-immunized mice (Figure 3C).
Figure 3.
Anti-influenza virus specific humoral immune responses and protection against homologous influenza virus. (A) Whole influenza virus-specific serum IgG antibody levels in ELISA OD values. (B) HAI titers. (C) Viral titers in mouse respiratory tract at day 3 after challenge with homologous H7N9 influenza virus.
3.2. The recombinant LAIV-RSV constructs efficiently protect mice against RSV infection
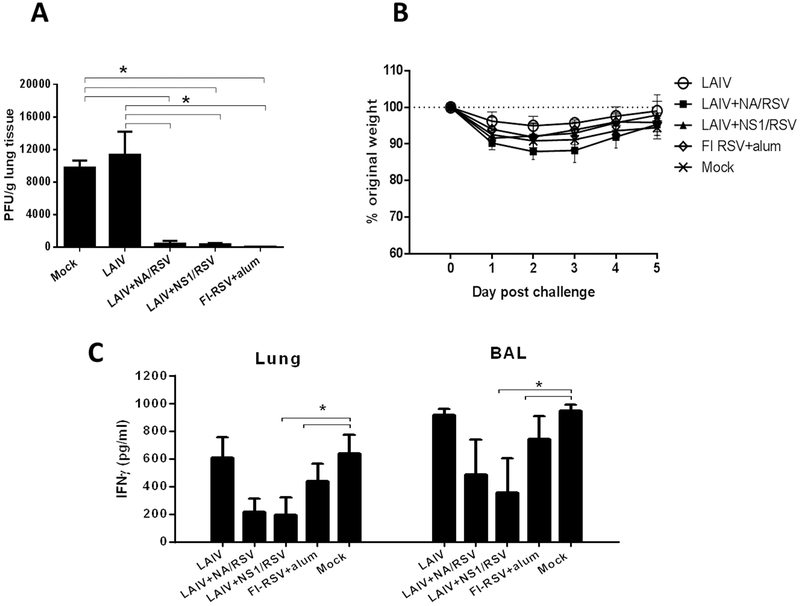
For the experiments of protective efficacy of the LAIV-RSV vaccine candidates, we included one additional control group of mice immunized with FI-RSV vaccine. To determine the efficacy of protection against RSV, the groups of mice that were prime boost immunized with LAIV, recombinant LAIV-RSV, or FI-RSV were challenged with RSV. RSV infection is not lethal in immune competent wild type mice as reported in previous studies (Hwang et al., 2014; Stokes et al., 2011; Yu et al., 2008). Five days after RSV challenge, high viral loads were detected in the Mock-immunized group, as well as in mice administered LAIV vector virus. No live virus was detected in FI-RSV vaccine group, and few RSV plaques were displayed in the titer culture plates from the LAIV-RSV vaccine groups. The three latter RSV vaccine groups showed lung RSV replication at significantly lower titers compared to the control Mock and LAIV groups (Figure 4A). Mice from all test groups slightly lost weight on days 2–4 after RSV infection, however all animals recovered by day 5 post-challenge, suggesting that the selected RSV dose (2×105 PFU) was not lethal (Figure 4B). The data of body weight changes and RSV titers suggest that there is no correlation between body weight loss and lung RSV replication in mice. The secretion of IFNγ, a pro-inflammatory cytokine, was significantly reduced in two groups immunized with LAIV-RSV vectored vaccines, whereas the levels of IFNγ were similar between the LAIV, FI-RSV and Mock-immunized groups (Figure 4B). Similar results of reduced IFNγ levels upon RSV challenge were observed for murine cytomegalovirus expressing the RSV M protein: the IFNγ level peaked on days 2–3 post-challenge and significantly dropped by day 4, which correlated with the RSV viral load in the lungs (Morabito et al., 2017).
Figure 4.
Protective efficacy of the vaccine viruses against RSV A2 strain. (A) RSV A2 virus titers in lungs as determined by plaque assay in Hep2 cells. (B) Body weight loss following RSV challenge. (C) IFNγ concentration in the lungs and BAL of immunized mice post-RSV challenge. (*) indicates a significant difference between indicated groups (p<0.05, Mann-Whitney U test).
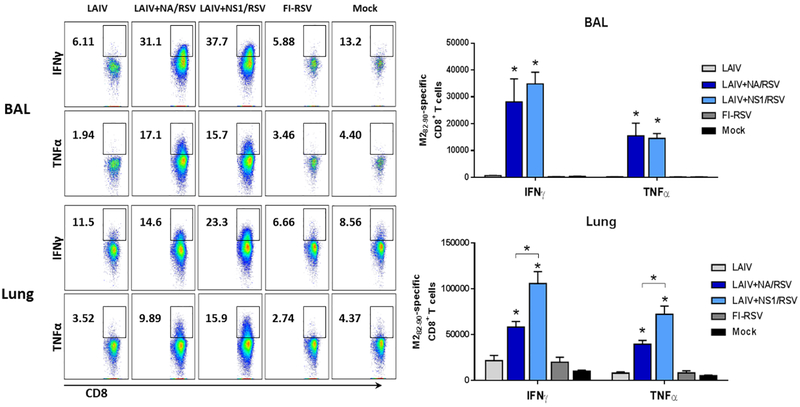
3.3. The recombinant LAIV-RSV vaccines prime RSV M282–90-specific CD8 T cells and induce the robust recall responses following RSV challenge
CD8 T cells play an important role in viral clearance from the infected individual. Previously we demonstrated that the LAIV+NA/RSV and LAIV+NS1/RSV viral vectored vaccines efficiently induced RSV M282–90-specific CD8 T cells after vaccination, and that these T cells possessed in vivo cytotoxic activity as measured by a CTL in vivo assay (Kotomina et al., 2018). In the present study, we determined the level of effector M282–90-specific CD8 T cells producing IFNγ and TNFα in the BAL and lungs of immunized mice at five days post-RSV challenge. As shown in Figure 5, when stimulating BAL cells with M282–90 peptide, the IFNγ- and TNFα-producing CD8 T cells were detected at similarly high levels in mice immunized with LAIV+NA/RSV and LAIV+NS1/RSV vaccines, whereas only few M282–90-specific CD8 T cells were seen in the groups immunized with FI-RSV and LAIV vector (Figure 5, upper panel). Although the RSV M282–90-specific CD8 T cells could also be detected in the lung cells from both LAIV-RSV-immunized groups, the LAIV+NS1/RSV candidate induced more robust recall responses, compared to the LAIV+NA/RSV group, both for IFNγ- and TNFα-producing CD8 T cell subsets (Figure 5, lower panel). These data suggest that the LAIV+NS1/RSV vaccine might be more effective in inducing M2-specific tissue-resident memory (TRM) population, which responds rapidly on RSV challenge. Nevertheless, both LAIV-RSV constructs were equally efficacious against RSV as measured by the reduction of virus titers in lungs (Figure 4).
Figure 5.
RSV M282–90-specific CD8 T-cell recall responses in immunized mice at 5 days post-RSV challenge. IFNγ and TNFα-producing CD8 T cells were determined after stimulating BAL (upper panel) and lung (lower panel) cells with RSV M282–90 peptide, followed by intracellular cytokine staining and flow cytometry. * p<0.05 (Mann-Whitney U test).
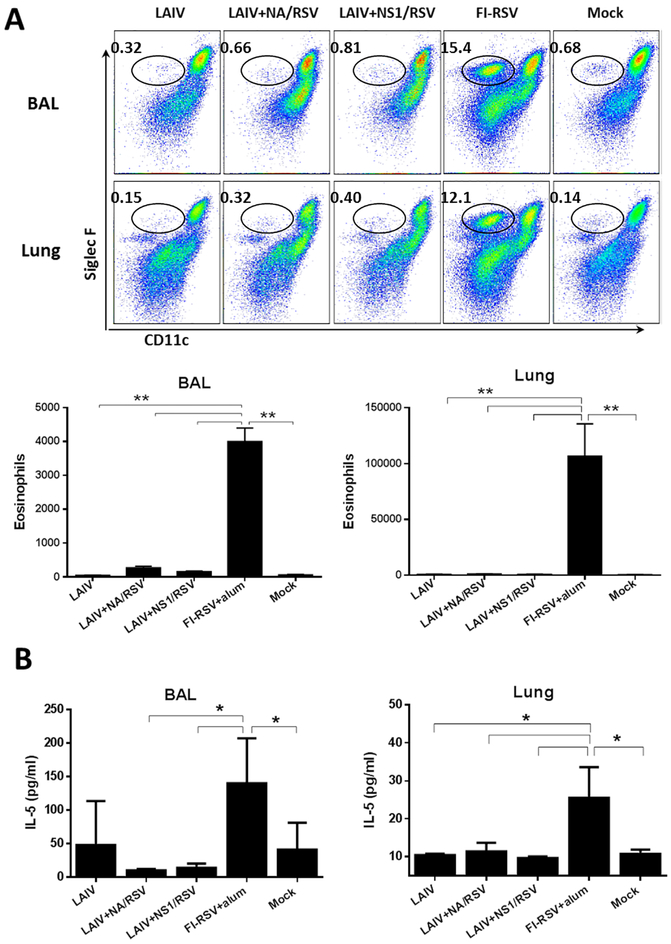
3.4. Vaccination with the recombinant LAIV-RSV constructs does not induce eosinophil infiltration and IL-5 cytokines after RSV challenge
Consistent with previous studies, FI-RSV vaccination of mice promoted massive infiltration of eosinophils into the airways of mice at day 5 after RSV challenge. Large quantities of infiltrating cells positive for eosinophil-specific surface marker CD11c−CD11b+Siglec F+ (Stevens et al., 2007), were observed in BAL and lungs of the FI-RSV immunized mice, whereas the LAIV, LAIV-RSV and the Mock groups did not show significant increases in this cell population after RSV challenge (Figure 6A).
Figure 6.
Evaluation of vaccine-induced lung pathology after RSV challenge by measuring (A) the levels of eosinophils and (B) IL-5 in BAL and lungs of immunized mice post-RSV challenge. * p<0.05; ** p<0.01 (Mann-Whitney U test)
In addition, we measured the levels of inflammatory cytokine IL-5 in BAL and lung tissues of immunized mice post-RSV challenge. We found that only FI-RSV vaccine promoted a pronounced secretion of IL-5 at day 5 after RSV infection, whereas the remaining vaccine groups including LAIVRSV displayed a background level of IL-5 similar to the Mock-immunized group (Figure 6B). These data clearly indicate that only FI-RSV vaccine induced Th2-polarized immune responses, whereas none of the live attenuated vaccines administered intranasally induced such eosinophilic infiltration and allergic Th2 cytokine IL-5 in the lung after RSV challenge.
3.5. Recombinant LAIV-RSV vaccination does not cause lung histopathology after RSV challenge
We also performed histological examination of lung tissues collected at 5 days after RSV challenge using a panel of dyes. One feature of lung histopathology associated with RSV infection includes overabundance of airway mucus production. The mucus is mainly composed of mucous glycoproteins, which can be assessed using PAS, staining mucins in magenta. Vaccine-exacerbated disease is accompanied by a significantly increased eosinophil release, as well as airway edema, the death of respiratory epithelial cells, as evidenced by the thickness of the epithelium and abundant infiltration of immune cells, including the prevalence of neutrophil infiltrates (Openshaw and Chiu, 2013; Peebles and Graham, 2005). H&E staining, commonly used to check the functional state of cells, shows the overall effect of the inflammatory process, which results in infiltrates consisting of macrophages, polymorphs, eosinophils, lymphocytes and multinucleate giant cells (Ponnuraj et al., 2001). In addition to routine H&E techniques, Congo-Red staining also shows sensitivity when staining eosinophils (Meyerholz et al., 2009). Since the secretion of eosinophilic proteins in a state of RSV infection involves an eosinophilic cationic protein and an eosinophil-derived neurotoxin that binds amyloid diagnostic dyes, therefore eosinophil granules appear in red-orange colors (Acharya and Ackerman, 2014).
Histologic examination of the lung tissues demonstrated that live RSV infection in the Mock group caused moderate pathological signs affecting the lung structure to some extent (Figure 7). The increased level of pulmonary pathology was displayed for the FI-RSV group, including airway obstruction, accompanied by narrowing of the peripheral airways and exudates of inflammatory cells and abundance of mucus covering the alveolar, bronchiole surfaces and around the blood vessels (Figure 7). Less histopathology scores were assigned to the Mock infection group and the LAIV groups, suggesting that vector alone did not cause enhanced disease. In contrast to others, recombinant vector LAIV-RSV vaccine groups displayed the lowest level of histopathology scores.
Figure 7.
Lung pathology in immunized mice after RSV challenge. (A) Representative photomicrographs of lung tissue sections stained with H&E, H&CR, and PAS. Scale bars indicate 100 μm. The arrows indicate representative spot area displaying severe H&E and PAS staining. The inserted boxes indicate the enlarged photos on H&CR stained eosinophilic cells The arrows mark representative inflamed area. (B) The histopathology was scored for inflammation blindly using a 1–5 scoring system. (C) The eosinophil infiltration in H&CR stained sections of individual lung tissue of the immunized mice. The infiltrated eosinophils per field were quantified in the lung airways. (D) The percentage of PAS stained lung section area with PAS positive expression from individual mouse.
4. Discussion
Currently there are experimental RSV vaccines and antibodies under pre-clinical and clinical development (Griffiths et al., 2017; Mazur et al., 2018; Neuzil, 2016). Early studies identified the RSV M2–1 protein as a target for the development of CTL-based RSV vaccines since M2-specific immunodominant CD8 T cells can inhibit vaccine-enhanced Th2-driven immunopathology after RSV challenge (Derscheid et al., 2013; Srikiatkhachorn and Braciale, 1997). Several viral vectors have been utilized for the delivery of RSV M2–1 protein to target cells (along or in combination with other RSV proteins), such as modified vaccinia Ankara (Pierantoni et al., 2015), genetically modified chimpanzee derived adenoviruses (Mazur et al., 2018; Taylor et al., 2015), baculoviruses (Lee and Chang, 2017), and murine cytomegalovirus (MCMV) (Morabito et al., 2017). Some of these vaccines are already in clinical development (Mazur et al., 2018). However, it was shown that the excessive induction of systemic RSV-specific memory CD8 T cells can result in the exacerbated morbidity and mortality following RSV infection, indicating that designing T cell-based RSV vaccines must be considered with caution (Schmidt et al., 2018). Importantly, Schmidt et al. demonstrated that local prime-boost immunization generated RSV-specific resident memory CD8 T cells, thus preventing fatal immunopathology following RSV challenge (Schmidt et al., 2018).
The use of an attenuated influenza virus as a viral vector for delivering RSV-specific CD8 T cell epitopes can be advantageous to other viral vectors for several reasons. Licensed LAIVs are clinically relevant and enable delivery of the desired foreign epitopes to the upper respiratory tract, thus inducing mucosal and cell-mediated immunity without the use of any adjuvant (Korenkov et al., 2018; Mohn et al., 2017). Intranasal administration of LAIVs is an easy, needle-free and painless way of vaccine delivery and does not require qualified medical personnel. There is no DNA synthesis during the life cycle of LAIV, implicating that viral genetic material would not be integrated into the cell genome. LAIVs are able to generate lung-localized virus-specific CD8+ tissue-resident memory T cells, similar to those generated by influenza virus infection (Zens et al., 2016). There is no need to avoid anti-vector immunity in using influenza virus as a viral vector. In this study, we demonstrated that LAIVRSV recombinant vaccines also protected mice against virulent influenza virus, as evidenced by the absence of virus replication in the upper and lower respiratory tracts of immunized mice after challenge.
It is likely that airway epithelial cells are primary target cells highly susceptible to RSV infection. Killing of RSV-infected cells is considered a primary immune protective mechanism via RSV M2-specific CD8+ T cells in mice immunized with LAIV+NA/RSV or LAIV+NS1/RSV. We found that significantly higher cell numbers of CD8 T cells secreting IFN-γ and TNF-α cytokines were induced in the BAL and lung samples from the LAIV+NA/RSV or LAIV+NS1/RSV group upon challenge. There was a correlation between high levels of RSV M2 specific CD8 cytotoxic T cell responses and effective control of lung viral replication of RSV, preventing the secretion of RSV replication-induced inflammatory cytokines including IFN-γ. These CD8 T cell infiltrates might be contributing to very minor histopathology in these groups, which is slightly higher than naïve mice without RSV infection. In contrast, RSV infection and high lung viral loads lead to the induction of acute inflammatory IFN-γ cytokines at high levels, as observed in the naïve mice with RSV infection or LAIV control mice with RSV infection. Although we did not measure the magnitude of RSV M2-specific CD8 TRM response prior to RSV challenge, there were significant levels of RSV epitope-specific effector CD8 T cells in BAL and lungs at day 5 after infection, along with expedited virus clearance and absence of lung immunopathology in mice vaccinated with LAIV-RSV vaccines. These data in this study suggest that the LAIV-vectored vaccines indeed induced local RSV-specific protective CTL immune responses.
The potential limitation of using influenza viruses as general viral vectors is the capacity of the viral genome which does not allow incorporation of large foreign antigens (Li et al., 2013). However, this obstacle is not so significant when the designed vaccines are targeted to the T-cell epitopes, as this does not require the incorporation of full viral proteins. Thus, in our LAIV-RSV constructs we inserted only selected epitopes of RSV M2–1 protein with the surrounding amino acids, 65 residues in total (Isakova-Sivak et al., 2016b; Kotomina et al., 2018), and this insertion had no negative effect on anti-influenza protective immunity, while inducing strong RSV-specific CTL immune response.
As a conclusion, here we demonstrate that new dual LAIV-RSV vaccines are safe and protect mice against influenza and RSV. Such vaccines provide a unique opportunity to fight the two most dangerous respiratory viral infections using a single vaccine preparation.
Highlights.
Two recombinant attenuated influenza viruses expressing CD8 T-cell epitopes of RSV M2–1 protein were studied in mice.
The LAIV-RSV vaccines induce functional RSV M282-specific cytotoxic T cell responses.
Intranasal vaccination protects mice against both influenza virus and RSV.
The bivalent vaccines do not induce pulmonary immunopathology after RSV challenge.
Funding
The current research was supported by Russian Science Foundation Grant №17-75-20054, and in part by National Institutes of Health/National Institute of Allergy and Infectious Diseases grants AI093772 (SMK), AI105170 (SMK), and AI134132 (SMK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
All authors have no conflicts to disclose.
References
- Acharya KR, Ackerman SJ, 2014. Eosinophil granule proteins: form and function. The Journal of biological chemistry 289, 17406–17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Fearns R, Graham BS, 2013. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Current topics in microbiology and immunology 372, 3–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derscheid RJ, van Geelen A, McGill JL, Gallup JM, Cihlar T, Sacco RE, Ackermann MR, 2013. Human respiratory syncytial virus Memphis 37 grown in HEp-2 cells causes more severe disease in lambs than virus grown in Vero cells. Viruses 5, 2881–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE, 2005. Respiratory syncytial virus infection in elderly and high-risk adults. The New England journal of medicine 352, 1749–1759. [DOI] [PubMed] [Google Scholar]
- FDA, 2017. FDA Briefing Document. Development of Vaccines for Prevention of Respiratory Syncytial Virus (RSV) Disease in RSV-Naïve Infants. 17 May 2017. Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM556495.pdf.
- Ferko B, Stasakova J, Sereinig S, Romanova J, Katinger D, Niebler B, Katinger H, Egorov A, 2001. Hyperattenuated recombinant influenza A virus nonstructural-protein-encoding vectors induce human immunodeficiency virus type 1 Nef-specific systemic and mucosal immune responses in mice. Journal of virology 75, 8899–8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG, 1993. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. Journal of immunology (Baltimore, Md. : 1950) 151, 2032–2040. [PubMed] [Google Scholar]
- Griffiths C, Drews SJ, Marchant DJ, 2017. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clinical Microbiology Reviews 30, 277–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HS, Kwon YM, Lee JS, Yoo SE, Lee YN, Ko EJ, Kim MC, Cho MK, Lee YT, Jung YJ, Lee JY, Li JD, Kang SM, 2014. Co-immunization with virus-like particle and DNA vaccines induces protection against respiratory syncytial virus infection and bronchiolitis. Antiviral research 110C, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HS, Lee YT, Kim KH, Ko EJ, Lee Y, Kwon YM, Kang SM, 2017. Virus-like particle vaccine primes immune responses preventing inactivated-virus vaccine-enhanced disease against respiratory syncytial virus. Virology 511, 142–151. [DOI] [PubMed] [Google Scholar]
- Isakova-Sivak I, Chen LM, Bourgeois M, Matsuoka Y, Voeten JT, Heldens JG, van den Bosch H, Klimov A, Rudenko L, Cox NJ, Donis RO, 2014. Characterization of reverse genetics-derived cold-adapted master donor virus A/Leningrad/134/17/57 (H2N2) and reassortants with H5N1 surface genes in a mouse model. Clinical and vaccine immunology : CVI 21, 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakova-Sivak I, Korenkov D, Smolonogina T, Kotomina T, Donina S, Matyushenko V, Mezhenskaya D, Krammer F, Rudenko L, 2018. Broadly protective anti-hemagglutinin stalk antibodies induced by live attenuated influenza vaccine expressing chimeric hemagglutinin. Virology 518, 313–323. [DOI] [PubMed] [Google Scholar]
- Isakova-Sivak I, Tretiak T, Rudenko L, 2016a. Cold-adapted influenza viruses as a promising platform for viral-vector vaccines. Expert review of vaccines 15, 1241–1243. [DOI] [PubMed] [Google Scholar]
- Isakova-Sivak IN, Korenkov DA, Fedorova EA, Tretiak TS, Matyushenko VA, Smolonogina TA, Rudenko LG, 2016b. Analysis of Immune Epitopes of Respiratory Syncytial Virus for Designing of Vectored Vaccines Based on Influenza Virus Platform. Bulletin of experimental biology and medicine 161, 533–537. [DOI] [PubMed] [Google Scholar]
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH, 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. American journal of epidemiology 89, 422–434. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, Kim MK, Shin BA, Choi SY, 2011. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PloS one 6, e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch R, 2013. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology--a systematic review. BMC veterinary research 9, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CJ, Hartwig SM, Meyerholz DK, Varga SM, 2015. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS pathogens 11, e1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenkov D, Isakova-Sivak I, Rudenko L, 2018. Basics of CD8 T-cell immune responses after influenza infection and vaccination with inactivated or live attenuated influenza vaccine. Expert review of vaccines 17, 977–987. [DOI] [PubMed] [Google Scholar]
- Kotomina T, Korenkov D, Matyushenko V, Prokopenko P, Rudenko L, Isakova-Sivak I, 2018. Live attenuated influenza vaccine viral vector induces functional cytotoxic T-cell immune response against foreign CD8+ T-cell epitopes inserted into NA and NS1 genes using the 2A self-cleavage site. Human vaccines & immunotherapeutics, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Chang J, 2017. Recombinant baculovirus-based vaccine expressing M2 protein induces protective CD8(+) T-cell immunity against respiratory syncytial virus infection. Journal of microbiology 55, 900–908. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee YT, Ko EJ, Kim KH, Hwang HS, Park S, Kwon YM, Kang SM, 2017. Soluble F proteins exacerbate pulmonary histopathology after vaccination upon respiratory syncytial virus challenge but not when presented on virus-like particles. Human vaccines & immunotherapeutics 13, 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Arevalo MT, Zeng M, 2013. Engineering influenza viral vectors. Bioengineered 4, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS, Englund JA, Mejias A, Karron RA, Simoes EA, Knezevic I, Ramilo O, Piedra PA, Chu HY, Falsey AR, Nair H, Kragten-Tabatabaie L, Greenough A, Baraldi E, Papadopoulos NG, Vekemans J, Polack FP, Powell M, Satav A, Walsh EE, Stein RT, Graham BS, Bont LJ, Respiratory Syncytial Virus Network, F., 2018. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. The Lancet. Infectious diseases 18, e295–e311. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Griffin MA, Castilow EM, Varga SM, 2009. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicologic pathology 37, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn KGI, Zhou F, Brokstad KA, Sridhar S, Cox RJ, 2017. Boosting of Cross-Reactive and Protection-Associated T Cells in Children After Live Attenuated Influenza Vaccination. The Journal of Infectious Diseases 215, 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS, 2017. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal immunology 10, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil KM, 2016. Progress toward a Respiratory Syncytial Virus Vaccine. Clinical and Vaccine Immunology 23, 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Varga SM, 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. Journal of immunology (Baltimore, Md. : 1950) 179, 5415–5424. [DOI] [PubMed] [Google Scholar]
- Openshaw PJ, Chiu C, 2013. Protective and dysregulated T cell immunity in RSV infection. Current opinion in virology 3, 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles RS Jr., Graham BS, 2005. Pathogenesis of respiratory syncytial virus infection in the murine model. Proceedings of the American Thoracic Society 2, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantoni A, Esposito ML, Ammendola V, Napolitano F, Grazioli F, Abbate A, Del Sorbo M, Siani L, D’Alise AM, Taglioni A, Perretta G, Siccardi A, Soprana E, Panigada M, Thom M, Scarselli E, Folgori A, Colloca S, Taylor G, Cortese R, Nicosia A, Capone S, Vitelli A, 2015. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Molecular therapy. Methods & clinical development 2, 15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnuraj EM, Hayward AR, Raj A, Wilson H, Simoes EA, 2001. Increased replication of respiratory syncytial virus (RSV) in pulmonary infiltrates is associated with enhanced histopathological disease in bonnet monkeys (Macaca radiata) pre-immunized with a formalin-inactivated RSV vaccine. The Journal of general virology 82, 2663–2674. [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Knudson CJ, Hartwig SM, Pewe LL, Meyerholz DK, Langlois RA, Harty JT, Varga SM, 2018. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS pathogens 14, e1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang DA, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lazaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccala G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida LM, Yu H, Zar HJ, Campbell H, Nair H, Network RSVGE, 2017. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390, 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikiatkhachorn A, Braciale TJ, 1997. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. The Journal of experimental medicine 186, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova EA, Kotomina TS, Matyushenko VA, Smolonogina TA, Shapovalova VS, Rudenko LG, Isakova-Sivak IN, 2019a. Amino Acid Substitutions N123D and N149D in Hemagglutinin Molecule Enhance Immunigenicity of Live Attenuated Influenza H7N9 Vaccine Strain in Experiment. Bulletin of experimental biology and medicine 166, 631–636. [DOI] [PubMed] [Google Scholar]
- Stepanova EA, Kotomina TS, Matyushenko VA, Smolonogina TA, Shapovalova VS, Rudenko LG, Isakova-Sivak IN, 2019b. Amino acid substitutions N123D and N149D in hemagglutinin molecule enhance immunogenicity of H7N9 live attenuated influenza vaccine strain in experiment. Bulletin of Experimental Biology and Medicine (accepted manuscript). [DOI] [PubMed] [Google Scholar]
- Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ, 2007. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. Journal of immunological methods 327, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS Jr., Moore ML, 2011. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. Journal of virology 85, 5782–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G, Thom M, Capone S, Pierantoni A, Guzman E, Herbert R, Scarselli E, Napolitano F, Giuliani A, Folgori A, Colloca S, Cortese R, Nicosia A, Vitelli A, 2015. Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci Transl Med 7, 300ra127. [DOI] [PubMed] [Google Scholar]
- Yu JR, Kim S, Lee JB, Chang J, 2008. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. Journal of virology 82, 2350–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zens KD, Chen JK, Farber DL, 2016. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI insight 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Li Y, Belser JA, Pearce MB, Schmolke M, Subba AX, Shi Z, Zaki SR, Blau DM, Garcia-Sastre A, Tumpey TM, Wentworth DE, 2010. NS-based live attenuated H1N1 pandemic vaccines protect mice and ferrets. Vaccine 28, 8015–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]