Abstract
Background.
Detection of chronic kidney disease (CKD) with urine albumin-to-creatinine ratio (UACR) among patients with hypertension (HTN) provides an opportunity for early treatment, potentially mitigating risk of CKD progression and cardiovascular complications. Differences in UACR testing patterns among racial/ethnic populations at risk for CKD could contribute to known disparities in CKD complications.
Methods.
We examined the prevalence of UACR testing among low-income adult primary care patients with HTN, defined by a new administrative code for HTN or two clinic blood pressures (BP) > 140/90 mmHg between January 1, 2014 and January 1, 2017 in one public healthcare delivery system with a high prevalence of end stage kidney disease among race/ethnic minorities. Logistic regression was used to identify odds of UACR testing within one year of a HTN diagnosis, overall, and by racial/ethnic subgroup, adjusted for demographic factors, estimated glomerular filtration rate, and hypertension severity. Models were also stratified by diabetes status.
Results:
The cohort (n=16,414) was racially/ethnically diverse (16% White, 21% Black, 34% Asian, 19% Hispanic and 10% other) and 51% female. Only 35% of patients had UACR testing within 1 year of a HTN diagnosis. Among individuals without diabetes, odds of UACR testing were higher among Asians, Blacks and Other subgroups compared to Whites [adjusted Odds Ratio (aOR)=1.19; 95% CI: 1.00–1.42 for Blacks; aOR=1.33; 1.13–1.56 for Asians; aOR=1.30; 1.04–1.60 for Other] but were not significantly different between Hispanics and Whites (aOR=1.17; 0.97–1.39). Among individuals with diabetes, only Asians had higher odds of UACR testing compared to Whites (aOR=1.35; 1.12–1.63).
Conclusions:
Prevalence of UACR testing among low-income patients with HTN is low in one public healthcare delivery system, with higher odds of UACR testing among racial/ethnic minority subgroups compared to Whites without diabetes and similar odds among those with diabetes. If generalizable, less albuminuria testing may not explain higher prevalence of kidney failure in racial/ethnic minorities.
Keywords: chronic kidney disease, albuminuria testing, CKD, hypertension, diabetes, racial disparities, CKD disparities, safety-net clinic, vulnerable populations
Introduction
Chronic Kidney Disease (CKD) currently affects an estimated 30 million Americans and is associated with high blood pressure, anemia, disorders of bone mineral metabolism, poor nutritional health, and cardiovascular disease.1 Implementation of evidence-based treatments for CKD can decrease the risk of CKD progression to End Stage Kidney Disease (ESKD), reduce cardiovascular complications, and decrease the risk of early death.2–4 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) and Kidney Disease Improving Global Outcomes (KDIGO) have published evidence-based guidelines to assist clinicians in treating patients with CKD to slow disease progression.5 To implement such therapies, providers must properly diagnose individuals with kidney disease, which may include testing individuals at highest risk of developing CKD, including those with hypertension (HTN) and diabetes mellitus (DM), the two most common causes of ESRD in the United States.1
One recommended diagnostic tool for CKD is Urinary Albumin-to-Creatinine ratio (UACR) testing. Unlike the urine dipstick test, which may be influenced by urine concentration and may lack sensitivity, UACR testing accurately detects small amounts of albumin in the urine prior to the development of more significant kidney damage.6 In addition to diagnosing early kidney damage and prompting initiation of CKD management, albuminuria is an independent prognostic risk factor for adverse outcomes, including all-cause mortality, myocardial infarction, and ESKD, among individuals with preserved estimated glomerular filtration rates (eGFRs) as well as those with abnormal eGFRs.7 For this reason, albuminuria screening among adults at highest risk for CKD, including those with HTN and DM, has been shown to be cost-effective.8,9 The National Committee for Quality Assurance (NCQA) recommends that patients with DM receive annual UACR testing due to the increased risk of kidney disease and cardiovascular complications associated with this condition but does not currently recommend UACR testing among individuals with HTN alone. By contrast, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends UACR testing among individuals with DM or HTN.10,11
Early CKD detection by UACR testing among individuals at high risk of CKD may not only enhance CKD treatment aimed at slowing its progression and managing associated complications, but it may also help reduce racial/ethnic and socioeconomic disparities in clinical outcomes among individuals with CKD. Abnormal UACR levels are more prevalent among Blacks compared to other racial/ethnic subgroups and data demonstrate that racial/ethnic minority groups suffer from ESKD at a rate between 1.6 to 4 times more than Whites, with Blacks experiencing the highest odds of ESKD followed by Hispanics, Native Americans and Asians.12,13 Similar trends are noted when viewing kidney disease by socioeconomic status. In the third National Health and Nutrition Examination survey, individuals below the 200% federal poverty line had a 35% increased odds of having microalbuminuria (a UACR value between 30–299 mg/g) and a 78% increased odds of having macroalbuminuria (a UACR value > 300mg/g) compared to those above the 200% federal poverty line.14 Additionally, data suggest that ESKD is more common among individuals living in low-income neighborhoods compared to higher income neighborhoods.15 Different UACR testing patterns among racial/ethnic and socioeconomic subgroups at risk for CKD could thus contribute to disparities in CKD diagnosis and subsequent complications.
We examined the prevalence of UACR testing among patients at risk for CKD due to hypertension overall, and by race/ethnic subgroup in a public healthcare delivery system that predominantly serves a low-income patient population. Because UACR testing among individuals with HTN is not currently recommended by the NCQA, we hypothesized that the overall prevalence of UACR testing would be low among individuals with HTN. We also thought that the odds of UACR testing would be similar across race and ethnicity subgroups given the uniformity in low socioeconomic status and widespread experience with fragmented health care delivery in this population.16
Methods
Study Design/Setting/Population
This is a cross-sectional study using data from the San Francisco Health Network (SFHN), the public healthcare delivery system that cares for San Francisco’s uninsured and underinsured populations and has previously demonstrated a high prevalence of ESKD among race/ethnic minorities compared to Whites.17 Census Bureau data suggest that racial and ethnic minorities are over represented in the SFHN when compared to the city of San Francisco. Similarly, zip codes associated with the city’s highest rates of residents living below 200% of the census poverty threshold represent nearly 40% of patient zip codes visiting the SFHN hospital (Zuckerberg San Francisco General Hospital and Trauma Center).18
Data were extracted from the Network’s electronic health record to create a cohort of active adult primary care patients over the age of 18 with hypertension. Active patients were defined by having contact with primary care at least once between 2014–2017 and hypertension was defined as being on the local hypertension disease registry, which required either a diagnosis code for hypertension or two clinic BPs > 140/90 mmHg recorded at separate clinic visits. Patients entered this cohort (and the hypertension registry) on the date of a new HTN diagnosis code or on the date of their second qualifying high BP measurement. Patients must have demonstrated at least one year of follow up with the healthcare system in order to be included in our analysis to allow time for UACR testing and have an eGFR > 15 ml/min/1.73m2. Under these criteria, the cohort consisted of 16,414 patients. The Institutional Review Board of UCSF approved this study.
Study variables
The outcome variable was the completion of UACR testing within one year of cohort entry, identified by a numerical value associated with the test name “microalbuminuria”. Need for UACR testing was determined by individual provider assessment at each clinic visit, as there was no established protocol for UACR testing among individuals with hypertension. The primary predictor was race/ethnicity listed in the electronic health record as a categorical variable (White, Black, Asian, Hispanic and Other).
Race/ethnicity is self-reported and is entered in the electronic health record when a patient is registered for care. Multi-ethnic patients and those with unknown race/ethnicity in the electronic health record were categorized as ‘Other’. Other covariates included sociodemographic variables (age, gender, and insurance status), primary care clinic, and co-morbid conditions thought to affect UACR testing: diabetes status (defined by a diagnosis code or glycosylated hemoglobin > 6.5%) and eGFR defined by the CKD-EPI equation19 at time of cohort entry. All BPs obtained during clinic visits were abstracted. In the SFHN, medical assistants use standard oscillometric devices to check BP in all ambulatory clinics (including primary and specialty care) with a standardized protocol. If the first BP is elevated, a second and third BP are obtained 3–5 minutes apart. While all BP measures are included in the medical record, only the lowest BP at each clinic visit (often the last one recorded) was used for treatment decisions and was thus used to define HTN in this study. While not consistent with American Heart Association guidelines, which recommend using an average BP measurement, we opted to use the BP measurement that was used for clinical purposes.
Statistical Analysis
Patient characteristics were compared by race/ethnicity using chi-squared and ANOVA. Multivariate logistic regression was used to identify odds of UACR testing by racial/ethnic subgroup, independent of sociodemographic variables, primary care clinic, eGFR, and systolic BP at cohort entry. We hypothesized that odds of UACR testing might differ by diabetes status because of national quality performance measures that recommend annual UACR testing among individuals with diabetes, so we tested for interaction.10 We found the presence of an interaction between race/ethnicity and diabetes among those of Asian race/ethnicity (p≤0.001 for Asian vs. White) so analyses were stratified by diabetes status.
Results
Characteristics of study population
The final study cohort (n=16,414) was racially/ethnically diverse (16% White, 21% Black, 33% Asian, 19% Hispanic and 10% Other) and geographically distributed across San Francisco, including patients who sought primary care from 11 different primary care clinics (Table 1). The study cohort was 51% female, with an average age of 59.4 (SD=11.7) years. On average, individuals of Black, Hispanic, and Other race/ethnicity with HTN were of younger age compared to Whites and Asians (P<0.01). A majority of patients (90%) were publicly insured, with Medicaid serving the majority of insured patients (45%), though differences in insurance coverage were noted by race/ethnicity (p<0.01). The prevalence of uninsured patients was highest among Hispanics (n=693; 22%) and lowest among Asians (n=263; 5%). Overall, 37% of the cohort had a diagnosis of diabetes. Diabetes prevalence was highest among those of Other race/ethnicity (n=687, 44%) and Hispanics (n=1352, 43%) and lowest among those with White race/ethnicity (n=722, 27%) (p<0.01). Mean eGFR in the cohort was 87.2 (SD 23.3) ml/min/1.73m2 and prevalence of CKD stage 3–5 ranged from 10% to 13% by race/ethnicity, with the highest prevalence among Blacks (p<0.001).
Table 1.
Characteristics of the study population.
| Characteristics | All | White | Asian | Hispanic | Black | Other | p-value |
|---|---|---|---|---|---|---|---|
| N=16414 | n=2680 | n= 5521 | n=3177 | n=3465 | n=1571 | ||
| Age, mean (SD) | 59.4 (11.7) | 59.4 (10.5) | 62.4 (10.5) | 57.7 (13.5) | 57.1 (11.2) | 57.5 (12.3) | <0.01 |
| Age, N (%) | <0.01 | ||||||
| 18–39 | 916 (5.6) | 122 (4.6) | 127 (2.3) | 305 (9.6) | 242 (7.0) | 120 (7.6) | |
| 40–59 | 6859 (41.8) | 1111 (41.5) | 1820 (33.0) | 1439 (45.29) | 1751 (50.53) | 738 (47.0) | |
| 60–79 | 7943 (48.4) | 1387 (51.8) | 3241 (58.7) | 1266 (39.9) | 1388 (40.1) | 661 (42.1) | |
| 80+ | 696 (4.2) | 60 (2.2) | 333 (6.0) | 167 (5.3) | 84 (2.4) | 52 (3.3) | |
| Female, N (%) | 8398 (51.2) | 970 (36.2) | 3319 (60.1) | 1683 (53.0) | 1599 (46.2) | 827 (52.6) | <0.01 |
| Health insurance, (N) % | <0.01 | ||||||
| Medicaid | 7235 (45.2) | 1185 (45.2) | 1882 (35.0) | 1476 (47.8) | 1975 (58.1) | 717 (47.1) | |
| Medicare | 4347 (27.1) | 723 (27.6) | 1604 (29.8) | 715 (23.1) | 963 (28.3) | 342 (22.4) | |
| Other public insurance | 2760 (17.2) | 545 (20.8) | 1623 (30.1) | 189 (6.1) | 206 (6.1) | 197 (13.0) | |
| None | 1599 (10.0) | 158 (6.0) | 263 (4.9) | 693 (22.4) | 233 (6.9) | 252 (16.5) | |
| Diabetes, % (N) | 6099 (37.2) | 722 (26.9) | 2208 (40.0) | 1352 (42.6) | 1130 (32.6) | 687 (43.7) | <0.01 |
| A1c, mean (SD) | 6.4 (1.6) | 6.2 (1.4) | 6.4 (1.4) | 6.7 (1.9) | 6.3 (1.6) | 6.8 (1.8) | <0.01 |
| SBP, mean (SD) | 135.1 (18.5) | 134.1 (18.2) | 133.1 (17.5) | 136.4 (18.3) | 137.7 (20.1) | 136.0 (18.3) | <0.01 |
| DBP, mean (SD) | 80.3 (11.1) | 80.9 (11.2) | 78.1 (10.2) | 80.4 (10.8) | 82.9 (12.0) | 80.9 (11.0) | <0.01 |
| eGFR, mean (SD) | 87.2 (23.3) | 84.1 (21.3) | 84.5 (21.1) | 90.9 (23.5) | 90.2 (27.1) | 89.1 (27.9) | <0.01 |
Abbreviations: SD = standard deviation; A1c = glycosylated hemoglobin; SBP = systolic blood pressure; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate
Total N = 16,414 for all rows, with exception of the following: Health Insurance (N=16,022); glycosylated hemoglobin (N=15,591); eGFR (N=16,232); SBP and DBP (N=16,411
Crude Prevalence of UACR testing
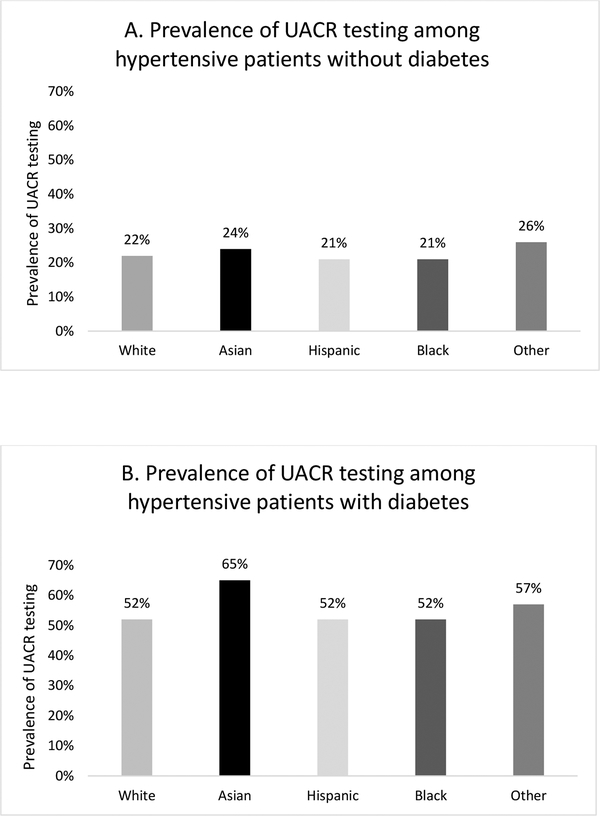
Approximately 35% of the cohort had UACR testing within 1 year of an abnormal BP or diagnosis of HTN, including 22.2% of patients without diabetes and 57.1% of patients with diabetes. Among those without diabetes, those of Other and Asian race/ethnicity had the highest prevalence of UACR testing (26% and 24% respectively), followed by Whites, Blacks and Hispanics (22%, 21% and 21% respectively) (Figure 1a). Among individuals with diabetes, the highest prevalence of UACR testing was among Asians (64.6%). Prevalence was approximately 52% for Whites, Blacks and Hispanics (Figure 1b).
Figure 1.
Prevalence of UACR testing by race/ethnicity within one year of an elevated blood pressure or HTN diagnosis in patients without diabetes (A) and with diabetes (B).
Odds of UACR testing by race/ethnicity
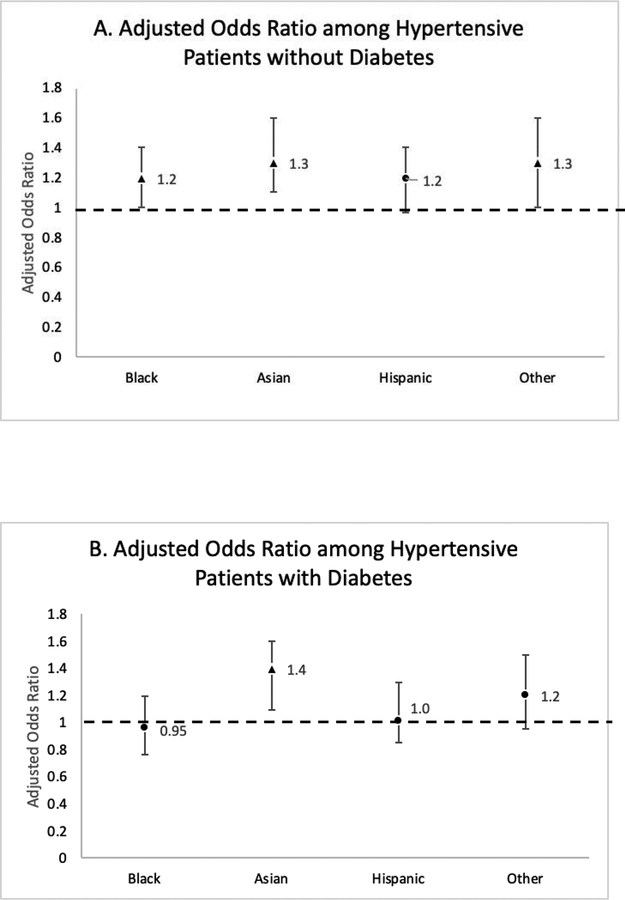
Among individuals without diabetes, Blacks, Asians and those of Other race/ethnicity experienced greater odds of UACR testing compared to Whites: aOR=1.19; (95% CI: 1.00–1.42) for Blacks; aOR=1.33 (1.13–1.56) for Asians; aOR=1.30 (1.04–1.60) for Other race/ethnicity. There was no statistically significant difference in odds of UACR testing among Hispanics and Whites [aOR=1.16 (0.97–1.39)] (Figure 2a). Among individuals with diabetes, Asians experienced a 35% greater odds of UACR testing when compared to Whites [aOR=1.35 (1.12–1.62)]. Odds of UACR testing were similar among Blacks, Hispanics, Other and Whites with hypertension and diabetes (Figure 2b).
Figure 2.
Adjusted odds ratios and 95% CI of UACR testing among hypertensive patients by race/ethnic subgroup compared to Whites without diabetes (A) and with diabetes (B). Odds ratios are adjusted for age, sex, insurance, eGFR and SBP at cohort entry. Triangle indicates p-values < 0.05.
Discussion
To our knowledge, this analysis is one of the first to look at differences in UACR testing among race/ethnic groups in a cohort of primary care patients with hypertension in a low-income urban setting. Overall, only 35% of our primary care study population received UACR testing within one year of having HTN, which is quite low. Additionally, contrary to our hypothesis that there would be no relationship between race/ethnicity and odds of UACR testing due to commonly shared experiences with fragmentation of healthcare delivery, our results demonstrated that in general, individuals of racial/ethnic minority groups with hypertension experienced higher odds of UACR testing compared to their White counterparts, in particular among those without diabetes. While this mirrors the higher prevalence of ESRD among racial/ethnic minority groups, this finding was not expected. Several studies have previously documented that preventive care such as routine examinations and disease screenings are low and often underutilized in racial and ethnic minority groups.20 For example, in a cohort of adults with diabetes, Black patients in an internal medicine patient centered medical home (PCMH) were less likely to receive hemoglobin A1C testing and influenza vaccinations, and less likely to achieve cholesterol management targets compared with their White peers in the same PCMH.21 Higher UACR testing among individuals of racial/ethnic minorities suggests that early identification of CKD may not substantially mitigate the higher risk of ESRD among racial/ethnic subgroups.
Differences in odds of UACR testing by race/ethnicity were more striking in the hypertensive patient population without diabetes. Among those without diabetes, odds of UACR testing were slightly higher among Asians, Blacks and Other compared to Whites. Odds of UACR testing among patients with diabetes and hypertension were similar across most of the racial/ethnic groups (with the exception of the Asian subgroup, which had persistently higher odds of UACR testing compared to Whites). While speculative, the observed leveling of race/ethnicity-based differences in UACR testing in the presence of diabetes may be attributable to the national Healthcare Employer Data and Information Set (HEDIS) and NCQA measures that recommend annual assessment of renal function, including UACR, in individuals with diabetes.10 A prior study found key improvements in completion of quality metrics before vs. after HEDIS implementation, suggesting that almost half of U.S. adults benefited from improved performance on at least one HEDIS quality measure after HEDIS implementation, regardless of race/ethnicity.22 Furthermore, Data from the 2015 National Impact Assessment of Quality Measures Report identified several improvements in racial/ethnic disparities upon the implementation of HEDIS measures. These included kidney function testing for members with diabetes, cholesterol screening for patients with heart disease, breast cancer screening in women aged 52 – 69 and several others.23 Since UACR testing is important for CKD identification and for accurate CKD staging, perhaps greater and more universal UACR testing would result if it were considered a quality measure among individuals with HTN, regardless of diabetes status.
While this was not the primary purpose of this study, we can identify several possible explanations for the somewhat higher rate of UACR testing in some minority groups. First, some of the clinics in this healthcare delivery system have training and academic physicians as part of their workforce. In such clinics, there may be increased awareness among primary care providers of disparities in existing CKD treatment and health outcomes, including a higher prevalence of ESRD in racial/ethnic minorities, thus leading to an increased emphasis on CKD identification in the highest at-risk groups (i.e., minority populations). This has been reported among patients with other chronic conditions. For example, a recent study demonstrated that patients with Hepatitis B who received care through an academic practice were more likely to have follow-up laboratory exams sooner after the initiation of anti-viral therapy and more frequent laboratory monitoring, when compared to community practice.24 Second, there must be agreement between patients and clinicians if UACR testing is to be completed. Agreement is likely fostered through greater trust in the medical system. In a series of interviews conducted by researchers at the University of Nebraska, patients identified three barriers to participating in follow-up visits and completing recommended laboratory testing: emotional barriers, perceived disrespect of patients’ beliefs and time, and distrust and misunderstanding.25 Greater trust in the healthcare system by Asians, as has been depicted in other studies,26,27 may have been associated with greater patient completion of tests ordered by clinicians and higher clinic show-rates, which could have led to greater UACR testing within one year of a hypertension diagnosis or elevated blood pressure.
UACR testing was similar among Hispanics and Whites, regardless of diabetes status. This finding may be explained by the broad categorization used to identify individuals of Hispanic ethnicity (i.e., categorizing Hispanic individuals with different nationalities into one ethnic subgroup), mirroring those methods often used by other large studies in the U.S.28 This categorization system has resulted in conflicting results among studies looking at healthcare utilization or health testing outcomes among Hispanic patients. For example, when looking at Hispanic subgroups by frequency in prenatal care, differences by subgroup exist. Hispanics of Mexican origin have the lowest rate of prenatal care while Cubans have a visit frequency that often exceeds that of non-Hispanic Whites.29 Similar to UACR testing, prenatal care routinely involves urine sample collection and follow up visits. It is possible that the differences in prenatal care previously documented amongst Hispanic subgroups also exist in Hispanic subgroups who require chronic hypertension care. If true, this variability within the Hispanic subgroups of our analysis would have been aggregated into one racial/ethnic group resulting in masked or skewed data.
The aforementioned reasons may explain some of the differences in UACR testing by race/ethnicity, but there are likely unmeasured confounders that are contributing to our results. It is important to note that CKD severity and general differences in the quality of care delivered at each primary care clinic, including referral to nephrology services, are not likely contributors to the observed racial/ethnic differences in UACR testing, as these variables were included in our multivariate logistic regressions. However, we did not have encounter visit data and could not adjust for nephrology care delivery. Limitations to this study also include the probable categorization of individuals with multiple different races and ethnicities and those with unknown race/ethnicity, in the ‘Other’ category in our analysis. The accuracy of race/ethnicity from the electronic health record is not known. Additionally, this analysis looked at completed UACR tests but could not consider differences in prescribed UACR testing by clinicians, including those that were not completed by patients. Observed differences in UACR testing could arise from differences in variable follow-up and test completion by racial/ethnic group rather than differences in clinician testing patterns. Additionally, we could not account for UACR testing that took place in clinics or hospitals outside of the SFHN.
Nevertheless, we demonstrate that prevalence of UACR testing among individuals with hypertension was low and that adjusted odds of UACR testing were different by race/ethnicity, particularly among individuals without diabetes. The higher odds of UACR testing among race/ethnic minorities suggest that differences in UACR testing cannot account for the increased burden of CKD and its complications among low-income racial and ethnic minority groups in SFHN. Importantly, the higher prevalence of UACR testing among individuals with diabetes and the observed equalization of odds of UACR testing among individuals with diabetes suggest that national quality measures influence care delivery. Including UACR testing as a quality measure among individuals with hypertension regardless of diabetes status could directly impact UACR testing among individuals at high risk of CKD.
Acknowledgements.
We thank the patients and providers of the San Francisco Health Network.
Financial Support information. This work was supported by R01DK104130 and Diversity Supplement R01DK104130–03S1 from the National Diabetes and Digestive and Kidney Diseases. CC is supported by T32DK007219–41 and VF is supposed by K23HL136899, both from National Diabetes and Digestive and Kidney Disease. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures. The authors have no conflicts of interest or financial disclosures to report.
References
- 1.United States Renal Data System. 2018 USRDS annual data report:Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2018. . [Google Scholar]
- 2.Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJ, Steinberg EP. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12(8):1713–1720. [DOI] [PubMed] [Google Scholar]
- 3.Ku E, Glidden DV, Johansen KL, et al. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney Int. 2015;87(5):1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett BJ. Applying multiple interventions in chronic kidney disease. Semin Dial. 2003;16(2):157–164. [DOI] [PubMed] [Google Scholar]
- 5.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. [DOI] [PubMed] [Google Scholar]
- 6.Park JI, Baek H, Kim BR, Jung HH. Comparison of urine dipstick and albumin:creatinine ratio for chronic kidney disease screening: A population-based study. PLoS One. 2017;12(2):e0171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. [DOI] [PubMed] [Google Scholar]
- 8.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290(23):3101–3114. [DOI] [PubMed] [Google Scholar]
- 9.Komenda P, Ferguson TW, Macdonald K, et al. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis. 2014;63(5):789–797. [DOI] [PubMed] [Google Scholar]
- 10.https://www.ncqa.org/hedis/measures/. Accessed October 1 2018.
- 11.National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/cg182. Accessed December 15 2018.
- 12.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68(3):914–924. [DOI] [PubMed] [Google Scholar]
- 13.Albertus P, Morgenstern H, Robinson B, Saran R. Risk of ESRD in the United States. Am J Kidney Dis. 2016;68(6):862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkova N, McClellan W, Klein M, et al. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19(2):356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuot DS, Grubbs V. Chronic kidney disease care in the US safety net. Adv Chronic Kidney Dis.2015;22(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall YN, Choi AI, Chertow GM, Bindman AB. Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol. 2010;5(5):828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.San Francisco General Hospital and Trauma Center Annual Report, Fiscal Year 2013–2014. Accessed on March 15th 2019: https://www.sfdph.org/dph/files/SFGHdocs/2013-2014-AnnualReport-141105.pdf.
- 19.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adepoju OE, Preston MA, Gonzales G. Health Care Disparities in the Post-Affordable Care Act Era. Am J Public Health. 2015;105 Suppl 5:S665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonetti JA, Fine MJ, Chen YF, Simak D, Hess R. Racial comparisons of diabetes care and intermediate outcomes in a patient-centered medical home. Diabetes Care. 2014;37(4):993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eddy DM, Pawlson LG, Schaaf D, et al. The potential effects of HEDIS performance measures on the quality of care. Health Aff (Millwood). 2008;27(5):1429–1441. [DOI] [PubMed] [Google Scholar]
- 23.Fiscella K, Sanders MR. Racial and Ethnic Disparities in the Quality of Health Care. Annu Rev Public Health. 2016;37:375–394. [DOI] [PubMed] [Google Scholar]
- 24.Lee HM, Ahn J, Kim WR, et al. A Comparison Between Community and Academic Practices in the USA in the Management of Chronic Hepatitis B Patients Receiving Entecavir: Results of the ENUMERATE Study. Dig Dis Sci. 2018. [DOI] [PubMed] [Google Scholar]
- 25.Lacy NL, Paulman A, Reuter MD, Lovejoy B. Why we don’t come: patient perceptions on no- shows. Ann Fam Med. 2004;2(6):541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97(7):1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngo-Metzger Q, Legedza AT, Phillips RS. Asian Americans’ reports of their health care experiences. Results of a national survey. J Gen Intern Med. 2004;19(2):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2014 and 2015–2016.
- 29.National Research Council. 2006. Hispanics and the Future of America. Washingon, DC: The National Academies Press. [PubMed] [Google Scholar]