Abstract
Lymphomatous brain lesions can represent primary central nervous system(CNS) lymphoma or secondary involvement as part of systemic disease(SCNSL). In this study, we characterize staging evaluations in a large patient cohort with newly-diagnosed brain lymphomas, to determine the frequency of SCNSL and secondary malignancies. This retrospective review includes 262 patients with newly-diagnosed lymphomatous CNS lesions evaluated at Memorial Sloan Kettering Cancer Center between 2006-2018. Staging procedures included PET scans in 180(69%) patients, CT scans of chest/abdomen/pelvis in 195(74%) and bone marrow biopsies(BMB) in 177(68%). PET scans were reported as abnormal in 34 of 180(19%), CT in 50 of 195(26%) and BMB in 15 of 177(8.5%). A total of 24 non-CNS malignancies were identified(11.8%; 19 systemic lymphomas and 5 secondary malignancies). Thus, in patients with new lymphomatous brain lesions, performing initial systemic staging procedures can identify systemic lymphoma and additional malignancies, highlighting the importance of staging evaluations, in particular PET and BMB.
Keywords: central nervous system, lymphoma, staging, secondary malignancy, FDG-PET
Introduction
Lymphomatous brain lesions can represent primary central nervous system (CNS) lymphoma (PCNSL) or metastatic disease from a non-CNS lymphoma (secondary CNS lymphoma; SCNSL). PCNSL is a rare form of aggressive extra-nodal non-Hodgkin’s lymphoma (NHL) that is found in approximately 4% of newly diagnosed CNS tumors [1–6]. It typically disseminates within the brain, cranial nerves, leptomeninges, cerebrospinal fluid (CSF), intraocular structures and spinal cord and does not have lesions outside the CNS [2,5,7,8]. SCNSL can be found in 5-10% of patients with non-CNS diffuse large B-cell lymphoma [3,4,8–11]. SCNSL can be found as leptomeningeal involvement or parenchymal brain lesions; rarely also as isolated ocular involvement.
Patients with CNS lymphoma and active systemic, non-CNS disease most often require the addition of chemotherapy targeting the non-CNS disease (e.g. R-CHOP) in addition to a methotrexate-based chemotherapy for the CNS disease [9,10,12]. To identify systemic, non-CNS lymphoma in patients with lymphomatous brain lesions, the International Primary CNS Lymphoma Group (IPCG) therefore recommends a systemic staging evaluation to identify non-CNS disease [1,2,8,13–15]. Staging investigations are recommended to include expert ophthalmologic assessment, conventional imaging such as computed tomography (CT) of the chest, abdomen and pelvis (CAP), Positron Emitted Tomography (PET) body scan and bone marrow biopsy (BMB) [1,2,7,8,13,14,16–18]. Staging evaluation (CT CAP and BMB) in patients with lymphomatous brain lesions typically identifies a site of systemic disease in about 4% of patients [9,15,19,20] and other secondary malignancies.
Positron emission tomography (PET) has become the standard study for assessment of disease burden and management of non-CNS NHL [16,21,22]. Fluorodeoxyglucose (FDG)-PET has the potential for identifying systemic, non-CNS disease better than CT CAP in CNS lymphoma patients [17,18,23,24]. Mohile et al [18] investigated the use of FDG-body-PET retrospectively in 49 PCNSL patients as part of systemic staging and observed that FDG-PET may be more sensitive than CT CAP in the detection of systemic, non-CNS lymphomatous lesions.
In this study, we characterize the systemic staging evaluation performed in a large patient cohort with newly diagnosed lymphomatous brain lesions without a prior lymphoma diagnosis. We report on the use of staging modalities and the frequency of identified non-CNS malignancies.
Materials and Methods
We performed an Institutional Review Board approved retrospective review of patients with a newly diagnosed lymphomatous brain lesions who were evaluated at Memorial Sloan Kettering Cancer Center from the 1/2006 to 1/2018. We identified patients through an automated search of our institutional database using keyword terms such as neurology visit or consultation in the inpatient or outpatient setting and primary CNS lymphoma. A confirmatory manual search of all charts was performed. Patient demographics including age, gender, and performance status (KPS) were recorded. The histologic diagnosis of lymphoma was confirmed in all patients. The records of identified patients were reviewed to determine the systemic staging evaluation as a part of their initial evaluation at the time of diagnosis. Clinical data points included results of MRI brain, FDG-body-PET, CT Chest/Abdomen/Pelvis (CT CAP), Bone Marrow biopsy (BMB) and pathology. A staging test was defined as positive, if the nuclear medicine physician/radiologist/pathologist reported an abnormal finding.
Results
We identified a total of 262 patients with newly diagnosed lymphomatous brain lesions. The median age was 63 (range 19-90) with a median Karnofsky Performance Score of 80 (range 40-100); 136 patients (52%) were men and 126 (48%) were women (Table 1).
Table 1:
Patient Demographics
| Age | Median (range)- yr | 63 (19-90) |
| Distribution- no.(%) | ||
| <65 yr | 137 (52%) | |
| 65-70 yr | 44 (17%) | |
| >70 yr | 81 (31%) | |
| Gender | ||
| Male | 136 (52%) | |
| Female | 126 (48%) | |
| KPS | ||
| >70 | 139 (53%) | |
| ≤70 | 123 (47%) | |
| Median | 80 |
Systemic staging included FDG-PET in 180/262 patients (69%), CT CAP in 195/262 (74%) and bone marrow biopsy (BMB) in 177/262 (68%) (Fig. 1A). In 122/262 patients (46.6%), both CT CAP and FDG-PET was done at staging. All three were performed in 91 (35%) patients. At least one of the staging assessments was performed in 195/262 (74%) patients; those without staging evaluations at initial diagnosis were patients that transferred care to MSKCC after initiation of chemotherapy at another institution.
Figure 1. Staging Evaluations.
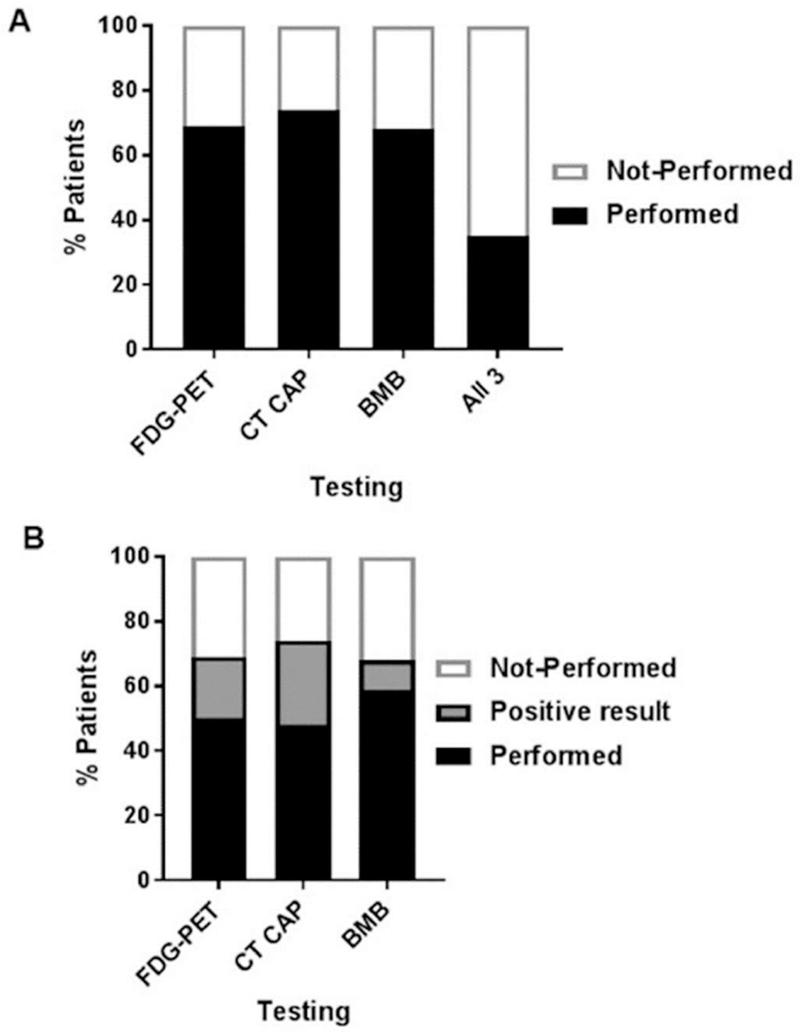
A) Percentage of patients with newly diagnosed lymphomatous brain lesions evaluated with Positron Emitted Tomography (FDG-PET) body scan, computed tomography (CT) of the chest, abdomen and pelvis (CT CAP), bone marrow biopsy (BMB) or all three modalities (all 3) for staging assessment. B) Percentage of abnormal test results by testing modality.
CT CAP demonstrated an abnormal finding in 50/195 (26%), FDG-PET in 34/180 (19%), and BMB in 15/177 (8.5%) patients (Fig. 1B). Abnormal CT CAP findings included thyroid and lung nodules, pulmonary emboli, possible pneumonia and lymphadenopathy. The abnormal PET scans most commonly identified hypermetabolism in the thyroid, lung and lymph nodes.
Fourteen abnormal BMBs identified indolent, low-grade lymphoma whereas one demonstrated diffuse large B-cell lymphoma (DLBCL) involvement. Two of the abnormal BMB findings had a PET correlate (13.3%) whereas none had a CT finding. The FDG-PET identified diffuse FDG uptake in the bone marrow in both cases.
Further work-up, mainly biopsies of the identified imaging abnormalities, identified 10 additional secondary malignancies (Table 2). The malignancies identified were thyroid follicular neoplasm, thyroid papillary carcinoma, peripheral nerve sheath tumor non-small cell lung cancer (NSCLC) and systemic involvement of DLBCL. One patient was found to have a low-grade lymphoma through BMB and a Hurthle cell thyroid cancer through PET. In only 1 patient all 3 studies identified the secondary malignancies (non-CNS DLBCL).
Table 2.
Identified Secondary Malignancies
| Malignancy | FDG-PET | CT | BMB |
|---|---|---|---|
| DLBCL (n=5) | 5 | 4 | 1 |
| Thyroid Cancer (n=3) | 3 | 2 | 0 |
| NSCLC (n=1) | 1 | 0 | 0 |
| Peripheral Nerve Sheath Tumor (n=1) | 1 | 1 | 0 |
| Isolated Bone Marrow Low Grade B Cell Lymphoma (n=14) | 2 | 0 | 14 |
To determine the imaging modality that identifies secondary malignancies most accurately, we compared the findings in those patients who underwent both CT CAP and FDG-PET (n=122) (Fig. 2). Nineteen patients had concordant abnormal CT CAP and PET findings of which only 7 where attributed to secondary malignances on further work-up. Of the 10 secondary malignancies not involving the bone marrow all were identified by FDG-PET. CT CAP did not identify three secondary malignancies (1 NSCLC, 1 DLBCL and 1 Hurthle cell). Abnormal non-malignant imaging lesions included benign thyroid nodules, nonspecific pulmonary nodules, pneumonia and nonspecific adenopathy. In the entire cohort, FDG-PET could identify non-bone marrow related secondary malignancies with a sensitivity of 100% and a specificity of 86% whereas CT CAP had a sensitivity of 70% and a specificity of 77%.
Figure 2. Comparison PET to CT.
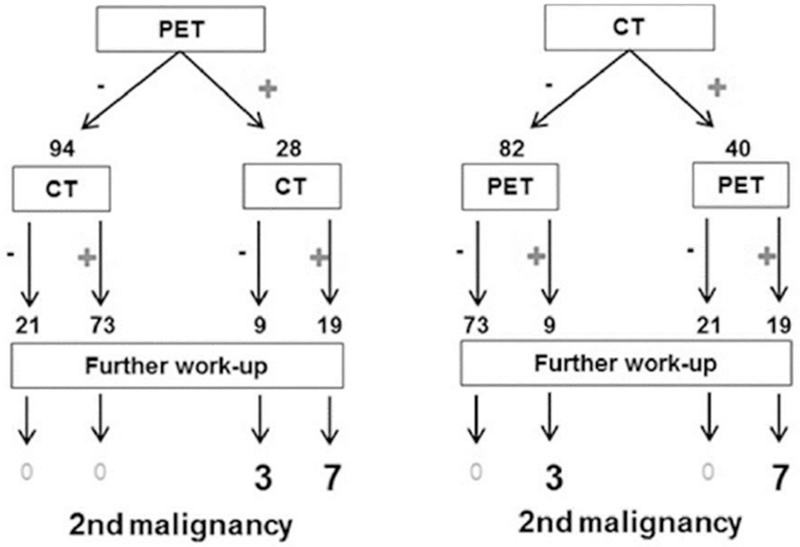
In 122 patients Positron Emitted Tomography (PET) body scan and computed tomography (CT) of the chest, abdomen and pelvis were performed. The flowchart summarizes the results of both imaging tests. In 19 patients both imaging modalities showed concordant results. All secondary malignancies were identified through PET imaging, 3/10 (30%) were not detected on CT.
Of note, in abnormal PET scans which identified a secondary malignancy the mean standard uptake values (SUV) was 10.55 (± 2.8) in contrast to 5.2 (±1.06) in those patients with an abnormal PET scan who did not have an additional malignancy identified (p value=0.0478).
In total, 23 (11.8%) patients had 24 additional secondary, non-CNS malignancies: 19 (80%) systemic involvement of lymphoma (5 DLBCL, 14 low grade lymphomas), 3 thyroid malignancies (follicular, papillary and Hurthle cell), 1 NSCLC, and 1 benign peripheral nerve sheet tumor (Table 2).
There has been an increase in identified secondary malignancies in PCNSL patients over the last decade; before 2009 only 5.3% (4/76) of patients with a secondary, non-CNS malignancy were identified in contrast to 13.8% after 2013 (11/80), which may correlate with a more frequent use of FDG-PET as part of the staging evaluation from 43% prior to 2009 to 72% after 2013(Fig 3).
Figure 3. Rates of Secondary Malignancies identified on Staging.
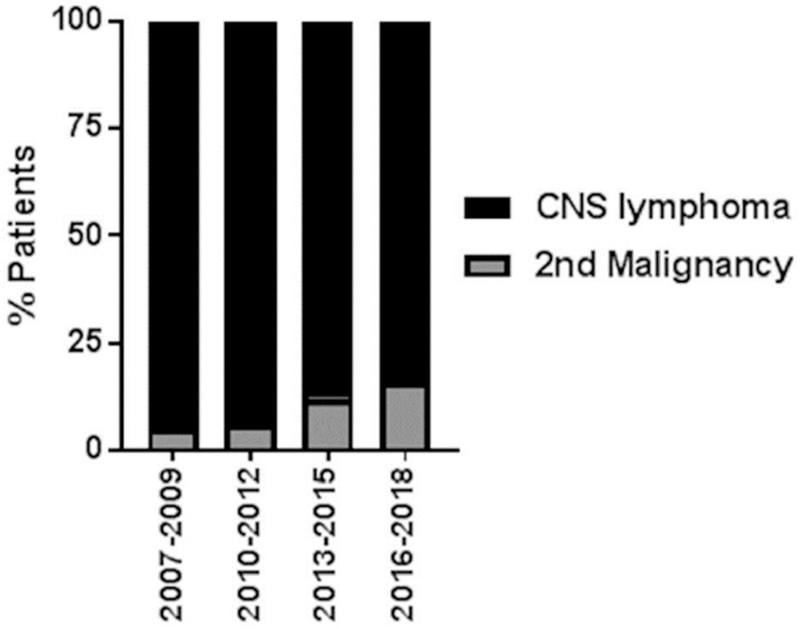
Shown is the percentage of patients with a secondary malignancy identified on staging since 2007 in three-year intervals. Identification of secondary malignancies has increased steadily since 2007.
Discussion
This study reviews the utilization of staging examinations for patients with newly found lymphomatous brain lesions. Staging evaluations play an important role in evaluating the extent of disease and for identifying secondary, non-CNS malignancies [11–15]. In our study staging evaluations identified secondary, non-CNS malignancies in 11.8% of patients which ranged from an indolent lymphoma and non-CNS DLBCL to thyroid malignancies. Compared to prior studies which reference detection rates of ~4% [3,5,10,25], we observed a higher rate of secondary malignancies. This may in part be due to the increased utilization of staging imaging/procedures over recent years as well as the increased use of FDG-PET.
Specific baseline evaluations and staging have been recommended by the IPCG [1,13] for patients with brain lymphoma and established guidelines include bone marrow biopsy and CT CAP [1,2,7,13,14]. Our study suggests, within the confines of a retrospective review, that FDG-PET imaging should be the preferred imaging modality to identify secondary malignancies. Moreover, the observed high sensitivity of FDG-PET in our study suggests the use of FDG-PET over CT CAP for staging to reduce the need of additional follow-up investigation to work-up identified imaging abnormalities. Further, the degree to which an SUV value is elevated is helpful in identifying secondary malignancies. The interpretation of SUV values on FDG-PET requires the expertise of a nuclear medicine physician in order to reduce the risk of false positive results as SUV values are dependent on many factors, including plasma glucose levels, patient habitus and respiratory motion.. BMB identified bone marrow involvement not detected on FDG-PET (except for in 2 patients) or CT CAP. In addition, concordantly abnormal results in all staging modalities was rarely observed. We therefore suggest to routinely include BMB as well as an imaging modality, preferably FDG-PET, into the staging evaluation for all newly diagnosed brain lymphoma patients.
Our study has limitations due in part to the nature of a retrospective review as well as the fact that not all patients in our cohort had complete staging evaluations and larger prospective studies need to be done to further define the benefits of staging and in particular the use of FDG-PET.
Acknowledgements
This research was supported by a NIH/NCI Cancer Center Support Grant (P30-CA008748) and supported by grants from Cycle for Survival Equinox and the Leukemia & Lymphoma Society
Footnotes
Disclosure: The authors report no conflict of interest
References
- 1.Ferreri AJ, Batchelor T, Zucca E, Cavalli F, Armitage J. International Collaborative Group against Primary CNS Lymphomas. J Clin Oncol 2003;21:1649–1650. [DOI] [PubMed] [Google Scholar]
- 2.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 2003;21:266–272. [DOI] [PubMed] [Google Scholar]
- 3.Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol 2017;35:2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotkin SR. Update on primary central nervous system lymphoma. Curr Opin Neurol 2005;18:645–653. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein J, Ferreri AJ, Pittaluga S. Primary lymphoma of the central nervous system: epidemiology, pathology and current approaches to diagnosis, prognosis and treatment. Leuk Lymphoma 2008;49 Suppl 1:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 2018;20:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006;24:5711–5715. [DOI] [PubMed] [Google Scholar]
- 8.Ferreri AJ, Reni M. Prognostic factors in primary central nervous system lymphomas. Hematol Oncol Clin North Am 2005;19:629–649, vi. [DOI] [PubMed] [Google Scholar]
- 9.Levitt LJ, Dawson DM, Rosenthal DS, Moloney WC. CNS involvement in the non-Hodgkin’s lymphomas. Cancer 1980;45:545–552. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill BP, Dinapoli RP, Kurtin PJ, Habermann TM. Occult systemic non-Hodgkin’s lymphoma (NHL) in patients initially diagnosed as primary central nervous system lymphoma (PCNSL): how much staging is enough? J Neurooncol 1995;25:67–71. [DOI] [PubMed] [Google Scholar]
- 11.Bollen EL, Brouwer RE, Hamers S, et al. Central nervous system relapse in non-Hodgkin lymphoma. A single-center study of 532 patients. Arch Neurol 1997;54:854–859. [DOI] [PubMed] [Google Scholar]
- 12.Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer 2002;95:1504–1510. [DOI] [PubMed] [Google Scholar]
- 13.Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034–5043. [DOI] [PubMed] [Google Scholar]
- 14.Ferreri AJ, Reni M, Zoldan MC, Terreni MR, Villa E. Importance of complete staging in non-Hodgkin’s lymphoma presenting as a cerebral mass lesion. Cancer 1996;77:827–833. [DOI] [PubMed] [Google Scholar]
- 15.International Non-Hodgkin’s Lymphoma Prognostic Factors P. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 1993;329:987–994. [DOI] [PubMed] [Google Scholar]
- 16.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med 2006;354:496–507. [DOI] [PubMed] [Google Scholar]
- 17.Karantanis D, O’Neill BP, Subramaniam RM, et al. . Contribution of F-18 FDG PET-CT in the detection of systemic spread of primary central nervous system lymphoma. Clin Nucl Med 2007;32:271–274. [DOI] [PubMed] [Google Scholar]
- 18.Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol 2008;10:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ, Radiation Therapy Oncology Group S. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 2002;20:4643–4648. [DOI] [PubMed] [Google Scholar]
- 20.Ferreri AJ. How I treat primary CNS lymphoma. Blood 2011;118:510–522. [DOI] [PubMed] [Google Scholar]
- 21.Schoder H, Noy A, Gonen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol 2005;23:4643–4651. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roelcke U, Leenders KL. Positron emission tomography in patients with primary CNS lymphomas. J Neurooncol 1999;43:231–236. [DOI] [PubMed] [Google Scholar]
- 24.Zou Y, Tong J, Leng H, Jiang J, Pan M, Chen Z. Diagnostic value of using 18F-FDG PET and PET/CT in immunocompetent patients with primary central nervous system lymphoma: A systematic review and meta-analysis. Oncotarget 2017;8:41518–41528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollack IF, Lunsford LD, Flickinger JC, Dameshek HL. Prognostic factors in the diagnosis and treatment of primary central nervous system lymphoma. Cancer 1989;63:939–947. [DOI] [PubMed] [Google Scholar]


