Abstract
Background:
We examined the association between administration of antineoplastic drugs (AD) and fecundity among female nurses.
Methods:
AD administration and use of exposure controls (EC) such as gloves, gowns, and needleless systems, were self-reported at baseline among 2,649 participants of the Nurses’ Health Study 3 who were actively attempting pregnancy. Every 6 months thereafter, nurses reported the current duration of their pregnancy attempt. Multivariable accelerated failure time models were used to estimate time ratios (TR) and 95% confidence intervals (CI) adjusted for age, race, body mass index (BMI), smoking, marital status, hours of work, and other occupational risk factors.
Results:
Mean (standard deviation) age and BMI at baseline were 30.7 years (4.7) and 26.0 kg/m2 (6.4). Forty-one percent of nurses reported ever administering AD; 30% only in the past and 11% currently. Former administration of AD (TR=1.02, 95% CI 0.93–1.12) was unrelated to the ongoing duration of pregnancy attempt. Among nurses currently administering AD, those who had administered AD for ≥6 years had a 27% (95% CI 6%−53%) longer duration of pregnancy attempt than nurses who never handled ADs in unadjusted analyses. This difference disappeared in multivariable analyses (TR=1.01, 95% CI 0.85–1.21). 93% (n=270) of the nurses currently administering ADs reported consistent use of EC. These nurses had a similar median duration of pregnancy attempt to those who never handled AD (TR=1.00, 95% CI 0.87–1.15).
Conclusions:
Administration of ADs did not appear to have an impact on fecundity in a cohort of nurses planning for pregnancy with a high prevalence of consistent ECs. Our results may not be generalizable to women who are less compliant with PPE use or with less availability to ECs. Therefore, it is possible that we did not observe an association between occupational exposure to AD and reduced fecundity because of lower exposure due to more prevalent use of effective ECs.
Key terms: antineoplastic drugs, time to pregnancy, occupational exposure, nurses, reproductive health, pregnancy, protective equipment, Nurses’ Health Study
1. Introduction
Antineoplastic drugs (ADs) are highly toxic agents with known and well-documented reproductive toxicity for patients receiving them as treatment1. Most workplaces where ADs are handled are contaminated with ADs and numerous studies have demonstrated worker exposure to these drugs2–5. Healthcare workers could be exposed to ADs by preparing or administering ADs; working in areas where ADs are used through contaminated work surfaces, drug vials, containers, clothing, or medical equipment; and in patient excreta and other secretions such as urine, feces, and sweat6. Healthcare workers with chronic, long-term exposure to low levels of ADs appear to have an increased risk of adverse reproductive outcomes6. Furthermore, there are no established occupational exposure limits for ADs and it is not possible to establish low-risk exposure levels for ADs because the carcinogenic, mutagenic and reproductive toxic effects are not dependent on a minimum dosage7. Therefore, nurses of reproductive age who administer these drugs could be specifically at risk because of the documented reproductive toxicity of these drugs.
Previous studies on occupational exposure to ADs have reported associations with fetal loss, spontaneous abortion, and malformations in the offspring6. Particularly, the association with fecundity as measured by time to pregnancy is understudied. To our knowledge, there is only one study8 that investigated the association between occupational exposure to ADs and time to pregnancy. Fransman et al.8 reported that Dutch nurses who were highly exposed to ADs had longer duration of pregnancy attempt than the nurses with no exposure. Because this study investigated reproductive outcomes collected retrospectively up to seven years after the pregnancy attempt, they could not exclude the possibility that differential recall of time to pregnancy might explain their findings.
To further evaluate the potential reproductive effects of occupational exposure to ADs, we evaluated the association between administration of ADs and fecundity as measured by time to pregnancy among female nurses in a large cohort study. We also aimed to assess whether duration, frequency, physical form of ADs, and exposure control (EC) use modify this association. We hypothesized that unprotected administration of ADs would be related to longer time to pregnancy among nurses.
2. Methods
2.1. Study population
The Nurses’ Health Study 3 (NHS3) is an ongoing, internet-based prospective cohort study of nurses in the United States and Canada which started recruitment in 2010 9. Female nurses (registered nurses, licensed practical/vocational nurses or nursing students) born on or after January 1st, 1965 were eligible to participate. As of October 15th, 2017, 44,077 female nurses had joined the study. Every six months, questionnaires are sent to participants to update information on lifestyle and medical characteristics. The response rate for the first follow-up questionnaire is 71%; for women who have completed ≥2 questionnaires, response rates range between 78 and 88%. Nurses were excluded from this analysis if they reported being postmenopausal (n=825), not employed outside the home during the last year on their baseline questionnaire (n=7,493), and not trying to get pregnant on any subsequent questionnaire (n=31,469) (Supplemental Figure I). From the 2,659 nurses who met these criteria, we excluded those who failed to report the duration of their ongoing pregnancy attempt (n=6) or failed to provide information on occupational exposure to ADs (n=4), leaving 2,649 nurses eligible for analysis.
2.2. Exposure assessment
The baseline questionnaire collects information about selected occupational exposures including ADs, ionizing radiation, aerosolized drugs, high-level disinfectants, and anesthetic gases, as well as work schedule, and heavy lifting. All eligible nurses were first asked “have you ever administered antineoplastic agents to patients? (Other terms used for antineoplastic agents include chemotherapeutic drugs, cytotoxic drugs and anticancer drugs.)?” Participants who indicate having administered ADs are then asked how long during their career they had been administering ADs (< 6 months, ≥6 and < 12 months, 1–5 years, 6–10 years, 11–20 years, or > 20 years), and how much total time was spent handling ADs over an average week in the past month (1, 2–3, 4–5, 6–10, or >10 times/ week). Additional questions on AD administration were added to the baseline questionnaire in 2012 (and were thus not asked of all participants), which included if administration was via infusion, pills, or both; and if the pills were usually crushed. Nurses reporting administering ADs in the past month were also asked to report how often they used engineering controls and personal protective equipment (PPE), collectively referred to as exposure controls (ECs) in this paper. Engineering controls included: use of a designated room or area, drug delivery system with Luer-lock (or other similar type) fittings, absorbent pads, and needleless systems. PPE included chemotherapy or latex gloves, water resistant gown or outer garment with closed front and tight cuffs, and eye protection (safety glasses, goggles, face shield). Response categories for EC use were “always”, “sometimes”, and “never”. We combined “sometimes” and “never” responses due to low frequency. To assess effect modification by EC use, we further cross-classified nurses currently administering ADs according to “never or sometimes use” and “always use” for EC. We also further subcategorized the “always use” according to the type of ECs individually that were always used and the physical form of the ADs.
2.3. Outcome assessment
Nurses were asked if they were currently pregnant or actively trying to become pregnant in each follow-up questionnaire. Those who reported actively trying to get pregnant were asked to report the current duration of their ongoing pregnancy attempt. Specifically, they were asked: “For how many months have you been actively trying to get pregnant?” Response categories included: ≤1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12 months, 1–2 years, and ≥3 years. We used a respondent’s first report of ongoing pregnancy attempt after the baseline questionnaire as the outcome. As such, the majority of current durations were reported on questionnaire 2 (51%) followed by questionnaire 3 (16%), questionnaire 4 (13%), questionnaire 5 (11%), and questionnaire 6 (9%). Self-report of duration of pregnancy attempt is considered a valid methodology for assessment of fecundity among pregnancy planners10,11.
2.4. Covariate assessment
We obtained information on potential confounding variables from the baseline questionnaire. We considered age, body mass index (BMI) based on self-reported weight and height, race/ethnicity, highest nursing degree, pregnancy history, smoking, marital status, and other occupational exposures including frequency of nursing work, frequency of lifting or moving a heavy load, and current exposure at baseline to ionizing radiation, high-level disinfectants, anesthetic gases, and aerosolized drugs.
2.5. Statistical analysis
Nurses were classified into three categories of AD administration: “never”, “administration prior to baseline”, and “current administration at baseline”. We tested for any differences in demographic, lifestyle, and reproductive characteristics across categories of AD administration using chi-square tests (or Fisher’s exact test where appropriate) for categorical variables and Kruskal-Wallis tests for continuous variables. Nurses reporting current administration of ADs were further classified according to physical form [pills, liquid (with or without pill) or not reported] of ADs administered, duration (<1, 1–5, or ≥ 6 years), and frequency (once/week or ≥ 2 times/week), and cross-classified according to use of EC.
We used the current duration approach in which information on current duration of ongoing pregnancy attempt collected cross-sectionally are used to make inferences about actually realized waiting times to pregnancy (TTP)12. This approach has been used before to assess fecundity13,14. We used accelerated failure time models with log normal distribution to estimate time ratios (TR) and 95% confidence intervals (95% CI) as a measure of relative fecundity14. TRs can be interpreted as the ratio of the median duration of pregnancy attempts between the compared groups, a TR greater than 1 indicates longer time to pregnancy and lower fecundity.
Models were adjusted for a priori selected demographic and occupational variables including age, race, BMI, smoking status, marital status, work hours per week, frequency of lifting or moving a heavy load, and current exposure at baseline to ionizing radiation, high-level disinfectants, anesthetic gases, and aerosolized drugs. We did not include the pregnancy history in the main analysis to avoid over-adjustment if ongoing work characteristics are related to the inability to get pregnant, which could manifest as nulligravidity15,16 but we included it in a sensitivity analysis. To assess the robustness of the results to modeling assumptions we conducted additional sensitivity analyses which included using a generalized gamma distribution for the outcome, restricting the analysis to nurses with current durations of pregnancy attempt reported within two years of the baseline exposure assessment, truncating the durations of pregnancy attempt at 12 months, and fitting models without adjusting for the other occupational exposures and further adjusting for the typical work schedule. We also conducted a sensitivity analysis using finer categories for the frequency of ADs administration. We explored effect modification by age (<37 or ≥37 yrs), BMI (<25 or ≥25 kg/m2), smoking status (never versus ever smokers), and gravidity (nulligravid versus gravid) by adding cross-product interaction terms in the final multivariable model. Data were analyzed using SAS 9.4 (SAS Institute, Inc., Cary, N.C.) and a significance level of P <0.05.
3. Results
The estimated proportions of women not pregnant after 12 and 24 months were 13% and 4%, respectively. Forty-one percent (n=1,077) of nurses reported ever administering ADs in their careers with 30% (n=786) only in the past and 11% (n=291) currently at baseline. The mean (standard deviation) age and BMI at baseline were 30.7 years (4.7) and 26.0 kg/m2 (6.4), respectively. On average, nurses who administered ADs prior to baseline were slightly older, had lower BMI, worked more hours, lifted heavy objects more frequently, and were slightly less likely to be nulligravid than nurses who never administered ADs (Table I). Nurses who administered ADs currently at baseline worked more hours, lifted heavy objects more frequently, and were more likely to be married and nulligravid than nurses who never administered ADs (Table I). 20.4% of the nurses who administered ADs currently at baseline were working in night shifts only.
Table I.
Age-standardized* baseline demographic and occupational characteristics of nurses attempting pregnancy according to antineoplastic drug administration (n=2,649).
| Never | Yes, prior to baseline | Yes, at baseline | |
|---|---|---|---|
| (n=1,572 nurses) | (n=786 nurses) | (n=291 nurses) | |
| Age, yrsa,b | 30.4 (4.7) | 31.2(4.6) | 30.5 (4.8) |
| BMI, kg/m2 | 26.1 (6.7) | 25.6 (6.1) | 26.2 (6.1) |
| Hispanic ethnicity, % | 4.7 | 3.2 | 3.9 |
| Race, % | |||
| White | 91.9 | 92.2 | 89.4 |
| Black | 1.4 | 2.5 | 2.5 |
| Asian | 2.2 | 2.6 | 3.4 |
| Smoking status, % | |||
| Never smoker | 79.2 | 78.3 | 77.8 |
| Former smoker | 4.5 | 3.1 | 4.7 |
| Current smoker | 16.3 | 18.4 | 17.5 |
| Married, % | 65.0 | 64.7 | 68.8 |
| Nulligravidity, % | 61.1 | 59.6 | 62.2 |
| Highest nursing degree, %b | |||
| PhD | 1.5 | 0.9 | 1.6 |
| MS | 23.1 | 22.1 | 13.1 |
| RN or B.S.N. | 69.9 | 74.8 | 82.9 |
| LPN/LVN | 2.6 | 0.4 | 1.7 |
| Associate’s degree | 1.0 | 1.3 | 0.7 |
| Nursing student | 1.9 | 0.4 | 0 |
| History of Thyroid disease | 1.1 | 0.8 | 0 |
| History of Diabetes | 1.0 | 1.0 | 1.0 |
| Typical work schedule, %b | |||
| Days only | 57.1 | 57.8 | 46.8 |
| Evenings only | 5.0 | 5.9 | 5.0 |
| Nights only | 17.4 | 16.4 | 20.4 |
| Rotating with nights | 16.0 | 15.9 | 24.5 |
| Rotating no nights | 4.4 | 4.1 | 3.2 |
| Hours/ week of nursing work, %b | |||
| 1–20 hrs/week | 9.5 | 6.2 | 1.9 |
| 21–40 hrs/week | 65.6 | 68.0 | 66.1 |
| > 40 hrs/week | 25.0 | 25.8 | 32.0 |
| Frequency of occupational moving or lifting heavy loads, %b | |||
| None | 34.6 | 28.0 | 13.6 |
| 1–5 times/day | 39.3 | 41.1 | 47.7 |
| 6–15 times/day | 20.6 | 24.2 | 30.5 |
| >15 times/day | 5.5 | 6.7 | 8.1 |
| Current co-exposures, % | |||
| High-level disinfectantsb | 17.5 | 20.1 | 27.2 |
| Ionizing radiationb | 5.3 | 6.9 | 9.5 |
| Aerosolized drugsb | 0.9 | 0.8 | 3.2 |
| Anesthetic gasesb | 11.5 | 7.9 | 5.3 |
| Menstrual cycle length, % | |||
| ≤ 25 days | 12.3 | 11.7 | 8.0 |
| 26–39 days | 86.6 | 88.2 | 91.6 |
| ≥ 40 days | 1.1 | 0.2 | 0.4 |
| Regular menstrual cycles, % | 82.1 | 78.7 | 80.3 |
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
High-level disinfectants included glutaraldehyde, orthophthalaldehyde, peracetic acid, and hydrogen peroxide.
Values for age are not age-adjusted.
There was statistically significant differences between categories.
Abbreviations: n, number of nurses; BMI, body mass index; BSN, Bachelor of Science in nursing; LPN, licensed practical nurse; LVN, licensed vocational nurse; MS, masters of Science; PhD, doctorate of philosophy; RN, registered nurse.
Of the nurses who had ever administered ADs (n=1,077), 507 (47%) had done so for 1 to 5 years. Among nurses for whom we had information about the physical form of the ADs administered (n=302), 52% handled only pills and 48% handled liquids with or without pills. The overall prevalence of the consistent use of each individual exposure control ranged from 17% for eye protection to 83% for gloves (Supplemental Table I). Most (93%) nurses consistently used some type of EC (Supplemental Table II) and most often, this EC was gloves. Use of ECs was high regardless of frequency of AD administration. Among women who used just one EC, 79% used gloves only and among women using two or more EC, 91% used gloves (Supplemental Table II).
Current, past, or ever administration of ADs was not associated with the ongoing duration of pregnancy attempt in unadjusted or adjusted analyses (Table II). When duration of AD administration was examined, nurses who currently administered ADs and had been doing so for ≥6 years had a 27% (95% CI: 6%−53%) longer duration of pregnancy attempt than nurses who never handled ADs. However, this difference disappeared after adjusting for age (TR=1.01, 95% CI: 0.85–1.21); multivariable adjusted analyses showed no association either (Table II). Among nurses currently administering ADs, frequency of administering ADs over the past month and the physical form of AD administration were unrelated to current duration of pregnancy attempt in all analyses (Table II).
Table II.
Association of occupational administration of antineoplastic drugs (ADs) with fecundity among women attempting pregnancy (n=2,649).
| n (%) | Median Pregnancy Attempt in Months [IQR] | Unadjusted TR | (95%CI) | Adjusted TR* | (95%CI) | |
|---|---|---|---|---|---|---|
| Ever administered AD | ||||||
| Never administered | 1572 (59.3) | 3[1,7] | 1(REF) | - | 1(REF) | - |
| Yes | 1077 (40.7) | 3[1,9] | 1.06 | 0.97–1.16 | 1.01 | 0.93–1.09 |
| Ever administered AD | ||||||
| Never administered | 1572 (59.3) | 3[1,7] | 1(REF) | - | 1(REF) | - |
| Yes, prior to baseline | 786 (29.7) | 3[1,9] | 1.08 | 0.98–1.18 | 1.02 | 0.93–1.12 |
| Yes, at baseline | 291 (11.0) | 3[1,8] | 1.02 | 0.89–1.17 | 0.98 | 0.85–1.12 |
| Baseline administered AD | ||||||
| Not at baseline (Never or prior) | 2358 (89.0) | 3[1,8] | 1(REF) | - | 1(REF) | - |
| Yes, at baseline | 291 (11.0) | 3[1,8] | 1.00 | 0.87–1.14 | 0.97 | 0.85–1.10 |
| Duration of AD administration | ||||||
| Never administered | 1572 (59.3) | 3[1,7] | 1(REF) | - | 1(REF) | - |
| <1year | 415(15.7) | 3[1,10] | 1.10 | 0.98–1.24 | 1.08 | 0.96–1.21 |
| 1–5years | 507 (19.1) | 3[1,8] | 0.97 | 0.87–1.09 | 0.95 | 0.85–1.06 |
| 6+years | 155 (5.9) | 4[1,12] | 1.27 | 1.06–1.53 | 1.01 | 0.85–1.21 |
| Frequency of AD administration in past month | ||||||
| Never administered | 1572 (59.3) | 3[1,7] | 1(REF) | - | 1(REF) | - |
| Yes, prior to baseline | 786 (29.7) | 3[1,9] | 1.08 | 0.98–1.18 | 1.02 | 0.93–1.12 |
| 1 time/week at baseline | 135 (5.1) | 3[1,11] | 1.16 | 0.96–1.41 | 1.14 | 0.95–1.37 |
| ≥2 time/ week at baseline | 156 (5.9) | 2.5[1,7] | 0.91 | 0.76–1.10 | 0.86 | 0.72–1.02 |
| Physical form of AD at baseline | ||||||
| Never administered | 1572 (59.3) | 3[1,7] | 1(REF) | - | 1(REF) | - |
| Pills only | 156 (5.9) | 3[1,8] | 1.05 | 0.87–1.26 | 1.05 | 0.88–1.26 |
| Typical pills only | 121(4.6) | 3[1,8] | 1.07 | 0.87–1.31 | 1.08 | 0.88–1.31 |
| Crushed pills only | 35 (1.3) | 3[1,7] | 0.98 | 0.68–1.42 | 0.97 | 0.68–1.39 |
| Liquids only | 37 (1.4) | 4[1,10] | 1.21 | 0.84–1.74 | 1.05 | 0.74–1.48 |
| Both pills and liquids | 109 (4.1) | 3[1,9] | 1.15 | 0.93–1.43 | 1.11 | 0.90–1.38 |
| Not reported | 775 (29.3) | 3[1,10] | 1.04 | 0.95–1.15 | 0.98 | 0.90−−1.08 |
Adjusted for age, race, BMI, smoking status, marital status, hours worked per week, frequency of occupational moving or lifting a heavy load, and current exposure to ionizing radiation, high-level disinfectants, anesthetic gas, and aerosol drugs.
Abbreviations: n, number of nurses; AD, antineoplastic drugs; IQR, inter-quartile range; TR, time ratio; REF, reference.
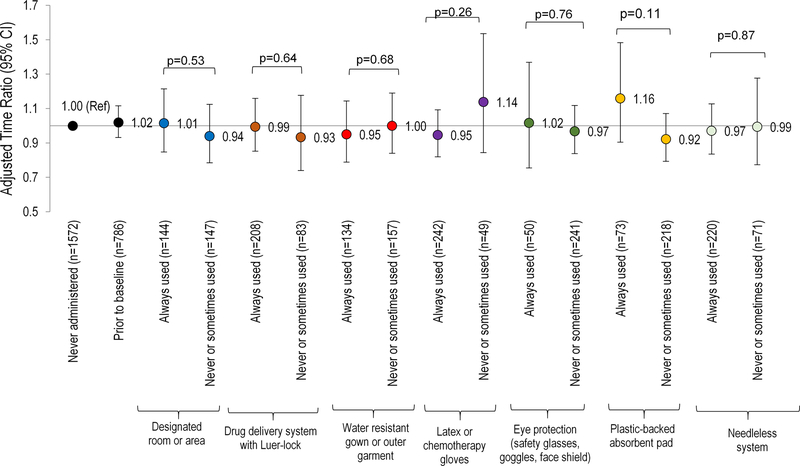
We then considered whether the use of EC modified the association of AD administration with fecundity (Table III). In these analyses, nurses who currently administered ADs but did not always use EC (n=21) had a longer median current duration of pregnancy attempt than nurses who had never handled ADs in the unadjusted and adjusted analyses, but none of the estimates were statistically significant (Table III). When we considered the type of EC individually used when administering ADs, no clear association patterns emerged (Figure I).
Table III.
Association between exposure control use during administration of antineoplastic drugs and fecundity among women attempting pregnancy (n=2,649).
| n (%) | Median Pregnancy Attempt in Months [IQR] | Unadjusted TR | (95%CI) | Adjusted TR* | (95%CI) | |
|---|---|---|---|---|---|---|
| Antineoplastic drug administration | ||||||
| Never | 1572 (59.3) | 3[1,7] | 1(REF) | - | 1(REF) | - |
| Yes, prior to baseline | 786 (29.7) | 3[1,9] | 1.08 | 0.98–1.18 | 1.02 | 0.93–1.12 |
| Yes, at baseline | ||||||
| Exposure controls not always used | 21 (0.79) | 4 [2,11] | 1.33 | 0.83–2.15 | 1.25 | 0.79–1.96 |
| Exposure controls always used | 270 (10.2) | 3[1,8] | 1.00 | 0.87–1.15 | 0.96 | 0.83–1.10 |
Adjusted for age, race, BMI, smoking status, marital status, hours worked per week, frequency of occupational moving or lifting a heavy load, and current exposure to ionizing radiation, high-level disinfectants, anesthetic gases, and aerosolized drugs.
Abbreviations: n, number of nurses; IQR, inter-quartile range; TR, time ratio; REF, reference.
Figure I. Association between each type of exposure control used during the administration of antineoplastic drugs (ADs) and fecundity among women attempting pregnancy (N=2,649).
Accelerated failure time models with log normal distribution [adjusted for age, race, body mass index, smoking status, marital status, hours worked per week, frequency of occupational lifting/moving a heavy load, and current exposure to radiation, disinfectants, anesthesia gas, and aerosol drugs] were used to estimate the time ratios (TR) and 95% confidence intervals.
Abbreviations: n, number of nurses; AD, antineoplastic drugs; EC, exposure control; TR, time ratio; Ref, reference.
The results were consistent in sensitivity analyses without co-adjustment for other occupational exposures, with further adjustment for gravidity, and the typical work schedule, restricting the follow-up period to two years after baseline, truncating time to pregnancy attempt to one year, or specifying a generalized gamma outcome distribution for current duration of pregnancy attempt (Supplemental Table III) or using finer categories of the exposure frequency (Supplemental Table IV).
4. Discussion
We prospectively evaluated the association between administration of antineoplastic drugs (AD), use of EC, and fecundity in a contemporary cohort of nurses in the U.S. and Canada. Contrary to our hypothesis, we found no association between AD administration and fecundity in this cohort. Given the high frequency of EC use in this population, these results cannot rule out the possibility that ADs have adverse effects on fecundity in the absence of consistent EC use, or among nurses who did not plan their pregnancies.
Two previous studies have evaluated the association of AD administration with fecundity with only one study using the time to pregnancy approach8. In a case-control study of nurses and pharmacists in the U.S. (405 cases and 1,215 controls), Valanis et al.,17 reported that occupational handling of chemotherapeutic drugs prior to the onset of infertility was associated with self-reported infertility (defined as having tried to have a child for at least two years without success). However, this study17 in addition to relying on retrospective report of exposure and outcome, did not consider EC use in their analysis. In a retrospective, pregnancy-based cohort of Dutch nurses, Fransman et al.8 assessed whether AD exposure was associated with the duration of pregnancy attempt prior to the nurses’ most recent pregnancy. AD exposure was estimated based on the measurement of dermal exposure to cyclophosphamide (glove pairs and handwashing samples) coupled with the self-reported frequency of tasks routinely performed by Dutch oncology nurses (preparation, administration, handling patient urine, washing a patient, removing bed sheets, cleaning toilets). The 177 Dutch nurses who were in the highest tertile of antineoplastic drug exposure took a longer time to conceive (median of 3 months) compared to nurses with no exposure to ADs (n=663) (median of 2 months) (adjusted hazard ratio = 0.8; 95% CI = 0.6–0.9). This study, however, could not exclude the possibility of differential recall and measurement error of time to pregnancy because the data on TTP were collected retrospectively up to 7 years in the past. This study also included only gravid women, which could cause bias if exposure to ADs is associated with sterility. In addition, Fransman et al.8, did not thoroughly investigate the use of ECs, other than gloves, which could have modified this association. Most important, in this study, the dermal exposure assessment was done after the outcome and therefore the temporality of exposure assessment in relation to outcome assessment is problematic for causal inference.
Previous studies on occupational exposure to ADs have suggested associations with fetal loss, spontaneous abortion, malformations in offspring, and lower mean birth weight of offspring6. The most consistent association with the reproductive outcomes wasa small incremental risk for spontaneous abortions in female staff working with cytotoxic agents, according to a systematic review and meta-analysis18. However, most of these studies occurred in earlier time periods when fewer ECs were available to protect the nurses from exposures to ADs6. It is possible that the reproductive toxicity of ADs was dramatically decreased by consistent use of ECs. This hypothesis is in agreement with our findings and with results from previous studies. For example, Skov et al.5, did not observe increased adverse reproductive outcomes including miscarriages, malformations, low birth weight, and preterm birth in occupationally exposed pregnant nurses to ADs in a retrospective cohort. Similar to our study, Skov et al.5, attributed the null results to the lower exposure compared to the other studies due to the safety measures implemented in the oncology departments in Denmark in the 1980s which protected healthcare personnel from adverse reproductive outcomes of ADs.
While true lack of association given the highly prevalent use of EC in this population is a plausible explanation for the results, alternate explanations must be considered. First, selection bias may arise if AD administration is associated with pregnancy planning and if unplanned pregnancies had longer or shorter waiting times to pregnancy. However, current waiting time to pregnancy is, by definition, undefined among non-planners making this assumption untestable. Furthermore, baseline prevalence of AD administration was comparable among women in this cohort who eventually became pregnant and who planned (41%) or did not plan (36%) their pregnancy, making this bias less likely. Second, it is important to consider the “infertile worker effect”19, whereby women who do not get pregnant are more likely to remain in the workforce and may be more exposed to occupational factors than women who become pregnant and may subsequently leave the workforce. However, the distribution of current duration of pregnancy attempt did not differ between women who were working in nursing (median [IQR] TTP= 3 [1–8] months) and women who were not employed outside home at baseline (median TTP= 3 [1–10] months), making this explanation unlikely.
Our study had limitations. First, we assessed AD administration only at baseline and the nurses’ pregnancy attempts occurred up to 3 years after the baseline questionnaire. In that time, nurses could have changed jobs or their use of AD or ECs when administering chemotherapy, leading to misclassification of exposure. However, other data from our cohort suggests that nurses are unlikely to change their occupational exposures because of a pregnancy or pregnancy attempt20. Second, we did not have detailed information about use of EC such as double versus single gloves, training on safe handling, engineering controls, barriers for not using EC, such as availability of EC, and if the nurses might have been exposed to ADs in any non-traditional work settings e.g., caring for someone at home. We do not know the nurses’ specialties, although we expect that they worked in a variety of settings as ADs are used in many departments besides just the oncology floors. However, it is possible that nurses working in oncology departments might be more likely to consistently use ECs assuming they received better safety training on hazardous drugs. We also lacked information about the facility type or size, which could also affect the safety training for AD administration. Third, although we adjusted for various confounders, the possibility of unmeasured or residual confounding cannot be completely ruled out, particularly due to other hazardous chemicals and physical hazards, frequency of sexual intercourse, and male factor fertility. We tried to minimize the possibility of bias that may arise from the very long duration of pregnancy attempts that might be due to over-representation of infertile women by conducting a sensitivity analysis in which we changed this cut-off to 1 year, which was consistent with the primary analysis. Another limitation is that we did not have information on what physical form of AD all nurses administered (pills, liquids, or both), which may have influenced what type of EC was used, because this question was added in 2012. Finally, although we had a large sample size overall, in the analyses involving specific ECs, we had small sample sizes in some groups which decreased the statistical power for us to detect a significant association. We conducted a post-hoc power analysis. The studyhad 80% power to detect at least12% change in the time ratio of the median time to pregnancy in the never vs ever analysis and 19% in the current vs non-current analysis. The detectable effect size was larger for the other sub-analysis (Supplemental Table V) but was large enough to detect associations of similar magnitude to those previously reported in the literature8.
Our study had several strengths. This is the first study in the U.S. to assess the association between AD administration in nurses and fecundity. Second, because administration of ADs in healthcare is not uncommon and nurses’ exposure to ADs is unlikely to be completely eliminated, collecting information on the use of ECs allowed us to address the more clinically relevant question of whether the association between administration of ADs and fecundity was modified by the use of ECs. Third, since our cohort was restricted to nurses many socio-economic factors that might be confounders were accounted for by design. Fourth, we were able to include both highly fertile women (who are excluded from many prospective cohorts) and involuntarily infertile women (who are excluded from retrospective pregnancy cohorts) by using the current duration approach, as compared to more traditional time to pregnancy approaches. Finally, assessment of the administration of the ADs by the nurses preceded the ascertainment of the ongoing duration of pregnancy minimizing the possibility of differential misclassification of the outcome.
In sum, administration of ADs did not appear to have an impact on fecundity in a cohort of nurses planning for pregnancy with a high prevalence of consistent ECs. Our results may not be generalizable to women who are less compliant with PPE use or with less availability to ECs. Therefore, it is possible that we did not observe an association between occupational exposure to AD and reduced fecundity because of lower exposure due to more prevalent use of effective ECs. Nevertheless, it is reassuring that ECs designed to reduce occupational exposure during administration of ADs may be effective in minimizing adverse health effects of these drugs.
Supplementary Material
Acknowledegements:
None
Funding: Grant sponsor: CDC/NIOSH; Grant number: contract 200–2013-M-54978 and Grant sponsor: NIEHS; Grant number: K99ES026648.
Footnotes
Institution at which the work was performed: Harvard T. H. Chan School of Public Health, Boston, MA.
Institution and Ethics approval and informed consent: The Institutional Review Boards of the Brigham and Women’s Hospital (Boston, Massachusetts) and the National Institute for Occupational Safety and Health (Cincinnati, Ohio) approved the study. Completion of the web-based questionnaires was considered as implied informed consent.
Disclosure (Authors): The authors declare no conflicts of interest.
Disclaimer: None
References
- 1.NTP. NTP Monograph: Developmental Effects and Pregnancy Outcomes Associated With Cancer Chemotherapy Use During Pregnancy. NTP monograph. 2013(2):i–214. [PubMed] [Google Scholar]
- 2.Davis J, McLauchlan R, Connor TH. Exposure to hazardous drugs in healthcare: an issue that will not go away. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2011;17(1):9–13. [DOI] [PubMed] [Google Scholar]
- 3.Valanis BG, Vollmer WM, Labuhn KT, Glass AG. Acute symptoms associated with antineoplastic drug handling among nurses. Cancer nursing. 1993;16(4):288–295. [PubMed] [Google Scholar]
- 4.Hon CY, Teschke K, Chu W, Demers P, Venners S. Antineoplastic drug contamination of surfaces throughout the hospital medication system in Canadian hospitals. Journal of occupational and environmental hygiene. 2013;10(7):374–383. [DOI] [PubMed] [Google Scholar]
- 5.Skov T, Maarup B, Olsen J, Rorth M, Winthereik H, Lynge E. Leukaemia and reproductive outcome among nurses handling antineoplastic drugs. British journal of industrial medicine. 1992;49(12):855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor TH, Lawson CC, Polovich M, McDiarmid MA. Reproductive health risks associated with occupational exposures to antineoplastic drugs in health care settings: a review of the evidence. Journal of occupational and environmental medicine. 2014;56(9):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp B, Schierl R, Nowak D. Evaluation of working practices and surface contamination with antineoplastic drugs in outpatient oncology health care settings. International archives of occupational and environmental health. 2013;86(1):47–55. [DOI] [PubMed] [Google Scholar]
- 8.Fransman W, Roeleveld N, Peelen S, de Kort W, Kromhout H, Heederik D. Nurses with dermal exposure to antineoplastic drugs: reproductive outcomes. Epidemiology. 2007;18(1):112–119. [DOI] [PubMed] [Google Scholar]
- 9.Bao Y, Bertoia ML, Lenart EB, et al. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20(1):56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielhuis GA, Hulscher MEJL, Florack EIM. Validity and Reliability of a Questionnaire on Fecundability. International Journal of Epidemiology. 1992;21(6):1151–1156. [DOI] [PubMed] [Google Scholar]
- 12.Hartvig H, Kvist K, Tvede M, Keiding N, Juul S. Estimating time to pregnancy from current durations in a cross‐sectional sample. Biostatistics (Oxford, England). 2002;3(4):565–578. [DOI] [PubMed] [Google Scholar]
- 13.Gaskins AJ, Chavarro JE, Rich-Edwards JW, et al. Occupational use of high-level disinfectants and fecundity among nurses. Scand J Work Environ Health. 2017;43(2):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slama R, Ducot B, Carstensen L, et al. Feasibility of the current-duration approach to studying human fecundity. Epidemiology. 2006;17(4):440–449. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg CR. Toward a clearer definition of confounding. American journal of epidemiology. 1993;137(1):1–8. [DOI] [PubMed] [Google Scholar]
- 16.Howards PP, Schisterman EF, Heagerty PJ. Potential confounding by exposure history and prior outcomes: an example from perinatal epidemiology. Epidemiology. 2007;18(5):544–551. [DOI] [PubMed] [Google Scholar]
- 17.Valanis B, Vollmer W, Labuhn K, Glass A. Occupational exposure to antineoplastic agents and self-reported infertility among nurses and pharmacists. Journal of occupational and environmental medicine. 1997;39(6):574–580. [DOI] [PubMed] [Google Scholar]
- 18.Dranitsaris G, Johnston M, Poirier S, et al. Are health care providers who work with cancer drugs at an increased risk for toxic events? A systematic review and meta-analysis of the literature. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2005;11(2):69–78. [DOI] [PubMed] [Google Scholar]
- 19.Joffe M Biases in research on reproduction and women’s work. Int J Epidemiol. 1985;14(1):118–123. [DOI] [PubMed] [Google Scholar]
- 20.Lawson CC, Johnson CY, Nassan FL, et al. CE: Original Research: Antineoplastic Drug Administration by Pregnant and Nonpregnant Nurses: An Exploration of the Use of Protective Gloves and Gowns. The American journal of nursing. 2019;119(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.