Abstract
Overeating of highly palatable (HP) foods in the ubiquitous HP food cue environment and under stress is associated with weight gain and contributes to the global obesity epidemic. However, subjective and biobehavioral processes that may increase HP overeating are not clear. Using a novel experimental approach, we examined HP food motivation and intake and neuroendocrine responses in the context of food cues, stress and a control neutral relaxing cue exposure in healthy individuals.
Methods:
Twenty individuals (12M; 8F; ages 18-45) with body mass index (BMI) in the lean (LN: N=8; 3/8 female BMI: 18-24.9) or overweight/obese (OW: N=12; 5/12 female; BMI: 25-37) range were enrolled in a controlled, hospital-based, 3-day laboratory experiment. On each day, subjects were exposed to a brief 5-minute individualized guided imagery of stress, food cue or an active neutral-relaxing control cue script, followed by a food snack test (FST), with one imagery condition per day and order of imagery exposure randomized and counterbalanced across subjects. Subjective HP food craving and caloric intake, anxiety, cortisol and total ghrelin was assessed repeatedly during each test day.
Results:
Significant condition and condition X group effects for food craving, anxiety and HP calorie intake were observed, with food cue relative to neutral condition increasing HP food craving and intake across all subjects (p<.001), but stress relative to neutral condition increased HP food craving and intake in the OW but not LN group (p<. 01). Pre-snack increases in food craving after exposure to food cues and to stress predicted greater subsequent HP food intake (p’s<.01). Furthermore, ghrelin increased in the food cue and stress conditions (p< .01), but stress-induced increases in ghrelin was associated with HP food intake only in the OW/OB condition (p<.01). Finally, cortisol increased during food cue exposure and increased cortisol responses were associated with greater HP food caving and with intake (p’s<.05).
Conclusions:
These findings, while preliminary, validate a laboratory model of HP food motivation and intake and identify specific subjective and neuroendocrine responses that may play a role in HP snacking with implications for weight gain and obesity risk.
Keywords: Food cues, stress, food craving, cortisol, ghrelin, highly palatable food intake
INTRODUCTION
The United States is at the forefront of the global obesity epidemic with 67% of its population classified as overweight or obese (body mass index (BMI) ≥ 25kg/m2) [1-3]. Despite the epidemic and its significant adverse medical sequelae [2, 3], mechanisms driving such increases in obesity are not well understood. Overeating of highly palatable (HP) foods in the ubiquitous HP food cue environment and in stressful life contexts are known to contribute to weight gain and obesity risk [4-6], but underlying processes in these environmental contexts that promote overeating in humans is not well understood.
Highly palatable (HP) foods are more liked, preferred and found to be rewarding in taste relative to less palatable foods. These HP foods include those high in sugar, with sweet taste, highly processed foods high in saturated fats or high carbohydrates making up savory tastes and combination of food groups prepared in ways that enhance taste and value or ‘salience’ of such foods to an individual. These foods are ubiquitous in our current obesogenic environment, and such highly palatable and processed foods and their related associations or‘cues’ stimulate the brain reward and motivation pathways similar to reinforcing drugs of abuse, and via learning/conditioning mechanisms increase the likelihood of HP food seeking and consumption [4, 7-11]. These HP foods are also associated with increased food cravings and intake [12], and consumption of fast foods such as potato chips, processed meats, sugar sweetened beverages all predict long term weight gain in large prospective cohorts of US men and women [13]. Despite such evidence that conditioned properties of HP foods increases greater food craving and promotes their intake, there are few experimental studies that examine the biobehavioral processes that may promote HP food craving and intake.
Stressful environments are also associated with greater food intake [14-16] and chronic stress levels are associated with greater weight gain and obesity risk [6, 17-19]. Stressful experiences may increase demand for energy and promote gluconeogenesis and energy intake, that may also increase risk of higher food craving and intake [20]. Previous experimental studies assessing acute stress exposure effects on food intake have reported variable results [21-25]. While some research shows stress exposure increases high calorie food intake [17, 24, 26], others have shown reduced food intake with acute stress exposure in people with obesity [23, 27]. However, this research varies by a number of experimental factors, including type of acute stressor utilized in the experiment, weight status of the participants, and type of food presented for intake and exposure time for food intake. More importantly, they have largely not addressed motivational status of the individual, i.e., food cravings, and whether stress increases food craving to promote HP food intake.
Building on the previous literature from addiction models, where stress and drug cues each promote drug craving and facilitate risk of increased drug use [18], we conducted a preliminary experimental study to assess whether exposure to acute stress and HP food cues each increase food craving and intake in healthy lean and overweight individuals. We utilized a previously well-validated and extensively utilized individualized guided imagery method [28-35] for inducing stress, food cues and control neutral relaxing state in the laboratory. This was followed by assessment of HP food craving and intake in a food snack test (FST) after each exposure. Furthermore, as both cortisol and ghrelin have been associated with stress and with HP food intake [36, 37], and are also altered by high BMI status [38], these hormones were repeatedly assessed throughout the study. We hypothesized that food cue and stress will each increase HP food cravings relative to the neutral control condition and that such increases in HP food craving will predict subsequent HP food intake. Furthermore, we hypothesized that significant changes in cortisol and ghrelin during food cue and with stress exposure, will be associated with HP food craving and intake.
MATERIALS AND METHODS
Screening and Intake Procedures
Healthy, non-smoking, non-substance using volunteers (12 men; 8 women; aged 18-45) were recruited from a community cohort of volunteers recruited from the greater New Haven area via flyers, radio, and social media advertisements. All volunteers completed medical, demographic, health assessments, including the Structured Clinical Interview for the Diagnostic and Statistical Manual IV-TR [39], Participants were excluded if they were cigarette smokers, had BMI above 38 due to risk of morbid obesity-related adverse health conditions, reported current dieting, met current DSM-IV-TR dependence criteria for any substance use disorders, or current other psychiatric disorders, including current eating disorders, had any current medical conditions and illnesses or if they were taking any medications. From 54 volunteers screened for this study, 16 were ineligible pertaining to these exclusions, while the others declined participation due to time constraints and changing their mind. Participants underwent breath alcohol testing, carbon monoxide testing, and urine toxicology screens to confirm being drug-free at each study appointment. Women who were pregnant, lactating or were on oral contraceptives as well as those who were peri or post-menopausal were also excluded. Participants received a physical examination by the admitting physician (JBG) to ensure all participants were in good health. Participants provided written informed consent and the study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Script Development and Imagery Training
Prior to participation in the experimental procedures, all subjects participated in a structured interview session for imagery script development for the provocation of stress, food cue and control neutral relaxing contexts for the later laboratory experiment, as per our previously validated methods and structured manualized procedures [30, 32, 33, 35, 40]. Briefly, the stress imagery script was based on participants’ descriptions of a recent personally stressful event which made them “sad, mad, or upset, and with little control over the situation in the moment,” and that was experienced as “most stressful.” “Most stressful” was determined by assessing perceived stress on a 10-point Likert scale where 1 = not at all stressful and 10 = the most stress they felt in the past year. Individual calibration of stressful situations was conducted by only accepting situations rated as 8 or above as appropriate for script development. Stressful situations involving food intake or eating contexts were not allowed. The food cue scripts were developed by participants identifying a recent situation that included their favorite HP food-related stimuli and subsequent intake of those HP foods. Favorite food intake situations that were associated with negative affect or psychological distress were not allowed. A neutral-relaxing, non-physiologically arousing, and non-food related script was developed from the participants’ description of a personally relaxing situation to serve as a control condition (See Appendix A for sample imagery scripts).
Imagery Training and Habituation Session:
Participants were also provided with training in progressive relaxation and mental imagery in a 40 minute session prior to experimental sessions on Day 1 of admission as described below. During this session, an Intravenous (IV) heparin-treated catheter was inserted by the research nurse in the antecubital region of the participant’s non-preferred arm for the participant to habituate to any potential stress of the IV insertion. No blood was drawn during this training session. The procedures for the training are outlined in the imagery training manual [40] and in previous work [30]. Briefly the progressive muscle relaxation procedure involved learning to monitor the tension in specific muscle groups by first tensing each muscle group, then releasing the tension, while directing attention towards the differences felt during tension and relaxation.
Experimental Procedures:
Participants were admitted for a three-day, 2-night stay at the Yale Center for Clinical Investigation-Hospital Research Unit (HRU) located at the Yale New Haven Hospital, to ensure a controlled environment during the 3-day experiment. Upon admission on day 1, subjects were trained in progressive muscle relaxation and guided imagery and habituated to the IV insertion, as per our previous work and as outlined in the personalized imagery procedures training manual [40]. On each experimental day, participants were brought into the testing room at 2:00PM, after eating a standard healthy lunch at noon. A heparin-treated catheter was inserted by the research nurse in the antecubital region of the participant’s non-preferred arm. A 45-minute adaptation period followed, after which baseline plasma samples were drawn and subjective anxiety and food craving assessments were conducted before and after a 20 minute progressive relaxation procedure. Next, participants were provided with headphones and exposed to an audio recording of the 5-minute personalized guided imagery script of either their stress, food cue, or neutral-relaxing scenarios, only one condition per day in a randomized and counterbalanced order of presentation across subjects. We instructed participants to: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation.” Immediately following imagery exposure on each day, and after blood draws and assessments, participants were presented with the food snack test (FST) which comprised 4 bowls of HP snacks of one each of chocolate pudding, potato chips, popcorn and mini chocolate chip cookies, and two bowls of healthy snacks of baby carrots and grapes, each portioned to ~500 calories (3000 calories per lab session). The presentation of the FST with HP foods serves as discrete HP food cues presented after imagery to assess effects on food motivation prior to food intake. Participants were then provided with the following instructions: “There are 6 bowls of different snacks on the tray. We will leave these here for the next hour and you can eat as much or as little as you like during the next hour”. All subjects were also videotaped for observed eating behavior coding. During this period, participants were not allowed to watch movies or do other activities but did have access to their phones. At the end of the hour, the tray was removed and the total calories consumed from each bowl of HP and healthy snacks was measured after each session as a measure of behavioral HP food motivation and intake. Food craving, anxiety, bloods draws for cortisol and ghrelin assessments were made at repeated timepoints throughout the session (Figure 1). All research staff were blind to the condition on each day as audiotapes were only marked by day and not by condition, which allowed for all assessments being conducted in a blinded manner.
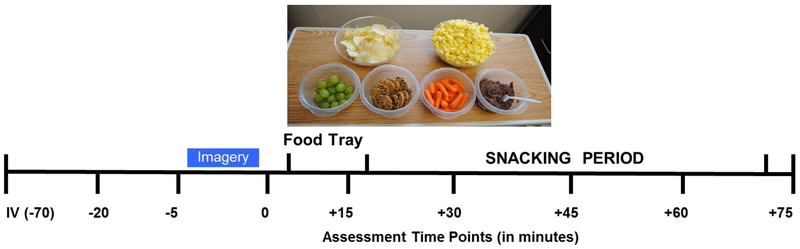
Figure 1:
The 3-day Experimental Schedule of Procedures: At 2:00 pm on each experimental day, the IV was inserted (−70) and after a 45 minute adaptation period at 2:45, the first blood draw and assessment was completed (−20 time point), followed by a standard 15-minute progressive relaxation protocol, after which the second baseline assessment was completed (−5) and instructions for stress (S), food cue (FC) or neutral (N) cue imagery was provided. Immediately following the imagery period, assessments were completed (0), and this was followed by presentation of the food snack tray placed in front of the subject, followed by pre-snacking assessments (+15). After this assessments subjects participated in a snacking period with assessments at +30, +45 and +60 minutes after which the tray was taken away and a final assessment was done at +75 minutes.
Dependent Measures
HP Food Craving
A brief 6-item food craving scale (FCS) was derived from the well-validated Food Craving Inventory (FCI, [41]), and included highly rated select high sugar, carbohydrate and fat items, for repeated assessment of food craving in an experimental setting. The specific FCI items rated included in the laboratory FCS were pizza, brownie, chocolate, cookies, ice cream and steak (on a 7 point scale). A total HP food craving score (sum of ratings) was computed at each timepoint of assessment as shown in Figure 1.
Subjective Anxiety Ratings
Subjective anxiety was rated on a 11-point scale anchored at 0 for “not at all” and 10 for “extremely anxious” measured at each assessment timepoint per session as shown in Figure 1.
Measurement of Cortisol and Ghrelin
Plasma samples (8 mL) were collected at the same 8 consecutive time-points as food craving on each day (Figure 1). All plasma samples were collected in heparinized tubes that were immediately placed on ice after drawing. Within 30 minutes of collection, the blood was centrifuged at 4°C and the plasma was pooled and aliquoted for cortisol and for total ghrelin assays. All tubes were stored at −70°C and analyzed using standard radioimmunoassay procedures as reported previously [19, 31].
Food Snack Test (FST) Calories Consumed:
All snacks were carefully weighed and 500 calories of the snack foods of chocolate pudding, potato chips, popcorn, mini chocolate chip cookies, baby carrots and grapes were portioned into their respective bowls prior to each session as shown in Figure 1. After the one-hour snacking period in each session, the amount of food snack consumed from each bowl was computed and the calories for each food was recorded, with a maximum of 500 calories per bowl for a total of 3000 calories for each laboratory session per day. HP food calories was a sum of the chocolate chip cookies, chocolate pudding, popcorn and potato chips, while the healthy food calories comprised the sum of calories of baby carrots and grapes consumed on each day.
Data Analytic Plan
All statistical analyses were performed using SAS software (SAS, Version 9.3) and all figures were created with GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA).
We used Linear mixed effect (LME) models with a compound symmetry matrix structure for all analyses. The study design included the Between-Subjects factor of BMI [Lean(LN): below 25; Overweight/Obese(OW): 25-28] and Within-Subjects repeated factors, of Condition (Food cue, stress, neutral-relaxing) and Timepoint (baseline before imagery(−5), immediately following imagery(0), pre-snack timepoint prior to FST(+15), and 4 additional timepoints during ((+30, +45, +60) and post (+75) FST). Using these models, we tested the effects of BMI and Condition (food cue, stress relative to neutral cues) on food craving, cortisol, ghrelin and FST intake across time points. LME models in SAS also provide simple effects estimates with corresponding t-tests to deconstruct significant main effects and interactions. The specific contrasts were Stress (S) relative to neutral (N), Food Cue (FC) relative to N, and FC relative to N to deconstruct Condition main effects. In addition, BMI X Condition interaction effects were deconstructed with Condition effects as outlined above for each BMI group, and also for each Condition contrasting BMI groups, i.e., LN: S-N, FC-N, FC-S; OW: S-N, FC-N, FC-S; and LN vs. OW for S, FC and N conditions. In addition, Pearson product-moment correlation analyses were conducted to assess the relationship of pre-snacking food craving, cortisol and ghrelin and amount of HP food consumed during the FST.
RESULTS
The subject sample comprised 12 men and 8 women, with 8 individuals with BMI’s in the lean (LN; 38% women) and 12 in the overweight/obese (OWOB - BMI: 25-38; 42% women) range, with a mean age of 31.35 (SD: 7.39), mean education level of 15.2 years (SD: 2.07), mean IQ of 111.4 (SD:7.9), who were 35% Caucasian, 35% African American, 20% Hispanic and 10% Other. There were no differences between BMI groups on any of these demographic characteristics.
Pre-Snack HP Food Craving (FCS) and Subjective Anxiety Ratings:
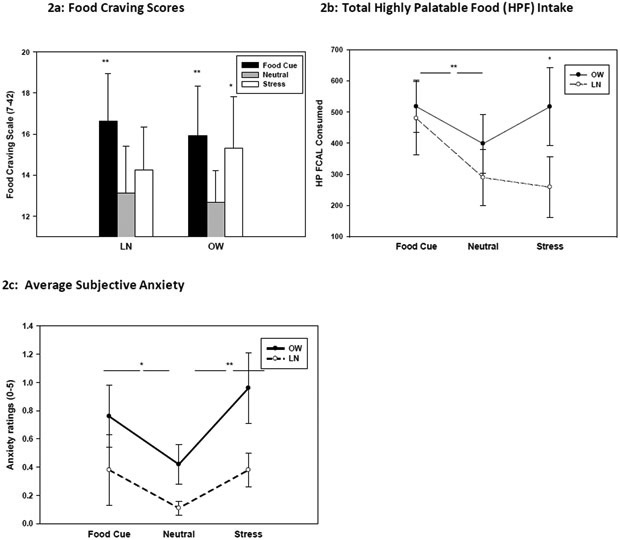
For subjective HP food craving, a significant effect of Condition (F(2,36)=12.74, p<.0001) and BMI Group X Condition (F(2,36)=4.93, p<.02) was observed. Simple effects t-test analyses indicated significantly higher food craving in the food cue relative to neutral (FC > N: t=4.92, p<.0001) and relative to stress(FC>S: t=3.45, p<.001). Simple effects for the BMI X Condition effect revealed the lean (LN) group showing greater craving only in the food cue relative to neutral condition (t=4.66, p<.0001), but the OW group showing food craving increases in both the food cue (t=2.27, p<.04) and in the stress (t=2.01, p<.05) relative to neutral conditions (see Figure 2a).
Figure 2:
Mean and Standard Error bars (SEs) are shown for the Stress (S), Food Cue (FC) and Neutral (N) conditions for individuals with BMI in the Lean (LN) and Overweight/Obese (OW) range. 2a: For HP food craving scale (FCS) with the score range of 7-42, simple effects for Condition Main effect indicated S>N (p<.05) and FC>N (p<.05), and the Group X Condition interaction indicated LN group showing FC>N (p<.0001) only, but OW group showing FC>N (p<.05) and S>N (p<.05). 2b: Mean and SEs for HP food calories (HP FCAL) consumed with a Condition main effect indicating FC>N (p<.01), and a trend for the Group X Condition effect with simple effects analysis showed FC> N across only for the LN (p<.04), and in the Stress Condition, OW>LN (p<.05). 2c: Mean and SEs for anxiety ratings are shown for significant BMI Group X Condition effects. Simple effects showed no significant increases in anxiety in the LN group, but increased anxiety in the FC> N (denoted by *p<.03) and the S>N (denoted by **p<.001) condition in the OW group.
For HP FST intake, a main effect of Condition (F(2,36)=3.86, p<.03) was observed, indicating significantly higher levels of HP food calories consumed in the food cue (t=2.99, p<.01) relative to the neutral condition (Figure 2b). A trend level interaction of BMI Group X Condition (F(2,36)=3.31, p<.08) was also observed, resulting from greater HP food calories (HP FCAL) consumed in the food cue relative to neutral condition for the Lean (t=2.14, p<.04) only, but higher HPF CAL consumed in the stress condition for the OW relative to the LN group (t= 1.91, p<.05) (see Figure 2b).
For subjective anxiety, a significant effect of Condition (F(2,36)=8.7, p<.0008) and BMI Group X Condition (F(2,36)=3.71, p<.03) was observed. Simple effect contrasts revealed overall significantly higher anxiety in the food cue (t=2.02, p<.05) and in stress (t=2.11, p<.04) relative to the neutral condition, but this arose from the people with Lean BMIs showing no significant increases in anxiety across any condition, but the people with OW/OB BMIs showing increased anxiety in the food cue (t=2.10, p<.03) and in the stress (t=4.35, p<.001) relative to the neutral condition (see Figure 2c).
Relationship between Pre-Snack HP Food Craving and HP FST Calorie Consumed:
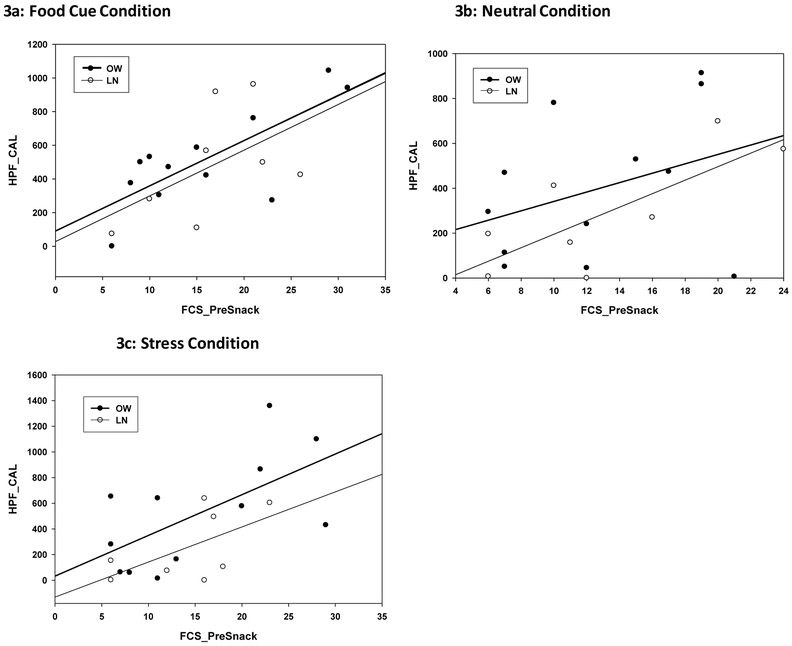
Pre-FST food craving scores (FCS_PreSnack) were found to be highly correlated with subsequent HP food calories (HPF_CAL) consumed in the food cue (r=.82, p<.0001) condition across groups but not in the neutral condition (r=.31, p<.18). On the other hand, stress-induced food craving predicted HP FST intake only in the OW group but not in the Lean group (OW: r=.68, p<.02; LN: r=.55, p<.12) (see Figure 3a - 3c).
Figure 3:
Pearson product moment correlations between the HP food craving pre-snack (FCS_PreSnack) and total HP food calorie consumed (HPF_CAL) in the (3a) food cue condition (r=.82, p<.0001), (3b) neutral (r= 0.31, p<.18) and (3c) stress (OW: r=.68, p<.02; LN: r=.55, p<.12).
Total Ghrelin Response and Relationship to HP Food Craving and Intake
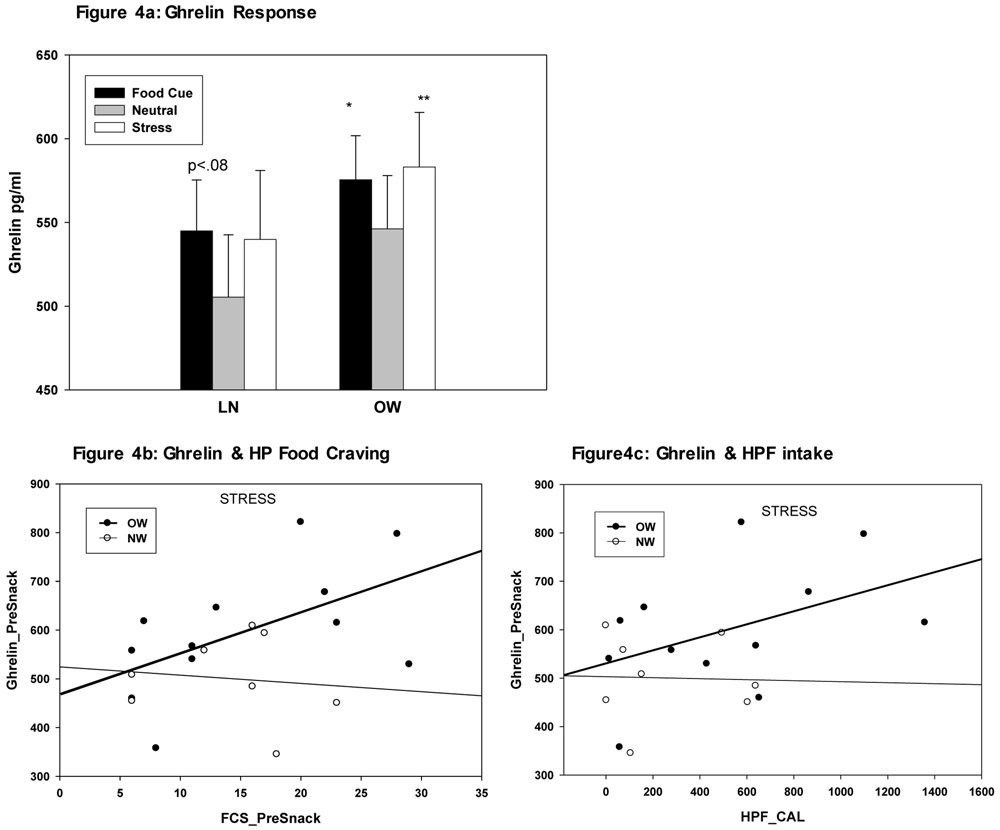
Overall LME analyses revealed a Condition main effect (F(2,35)=5.61, p<.008), but no significant BMI Group X Condition interaction (F(2,35)=0.75, p<.48). The Condition main effect resulted from stress (t=2.88, p<.007) and food cue (t=2.97, p<.005) exposure each increasing ghrelin relative to the neutral condition (see Figure 4a). Secondary exploratory effect of group differences showed that ghrelin increases during both stress and in food cue relative to neutral conditions was due to increases in those in the OW/OB BMI group (FC vs N: t=2.54, p<.02 S vs N: t=3.25, p<.003) and not the lean group (FC vs N: t= 1.78, p<.08; S vs N: t=1.12, p<.27) (see Fig 4a). Association between total ghrelin increases in each condition and food craving and intake were also conducted and revealed that stress-induced, but not food cue-induced increases in ghrelin were significantly associated with HP food craving and intake, and only in the OW group (see Figure 4b - 4c).
Figure 4:
4a: Mean and Standard Error bars (SE) for total ghrelin (pg/ml) by Group and Condition are shown. Condition effect arises from FC>N (p<.007) and S>N (p<.005) for ghrelin responses in each condition relative to the neutral condition. Exploratory analyses indicated that this effect was driven by the OW group showing FC>N (t=2.54, p<.02) and S>N (t=3.25, p<.003), but only a trend in the LN group for the FC>N (t= 1.78, p<.08) contrast. 4b: Significant association in stress-induced increases in Ghrelin Pre-Snack time point and HP food craving responses pre-snack (FCS_PreSnack) only in the OW group (r=.68, p<.01) and not in the lean group (r=.013, p<.75).4c: Significant association in stress-induced increases in Ghrelin Pre-Snack and subsequent HP food intake calories (HPF_CAL) was also observed only in the OW group (r=0.49, p<.05) and not in the lean group (r=.16, p<.70).
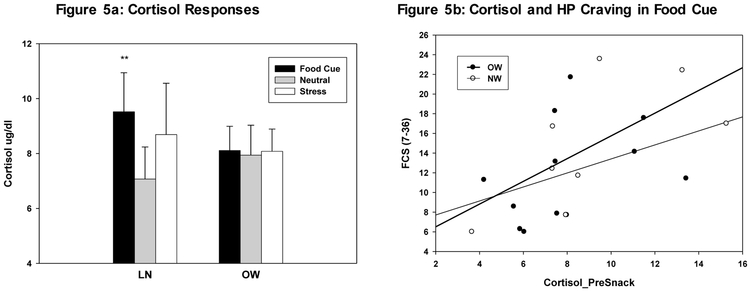
Cortisol Response and Relationship to HP Food Craving and Intake
Overall LME results indicated a Condition main effect (Condition: F(2,35)=3.58, p<.04) for the Cortisol response. Simple effect t tests contrasts indicated significant cortisol increases overall in the food cue relative to the neutral condition (t=2.66, p<.01). However, exploratory analysis by BMI Group indicated that this food cue-related increase in Cortisol was only significant for the Lean (LN) group (t=3.05, p<.004), and no significant differences between conditions were evident in the OW group (see Figure 5a). This overall blunted response in the OW group suggests BMI-related adaptations in cortisol responses. Finally, regarding correlations, cortisol responses during food cue imagery pre-snack was associated with food cue-induced HP craving (r=.54, p<.01) (see Figure 5b).
Figure 5:
Mean and Standard Error bars (SEs) for responses during stress, food cue and neutral conditions. 5a. Increased food cue-induced cortisol responses (p<.01), significant only in the LN (Lean) group (p<.004), and blunted food cue and stress cortisol responses in the OW (Overweight/Obese) group (p=.68). 5b. Significant correlations in the food cue condition between cortisol responses pre-snack (Cortisol_PreSnack) and subsequent HP food caving levels (FCS scores) for the LN and OW groups combined (r=.54, p<.01) was observed.
DISCUSSION
This preliminary study is the first to directly compare the effects of both food cue and stress exposure on HP food craving and HP food intake in a 3-day human laboratory experiment conducted within a controlled hospital-based setting with healthy community adults. As both the ubiquitous HP food environment and stressors are known to increase HP food intake and obesity risk, a direct comparison of these contexts could identify similar and differential processes that may underlie food motivation and intake. As hypothesized, and similar to our previous work in addiction [18], we found that exposure to both personal food cues and to personal stressors relative to a neutral relaxing control cue resulted in increases in HP food craving and subsequent increased snacking of HP foods in a standardized Food Snack Test (FST), but with differential effects in lean and overweight BMI groups. Furthermore, we found that increased food craving in these contexts was highly associated with subsequent amounts of HP food snack intake. Finally, both ghrelin and cortisol were found to significantly impact food motivation but differentially across lean and overweight individuals. Together, these results support the need for further investigation of biobehavioral processes underlying HP food motivation and intake, especially in the context of weight-related physiological adaptations.
Exposure to food cue imagery led to significant increases in HP food craving prior to the presentation of the FST at the pre-snack timepoint in both lean and OW individuals. Moreover, food cue-related increases in food craving predicted significant increases in HP snack intake across both lean and OW individuals. These data are consistent with a significant literature indicating that rewarding stimuli and their related contexts increase motivation and wanting of rewarding stimuli via conditioning and/or incentive salience mechanisms [4, 20, 42]. Also, a number of basic science and human neuroimaging studies have shown that food cue stimulus exposure increases food craving and activate brain reward and motivation regions [4, 7-11, 18, 43, 44]. Current findings further indicate that such food cue-related increases in HP food craving promote HP snack intake with greater intake of HP foods in the food cue context relative to the neutral relaxing contexts across individuals. These data validate the real-world contexts of advertising of HP foods to promote greater sales and intake of HP foods.
In contrast, we found a differential impact of stress on HP food craving and intake by BMI group where stress significantly increased food craving relative to the neutral condition, but only in the overweight and not the lean group. Furthermore, stress-induced HP food craving predicted subsequent HP snack intake in the overweight but not the lean group, suggesting that food motivational processes may be relevant in identifying those who are vulnerable to stress-induced eating. Previous laboratory studies have shown mixed results with some showing stress increasing food intake [17, 24], but others reporting no consistent increases in intake [23, 45]. While the methodology has varied across these studies, current findings suggest the need for direct comparison of BMI groups and consideration of weight-related adaptations in assessing stress effects on HP food motivation and intake. Current findings are also supported by a growing literature suggesting that BMI-related alterations impact not only metabolic hormones but also stress and reward processes that may contribute to increased food motivation and intake [18, 20, 44]. Together our findings on food cue- and stress- related effects on HP food motivation and intake suggests differential effects by BMI, that may in turn, point to different mechanisms that may drive weight gain and obesity risk in individuals who are lean versus those who are overweight.
Our findings also show differential effects of food cue and stress exposure on total ghrelin responses and their contribution to HP food motivation and intake. Stress and food cue exposure increased ghrelin responses, but significantly for the OW and not the LN group. Furthermore, only in the OW group was stress-induced ghrelin increases significantly associated with both HP food craving and subsequent HP food intake. While ghrelin has well known homeostatic effects on feeding function [46, 47], recent focus has been on its role in both stress and mood regulation as well as in hedonically motivated feeding behavior, partially via its effects on mesolimbic reward circuits [37, 48]. Findings indicate that ghrelin can shift preferences to high palatable foods and increase the motivational aspects of food reward [48]. Ghrelin is also known to increase with stress and is involved in regulating anxiety and mood (36, 49-51). Neuroimaging studies have demonstrated that ghrelin administration increases the neural response to food pictures in areas associated with hedonic eating (i.e., amygdala, hippocampus, orbitofrontal cortex, striatum, and ventral tegmental area) [52]. Additionally, there is growing evidence that feed-forward circuitry between stress and food reward maybe partly mediated by ghrelin [52, 53]. In the current study, as the effects were specific to the OW group, they suggest that weight-related adaptations in ghrelin modulated pathways could potentially shift food motivation towards hedonic regulation, especially under stress conditions.
Finally, our findings also showed differential effects of cortisol responses to stress and food cue exposure. Notably, there was a significant increase in cortisol in the food cue relative to the neutral condition that was due to significant increases in cortisol in the lean group and not so in the OW group. Furthermore, food cue-related increases in cortisol was associated with greater food cue-related HP craving, suggesting a role for cortisol in food motivation. While glucocorticoids are often associated with the stress response, they are also known to play an important role in energy intake and regulation. Cortisol increases have been noted with intake of specific macronutrients, including carbohydrates, fats and proteins in animals and humans [6, 54], with the source of cortisol increases to be via both adrenal and extra-adrenal production [54]. Furthermore, in our previous neuroimaging study, increases in cortisol during a mild hypoglycemic state was associated with greater activation of brain reward regions that promoted greater food craving [44]. Together, this previous research supports current findings of the association between greater cortisol responses to food cue exposure with food cue-induced HP food craving.
We did not see an overall significant increase in cortisol during stress exposure, but this was largely due to a lack of response in the OW group. Remarkably, the OW group showed blunted cortisol responses during stress and food cue relative to neutral conditions. This finding is consistent with growing evidence of blunted cortisol and autonomic responses to stress challenge in people who are OW or obese, along with altered autonomic and peripheral catecholamine responses to stress challenge in those weight groups [18, 21-25, 55]. HPA axis adaptations with low cortisol awakening responses have been reported in higher BMI groups, along with blunted responses to stress and reduced dopamine activity [20, 56]. Thus, the current findings of lack of cortisol increases in the OW group leads one to speculate that such HPA axis adaptations may contribute to higher HP food craving and intake in food cue and in stress contexts. However, this is speculation and would need further testing in future studies.
Several limitations of the current study need to be considered. First, the sample size was small and thus the findings are preliminary and need further validation. Also, due to the small sample size, sex differences and differential responses within the overweight groups were not assessed. It is possible that men and women differ in their food motivation and intake responses in food cue and stress contexts as shown in previous work [26,27]. Also, continuous assessment of BMI rather than arbitrary cutoffs for individuals in the lean, overweight and obese BMI range may provide greater sensitivity in assessing weight related adaptations. Thus, research with larger samples is required to replicate current findings and address these issues. Finally, as this study was conducted while participants were in a hospital, it is not clear whether the findings are generalizable to an outpatient experimental context or to real world food motivation and intake settings. On the other hand, as food cues and stress are among the most common contexts in which overeating of HP foods occur in the real world, the current study presents a well-controlled experimental model to study the biobehavioral processes that may contribute to increased food motivation and intake of highly palatable foods. Evidence of HP food motivation and increased snacking in these contexts is consistent with reports of obesogenic environments promoting increased HP food intake and weight gain in the real world. Furthermore, positive associations of HP food craving and intake by total ghrelin and cortisol responses suggests a role for metabolic and neuroendocrine adaptations in increased HP food motivation and intake.
HIGHLIGHTS:
This paper directly compares the effects of both food cue and stress exposure on highly palatable food craving and intake in a 3-day human laboratory experiment conducted within a controlled hospital-based setting with healthy community adults.
Results show that food cues and stress exposure increases food craving and subsequent snacking of highly palatable foods, and food cravings predict subsequent highly palatable food intake.
Ghrelin and cortisol hormones appear to play a role in food cue and stress related food motivation and intake.
These findings suggest the need for further carefully controlled studies to understand the biobehavioral processes that drive overeating and weight gain.
Acknowledgements:
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health grant R01-DK099039 and the National Institutes of Health Roadmap for Medical Research Common Fund Grants UL1-DE019586, UL1-RR024139 (Yale Clinical and Translational Science Award), and the PL1-DA024859. Dr. Sinha is on the Scientific Advisory Board for Embera Neurotherapeutics.
Appendix A
Sample Stress, Favorite Food Cue, and Neutral Imagery Scripts
Stress:
Its 6:00 on an October evening. You've just come home from the city. You see Monique* sitting on the couch, watching TV. Your heart beats faster. You look at her face and feel disgusted. You tense the muscles in your face and forehead. "What am I gonna say to her?" you think. You don’t even want to be there. You clench your fists. You feel like you just have to get this over with. “I can’t take this anymore, you say to her, “I think its time you looked for an apartment.” Your palms feel sweaty. You see her face look sad and then mad. Your stomach is in a knot. You can’t stand to even look at her. Your heart pounds. You have been staying out all night so you don’t have to see her. This has to end. You tense the muscles in your neck and shoulders. “I don’t wanna be here if you don’t want me to be here,” she says. You grit your teeth. You can’t understand why she can’t pay the $300 for her half of the bills. She has a job- why can’t she help you out? It’s like she is a kid and you have to be the parent. The thoughts are racing through your head. You want to scream or hit some one. You’re just spendin’, spendin’, spendin’ and she’s free loading. You tense the muscles all over your body. You are so pissed off at her. Your head is pounding. Maybe she’s not paying the bills because she’s saving up to leave me, you think. Has she been using me all this time? You start getting worried. You feel hot all over. How could you have thought she might even be your future wife? You feel choked up. After all this time, she doesn’t even care about what you think. You feel betrayed and alone. You feel like crying. Monique says, okay, I guess you are right. She doesn’t even try to make it right with you. It’s really over now. How could she do this to you? Tears come to your eyes. You feel empty, drained, and hollow. There is a deep intense pain sensation inside you. You just want to get away, away from her and all these awful feelings inside you. It hurts to be alive.
Favorite Food Cue:
It is a warm Spring evening in May. You just got home from school and are hanging out with David. You think about eating sundaes from Ashley’s and watching 90210. Your heart skips a beat. “Want to get sundaes from Ashley’s?” you ask David. “Yes!” he says. Your heart quickens. You think about what kind of ice cream you want. They have so many good flavors there. There are butterflies in your stomach. You and David get in the car and you drive to Ashley’s. Something chocolate would be really good right now. Your mouth waters. You walk into Ashley’s. It is crowded. Your eyes scan the selection of ice cream as you wait. You tense the muscles in your face and forehead. You see a chocolate raspberry flavor. You’ve never had that before. Your heart quickens. “That sounds really good,” you think. You feel a sense of excitement inside of you. You listen as David orders his mint chocolate chip. Mint chocolate chip sounds really good, too! Your heart beats faster. Now it is your turn to order. You want one of everything! You order mint chocolate chip and chocolate raspberry with peanut butter on top and snow caps. There are butterflies in your stomach. You watch as the server scoops in the ice cream, then pours on the peanut butter topping and snow caps. It looks so good. You haven’t had snow caps in so long. You think about the sweet chocolate taste. Now your mouth is really watering. You pay and take the bag of sundaes. You hurry home, eager to try your ice cream. You walk into your house and put the bag in the freezer. You go into the living room and turn on the TV. You get the show ready. You look up and see David coming in the living room with the sundaes. Your heart beats faster. He hands you your sundae. You take the lid off the cup and look at the chocolate raspberry and mint chocolate chip ice cream and all the peanut butter and snow caps toppings. Your mouth waters. You scoop up a big bite, aiming to get a little of everything into the spoon. You raise the spoon to your lips. You take a big bite, tasting the mint, chocolate raspberry, peanut butter, and snow caps. It tastes so good. You are ready for another bite. Before you know it, you scoop up another big spoonful and bring it to your lips.
Neutral/Relaxing:
It is 11am on a cool Spring morning. You are walking on Neck Road in Clinton. You breathe in deeply as you walk. The area is calm. You feel a general sense of release as you slowly exhale. You walk slowly down the road, listening to the soft crunch of gravel below your feet. You look up and take in the large colonial houses along the road. Your eyes scan the houses, noticing all the different colors - white, grey, yellow and blue. Your eyes follow the landscaping, noticing the green grass and plants in front of each home. You come upon a creek running in between two properties. You pause for a moment. You listen to the rhythmic sound of water running through the creek. You take in a deep breath. You look up to the sky, stretching the muscles in your neck and shoulders. Your eyes follow the tree branches into the sky. You notice the different patterns the branches make against the blue sky. You watch as a few fluffy white clouds move across the sky, in and out of the trees. You smile and look back down at the road. You begin to walk again. A soft gentle breeze blows across your face and body. The wind feels nice against your neck and face. You take a few deep breaths. You feel a sense of lightness, buoyancy, and upsurge of your body. As you continue to walk, you reach your arms out in front of you and stretch the muscles in your shoulders and back. You feel your muscles becoming more and more relaxed. You feel comfortable and at ease. You feel a release of tension in your whole body. Your heart beats slower. Your breathing slows down and all your worry thoughts seem to fade away. You wish you could stay on this walk and enjoy this feeling forever. You want to hold time and capture this moment. A feeling of peace comes over you.
*Note: Names and identifying information have been changed throughout the scripts to protect participant confidentiality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.World Health Organization (2011): Obesity and Overweight Fact Sheet no. 311. [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL (2016): Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Fryar CD, Flegal KM (2015): Prevalence of Obesity Among Adults and Youth: United States, 2011-2014. NCHS Data Brief.1–8. [PubMed] [Google Scholar]
- 4.Berthoud HR (2012): The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc.1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ (2009): Psychosocial stress and change in weight among US adults. Am J Epidemiol. 170:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallman MF (2010): Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 21:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weingarten HP (1983): Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 220:431–433. [DOI] [PubMed] [Google Scholar]
- 8.Alsio J, Olszewski PK, Levine AS, Schioth HB (2012): Feed-forward mechanisms: Addiction-like behavioral and molecular adaptations in overeating. Front Neuroendocrinol. 33:127–139. [DOI] [PubMed] [Google Scholar]
- 9.Lutter M, Nestler EJ (2009): Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 139:629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avena NM, Rada P, Hoebel BG (2009): Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 139:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coelho JS, Jansen A, Roefs A, Nederkoorn C (2009): Eating behavior in response to food-cue exposure: examining the cue-reactivity and counteractive-control models. Psychol Addict Behav. 23:131–139. [DOI] [PubMed] [Google Scholar]
- 12.Chao A, Grilo CM, White MA, Sinha R (2014): Food cravings, food intake, and weight status in a community-based sample. Eat Behav. 15:478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB (2011): Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 364:2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greeno CG, Wing RR (1994): Stress-induced eating. Psychol Bull. 115:444–464. [DOI] [PubMed] [Google Scholar]
- 15.Adam T, Epel E (2007): Stress, eating and the reward system. Physiol Behav. 91:449–458. [DOI] [PubMed] [Google Scholar]
- 16.Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ (2008): Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiol Behav. 94:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epel E, Lapidus R, McEwen B, Brownell K (2001): Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 26:37–49. [DOI] [PubMed] [Google Scholar]
- 18.Sinha R, Jastreboff AM (2013): Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 73:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao AM, Jastreboff AM, White MA, Grilo CM, Sinha R (2017): Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity (Silver Spring). 25:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha R (2018): Role of addiction and stress neurobiology on food intake and obesity. Biol Psychol. 131:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keltikangas-Jarvinen L, Ravaja N, Raikkonen K, Lyytinen H (1996): Insulin resistance syndrome and autonomically mediated physiological responses to experimentally induced mental stress in adolescent boys. Metabolism. 45:614–621. [DOI] [PubMed] [Google Scholar]
- 22.Benson S, Arck PC, Tan S, Mann K, Hahn S, Janssen OE, et al. (2009): Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology. 34:181–189. [DOI] [PubMed] [Google Scholar]
- 23.Appelhans BM, Pagoto SL, Peters EN, Spring BJ (2010): HPA axis response to stress predicts short-term snack intake in obese women. Appetite. 54:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyrka AR, Walters OC, Price LH, Anderson GM, Carpenter LL (2012): Altered response to neuroendocrine challenge linked to indices of the metabolic syndrome in healthy adults. Horm Metab Res. 44:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillman JB, Dorn LD, Loucks TL, Berga SL (2012): Obesity and the hypothalamic-pituitary-adrenal axis in adolescent girls. Metabolism. 61:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, et al. (2006): Food selection changes under stress. Physiol Behav. 87:789–793. [DOI] [PubMed] [Google Scholar]
- 27.Zellner DA, Saito S, Gonzalez J (2007): The effect of stress on men's food selection. Appetite. 49:696–699. [DOI] [PubMed] [Google Scholar]
- 28.Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ (2003): Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl). 170:62–72. [DOI] [PubMed] [Google Scholar]
- 29.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006): Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 63:324–331. [DOI] [PubMed] [Google Scholar]
- 30.Sinha R (2009): Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 14:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R (2013): Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 70:727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jastreboff AM, Potenza MN, Lacadie C, Hong KA, Sherwin RS, Sinha R (2011): Body mass index, metabolic factors, and striatal activation during stressful and neutral-relaxing states: an FMRI study. Neuropsychopharmacology. 36:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN (2013): Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care. 36:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jastreboff AM, Sinha R, Lacadie CM, Balodis IM, Sherwin R, Potenza MN (2015): Blunted striatal responses to favorite-food cues in smokers. Drug Alcohol Depend. 146:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yip SW, Lacadie CM, Sinha R, Mayes LC, Potenza MN (2016): Prenatal cocaine exposure, illicit-substance use and stress and craving processes during adolescence. Drug Alcohol Depend. 158:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang JC, Zigman JM (2010): Ghrelin's Roles in Stress, Mood, and Anxiety Regulation. Int J Pept. 2010, pii: 460549. Epub 2010 February 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, et al. (2011): Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 121:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Name M, Giannini C, Santoro N, Jastreboff AM, Kubat J, Li F, et al. (2015): Blunted suppression of acyl-ghrelin in response to fructose ingestion in obese adolescents: the role of insulin resistance. Obesity (Silver Spring). 23:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JB (1996): Structured clinical interview for DSM-IV Axis I disorders patient edition. [DOI] [PubMed] [Google Scholar]
- 40.Sinha R, Tuit K (2012): Imagery Script Development Procedures Manual. CreateSpace Independent Publishing [Google Scholar]
- 41.White MA, Grilo CM (2005): Psychometric properties of the Food Craving Inventory among obese patients with binge eating disorder. Eat Behav. 6:239–245. [DOI] [PubMed] [Google Scholar]
- 42.Robinson TE, Berridge KC (2008): Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 363:3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jastreboff AM, Lacadie C, Seo D, Kubat J, Van Name MA, Giannini C, et al. (2014): Leptin is associated with exaggerated brain reward and emotion responses to food images in adolescent obesity. Diabetes Care. 37:3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, et al. (2011): Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 121:4161–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appelhans BM, Whited MC, Schneider KL, Oleski J, Pagoto SL (2011): Response style and vulnerability to anger-induced eating in obese adults. Eat Behav. 12:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. (2001): A role for ghrelin in the central regulation of feeding. Nature. 409:194–198. [DOI] [PubMed] [Google Scholar]
- 47.Castaneda TR, Tong J, Datta R, Culler M, Tschop MH (2010): Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 31:44–60. [DOI] [PubMed] [Google Scholar]
- 48.Schmid DA, Held K, Ising M, Uhr M, Weikel JC, Steiger A (2005): Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology. 30:1187–1192. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr.(1992): Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 13:387–414. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, et al. (1996): Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 45:531–535. [DOI] [PubMed] [Google Scholar]
- 51.Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR (2005): Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol. 289:R353–R358. [DOI] [PubMed] [Google Scholar]
- 52.Malik S, McGlone F, Bedrossian D, Dagher A (2008): Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 7:400–409. [DOI] [PubMed] [Google Scholar]
- 53.Schellekens H, Finger BC, Dinan TG, Cryan JF (2012): Ghrelin signalling and obesity: at the interface of stress, mood and food reward. Pharmacol Ther. 135:316–326. [DOI] [PubMed] [Google Scholar]
- 54.Stimson RH, Mohd-Shukri NA, Bolton JL, Andrew R, Reynolds RM, Walker BR (2014): The postprandial rise in plasma cortisol in men is mediated by macronutrient-specific stimulation of adrenal and extra-adrenal cortisol production. J Clin Endocrinol Metab. 99:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Packard AE, Egan AE, Ulrich-Lai YM (2016): HPA Axis Interactions with Behavioral Systems. Compr Physiol. 6:1897–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang GJ, Volkow ND, Thanos PK, Fowler JS (2004): Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 23:39–53. [DOI] [PubMed] [Google Scholar]