Abstract
Though theory suggests that individual differences in neuroticism (a tendency to experience negative emotions) would be associated with altered functioning of the amygdala (which has been linked with emotionality and emotion dysregulation in childhood, adolescence, and adulthood), results of functional neuroimaging studies have been contradictory and inconclusive. We aimed to clarify the relationship between neuroticism and three hypothesized neural markers derived from fMRI during negative emotion face processing: amygdala activation, amygdala habituation, and amygdala-prefrontal connectivity, each of which play an important role in the experience and regulation of emotions. We used general linear models (GLMs) to examine the relationship between trait neuroticism and the hypothesized neural markers in a large sample of over 500 young adults. Although neuroticism was not significantly associated with magnitude of amygdala activation or amygdala habituation, it was associated with amygdala–ventromedial prefrontal cortex (vmPFC) connectivity, which has been implicated in emotion regulation. Results suggest that trait neuroticism may represent a failure in top-down control and regulation of emotional reactions, rather than overactive emotion generation processes, per se. These findings suggest that neuroticism, which has been associated with increased rates of transdiagnostic psychopathology, may represent a failure in the inhibitory neurocircuitry associated with emotion regulation.
Keywords: Neuroticism, emotion dysregulation, amygdala, fMRI, negative emotion processing
Trait neuroticism is a relatively stable personality domain reflecting individual differences in threat and punishment sensitivity as well as the tendency toward negative affect, including emotions such as fear, anger, worry, frustration, sensitivity to criticism, hostility, vulnerability, self-consciousness and frustration (DeYoung, 2015; Widiger, 2009). Individuals high in neuroticism tend to interpret events as more threatening, react more negatively to events, and utilize avoidant and defensive coping strategies, such as anger, irritability, depression, panic, and anxiety, in the face of negative events (McCrae & Costa, 2003; Tackett & Lahey, 2016). There is continuity between personality/temperament constructs assessed from childhood into adulthood (e.g., Caspi, 2000), and trait neuroticism in adulthood has its origins in related constructs that have been well documented in childhood (e.g., behavioral inhibition/trait anxiety; Caspi et al., 2003; Kagan, Reznick, Clarke, Snidman, Garcia-Coll, 1984).
Trait neuroticism has been associated with increased rates of transdiagnostic psychopathology in childhood, adolescence, and adulthood (e.g., Tackett, 2006; Ormel, Jeronimus, et al., 2013; Ormel, Rosmalen, & Farmer, 2004). Additionally, this proneness toward negative emotionality and maladaptive behavior that are associated with neuroticism predict a multitude of adverse functional outcomes; neuroticism is inversely correlated with marital satisfaction, occupational success, and quality of life (Lahey, 2009). As a result, trait neuroticism has an enormous economic cost on society; a cost that exceeds even that of common mental disorders (Cuijpers et al., 2010). Given its important implications for both individuals and society, there has been considerable interest in investigations of how neuroticism relates to both psychological processes and biological systems (DeYoung et al., 2010; Ormel, Bastiaansen, et al., 2013; Patrick et al., 2002). Substantial evidence supports the heritability of personality traits, including neuroticism (Lahey, 2009; Van Den Berg et al., 2014; Widiger, 2009), suggesting the likely existence of underlying biological processes that explain individual differences in trait neuroticism.
Early Biological Studies on Trait Neuroticism
Early studies into the biological underpinnings of trait neuroticism were largely based on Eysenck’s arousal theory of personality and Gray’s reinforcement sensitivity theory of personality. These theories suggest that neuroticism stems from the hyperarousal of certain key brain systems (e.g., limbic structures) with downstream effects on autonomic arousal and the hypothalamic-pituitary-adrenal (HPA) axis. This system is responsible for regulating the body’s stress reaction in response to threats or danger. Both Eysenck and Gray’s early theories, as well as more recent conceptualizations of trait neuroticism (e.g., DeYoung & Gray, 2009), posit a key role for altered functioning in the amygdala. The amygdala, an almond shaped mass of gray matter, is found in the medial temporal lobe of the brain, and is the brain region most closely associated with fear processing (Aggleton, 1992). A wealth of research from the animal literature has provided the basis for understanding the association between the amygdala and its projections to other brain areas in response to threatening stimuli (Davis, 1992). The amygdala connects to downstream brain regions involved in coordinating behavioral, neuroendocrine, and autonomic responses to emotional stimuli, including the hypothalamus and the brain stem (Depue, 2009; Heimer, 2003; LeDoux, 1998; Ormel, Bastiaansen, et al., 2013). The amygdala also has connections to higher cortical brain regions including the anterior cingulate cortex (ACC) and regions in the prefrontal cortex (PFC) involved in self-referential processing and the cognitive control of emotions (Kim et al., 2011; Ochsner & Gross, 2005).
Early biological studies testing Eysenck and Gray’s theories primarily utilized electrophysiological methods, such as skin conductance and salivary and urinary cortisol, which serve as global measures of central nervous system arousal and were understood to be proxies for amygdala activation (Cheng, Richards, & Helmstetter, 2007; Urry et al., 2006). These studies found that individuals higher in neuroticism exhibited greater reactivity in a variety of these psychophysiological markers, such as skin conductance (Norris, Larsen, & Cacioppo, 2007), greater startle reactions to fearful stimuli (Wilson, Kumari, Gray, & Corr, 2000), and greater salivary cortisol upon waking (Portella, Harmer, Flint, Cowen, & Goodwin, 2005). Taken together, these studies suggest that there is a higher level of arousability and reactivity among individuals higher in neuroticism and suggest that neuroticism might be associated with hyperarousal in the upstream limbic system.
Magnitude of Brain Activation and Neuroticism
Basic emotion research with humans has provided evidence for the important role of the amygdala in emotional learning and memory, negative emotion processing, and threat appraisal (Britton, Lissek, Grillon, Norcross, & Pine, 2011; Davis & Whalen, 2001; Morris et al., 1998). In turn, a growing body of research has employed functional magnetic resonance imaging (fMRI) to identify functional neural correlates of trait neuroticism, with a focus on the amygdala, particularly during negative emotion processing (Ormel, Bastiaansen, et al., 2013; Servaas et al., 2013). A number of fMRI studies have found evidence of a positive correlation between trait neuroticism and amygdala activation, measured by quantifying the magnitude in task-related change in the BOLD (blood-oxygen-level dependent) signal. These studies employ diverse paradigms involving affective content, such as emotional scenes and faces, the emotional Stroop task, and an emotional prosody task (Brück, Kreifelts, Kaza, Lotze, & Wildgruber, 2011; Chan, Norbury, Goodwin, & Harmer, 2009; Cunningham, Arbuckle, Jahn, Mowrer, & Abduljalil, 2011; Haas, Omura, Constable, & Canli, 2007; Harenski, Kim, & Hamann, 2009). However, other studies using similar fMRI paradigms have failed to find such an association (Cremers et al., 2010; Drabant, McRae, Manuck, Hariri, & Gross, 2009; Haas, Constable, & Canli, 2008; Hyde, Gorka, Manuck, & Hariri, 2011; Thomas et al., 2011). Most of these studies have been conducted in relatively small samples, which may account for these inconsistent findings (Yarkoni, 2009). However, a recent quantitative, parametric coordinate-based meta-analysis of fMRI and PET studies investigating neural activity associated with neuroticism also failed to find a significant positive association between likelihood of amygdala activation and neuroticism across 15 studies with a total sample size of 485 participants (Servaas et al., 2013). These inconsistencies in the literature compelled us to ask whether there might be an association between neuroticism and other amygdala-based indicators, such as amygdala habituation or amygdala functional connectivity, which might support the hypothesized relationship between neuroticism and amygdala function.
Time Course of Activation and Neuroticism
Some recent research has shifted focus from examining the magnitude of brain activation in regions of interest, such as the amygdala, to studying the temporal dynamics of activation in brain regions as a possible neural marker of neuroticism. For example, during sustained processing of negative information, Haas and colleagues (2008) found neuroticism to be associated with ongoing activation in the medial PFC while responding to sad emotional faces, but not fearful or happy faces. The medial PFC has been associated with higher-order cognitive control of emotional reactions. One study by Schuyler and colleagues (2014), in a relatively large sample of 120 individuals, found that while initial amygdala activation magnitude after seeing negative images was not predictive of trait neuroticism, a slower recovery time for the amygdala to return to baseline was predictive of neuroticism. Time to recovery, or habituation, has been defined as differential response amplitude to repeated stimuli over time. Neural habituation enables an individual to ignore known information and to focus on novel information. Failure to habituate indicates that the individual may have difficulty learning that an environment is familiar or predictable. Likely, a longer time course for habituation of amygdala activation may suggest greater difficulty recovering from emotionally evocative stimuli, which is in line with the phenotypic presentation of trait neuroticism.
There is some initial evidence that habituation may be a sensitive individual difference marker. One study found that differences in magnitude between left and right amygdala activation could actually be explained by differences in habituation rates (Phillips et al., 2001). Differences in habituation may, partially, explain genetic differences seen in individuals with certain polymorphisms (5-HTTLPR genotype group) associated with amygdala reactivity (Lonsdorf et al., 2011). One recent study found that amygdala habituation (during negative emotion processing) was a more reliable neural marker, exhibiting higher within-subject reliability in test-retest, than magnitude of amygdala activation. This finding suggests that amygdala habituation might be more well-suited than magnitude of amygdala activation to individual difference research on dimensional constructs (Plichta et al., 2014).
Brain Functional Connectivity and Neuroticism
Multiple brain regions are often involved in cognitive and affective processes. As such, interactions between brain regions during task-based fMRI may reflect reliable patterns of neural activity associated with psychological constructs (Buckholtz & Meyer-Lindenberg, 2012; Friston, 2005; Sporns, 2011). Network connectivity models, instead of studying the specialized processing occurring in specific brain regions, examine the co-activation of distributed brain systems (Mesulam, 1998). Evidence using both structural and functional data suggests that dysconnectivity between limbic and prefrontal regions may be a useful neural correlate for examining individual differences in neuroticism (Bjørnebekk et al., 2013; Cremers et al., 2010; Servaas et al., 2015; Servaas et al., 2013; Xu & Potenza, 2012). These studies suggest that inefficiencies in the prefrontal regions associated with top down control of emotion generating regions (such as the amygdala) might account for individual differences in levels of trait neuroticism.
There is strong support for an association between amygdala-prefrontal connectivity, as it relates to emotion dysregulation in the developmental psychopathology literature, suggesting that this might be a transdiagnostic vulnerability factor for the development in childhood and adolescence and maintenance into adulthood of both internalizing and externalizing psychopathology (Beauchaine & Zisner, 2016). Beauchaine and Gatke-Kopp (2012) define emotion dysregulation as “a pattern of emotional experience and/or expression that interferes with appropriate goal-directed behavior.” In this framework, studies have found that altered connectivity between the amygdala and regions of the frontal cortex, such as the medial prefrontal cortex or the orbitofrontal cortex have been implicated in emotional lability and failures in self-regulation (Churchwell et al., 2009; Hilt, Hanson, and Pollak, 2011). These altered patterns of connectivity have also been associated with adolescent psychopathology, such as higher anxiety and generalized anxiety disorder (Monk et al., 2008; Kujawa et al., 2017).
In the adult literature, studies have found evidence for an association between the patterns of connectivity in the amygdala and certain prefrontal regions as they relate to neuroticism and other related constructs. Using connectivity analyses (psychophysiological interactions [PPI]) during an event-related negative emotion processing task, Cremers and colleagues (2010) found that amygdala-ACC connectivity was inversely correlated with trait neuroticism in a community sample of 60 individuals. Another study, examining trait anxiety, found a similar association between amygdala-ACC dysconnectivity in a sample of 13 men (Kienast et al., 2008). Given the important role that these prefrontal brain regions play in cognitive control of emotions, altered connectivity between the amygdala and areas of the prefrontal cortex could provide a neural basis for the negative emotionality that is characteristic of neuroticism (Ochsner & Gross, 2005). Such findings suggest that dysconnectivity between brain regions involved in emotion generation and brain regions involved in emotion regulation might underlie the experience of negative emotions characterized by neuroticism. These functional dysconnectivity models are supported by structural connectivity studies, typically measured using diffusion tensor imaging (DTI), which show that there is a similar pattern of structural dysconnectivity associated with neuroticism (Xu & Potenza, 2012).
The Current Study
Activation, habituation, and dysconnectivity of the amygdala are all hypothesized neural correlates of trait neuroticism, but the evidence, to date, on the associations between these neural phenomena and trait neuroticism remains, at times, contradictory and inconclusive. The goal of the current study was to clarify the relationship between neuroticism and these three hypothesized neural markers, each of which have been found to play an important role in the experience and regulation of emotionality, using by far the largest sample (N = 663 twins) ever examined for this purpose. Twin participants underwent a neuroimaging assessment and completed a battery of self-report psychological measures, including several measures of neuroticism, allowing us to produce a robust index of trait neuroticism. Participants completed an emotional face-matching fMRI task known to activate the amygdala. We separated twin pairs for the group level analyses, resulting in two large twin groups (both with n > 270 individuals) and enabling a within-study replication attempt of the neuroticism-neural marker associations in both twin groups.
Based on previous research, we expected to find an association between one or more of the investigated neural markers and trait neuroticism. If we confirmed an association between neuroticism and altered patterns of amygdala activation, this would suggest that the trait might be characterized by differences in perception and processing of emotional information, whereas if we found an association between neuroticism and alterations in habituation or in patterns of brain connectivity to other brain regions, this would suggest deficiencies in the emotion regulation neurocircuitry across the neuroticism continuum. Better characterization of the neural correlates of neuroticism could help improve the current understanding of this key transdiagnostic trait by incorporating meaningful neurobiological data into a construct typically measured using self-report
Methods
Participants and Procedures
The current study included 663 same-sex male and female twin participants from the Minnesota Center for Twin and Family Research (MCTFR), which comprises several ongoing, population-based longitudinal twin and family studies. Participants were drawn from two twin cohorts in the Minnesota Twin Family Study (MTFS; Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Iacono & McGue, 2002; Iacono, McGue, & Krueger, 2006; Keyes et al., 2009). Both cohorts were first assessed at age 11, with follow-up assessments every 3 to 7 years. The average age across the sample in the current study was 30.40 (range = 23.36– 37.48; sd = 5.19) and the average age of the older cohort was 34.79 (range = 33.01–37.48; sd = 1.18) and of the younger cohort was 24.49 (range= 23.36–26.27; sd = .66). The sample is 54% female. Both cohorts underwent a comparable comprehensive, multimodal assessment that included interview, questionnaire, laboratory, and neuroimaging components. The neuroimaging assessment included a structural scan and an fMRI scan, during which participants completed an emotion processing task. The study was approved by the University of Minnesota’s Institutional Review Board and all participants were given monetary compensation for their participation.
Trait Neuroticism
Participants completed three self-report personality measures that assess indicators of trait neuroticism: the Inventory for Depression and Anxiety Symptoms-II (IDAS-II; Watson et al., 2012), the Multidimensional Personality Questionnaire (MPQ; Tellegen & Waller, 2008), and the Personality Inventory for DSM-5 (PID-5; Krueger, Derringer, Markon, Watson, & Skodol, 2012).
Inventory for Anxiety and Depression Symptoms-II.
We used a shortened version of the IDAS-II (Watson et al., 2012) that includes 38 items (rated from 1, Not at all to 5, Extremely) that yielded scores on Dysphoria, Panic, Suicidality, Social Anxiety, and Traumatic Avoidance and Traumatic Intrusions associated with PTSD. We selected the Dysphoria scale (which included questions such as, “I found myself worrying all the time”) as an indicator of trait neuroticism; the Dysphoria scale reflects general distress and negative affect and correlates highly with Big Five measures of neuroticism (David Watson, Gamez, & Simms, 2005; Watson & Naragon-Gainey, 2014; Simms, Grös, Watson, & O’Hara, 2008). Internal consistency for the Dysphoria scale in the present sample was excellent (Cronbach’s alpha = .92).
Multidimensional Personality Questionnaire (MPQ).
We used a brief version of the MPQ (Tellegen & Waller, 2008) that includes 138 items (rated from 1, Definitely true, to 4, Definitely false) that yielded scores on Stress Reactivity, Alienation, Aggression, Control, Harm Avoidance, and Well-Being. We selected the Stress Reactivity scale (which included questions such as, “I easily get upset”) as an indicator of trait neuroticism. The Stress Reactivity scale reflects a tendency toward being easily upset, having unaccountable mood changes, being nervous/tense, being prone to feeling guilty, being sensitive/vulnerable, and being worry-prone/anxious and correlates highly with Big Five measures of neuroticism (Hankin, Lakdawalla, Carter, Abela, & Adams, 2007; Tellegen & Waller, 2008). Internal consistency for the Stress Reactivity scale in the present sample was excellent (Cronbach’s alpha = .91).
Personality Inventory for DSM-5 (PID-5).
The PID-5 (Krueger et al., 2012) includes 220 items (rated from 0, Very false or often false, to 3, Very true or often true) that yielded scores on the domains Negative Affect, Detachment, Antagonism, Disinhibition, and Psychoticism. We selected the Negative Affect scale (which included questions such as, “I worry a lot about terrible things that might happen”) as an indicator of trait neuroticism; the Negative affect scale is comprised of the facets of anxiousness, emotional lability, hostility, perseveration, (lack of) restricted affectivity, separation insecurity, depressivity, suspiciousness, and submissiveness and the Negative Affect scale correlates highly with Big Five measures of neuroticism (De Fruyt et al., 2013; Gore & Widiger, 2013; Krueger et al., 2012). Internal consistency for the Negative Affect scale in the present sample was excellent (Cronbach’s alpha = .94).
Neuroticism composite.
We computed a neuroticism composite score comprising scores on the IDAS Dysphoria, MPQ Stress Reactivity, and PID-5 Negative Affect scales. Based on the instructions delineated in the PID-5, facet and domain-level scores were only calculated if at least 75% of items for a given facet or domain were completed (American Psychiatric Association, 2013). This same rule was also applied to the IDAS-II scales and the MPQ facets, though not explicitly specified in the scoring instructions for those measures, to ensure continuity across measures. Participants were excluded if we could not derive factor scores (i.e. due to one or more missing domain scores) through a factor analysis without data imputation (n = 50). The version of the PID-5 used in the MCTFR protocol includes two validity items that are unlikely to be endorsed to ensure careful responding (e.g., “I was born on the moon,” and “Two plus two equals five”). Because there were no validity items in the other two measures (IDAS-II or MPQ), but all three measures were typically completed during the same assessment session, if a participant failed to respond correctly to one or both of the PID-5 validity items, their data for all three personality measures was removed from the final dataset (n = 25).
Not surprisingly, because the maladaptive traits in the PID-5 and symptoms of anxiety and depression from the IDAS-II are unlikely to be endorsed in a normative population, IDAS-II Dysphoria and PID-5 Negative Affectivity were significantly, positively skewed as measured by the Kolmogorov-Smirnov (KS) test. Additionally, even though it was developed for use with normative populations, MPQ Stress Reactivity was also positively skewed, using the KS test. To correct for robust positive skew, the Blom transformation, a rank-based transformation, was applied to IDAS-II Dysphoria, MPQ Stress Reactivity, and PID-5 Negative Affectivity scores (Blom, 1958). Before and after skew values were IDAS-II Dysphoria (pre =.699, post = .418), MPQ Stress Reactivity (pre = .466, post = .379), and PID-5 Negative Affectivity (pre = .907, post = .085).
Zero-order correlations among the three neuroticism indicators were moderate to strong (IDAS Dysphoria and PID-5 Negative Affect, r = .48, p < .001; MPQ Stress Reactivity and PID-5 Negative Affect, r = .79, p < .001; IDAS Dysphoria and MPQ Stress Reactivity, r = .54, p < .001). A neuroticism factor score was derived from the three neuroticism indicators using exploratory factor analyses conducted in R (R Core Team, 2015). IDAS-II Dysphoria had a factor loading of 0.57, MPQ Stress Reactivity had a factor loading of 0.94, and PID-5 Negative Affectivity had a factor loading of 0.83 on the general neuroticism factor. Regression analyses were conducted to control for the linear effects of age and sex on the neuroticism factor score and subsequent analyses used this age/sex adjusted neuroticism score. This decision was based on previous studies which have shown age and gender based differences in levels of neuroticism (Soto, John, Gosling, & Potter, 2011; Weisberg, DeYoung, & Hirsh, 2011). Individuals’ factor scores from this age- and sex-corrected neuroticism factor were extracted and included in subsequent fMRI analyses, to determine whether robustly-measured trait neuroticism related to the hypothesized neural markers of interest.
fMRI Acquisition and Pre-processing
Imaging was performed using 3T Siemens Trio (n = 102) and Prisma (n = 561) MRI Scanners at the Center for Magnetic Resonance Research (CMRR) at the University of Minnesota. Images were collected with a 32-channel head coil, with foam placed between the participant and the coil to reduce head motion. High-resolution structural scans were collected and were used in the current study for localization of function. The images were acquired using the following sequence: MPRAGE, TE = 3.65 ms, TR = 2530 ms, flip angle = 7 degrees, FOV=256mm, matrix=256×256, in-plane resolution = 1.0 mm × 1.0 mm, slice thickness = 1 mm, 240 slices, acceleration factor of 2 (GRAPPA). During the structural scan, participants watched a movie of their choice. Each participant’s structural scan was reviewed for radiological abnormalities and participants with clinically significant findings, as determined by radiological review, were removed from the final dataset, as were participants with brain anatomical deviations deemed to be significant enough to potentially alter brain functionality (e.g., sizable visible cysts) (n = 32). Additionally, n = 8 participants were removed from the final dataset because of problems associated with the fMRI task (e.g., the task did not start properly, the participant was unable to see the task due to poor eyesight).
A mirror on the head coil enabled participants to view the behavioral task projected onto a rear-projection screen at the head of the scanner bore. Functional scans were collected on the Trio and Prisma scanners using a T2*-sensitive echo planar sequence (EPI, TE = 27 ms, TR=2.5s, flip angle=80◦, FOV=200mm, matrix=64×64, slice thickness=3.1mm with a 20% gap, in-plane resolution = 3.1 mm × 3.1 mm, 43 transversal slices, interleaved slice acquisition, 120 volumes). The phase encoding direction was posterior to anterior. Immediately prior to acquiring the task data, a short, 10-volume echo-planar scan was collected using the same parameters and positioning as the task data, but with opposite phase encoding (anterior to posterior). This opposite phase encoded scan was later used to correct geometric distortions in the task data.
Functional data were analyzed using FMRIB’s Software Library (FSL 5.0.9; www.fmrib.ox.ac.uk/fsl/). Each functional data set was registered to the relevant structural dataset using a rigid-body (6 DOF) linear transformation, and each participant’s structural data were registered to a standard coordinate space (Montreal Neurological Institute’s MNI 152 2-mm volume) using a full affine transformation to allow cross participant comparisons in a common space.
Functional data were preprocessed using the following steps: 1) geometric distortion correction using FSL’s topup and applytopup. For this process, the first 10 images from the task data were extracted from the data set. They were combined with the 10 images from the AP data set and submitted to the topup algorithm to create an unwarping field. That unwarping field was then applied to the full task data set to produce an unwarped data set; 2) motion correction using the first volume in the functional series as the reference volume; 3) slice timing correction; 4) skull stripping; 5) spatial smoothing using a 6 mm FWHM Gaussian filter; 6) grand-mean scaling; and 7) high-pass temporal filtering with a 100s cutoff. After preprocessing, each participant’s data run was evaluated for excessive motion using a tool in FSL, fsl_motion_outliers, which can detect time points in an fMRI dataset that have been corrupted by large motion (any volume exceeding the 75th percentile + 1.5x the interquartile range). The default metric was utilized, which involves examining the root mean squared (RMS) intensity difference of volume N relative to the reference volume. No participants showed motion outliers on greater than 25% of the volumes across all task conditions (the pre-determined exclusion cut-off); as such, no participants were excluded for excessive motion. Additionally, a covariate of non-interest file was produced for each participant which included the three linear and three rotational motion estimates produced by the motion correction step.
Brain masks were prepared for use in subsequent data extraction and small volume correction analyses. A mask was created for the bilateral amygdala and was defined structurally by the Harvard-Oxford Subcortical Atlas (at 100% probability). Based on previous studies which have found altered neuroticism to be associated with altered connectivity between the amygdala and the medial PFC, a medial PFC region of interest was selected using coordinates from a study of the regulation of negative emotions (Diekhof, Geier, Falkai, & Gruber, 2011) This region in the ventral-medial PFC (vmPFC) is the result of a coordinate-based quantitative meta-analysis on 49 studies examining neural correlates of emotion regulation in response to negative emotions. In the meta-analysis, the authors found that down-regulation of emotion in this region was associated with reduced activation in the amygdala, suggesting that it might be a central hub for amygdala-cortical emotion regulation circuitry. Though studies looking at emotion regulation circuitry in the developmental literature have identified alternative regions of interest such as the anterior cingulate cortex or the ventrolateral PFC, the region identified by Diekhof and colleagues represents a consensus in healthy adults of a primary region associated with the downregulation of emotion from the PFC to the amygdala across various emotion regulation strategies (Kujawa et al., 2017; Monk et al., 2008). Using FSL, a spherical mask of the region identified by Diekhof and colleagues was constructed, with an 8 mm radius around the coordinate (x=0, y=40, z=−18 in MNI space).
fMRI Task
During the fMRI session, participants completed a version of an emotion processing task adapted from Hariri and colleagues (Hariri et al., 2002). The task has been shown to reliably activate the amygdala in response to negative emotion viewing (Sauder, Hajcak, Angstadt, & Phan, 2013). During the task, participants completed shape-matching and emotional face-matching trials in 30-second blocks. During shape trials, participants saw a shape at the top of the screen and were instructed to choose which of two shapes at the bottom of the screen matched the shape at the top of the screen. Stimuli were solid black shapes (circle, horizontal ellipse, vertical ellipse) on a backing white rectangle. Participants used their right hand to respond, pressing one of two buttons on a Current Designs button box to indicate whether the left or right stimulus matched the top stimulus. Emotional face-matching blocks were identical to shape-matching blocks other than the stimuli presented. Face stimuli included black and white photos of White male and female actors posing facial expressions of anger or fear (Ekman & Friesen, 1977). Participants could not use identity to match the faces as three separate actors (same sex) were presented on each trial. Instead, participants were instructed to choose the actor on the bottom of the screen “who feels the same way” as the target actor at the top of the screen. Only fear and anger expressions were included. A total of 4 female actors and 4 male actors were used, with both anger and fear expressions for each actor. The selection and order of stimuli was random across participants, with the constraint that an equal number of fear and anger targets and an equal number of male and female actors were included in each block. Each block included 6 trials of the same condition (shape-matching or emotional face-matching). On each trial, the three stimulus images were presented on a solid gray background for 4500 ms. Each trial ended with a 500 ms interstimulus interval that included only the gray background. Responses were allowed for 4850 ms from the onset of the stimulus. In addition to shape and emotional face trials, three 20-second blocks of rest trials were included. Rest blocks included 4 trials. Stimuli included 3 white rectangles, identical in size and location to the shape and face stimuli. Rest stimuli were presented for 4500 ms, followed by a 500 ms interstimulus interval presenting a solid gray screen. No response was required from participants during rest trials. Block types alternated throughout the task, following a fixed order of rest (R), shape (S), and emotional face (E) blocks across all participants (R-S-E-S-E-R-S-E-S-E-R). The task lasted 5 minutes in total. An example image of the task is displayed in Figure 1.
Figure 1.
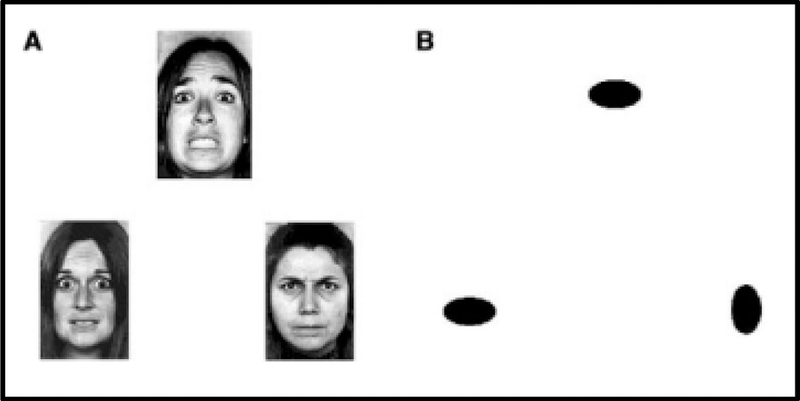
Experimental paradigm. Participants performed a matching task, with two trial types, emotion trials and shape trials, while undergoing fMRI. In order to identify amygdala responses to facial expressions and to compare these patterns to amygdala responses during a neutral shape matching condition, participants were asked to match A) during the emotion blocks, the face on the bottom of the screen that matches the emotion of the face at the top of the screen (e.g., the face at the bottom left of the image matches the face at the top of the A side of the image) and B) during the shape blocks, the shape on the bottom of the screen that matches the shape on the top of the screen (e.g., the oval on the bottom left of the image that matches the oval at the top of the B side of the image).
Subject and Group-Level fMRI Task Analyses
The final sample for subject level analyses was n = 548 individuals after excluding participants for brain anatomical abnormalities, missing personality data, invalid personality data, and rare problems with the fMRI task or data collection (see above for more information about exclusion criteria). There were commonalities across the processing stream used for all subject-level data, regardless of which neural marker (response magnitude, habituation or functional dysconnectivity) we were analyzing. General linear model (GLM) analyses were used to model the task, and while the modeling of the task differed across the three neural markers of interest, rest trials were not modeled in any of the analyses so that they could serve as a baseline. Task predictors included in the different models were convolved with a prototypical gamma-function approximation of the hemodynamic response. The temporal derivative for each task predictor was also added to the models. Predictors of no interest included three linear translation and three rotation motion predictors, as well as participant-specific nuisance predictors for each volume for that participant that exceeded the motion criterion (mean number of volumes that exceeded the motion criteria across participants = 5.2, SD = 3.2). Task predictors were coded as the start and duration of all of the emotion and shape portions of the task. All task predictors were coded in seconds.
The twin data enabled us to conduct a within-study replication attempt of the analyses, in two large sub-samples. That is, once analyses were completed at the subject-level, they were submitted to separate group level GLM analyses for the three neural markers of interest in the first-born twin group (n = 275) and second-born twin group (n = 273). While not independent samples, confirming the findings in both of these twin groups strengthens the results.
This analytic approach resulted in 6 total group-level GLMs, with 2 group-level analyses for each neural marker of interest (magnitude of amygdala activation across the task, habituation of amygdala activation across the task, and amygdala-prefrontal dysconnectivity) in each twin group. Three regressors were included in each of these analyses: a constant regressor of all 1’s (to assess the group mean), as well as one predictor of interest, each individual’s neuroticism factor score, and one regressor of non-interest for scanner type (Prisma or Trio), to control for the fact that two different scanner types were used over the course of the study (Han et al., 2006; Jovicich et al., 2009). Scanner type was identified by a 1 or a 0. The values for neuroticism and scanner type were grand mean-centered. Both the activation and habituation models were performed using a small-volume correction with the mask of the bilateral amygdala (defined structurally using the Harvard-Oxford Subcortical Atlas with a probabilistic threshold of 1.0) and the group-level statistical maps were then cluster-thresholded at z = 2.3, p < .05. The amygdala-prefrontal dysconnectivity analysis was performed using a small volume correction with the mask of the vmPFC (defined based on the coordinates from Diekhof et al., 2011) and the group-level statistical map was cluster-thresholded at z = 2.3, p < .05.
Magnitude of amygdala activation.
The first subject-level analysis modeled magnitude of amygdala activation across the task. In this model, two task predictors of interest were included: shape and emotion. The contrast of interest compared activation during emotion blocks to activation during shape blocks (emotion > shape). The within-subject output from the first subject-level GLM was passed to two separate group-level GLMs, one comprised of first-born twins and one comprised of second-born twins. Next, these analyses were run with trait neuroticism as a correlate and controlling for scanner type.
Habituation of amygdala activation.
The second subject-level analysis modeled habituation of amygdala activation over the course of the task. Habituation is defined as the reduction in response over time. In this case, it refers to a decrease in BOLD signal in the amygdala over the course of the emotion blocks during the task. In order to model this, the task session was divided into 4 emotion blocks and the BOLD response for each of the 4 blocks was averaged within each block. The 4 shape blocks during the session were averaged into a single value, based on the goal of reducing the number of predictors in the model and the premise that any neural habituation to the shape stimuli over the course of the task was not of interest in the analysis. In this model, 5 predictors of interest were included: 1 shape predictor (accounting for all 4 shape blocks) and 4 weighted predictors modeling a different level of emotional arousal (1.50, .50, −.50, −1.50) separately for each emotion block. Task predictors were coded as the start and duration of each of the four emotion blocks and all of the shape blocks. The contrast of interest examined the negative linear trend of the data (habituation), modeling the response to emotion decreasing, relative to fixation, across blocks. Figure 3 depicts a graphical representation of mean activation across the four emotion blocks in the bilateral amygdala in all participants included in fMRI analyses. As hypothesized, it indicates a generally linear trajectory of BOLD activation decreasing across emotion blocks over the course of the task.
Figure 3.
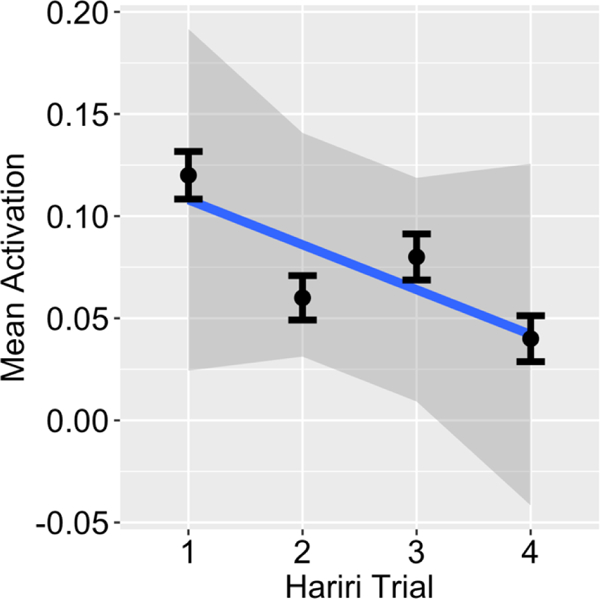
Hariri emotion task habituation across all Participants. Graphical depiction of mean activation across all participants from the four emotion trials in the Hariri task. As predicted, we observed a generally linear decrease in BOLD activation across the emotion blocks over the course of the task.
Next, the within-subject output was passed to two separate group-level GLMs, one comprised of first-born twins and one comprised of second-born twins. Next, these analyses were run with trait neuroticism as a correlate and controlling for scanner type.
Amygdala-vmPFC PPI analysis.
The third subject-level analysis modeled amygdala-vmPFC dysconnectivity during the emotion block relative to shape blocks using a psychophysiological interaction (PPI) model. PPIs measure the relationship between activity in a seed region and activity throughout the entire brain related to a specific task component (O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012). It is one of the primary ways to measure task-relevant functional connectivity. Based on the previous literature, the amygdala was selected as the seed region. In order to conduct the PPI, the bilateral structural amygdala mask was transformed into each participant’s functional space using the FLIRT tool found in FSL. The mean time-series within the amygdala mask was then extracted for each individual (using the fslmeans tool found in FSL). Subject level connectivity analyses were conducted using the FEAT tool in FSL. Using the same preprocessing as above, three task predictors were included in the model: 1) a single task predictor for the start and duration times for all emotion and shape blocks (where emotion blocks were coded with a 1 and shape blocks were coded with a −1), 2) a predictor with each individual’s bilateral amygdala mean time-series, and 3) a predictor quantifying the interaction between the amygdala time-course and the task predictor. The predictor of interest results from the interaction between the amygdala time-course and the task predictor. This predictor identifies regions that display stronger functional connectivity (or task-related co-activation) with the amygdala for negative emotional faces compared to shapes.
Next, the within-subject output was passed to two separate group-level GLM’s, one comprised of first-born twins and one comprised of second-born twins. These analyses produced mean statistical maps of brain regions showing greater connectivity with the bilateral amygdala during the emotion blocks relative to the shape blocks. Next, these analyses were run with trait neuroticism as a correlate and controlling for scanner type, with the small-volume correction of the vmPFC.
Results
Brainwise maps were produced for both twin groups for magnitude of activation, habituation, and amygdala-whole brain dysconnectivity during the emotion > shape trials of the task. The results of these are presented in Figure 2A, 2B, and 2C. Additionally, the regions that displayed significant activation, habituation, and amygdala-whole brain dysconnectivity, along with the maximum Z-value, and the coordinates of peak significance are identified in Table 1. These maps identify activation, habituation, and amygdala-whole brain connectivity in regions that would be expected across large groups of individuals during the task, in the absence of hypothesized predictors (such as neuroticism) or constrained, small volume-correction analyses. These findings suggest that the task was performing as to be expected for all three task analysis methods in both twin groups.
Figure 2.
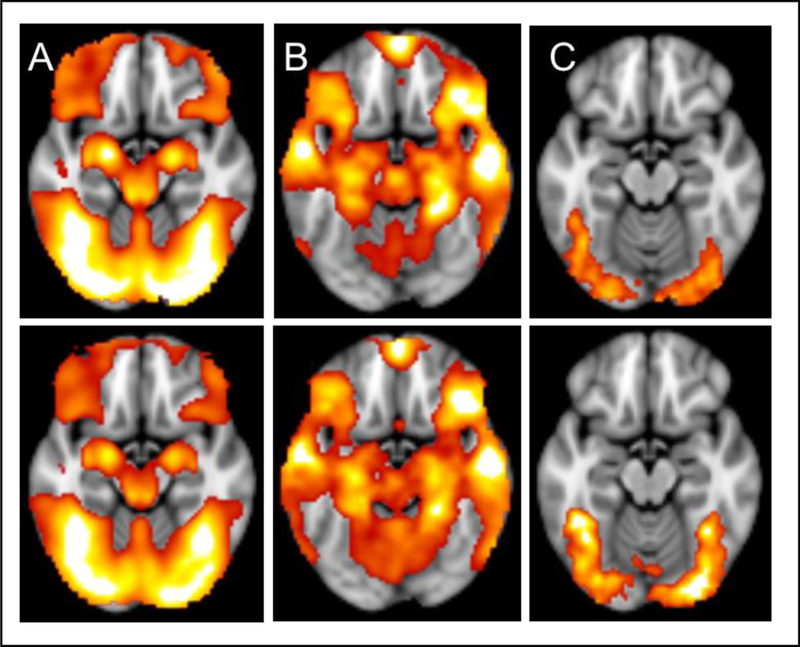
A) Functional activation in emotion > shape contrast in first-born twins (top) and second-born twins (bottom) B) Model of habituation over four emotion > shape blocks in first-born twins (top) and second-born twins (bottom) C) Functional connectivity seeding from the amygdala using a PPI in emotion > shape contrast in first-born twins (top) and second-born twins (bottom).
Table 1.
Brainwise Patterns of Activation, Habituation, and Connectivity in Both Twin Groups
| Region | Voxels | Z | x | y | z |
|---|---|---|---|---|---|
| Activation Twin 1 Emotion>Shape | |||||
| Right Occipital Pole | 112643 | 25.3 | 28 | −92 | −6 |
| Left Occipital Pole | 25 | −20 | −96 | −6 | |
| Right Occipital Pole | 24.8 | 22 | −96 | −2 | |
| Right Fusiform Gyrus | 22.8 | 42 | −50 | −20 | |
| Cerebellum | 22.8 | −6 | −80 | −36 | |
| Cerebellum | 22.3 | −6 | −78 | −28 | |
| Activation Twin 2 Emotion>Shape | |||||
| Left Occipital Pole | 111604 | 26.1 | −24 | −94 | −6 |
| Right Occipital Pole | 25.7 | 28 | −94 | −2 | |
| Left Fusiform Gyrus | 23.2 | −40 | −56 | −18 | |
| Right Fusiform Gyrus | 23.2 | 40 | −46 | −22 | |
| Cerebellum | 23.2 | −6 | −80 | −32 | |
| Cerebellum | 23.1 | −6 | −78 | −26 | |
| Habituation Twin 1 Emotlon>Shape | |||||
| Left Superior Frontal Gyrus | 100676 | 13.4 | −6 | 56 | 22 |
| Left Paracingulate Gyrus | 13.2 | −4 | 52 | 6 | |
| Cerebellum | 12 | 26 | −82 | −38 | |
| Left Middle Temporal Gyrus | 11.9 | −56 | −16 | −12 | |
| Left Superior Temporal Gyrus | 11.9 | −58 | −16 | −8 | |
| Left Middle Temporal Gyrus | 11.9 | −54 | −4 | −22 | |
| Habituation Twin 2 Emotlon>Shape | |||||
| Left Precuneus | 120909 | 14.4 | −4 | 58 | 22 |
| Left Paracingulate Gyrus | 14.3 | −4 | 50 | 16 | |
| Left Paracingulate Gyrus | 14.1 | −6 | 54 | 6 | |
| Left Superior Frontal Gyrus | 14.1 | −4 | 50 | 36 | |
| Left Middle Temporal Gyrus | 14 | −56 | −4 | −22 | |
| Right Paracingulate Gyrus | 13.9 | 4 | 52 | 8 | |
| Connectivity Twin 1 Emotion>Shape | |||||
| Right Lateral Occpital Cortex | 3308 | 6.63 | 28 | −90 | −2 |
| Right Occpital Pole | 5.93 | 22 | −98 | 4 | |
| Right Fusiform Gyrus | 4.6 | 40 | −62 | −12 | |
| Right Lateral Occpital Cortex | 4.47 | 44 | −78 | −8 | |
| Right Lateral Occpital Cortex | 4.46 | 38 | −78 | −8 | |
| Right Fusiform Gyrus | 4.34 | 44 | −46 | −20 | |
| Left Occipital Pole | 2783 | 6.73 | −24 | −92 | −2 |
| Left Lateral Occipital Cortex | 5.99 | −34 | −88 | −6 | |
| Left Lateral Occipital Cortex | 4.86 | −40 | −76 | −10 | |
| Left Occipital Pole | 4.71 | −14 | −102 | −2 | |
| Left Fusiform Gyrus | 4.42 | −32 | −82 | −14 | |
| Left Fusiform Gyrus | 3.89 | −24 | −88 | −18 | |
| Connectivity Twin 2 Emotion>Shape | |||||
| Left Occipital Pole | 8595 | 6.96 | −26 | −96 | 2 |
| Right Occipital Pole | 6.79 | 36 | −90 | 2 | |
| Right Occipital Cortex | 6.64 | 42 | −82 | −6 | |
| Right Fusiform Gyrus | 6.25 | 28 | −90 | −6 | |
| Right Occipital Pole | 6.17 | −26 | −92 | −8 | |
| Left Fusiform Gyrus | 5.82 | −32 | −84 | −16 | |
| Right Middle Frontal Gyrus | 563 | 4.44 | 42 | 12 | 30 |
| Right Inferior Frontal Gyrus | 4.12 | 48 | 20 | 18 | |
| Right Inferior Frontal Gyrus | 3.41 | 38 | 10 | 22 | |
| Right Inferior Frontal Gyrus | 3.22 | 58 | 18 | 8 | |
| Right Precentral Gyrus | 2.71 | 50 | 6 | 18 | |
| Right Precentral Gyrus | 2.46 | 46 | 4 | 22 |
Table 1.: Coordinates listed are in MNI space.
Amygdala Activation
Using a whole-brain analysis and a cluster correction of z = 2.3, p = .050 for the emotion > shape contrast, we observed significant activation in the amygdala, along with multiple other brain regions of the task (suggesting that the task was activating relevant regions, as it has in previous studies). These analyses produced mean statistical maps and clusters of significant regions for each twin group (Figure 2A and Table 1). However, using a small-volume correction (from the bilateral amygdala mask based on the Harvard-Oxford Subcortical Atlas) with a cluster correction of z = 2.3, p = .050, and with neuroticism included in the model as a correlate (and controlling for scanner type), no voxels were shown to be activated at a significant cluster threshold in either twin group. Therefore, we determined no significant relationship between trait neuroticism and activation in the amygdala to the emotion > shape contrast.
Amygdala Habituation
Using a whole-brain analysis and a cluster correction of z = 2.3, p = .050 for the emotion > shape contrast, we observed a number of brain regions, including the amygdala, involved in habituation and these analyses produced mean statistical maps and clusters of significant regions for each twin group (Figure 2B and Table 1). In addition, a negative linear trend of amygdala activation was observed, by examining change in mean amygdala activation over the four emotion blocks of the task (suggesting that indeed, as hypothesized, amygdala habituation was occurring to the emotional faces across participants over the course of the task) (Figure 3). However, using a small-volume correction (from the bilateral amygdala mask based on the Harvard-Oxford Subcortical Atlas) with a cluster correction of z = 2.3, p = .050, and with neuroticism included in the model as a correlate (and controlling for scanner type), no cluster-corrected voxels showed a significant association between trait neuroticism and amygdala habituation in either twin group. Therefore, we determined no significant relationship between trait neuroticism and habituation of the amygdala over the course of the task during emotion > shape trials.
Amygdala-vmPFC Functional Dysconnectivity
Using a whole-brain analysis and a cluster correction of z = 2.3, p < .050, we observed significant connectivity between the time course of the amygdala and the bilateral occipital cortex during the emotion component of the task. These analyses produced mean statistical maps and clusters of significant connectivity for each twin group (Figure 2C and Table 1). Using a small-volume correction (defined functionally from the coordinates from Diekhof et al., 2011) with a cluster correction of z = 2.3, p = .050 and with neuroticism included in the model as a correlate (and controlling for scanner type), we observed increased task-related functional connectivity during the emotion component of the task between the amygdala and the vmPFC, which was significantly correlated with increasing levels of trait neuroticism. This finding replicated across both twin groups. This finding can be seen visually in Figure 4, which displays an axial brain slice. In this image, the vmPFC region that was constructed based on the region found to be responsible for negative emotion regulation across studies from a recent meta-analysis (Diekhof et al., 2011) is outlined in red. Overlaid on this region, the green area represents the sub-region within this vmPFC region in which neuroticism correlated with significant functional connectivity with the amygdala in the first-born twin group, during the negative emotional task component (x = 6, y = 40, z = −18). Also, within the vmPFC region, the blue sub-region represents the area of the vmPFC in which neuroticism correlated with significant functional connectivity with the amygdala in the second-born twin group, during the emotional task component (x = 0, y = 32, z = −18). Thus, we determined a significant association between trait neuroticism and amygdala-prefrontal dysconnectivity over the course of the task during emotion > shape contrasts.
Figure 4.
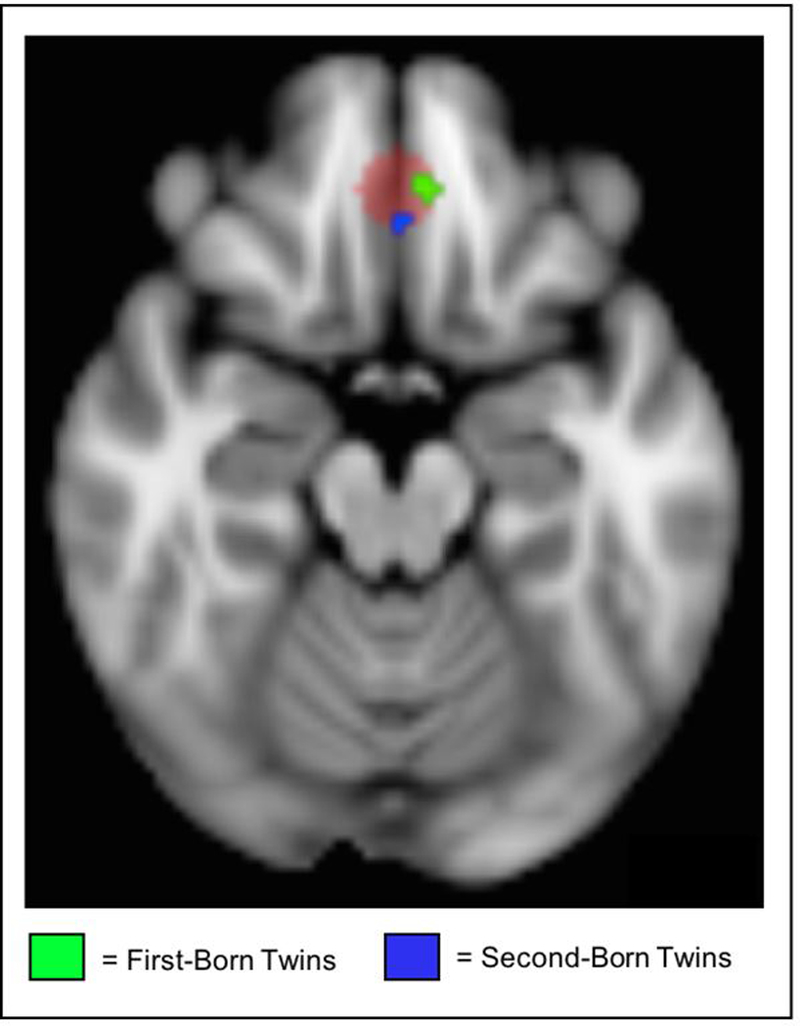
Amygdala-vmPFC dysconnectivity. In this figure, the red indicates the vmPFC region of interest based on the meta-analysis of emotion regulation (Diekhof et al., 2011). Overlaid are the regions of significant task-related functional connectivity between the amygdala and vmPFC during the emotion component of the task that correlate with trait neuroticism in each of the twin groups.
Discussion
The present study examined whether neural markers (amygdala activation, amygdala habituation, and amygdala-vmPFC dysconnectivity) derived from task-based fMRI during negative emotion processing were related to trait neuroticism, as defined using multiple self-report measures. These neural markers were selected based on previous literature, which has been limited by small sample sizes and inconsistent results. In a much larger sample than has typically been used in analyses of these kind, we failed to find evidence of an association between trait neuroticism and activation magnitude or habituation of amygdala activation during negative emotion processing. However, we did find evidence of increased task-related functional connectivity between the amygdala and vmPFC during the emotional component of the face-matching task, which correlated with increasing trait neuroticism. Taken together, results of the present study suggest that increasing levels of neuroticism represent alterations in top-down control and regulation of emotions, as evidenced by greater amygdala-vmPFC dysconnectivity, rather than from overactive emotion reaction processes, per se.
Magnitude of Amygdala Activation
Although several studies have reported significant associations between magnitude of amygdala activation and trait neuroticism (Brück et al., 2011; Chan et al., 2009; Cunningham et al., 2011; Haas et al., 2007; Harenski et al., 2009), a number have also reported nonsignificant results (Cremers et al., 2010; Drabant et al., 2009; Haas et al., 2008; Hyde et al., 2011; Thomas et al., 2011). Much of the existing research has been conducted in relatively small samples, which increases the likelihood of both Type I and Type II errors, and may account for these inconsistent findings. The non-significant association between magnitude of amygdala activation and trait neuroticism found in the current study, which included a much larger sample than previous studies, is consistent with results of a recent meta-analysis that also failed to find an association across 18 studies examining neuroticism and brain activation during emotion processing tasks (Servaas et al., 2013). Amygdala activation indexes the change in BOLD signal in the amygdala in response to the emotional face stimuli and higher activation is understood to reflect heightened emotional reactivity in response to emotional stimuli. Servaas and colleagues suggest that while the amygdala plays an important role in threat detection in response to salient stimuli in the environment, the regions that show alterations across the studies in the meta-analysis are those involved in fear learning (e.g., hippocampus and parahippocampus), anticipation of aversive stimuli (e.g., anterior cingulate cortex, posterior cingulate cortex), and emotion processing (e.g., middle cingulate gyrus, dorsal medial prefrontal cortex).
Amygdala Habituation
In the current study, we found no association between amygdala habituation and trait neuroticism. Only one previous study found an association between neuroticism and habituation (Schuyler et al., 2014). Habituation refers to the reduction in neural response to a repeatedly presented stimulus over time and it can be understood as a type of neural regulatory process, as activation goes down as the brain grows accustomed to a certain stimulus (Thompson & Spencer, 1966). Though previous research has shown that amygdala reactivity is generally consistent over multiple scanning sessions to the same stimulus (Johnstone et al., 2005), there is also evidence that within a scan session, amygdala reactivity reduces with time to emotional stimuli (Breiter et al., 1996; Strauss et al., 2005). Breiter and colleagues found that the amygdala habituates rapidly (i.e. within one minute) to happy and fearful faces and that these habituation effects were maintained after an inter-stimulus delay of four minutes. Thus, it is possible that the length of the task in the current study obscures the habituation process, which may be occurring more rapidly. However, given that other studies examining the relationship between habituation and psychopathology have used a longer time window and have found positive associations (e.g., Koeningsberg et al., 2014), the current findings, in this large sample, suggest that amygdala habituation does not significantly relate to trait neuroticism.
Amygdala-vmPFC Dysconnectivity
The results of the PPI analysis examining the co-activation of the amygdala and the vmPFC were significant, suggesting that the time courses of activation in these two brain regions are correlated and that this correlation is positively associated with individual differences in trait neuroticism. As the vmPFC-amygdala pathway is understood to be central to the neural mechanisms associated with emotion regulation, this significant finding suggests that altered connectivity between brain regions with emotion perception and brain regions associated with emotion regulation may be key to the neural underpinnings of trait neuroticism (Delgado, Nearing, LeDoux, & Phelps, 2008; Phelps & LeDoux, 2005; Stein et al., 2007). This finding is also consistent with the results of the Servaas et al. (2013) meta-analysis. Servaas and colleagues offer a model of the relationship between neuroticism and neural activation, based on their meta-analysis, in which individuals high in neuroticism have an over-active fear learning system coupled with difficulties anticipating or predicting negative outcomes. This combination of neural patterns of activation results in uncertainty and higher levels of stress, or a “neurotic cascade” (Suls & Martin, 2005), which is characterized by increased daily problems, higher emotional reactivity to these problems, more mood “spillover” from previous problems, and stronger emotional reactions to recurring problems. As a result of this increased emotional reactivity, there is a need for greater regulatory control in individuals higher in neuroticism. In such a model, the neural activation patterns associated with neuroticism might be better conceptualized as relating to the brain systems involved in down-regulating emotions, as opposed to primarily emotion perception and lower-order systems, such as threat detection, centered in the amygdala (Haas et al., 2008; Lemogne et al., 2011; Williams et al., 2006).
Previous studies using task-based fMRI provide support for this pattern of neural connectivity associated with individual differences in trait neuroticism. Using fMRI during negative emotion processing in a healthy sample Heinz et al. (2005) found that individuals with the short (s) allele of the human serotonin transporter gene (SLC6A4), a specific polymorphism associated with major depression, showed increased coupling of the amygdala and the vmPFC. In a sample of healthy adolescents, increased coupling of the amygdala and the vmPFC (as well as the amygdala and the ACC and the dorsal-lateral PFC) was associated with trait neuroticism during fear learning (Tzschoppe et al., 2014). Cremers and colleagues found that trait neuroticism was positively associated with amygdala and dorsal-medial PFC connectivity during angry and fearful face processing, though they found an inverse correlation between amygdala-ACC connectivity and neuroticism during sad face processing (2010). In individuals with borderline personality disorder, a disorder which has been characterized as an extreme maladaptive version of neuroticism (Samuel, Carroll, Rounsaville, & Ball, 2013), increased vmPFC-amygdala connectivity was found during fear processing, suggesting one hypothesized mechanism of exaggerated amygdala response, as the vmPFC may not be functioning in its inhibitory role over amygdala activity (Kamphausen et al., 2013).
Another possible explanation for this positive association between the amgydala-vmPFC and trait neuroticism and associated psychopathology is that the process of emotion regulation involves more effortful cognitive control in individuals higher in neuroticism and that the regulatory function may not be as effective at tempering amygdala reactivity, relative to individuals lower in neuroticism (Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Ochsner, Silvers, & Buhle, 2012; Urry, 2006). It may also be that this coupling reveals a more intertwined set of functional processes between the limbic emotion centers of the brain and the vmPFC, suggestive of more over-thinking, self-referential processing, and ruminating about emotional stimuli (Blair et al., 2008; Cremers et al., 2010; Disner, Beevers, Haigh, & Beck, 2011). These failures in neural processes of emotion regulation associated with neuroticism align with a relatively recent and growing understanding of transdiagnostic psychopathology in some part stemming from emotion dysregulation (Campbell-Sills & Barlow, 2007). The trend toward this thinking has been underscored by the growing use of emotion regulation-based treatments for various forms of psychopathology, such as dialectical behavioral therapy, emotion-regulation skills, and emotion-focused therapy (Berking et al., 2008; Gratz & Tull, 2010; Greenberg, 2017; Lynch et al., 2007). As such, the relationship among neuroticism, transdiagnostic psychopathology, and emotion dysregulation could be explained, in part, by alterations in this neural circuitry associated with emotion regulation.
Altered coupling between the amygdala and the prefrontal cortex is increasingly being understood as part of the neural pathway associated with emotion dysregulation and as a transdiagnostic marker for psychopathology both in adulthood and during development (Hardee et al., 2013; Monk et al., 2008; Kim et al., 2011; Beauchaine & Zisner, 2017; Bruhl et al., 2014). Still, the exact prefrontal regions implicated and the directionality of the relationship have been debated and there is some research which suggests that there may also be a developmental influence on the directionality of the relationship. Previous studies have found hypoconnectivity between the amygdala and vmPFC in adolescents with anxiety disorders (Hamm et al., 2014) during a resting-state fMRI study. Additionally, one previous study found a similar negative coupling between the amygdala and the vmPFC in anxious children (ages 11–19) during a task that required paying attention to threat vs. non-threat features, whereas in anxious adults (ages 24–48) they found positive coupling between the amygdala and the vmPFC (Gold et al., 2016). This finding contrasts with the current study and other studies which have found a negative coupling between the amygdala and the vmPFC in adults higher in anxiety (Kim et al., 2011). This difference in directionality between the findings in the current study and other resting state studies which have found negative coupling in adults to be associated with higher levels of anxiety or neuroticism, as opposed to the positive coupling between the amygdala and vmPFC found by Gold and colleagues (2016) might reflect different altered pathways associated with passive observation of emotional information (as in the current study) as opposed to a more active condition of threat detection (as in the study by Gold and colleagues).
Strengths and Limitations
The present study had a number of strengths, including its large, population-based sample. Because of both the size and composition of the sample, the data are well-suited for answering questions about neuroticism, which has been shown to be normally distributed in the general population. In addition, this study uses multiple self-report measures to assess trait neuroticism, in conjunction with multiple fMRI indicators – in this way, it is strengthened by its use of multiple measures across two methodological domains and serves to improve our understanding of the underlying construct of neuroticism. Particularly for the research area of neuroticism, this approach is novel, as the lion’s share of extant research on trait neuroticism relies on self-report alone. The current approach is in line with methods advocated by the Research Domain Criteria (RDoC) for the National Institute of Mental Health funding and lays the ground work for additional research integrating behavioral constructs and neurobiological approaches (Patrick et al., 2013).
The study design allowed for a within-study replication attempt, in that we were able to attempt to reproduce results found in first-born twins in the second-born twins from the twin pair. Though not based on independent samples, this method strengthens confidence in the conclusions that can be drawn from this project, as findings are generally consistent across the first-born and second-born twins. Notably, even when separated into first- and second-born twin groups, the present sample is much larger than the extant studies on amygdala activation, habituation, and dysconnectivity associated with neuroticism. Still, conducting analyses separately in the first-born twin group and the second-born twin group does not represent a true replication attempt, as the twins are related and therefore non-independent data points. Of note, there are currently limited tools for fitting biometrical models to interdependent twin data and there is no way to correct for the non-independent nature of twin data when all twins are included in a whole-group neuroimaging analysis.
It is important to acknowledge additional limitations of this study method. While the sample was much larger than typical fMRI samples, it was a predominantly White sample, mapping onto the demographics of Minnesota at the time the twins were born. Therefore, the results might not be generalizable to non-White populations. Additionally, the sample is population-based, suggesting that it might be generalizable to most individuals, but might not necessarily reflect the extreme poles of trait neuroticism in the population. This is an important caveat for trait neuroticism, which might have different neural features at the higher ranges, as some studies have shown the opposite relationship between amygdala-vmPFC in individuals with diagnosed psychopathology associated with high levels of neuroticism (Kim & Whalen, 2009).
Additionally, in the current study, the personality measures employed to derive the neuroticism factor scores were not standard Big Five measures. Rather, the PID-5 Negative Affect scale was designed to assess maladaptive personality traits and the IDAS-II Dysphoria scale was developed to assess dysphoria associated with DSM-IV diagnoses of depression and anxiety. While evidence broadly finds that these measures are associated with normal-range personality measures (Gore & Widiger, 2013; Watson, Stasik, Ro, & Clark, 2013), due to their clinical focus, these measures might fail to adequately characterize the range of neuroticism-like features in the community sample used in the current study.
Conclusion
Using multiple methods across two measurement domains, we found evidence for a relationship between trait neuroticism and vmPFC-amygdala co-activation during negative emotion processing in a large sample, while failing to find evidence of a relationship between neuroticism and amygdala activation or habituation. These findings suggest that a key element in the neural underpinnings of neuroticism is related to the interplay between the vmPFC, a central brain region involved in emotion regulation, and the amygdala, a brain region involved in emotion perception and processing. This neural interaction indicates that a crucial mechanism underlying neuroticism may be a failure in emotion regulation, rather than a problem with gating emotional information. Failures in emotion regulation have been shown to confer vulnerability for psychopathology, especially in the context of environmental risk. The present study may help explain the utility and increased use of treatments focusing on emotion regulation for various transdiagnostic mental illnesses that are associated with elevated levels of neuroticism. This finding further suggests that focusing on mechanisms of emotion regulation in relationship to trait neuroticism may prove fruitful for identifying additional neuroscientifically derived biomarkers.
Acknowledegements:
This research was supported by resources from the University of Minnesota’s Center for Magnetic Resonance Research (P41-RR008079, P41-EB015894, P30-NS076408) and the Minnesota Supercomputing Institute. Research reported in this paper was supported by the National Science Foundation under Award NSF00039202 (to M. H. S.), the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers K01DA037280 (to S. W.), R01DA036216 (to W. G. I.), and R37DA005147 (to W. G. I.).
References
- Aggleton JP (Ed.). (1992). The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: John Wiley & Sons, Inc. [Google Scholar]
- Allen TA, & Deyoung CG (2016). Personality Neuroscience and the Five Factor Model. Oxford: Handbook of the Five Factor Model; 10.1093/oxfordhb/9780199352487.013.26 [DOI] [Google Scholar]
- American Psychiatric Association. (2013). Personality Inventory for the DSM-5, 5, 9. Retrieved from https://www.psychiatry.org/FileLibrary/Psychiatrists/Practice/DSM/APA_DSM5_The-Personality-Inventory-For-DSM-5-Full-Version-Adult.pdf
- Beauchaine TP, & Gatzke-Kopp LM (2012). Instantiating the multiple levels of analysis perspective in a program of study on externalizing behavior. Development and Psychopathology, 24, 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, & Zisner A (2017). Motivation, emotion regulation, and the latent structure of psychopathology: An integrative and convergent historical perspective. International Journal of Psychophysiology, 119, 108–118. [DOI] [PubMed] [Google Scholar]
- Berking M, Wupperman P, Reichardt A, Pejic T, Dippel A, & Znoj H (2008). Emotion-regulation skills as a treatment target in psychotherapy. Behaviour Research and Therapy, 46(11), 1230–1237. 10.1016/j.brat.2008.08.005 [DOI] [PubMed] [Google Scholar]
- Bjørnebekk A, Fjell AM, Walhovd KB, Grydeland H, Torgersen S, & Westlye LT (2013). Neuronal correlates of the five factor model (FFM) of human personality:Multimodal imaging in a large healthy sample. NeuroImage, 65(October), 194–208. 10.1016/j.neuroimage.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, … Pine DS (2008). Neural Response to Self- and Other Referential Praise and Criticism in Generalized Social Phobia. Archives of General Psychiatry, 65(10), 1176 10.1001/archpsyc.65.10.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, … Rosen BR (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17(5), 875–887. 10.1016/S0896-6273(00)80219-6 [DOI] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross BS, & Pine DS (2011). Development of anxiety: the role of threat appraisal and fear learning. Depression and Anxiety, 28(1), 5–17. 10.1002/da.20733.Development [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück C, Kreifelts B, Kaza E, Lotze M, & Wildgruber D (2011). Impact of personality on the cerebral processing of emotional prosody. NeuroImage, 58(1), 259–268. 10.1016/j.neuroimage.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Bruhl AB, Delsignore A, Komossa K, & Weidt S (2014). Neuroimaging in social anxiety disorder -- a meta-analytic review resulting in a new neurofunctional model. Neuroscience and Biobehavioral Reviews, 47, 260–280. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, & Meyer-Lindenberg A (2012). Psychopathology and the human connectome: Toward a transdiagnostic model of risk for mental illness. Neuron, (74), 990–1004. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, & Barlow DH (2007). Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders In Gross JJ (Ed.), Handbook of emotion regulation (pp. 542–559). New York: Guilford Press. [Google Scholar]
- Caspi A, Harrington H, Milne B, Amell JW, Theodore RF, & Moffitt TE (2003). Children’s behavioral styles at age 3 are linked to their adult personality traits at age 26. Journal of Personality, 71, 495–514. [DOI] [PubMed] [Google Scholar]
- Chan SWY, Norbury R, Goodwin GM, & Harmer CJ (2009). Risk for depression and neural responses to fearful facial expressions of emotion. British Journal of Psychiatry, 194(2), 139–145. 10.1192/bjp.bp.107.047993 [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards JR, & Helmstetter FJ (2007). Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory, 14(7), 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, and Kesner RP (2009). Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Beahvioral Neuroscience, 123, 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee N. J. a, … Roelofs K (2010). Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. NeuroImage, 49(1), 963–970. 10.1016/j.neuroimage.2009.08.023 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Smit F, Penninx B, de Graaf R, ten Have M, & Beekman A (2010). Economic costs of neuroticism: A population-based study. Archives of General Psychiatry, 67, 1086–1093. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Arbuckle NL, Jahn A, Mowrer SM, & Abduljalil AM (2011). Reprint of: Aspects of neuroticism and the amygdala: Chronic tuning from motivational styles. Neuropsychologia, 49(4), 657–662. 10.1016/j.neuropsychologia.2011.02.027 [DOI] [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annual Reviews Neuroscience, 15, 353–375. Retrieved from http://www.annualreviews.org/doi/pdf/10.1146/annurev.ne.15.030192.002033 [DOI] [PubMed] [Google Scholar]
- Davis M, & Whalen PJ (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. 10.1038/sj.mp.4000812 [DOI] [PubMed] [Google Scholar]
- De Fruyt F, De Clercq B, De Bolle M, Wille B, Markon K, & Krueger RF (2013). General and maladaptive traits in a five-factor framework for DSM-5 in a university student sample. Assessment, 20(3), 295–307. 10.1177/1073191113475808 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, & Phelps EA (2008). Neural Circuitry Underlying the Regulation of Conditioned Fear and Its Relation to Extinction. Neuron, 59(5), 829–838. 10.1016/j.neuron.2008.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue R (2009). Genetic, environmental, and epigenetic factors in the development of personality disturbance. Development and Psychopathology, 21, 1031–1063. [DOI] [PubMed] [Google Scholar]
- DeYoung CG (2015). Cybernetic Big Five Theory. Journal of Research in Personality, 56, 33–58. 10.1016/j.jrp.2014.07.004 [DOI] [Google Scholar]
- DeYoung CG, & Gray JR (2009). Personality neuroscience: Explaining individual differences in affect, behaviour, and cognition. In The Cambridge Handbook of Personality Psychology (pp. 323–346). [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, & Gray J (2010). Testing predictions from personality neuroscience: Brain structure and the Big Five. Psychological Science, 21(6), 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, & Gruber O (2011). Fear is only as deep as the mind allows. A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage, 58(1), 275–285. 10.1016/j.neuroimage.2011.05.073 [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, & Beck AT (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12(8), 467–477. 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, & Gross JJ (2009). Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry, 65, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, & Friesen W (1977). Facial Action Coding System. [Google Scholar]
- Eysenck H (1967). The biological basis of personality. Springfield, IL: Charles C. Thomas. [Google Scholar]
- Friston KJ (2005). Models of brain function in neuroimaging. Annual Review of Psychology, 56, 57–87. 10.1146/annurev.psych.56.091103.070311 [DOI] [PubMed] [Google Scholar]
- Gore WL, & Widiger T. a. (2013). The DSM-5 dimensional trait model and five-factor models of general personality. Journal of Abnormal Psychology, 122(3), 816–21. 10.1037/a0032822 [DOI] [PubMed] [Google Scholar]
- Gratz KL, & Tull MT (2010). Emotion Regulation as a Mechanism of Change in Acceptance- and Mindfulness-based Treatments. (Baer RA, Ed.) (Assessing). Oakland, CA: New Harbinger Publications. [Google Scholar]
- Gray J (1982). The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. New York: Clarendon Press/Oxford University Press. [Google Scholar]
- Gray J (1991). Neural systems, emotion, and personality In Madden J (Ed.), Neurobiology of learning, emotion, and affect (pp. 273–306). New York: Raven Press. [Google Scholar]
- Greenberg LS (2017). Emotion-focused therapy of depression. Person-Centered and Experiential Psychotherapies, 16(2), 106–117. 10.1080/14779757.2017.1330702 [DOI] [Google Scholar]
- Haas BW, Constable R, & Canli T (2008). Stop the sadness: Neuroticism is associated with sustained medial prefrontal cortex response to emotional facial expressions. NeuroImage, 42, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, & Canli T (2007). Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience, 121(2), 249–56. 10.1037/0735-7044.121.2.249 [DOI] [PubMed] [Google Scholar]
- Hamm LL, Jacobs RH, Johnson MW, Fitzgerald DA, Fitzgerald KD, … Phan KL (2014). Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biology of Mood and Anxiety Disorders, 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, … Fischl B (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32(1), 180–194. 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Lakdawalla Z, Carter IL, Abela JRZ, & Adams P (2007). Are neuroticism, cognitive vulnerabilities and self–esteem overlapping or distinct risks for depression? Evidence from exploratory and confirmatory factor analyses. Journal of Social and Clinical Psychology, 26(1), 29–63. 10.1521/jscp.2007.26.1.29 [DOI] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, … Perez-Edgar K (2013). Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological Psychiatry, 74(4), 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Kim SH, & Hamann S (2009). Neuroticism and psychopathy predict brain activation during moral and nonmoral emotion regulation. Cognitive, Affective & Behavioral Neuroscience, 9(1), 1–15. 10.3758/cabn.9.1.1 [DOI] [PubMed] [Google Scholar]
- Heimer L (2003). A new anatomical framework for neuropsychiatric disorders and drug abuse. American Journal of Psychiatry, 160, 1726–1739. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, … Büchel C (2005). Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience, 8(1), 20–21. 10.1038/nn1366 [DOI] [PubMed] [Google Scholar]
- Hilt LM, Hanson JL, Pollak SD (2011). Emotion dysregulation In Brown BB & Prinstein MJ (Eds.), Encyclopedia of Adolescence (Vol. 3, pp 160–169). New York, NY: Elsevier. [Google Scholar]
- Hyde LW, Gorka A, Manuck SB, & Hariri AR (2011). Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. NeuropsychologiaNeuropsychologia, 49, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, & Whalen PJ (2005). Stability of amygdala BOLD response to fearful faces over multiple scan sessions. NeuroImage, 25(4), 1112–1123. 10.1016/j.neuroimage.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, & Davidson RJ (2007). Failure to Regulate: Counterproductive Recruitment of Top-Down Prefrontal-Subcortical Circuitry in Major Depression. Journal of Neuroscience, 27(33), 8877–8884. 10.1523/JNEUROSCI.2063-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, Kouwe A Van Der, Quinn B, … Fischl B (2009). MRI-derived measurements of human subcortical, ventricular, and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage, 46(1), 177–192. 10.1016/j.neuroimage.2009.02.010.MRI-derived [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick S, Clarke C, Snidman N, & Garcia-Coll C (1984). Behavioral inhibition to the unfamiliar. Child Development, 55, 2212–2225. [Google Scholar]
- Kamphausen S, Schröder P, Maier S, Bader K, Feige B, Kaller CP, … Tüscher O (2013). Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. The World Journal of Biological Psychiatry, 14(4), 307–318. 10.3109/15622975.2012.665174 [DOI] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, … Heinz A (2008). Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nature Neuroscience, 11(12), 1381–2. 10.1038/nn.2222 [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, & Whalen PJ (2011). The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–410. 10.1016/j.bbr.2011.04.025.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, & Whalen PJ (2009). The Structural Integrity of an Amygdala-Prefrontal Pathway Predicts Trait Anxiety. Journal of Neuroscience, 29(37), 11614–11618. 10.1523/JNEUROSCI.2335-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeningsberg HW, Denny BT, Fan J, Liu X, Guerreri S, Mayson SJ, … Siever LJ (2014). The Neural Correlates of Anomalous Habituation to Negative Emotional Pictures in Borderline and Avoidant Personality Disorder Patients. American Journal of Psychiatry, 171(January), 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Derringer J, Markon KE, Watson D, & Skodol a E. (2012). Initial construction of a maladaptive personality trait model and inventory for DSM-5. Psychological Medicine, 42(9), 1879–90. 10.1017/S0033291711002674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Wu M, Klumpp H, Pine DS, Swain JE, Fitzgerald KD, Monk CS, Phan KL (2016). Altered development of amgydala-anterior cingulate cortex connectivity in anxious youth and young adults. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(4), 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB (2009). Public health significance of neuroticism. The American Psychologist, 64, 241–256. 10.1037/a0015309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (1998). The emotional brain: The mysterious underpinnings of emotional life. New York: Simon & Schuster. [Google Scholar]
- Lemogne C, Gorwood P, Bergouignan L, Pélissolo A, Lehéricy S, & Fossati P (2011). Negative affectivity, self-referential processing and the cortical midline structures. Social Cognitive and Affective Neuroscience, 6(4), 426–433. 10.1093/scan/nsq049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Golkar A, Lindstöm KM, Fransson P, Schalling M, Öhman A, & Ingvar M (2011). 5-HTTLPR and COMTval158met genotype gate amygdala reactivity and habituation. Biological Psychology, 87(1), 106–112. 10.1016/j.biopsycho.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Lynch TR, Trost WT, Salsman N, Linehan MM, T.R. L, W.T. T, … M.M. L (2007). Dialectical behavior therapy for borderline personality disorder. Annual Review of Clinical Psychology, 3(1548–5943 (Print)), 181–205. 10.1146/annurev.clinpsy.2.022305.095229 [DOI] [PubMed] [Google Scholar]
- McCrae R, & Costa P (2003). Personality in adulthood: A five-factor theory perspective. New York: Guilford Press. [Google Scholar]
- Mesulam M (1998). From sensation to cognition. Brain, 121, 1013–1052. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, … Pine DP (2008). Amygdala and ventrolateral pre-frontal cortex activation to masked-angry faces in children and adolescents with generalized anxiety disorder. Archives in General Psychiatry, 65(5), 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, & Dolan RJ (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain, 121, 47–57. 10.1093/brain/121.1.47 [DOI] [PubMed] [Google Scholar]
- Norris CJ, Larsen JT, & Cacioppo JT (2007). Neuroticism is associated with larger and more prolonged electrodermal responses to emotionally evocative pictures. Psychophysiology, 44(5), 823–826. 10.1111/j.1469-8986.2007.00551.x [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, & Johansen-Berg H (2012). Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7(5), 604–609. 10.1093/scan/nss055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, (1251), E1–E24. 10.1111/j.1749-6632.2012.06751.x.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Bastiaansen a., Riese HH, Bos EH, Servaas M, Ellenbogen M, … Aleman AA (2013). The biological and psychological basis of neuroticism: Current status and future directions. Neuroscience and Biobehavioral Reviews, 37(1), 59–72. 10.1016/j.neubiorev.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Ormel J, Jeronimus BF, Kotov R, Riese H, Bos EH, Hankin B, … Oldehinkel AJ (2013). Neuroticism and common mental disorders: Meaning and utility of a complex relationship. Clinical Psychology Review, 33(5), 686–697. 10.1016/j.cpr.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Rosmalen J, & Farmer A (2004). Neuroticism: A non-informative marker of vulnerability to psychopathology. Social Psychiatry and Psychiatric Epidemiology, 39(11), 906–912. 10.1007/s00127-004-0873-y [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, & Tellegen A (2002). Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment, 14(2), 150–163. 10.1037/1040-3590.14.2.150 [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, & Kramer MD (2013). A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122(3), 902–16. 10.1037/a0032807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, & LeDoux JE (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48(2), 175–187. 10.1016/j.neuron.2005.09.025 [DOI] [PubMed] [Google Scholar]
- Phillips M, Medford N, Young A, Williams L, Williams S, Bullmore E, … Brammer M (2001). Time courses of left and right amygdalar responses to fear- ful facial expressions. Human Brain Mapping, (12), 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Grimm O, Morgen K, Mier D, Sauer C, Haddad L, … Meyer-Lindenberg A (2014). Amygdala habituation: A reliable fMRI phenotype. NeuroImage, 103, 383–390. 10.1016/j.neuroimage.2014.09.059 [DOI] [PubMed] [Google Scholar]
- Portella MJ, Harmer CJ, Flint J, Cowen P, & Goodwin GM (2005). Enhanced early morning salivary cortisol in neuroticism. American Journal of Psychiatry, 162(4), 807–809. 10.1176/appi.ajp.162.4.807 [DOI] [PubMed] [Google Scholar]
- Samuel DB, Carroll KM, Rounsaville BJ, & Ball SA (2013). Personality disorders as maladaptive, extreme variants of normal personality: borderline personality disorder and neuroticism in a substance using sample. Journal of Personality Disorders, 27(5), 625–35. 10.1521/pedi.2013.27.5.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler BS, Kral TRA, Jacquart J, Burghy CA, Weng HY, Perlman DM, … Davidson RJ (2014). Temporal dynamics of emotional responding: Amygdala recovery predicts emotional traits. Social Cognitive and Affective Neuroscience, 9(2), 176–181. 10.1093/scan/nss131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, Geerligs L, Renken RJ, Marsman JB, Ormel J, Riese H, & Aleman a. (2015). Connectomics and neuroticism: an altered functional network organization. Neuropsychopharmacology, 40(2), 296–304. 10.1038/npp.2014.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, Riese H, Renken RJ, Marsman J-BC, Lambregs J, Ormel J, & Aleman A (2013). The effect of criticism on functional brain connectivity and associations with neuroticism. PloS One, 8(7), e69606. 10.1371/journal.pone.0069606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, van der Velde J, Costafreda SG, Horton P, Ormel J, Riese H, & Aleman A (2013). Neuroticism and the brain: A quantitative meta-analysis of neuroimaging studies investigating emotion processing. Neuroscience and Biobehavioral Reviews, 37(8), 1518–1529. 10.1016/j.neubiorev.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Simms LJ, Grös DF, Watson D, & O’Hara MW (2008). Parsing the general and specific components of depression and anxiety with bifactor modeling. Depression and Anxiety, 25(7), 34–46. 10.1002/da.20432 [DOI] [PubMed] [Google Scholar]
- Soto CJ, John OP, Gosling SD, & Potter J (2011). Age differences in personality traits from 10 to 65: Big Five domains and facets in a large cross-sectional sample. Journal of Personality and Social Psychology, 100(2), 330–348. 10.1037/a0021717 [DOI] [PubMed] [Google Scholar]
- Sporns O (2011). The human connectome: A complex network. Annals of the New York Academy of Sciences, 1224(1), 109–125. 10.1111/j.1749-6632.2010.05888.x [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, & Meyer-Lindenberg A (2007). A validated network of effective amygdala connectivity. NeuroImage, 36(3), 736–745. 10.1016/j.neuroimage.2007.03.022 [DOI] [PubMed] [Google Scholar]
- Strauss MM, Makris N, Aharon I, Vangel MG, Goodman J, Kennedy DN, … Breiter HC (2005). fMRI of sensitization to angry faces. NeuroImage, 26(2), 389–413. 10.1016/j.neuroimage.2005.01.053 [DOI] [PubMed] [Google Scholar]
- Suls J, & Martin R (2005). The daily life of the garden-variety neurotic: Reactivity, stressor exposure, mood spillover, and maladaptive coping. Journal of Personality, 73(6), 1485–1509. 10.1111/j.1467-6494.2005.00356.x [DOI] [PubMed] [Google Scholar]
- Tackett JL (2006). Evaluating models of the personality-psychopathology relationship in children and adolescents. Clinical Psychology Review, 26, 584–599. [DOI] [PubMed] [Google Scholar]
- Tackett JL, & Lahey BB (2016). Neuroticism (Vol. 1). 10.1093/oxfordhb/9780199352487.013.14 [DOI] [Google Scholar]
- Tellegen A, & Waller N (2008). Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. In The SAGE handbook of personality theory and assessment 2 (pp. 261–292). [Google Scholar]
- Thomas EJ, Elliott R, McKie S, Arnone D, Downey D, Juhasz G, … Anderson IM (2011). Interaction between depression and rumination on neural responses to emotional faces. Psychological Medicine, 41, 1845–1855. [DOI] [PubMed] [Google Scholar]
- Thompson RF, & Spencer W. a. (1966). Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological Review, 73(1), 16–43. 10.1037/h0022681 [DOI] [PubMed] [Google Scholar]
- Tzschoppe J, Nees F, Banaschewski T, Barker GJ, Büchel C, Conrod PJ, … Flor H (2014). Aversive Learning in Adolescents: Modulation by Amygdala–Prefrontal and Amygdala–Hippocampal Connectivity and Neuroticism. Neuropsychopharmacology, 39(4), 875–884. 10.1038/npp.2013.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, & Davidson RJ (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience, 26(16), 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg SM, De Moor MHM, McGue M, Pettersson E, Terracciano A, Verweij KJH, … Boomsma DI (2014). Harmonization of neuroticism and extraversion phenotypes across inventories and cohorts in the Genetics of Personality Consortium: An application of item response theory. Behavior Genetics, 44(4), 295–313. 10.1007/s10519-014-9654-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. 10.1177/1073191112449857 [DOI] [PubMed] [Google Scholar]
- Watson D, Stasik SM, Ro E, & Clark LA (2013). Integrating Normal and Pathological Personality. Assessment, 20(3), 312–326. 10.1177/1073191113485810 [DOI] [PubMed] [Google Scholar]
- Weisberg YJ, De Young CG, & Hirsh JB (2011). Gender differences in personality across the ten aspects of the Big Five. Frontiers in Psychology, 2(August), 1–11. 10.3389/fpsyg.2011.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiger TA (2009). Neuroticism In Leary M & Hoyle R (Eds.), Handbook of individual differences in social behavior (pp. 129–146). New York, NY: Guilford Press. [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, … Gordon E (2006). The Mellow Years?: Neural Basis of Improving Emotional Stability over Age. Journal of Neuroscience, 26(24), 6422–6430. 10.1523/JNEUROSCI.0022-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GD, Kumari V, Gray JA, & Corr PJ (2000). The role of neuroticism in startle reactions to fearful and disgusting stimuli. Personality and Individual Differences, 29(6), 1077–1082. 10.1016/S0191-8869(99)00255-X [DOI] [Google Scholar]
- Xu J, & Potenza M (2012). White matter integrity and five-factor personality measures in healthy adults. NeuroImage, 59, 800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T (2009). Big correlations in little studies: Inflated fMRI correlations reflect low statistical power-commentary on Vul et al. Perspectives on Psychological Science, 4(3), 294–298. [DOI] [PubMed] [Google Scholar]


