Abstract
RATIONALE:
The feasibility and clinical outcomes of conservative fluid management after sepsis resuscitation remain unknown.
OBJECTIVES:
To evaluate the effect of a conservative fluid management protocol on fluid balance and intensive care unit (ICU)-free days among patients with sepsis.
METHODS:
In a single-center phase II/III randomized trial, we enrolled adults with suspected infection, ≥ 2 systemic inflammatory response syndrome criteria, and either shock (mean arterial pressure < 60 mmHg or vasopressors) or respiratory insufficiency (mechanical ventilation or oxygen saturation < 97% and fraction of inspired oxygen ≥ 0.3). Patients were randomized 1:1 to usual care or a conservative fluid management protocol. The protocol restricted intravenous fluid administration during shock to treatment of oliguria or increasing vasopressor requirement. In the absence of shock, loop diuretic infusion targeted equal fluid input and output each study day. The primary outcomes were mean daily fluid balance (Phase II) and ICU-free days (Phase III).
RESULTS:
At the completion of Phase II (n=30), the difference in mean daily fluid balance between groups (−398 mL) was less than the pre-specified threshold (−500 mL) and the trial was stopped. Patients in the conservative fluid management (n=15) and usual care (n=15) groups experienced similar cumulative fluid input (8,450 vs 7,049 mL; P = .90), of which only 14% was intravenous crystalloid or colloid. Loop diuretic infusion occurred more frequently in the conservative fluid management group (40% vs 0%; P = 0.02) and cumulative fluid output was 10,645 mL in the conservative fluid management group compared with 6,286 mL in the usual care group (P = .39). Hemodynamic, respiratory, and renal function did not differ between groups.
CONCLUSIONS:
In this Phase II trial, a conservative fluid management protocol did not decrease mean daily fluid balance by more than 500 mL among patients with sepsis.
REGISTRATION:
Keywords: Sepsis, intravenous fluid, acute kidney injury
INTRODUCTION
Early intravenous fluid administration is a fundamental therapy for sepsis1,2 and has been the subject of significant recent research3–7. In contrast, few data are available to inform the optimal approach to fluid management after initial sepsis resuscitation8,9. Guidelines have recommended fluid administration be continued as long as hemodynamic improvements persist10 and observational studies report net fluid balances of 5–11 liters positive during the first week of sepsis management11–15. After resuscitation, however, the potential benefits of fluid administration are weighed against the risks of organ edema and detrimental effects of the fluid constituents16–18. Numerous observational studies have associated positive fluid balance in sepsis with organ dysfunction and mortality11,15,19. However, only two pilot trials have prospectively examined post-resuscitation fluid management in sepsis8,9. Whether a conservative fluid management strategy after sepsis resuscitation results in a clinically meaningful difference in fluid balance or impacts outcomes compared to usual care remains unknown20.
We designed a phase II/III randomized trial to assess the feasibility (Phase II) and clinical effects (Phase III) of a protocol targeting neutral fluid balance after sepsis resuscitation. We hypothesized that this conservative fluid management protocol would decrease mean daily fluid balance (Phase II) and increase days alive and free of the intensive care unit (ICU) (Phase III) compared with usual care.
METHODS
Study Design and Oversight
The BALANCE (“Phase II/III Randomized Controlled Trial of a Conservative Fluid Balance Strategy for Patients with Sepsis and Cardiopulmonary Dysfunction”) study was a single-center, un-blinded, parallel-group, randomized trial comparing a conservative fluid management protocol to usual care among adults with sepsis and shock or respiratory insufficiency. Phase II compared conservative fluid management to usual care with regard to mean daily fluid balance among 30 patients. If the conservative fluid management protocol produced a mean daily fluid balance at least 500 mL less than usual care in Phase II, Phase III planned to enroll an additional 120 patients to compare ICU-free days between groups. The study protocol (available in the online supplement) was approved by the institutional review board at Vanderbilt University (IRB#140582) and the trial was registered online prior to initiation (NCT02159079).
Patient Population
From August 23, 2014 to February 25, 2016 we recruited adults (age ≥ 18 years) admitted to the medical ICU at Vanderbilt University Medical Center who met two or more criteria for systemic inflammatory response syndrome21, were receiving antimicrobial therapy, and met criteria either for shock (defined as a mean arterial pressure < 60 mmHg or vasopressor receipt) or respiratory insufficiency (defined as receipt of invasive or non-invasive mechanical ventilation or an arterial oxygen saturation < 97% while receiving a fraction of inspired oxygen (FiO2) ≥ 0.3). Exclusion criteria are described in the online supplement. All patients or their legally authorized representatives provided written informed consent prior to enrollment.
Randomization
Patients were randomized in a 1:1 ratio to conservative fluid management or usual care using computer-generated blocks of 2, 4 and 6, stratified by the presence or absence of shock. Group assignment remained concealed until a patient had qualified for enrollment, consent had been provided, and at least 12 hours had elapsed since ICU admission.
Study Interventions
In both groups, the study began at randomization (which occurred at the later of informed consent or 12 hours after ICU admission) and ended at study termination (which occurred at the first of ICU discharge, 14 days after enrollment, cessation of vasopressors and return of the patient’s FiO2 to pre-admission baseline, or death).
For patients assigned to usual care, all aspects of patient care including fluid management were deferred to treating clinicians. For patients assigned to the conservative fluid management group, fluid and diuretic therapy were governed by a fluid management protocol (available in the online supplement). This protocol was modeled on the simplified conservative fluid management protocol developed by the National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network targeting equal fluid intake and output in the 7 days after enrollment22,23 and refined by a multidisciplinary expert panel (see online supplement).
Upon initiation of the protocol, intravenous fluids being administered to maintain intravascular volume or replace insensible losses (“maintenance fluids”) were discontinued and the pharmacy maximally concentrated all intravenous medications. For patients in shock (mean arterial pressure < 60 mmHg or vasopressor receipt in the prior 12 hours), intravenous fluid boluses were administered only for oliguria (urine output < 30 mL/h for 6 hours) or increasing vasopressor requirement. For patients without shock, intravenous fluid boluses were permitted only for oliguria, and continuous furosemide infusion beginning at 3 mg/h and titrated as high as 24 mg/h was administered, as needed, to achieve a total fluid output greater than total fluid input for each 24 hour study day. The protocol was suspended only for the initiation of renal replacement therapy (RRT), receipt of at least 60 mcg/min of norepinephrine or increase in norepinephrine of greater than 20 mcg/min over 6 hours, or acute clinical decompensation (see online supplement). Patients, treating clinicians, and investigators were not blinded to study group assignment.
Data Collection
Data were collected at enrollment, daily for 14 days, and at study termination. Collected data included: baseline demographics, comorbidities, pre-enrollment sepsis management, measures of hemodynamic, respiratory, and renal function, severity of illness; hourly assessments of blood pressure, vasopressor receipt, and urine output; daily assessments of fluid input, fluid output, and diuretic receipt; daily assessments of hemodynamic and respiratory function, plasma laboratory values, and receipt of mechanical ventilation, vasopressors, and RRT; and blinded assessment of vital status, timing of liberation from mechanical ventilation, ICU transfer, and hospital discharge at 28 days after enrollment. Each patient’s chest radiographs were independently reviewed by two pulmonologists blinded to study group assignment for radiographic evidence of acute respiratory distress syndrome (ARDS) by Berlin criteria24.
Study Outcomes
The primary outcomes were mean daily fluid balance for Phase II and days alive and free of ICU admission in the 14 days after enrollment (ICU-free days) for Phase III. Daily fluid balance was calculated as total fluid input minus total fluid output between enrollment and study termination, divided by the number of days between enrollment and study termination. Secondary outcomes included in-hospital mortality, vasopressor-free days, ventilator-free days, renal-failure free days, highest stage of acute kidney injury by Kidney Disease Improving Global Outcomes (KDIGO) criteria25, highest plasma creatinine, change from baseline to highest plasma creatinine, and change from enrollment to highest plasma creatinine.
Statistical Analysis
For Phase II, a minimum difference between groups in mean daily fluid balance of 500 mL was selected as the threshold to continue to Phase III. This 500 ml threshold was based on a prior trial of conservative fluid management in ARDS in which a 1,000 mL difference in daily fluid balance between groups resulted in an absolute increase in ICU-free days of 2 days26. When designing the current trial, we felt that a difference between groups in mean daily fluid balance less than 500 mL was unlikely to produce a significant difference in clinical outcomes.
For Phase III, we calculated that enrollment of 150 patients would provide 80% power at an alpha level of 0.05 to detect a 2.0 day absolute increase in ICU-free days in the conservative fluid group compared to the usual care group, assuming the mean for ICU free days in the usual care group was 6.8 days and the standard deviation was 4.3 days. These assumptions were based on a prior sepsis cohort study in the same ICU27.
All analyses were conducted in an intention-to-treat fashion. Continuous variables were reported as mean ± standard deviation or median and interquartile range; categorical variables as frequencies and proportions. Between-group comparisons were made with the Mann-Whitney rank sum test for continuous variables, Fisher’s exact test or chi-square test for categorical variables, and multivariable regression for adjusted analyses and tests of interaction. A two-sided P value < 0.05 determined significance. Analyses were performed using SPSS Statistics v.24 (IBM Corp., Armonk, NY, USA) or R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Enrollment and Baseline Characteristics
Of 192 patients screened, 30 patients (15.6%) were eligible, provided informed consent, and were enrolled; an average rate of enrollment of 1.6 patients per month. After enrollment of 30 patients in the Phase II portion of the trial, the difference in mean daily fluid balance between groups was −398 mL (95% CI −1,227 to 430 mL; P = .33). This difference in mean daily fluid balance did not meet the pre-specified 500 ml threshold for continuing to Phase III and the trial was stopped (Figure 1).
Figure 1. Flow of participants through the trial.
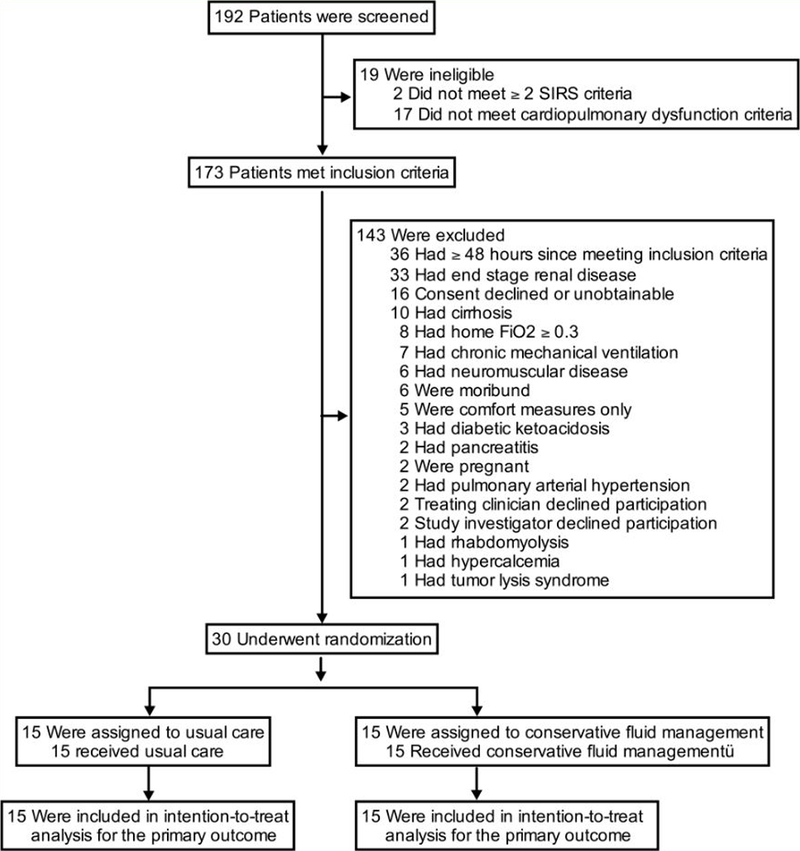
Of 192 patients screened, 30 met inclusion criteria without meeting exclusion criteria and were enrolled, randomized, followed, and analyzed. Two potentially eligible patients were excluded by study investigators, one receiving scheduled fluid boluses prior to amphotericin administration and one with septic shock in the context of severe mitral stenosis. SIRS = systemic inflammatory response syndrome, cardiopulmonary dysfunction criteria = shock (mean arterial pressure < 60 mmHg or vasopressor receipt) or respiratory insufficiency (mechanical ventilation or oxygen saturation < 97% with fraction of inspired oxygen ≥ 0.3), FiO2 = fraction of inspired oxygen
Patients randomized to conservative fluid management (n=15) and usual care (n=15) were similar at baseline (Table 1). All patients received antibiotics before enrollment (Table E1). The median time from ICU admission to enrollment was 13.8 hours, median time from enrollment to randomization was 3.5 minutes, and median time from randomization to protocol initiation in the conservative fluid management group was 2.0 minutes (Table E1).
Table 1.
Patient characteristics at baseline.
| Patient Characteristics | Usual Care (n = 15) |
Conservative (n = 15) |
|---|---|---|
| Age, median [IQR], years | 65 [57 – 77] | 61 [54 – 75] |
| Men, No. (%) | 8 (53.3) | 7 (46.7) |
| Caucasian, No. (%) | 14 (93.3) | 14 (93.3) |
| Body mass index, median [IQR], kg/m2 | 27.8 [26.0 – 32.7] | 28.9 [22.2 – 37.0] |
| Comorbidities, No. (%) | ||
| Congestive heart failure | 1 (6.7) | 3 (21.4) |
| Chronic kidney disease, stage III or greater* | 3 (20.0) | 1 (6.7) |
| Immunosuppression | 7 (46.7) | 5 (33.3) |
| Suspected site of infection, No. (%) | ||
| Pulmonary | 9 (60.0) | 8 (53.3) |
| Urinary | 1 (6.7) | 2 (13.3) |
| Bacteremia | 2 (13.3) | 1 (6.7) |
| Intra-abdominal | 0 (0.0 | 2 (13.3) |
| Central nervous system | 0 (0.0) | 2 (13.3) |
| Bone and joint | 1 (6.7) | 0 (0.0) |
| Unknown | 2 (13.3) | 0 (0.0) |
| Source of admission to ICU, No. (%) | ||
| Emergency department | 7 (46.7) | 6 (40.0) |
| Transfer from another hospital | 5 (33.3) | 8 (53.3) |
| Hospital ward | 3 (20.0) | 0 (0.0) |
| Another ICU within the hospital | 0 (0.0) | 1 (6.7) |
| Invasive mechanical ventilation, No. (%) | 4 (26.7) | 7 (46.7) |
| Noninvasive mechanical ventilation, No. (%) | 2 (13.3) | 1 (6.7) |
| Fraction of inspired oxygen, median [IQR] | 0.36 [0.27 – 0.50] | 0.40 [0.27 – 0.50] |
| Oxygen saturation, median [IQR], % | 96 [94–100] | 95 [93 – 98] |
| Heart rate, median [IQR], beats per min | 84 [78 – 109] | 95 [78 – 102] |
| Mean arterial pressure, median [IQR]. mmHg | 75 [67 – 80] | 70 [66 – 76] |
| Vasopressors, No. (%) | 9 (60.0) | 9 (60.0) |
| Norepinephrine, median [IQR], mcg/min | 8 [5 – 12] | 8 [7 – 24] |
| Creatinine, median [IQR], mg/dL | 1.6 [1.0 – 2.9] | 0.9 [0.7 – 2.1] |
| Lowest in 12 months prior to hospitalization | 0.9 [0.7 – 1.0] | 1.0 [0.9 – 1.4] |
| Stage of acute kidney injury at enrollment†, No. (%) | ||
| None | 5 (33.3) | 5 (33.3) |
| Stage I | 1 (6.7) | 4 (26.7) |
| Stage II | 5 (33.3) | 3 (20.0) |
| Stage III | 4 (26.7) | 3 (20.0) |
| APACHE II score, median [IQR] | 29 [23 – 31] | 24 [18 −30] |
| Fluid input in 24h prior to enrollment, median [IQR] mL | 2,740 [441 – 4,599] | 1,496 [325 – 2,448] |
Baseline characteristics are compared between patients randomized to usual care versus conservative fluid management. IQR = interquartile range, ICU = intensive care unit, ARDS = acute respiratory distress syndrome, APACHE II = Acute Physiology and Chronic Health Evaluation II – ranging from 0 to 71 with higher scores indicating higher severity of illness
Chronic kidney disease stage III or greater is defined as a glomerular filtration rate less than 60 ml/min per 1.73 m2 as calculated by the Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration equation32 using the patient’s baseline creatinine value.
Acute kidney injury is defined according to Kidney Disease Improving Global Outcomes (KDIGO) criteria25.
Fluid Balance
The difference between groups in mean daily fluid balance, the primary outcome for Phase II, was −398 mL (95% CI −1,227 to 430 mL; P = .33) (Figure 2–3). Cumulative fluid balance (total fluid input minus total fluid output) over the course of the study was −2,195 ± 4,313 mL in the conservative fluid management group compared with 764 ± 4,352 mL in the usual care group (mean difference −2,959 mL; 95% CI −6,199 to 282 mL; P = .11) (Figure 2). A total of 10 patients (66.7%) in the conservative fluid management group and 6 patients (40.0%) in the usual care group had a net negative fluid balance at 14 days (P = .14).
Figure 2. Fluid management by study group.

Cumulative fluid input (upper left panel), fluid output (upper right panel), and fluid balance (lower left panel) among patients in the conservative fluid management group (dotted line) and usual care group (solid line). The lower right panel displays the mean difference between groups in fluid balance on each study day. The diamond (mean) and bars (standard deviation) display the primary outcome of the difference between groups in mean daily fluid balance over the course of the trial. The numbers of patients in the conservative fluid management and usual care groups, respectively, were: 15 and 15 (day of enrollment), 14 and 14 (day 1), 10 and 9 (day 2), 7 and 8 (day 3), 5 and 3 (day 4), 4 and 3 (day 5), 2 and 1 (day 6), and 2 and 1 (day 7). The increase in cumulative fluid input in the conservative fluid management group on study days 7 and 8 resulted from a single patient who received 5,850 mL of oral free water over 48 hours for a sodium of 150 mmol/L.
Figure 3. Cumulative Fluid Balance for Each Individual Patient.

For patients in the conservative fluid management group (left) and usual care group (right), the cumulative fluid balance is displayed for each study day. Data are censored at the time of death or transfer out of the intensive care unit.
Fluid Administration
In the 3 days after enrollment, a total of 14 IV fluid boluses were administered among the 15 patients in usual care group, compared with 5 IV fluid boluses among the 15 patients in the conservative group (Table E2). The only patient to receive a fluid bolus more than 3 days after enrollment was a patient in the conservative arm who received three 500 mL IV fluid boluses on study day 8 for oliguria. All fluid boluses administered in the conservative fluid management group were compliant with study protocol and administered for either oliguria (n=6) or increase in vasopressor requirement (n=2). Of the 14 fluid boluses given to patients in the usual care arm, 2 were for oliguria, 3 were for increasing vasopressor requirement, and 9 were administered in the absence of oliguria or increasing vasopressor requirement. Over the course of the trial, patients in the usual care group received a mean volume of fluid from IV boluses of 733 ± 1,083 compared with 300 ± 560 in the conservative fluid management group (P = .30).
Between enrollment and study day 14, the pharmacy concentrated medication infusions for 15 (100%) patients in the conservative fluid management group based on study protocol and 1 patient (6.7%) in the usual care group based on clinician request (P < .001).
Cumulative fluid input between enrollment and study termination was similar between the conservative fluid management group (8,450 ± 10,103 mL) and usual care group (7,049 ± 6,459) (P = .90) (Figure 2). Most fluid input derived from IV mediations (37%), enteral nutrition (30%), and enteral free water (17%) rather than administration of IV crystalloids (14%), colloids (0%), or blood products (2%) (Table E3).
Diuretic Administration and Fluid Output
The mean cumulative furosemide dose received in the conservative fluid management group was 133 ± 361 mg versus 33 ± 68 mg in the usual care group (P = 0.34) (Table E2). Receipt of loop diuretic as a continuous infusion was more common for patients in the conservative fluid management group than in the usual care group (40% vs 0%; P = 0.02).
The 15 patients in the conservative fluid management group contributed a total of 56 patient-days alive and in the intensive care unit, on 23 (41.1%) of which vasopressors were administered such that study protocol did not dictated diuretic infusion. Of the remaining 33 patient-days, on 15 patient-days (45.5%) a diuretic infusion was administered (compliant with protocol), on 12 patient-days (36.4%) a diuretic infusion was not administered because the patient was net negative without diuretic infusion (compliant with protocol), and on 5 patient-days (15.2%) a diuretic infusion was not administered and the patient was net positive (non-compliant with protocol). The total volume net positive on each of these five non-compliant patient-days was 40 mL, 225 mL, 306 mL, 388 mL, and 934 mL, respectively. For patient-days on which a diuretic infusion was administered, the mean daily dose of loop diuretic in furosemide equivalents was 130 ± 116 mg/day, and the mean maximum hourly infusion rate was 10 ± 9 mg/hour.
Cumulative fluid output over the course of the study was 10,645 ± 11,733 mL in the conservative fluid management group compared with 6,286 ± 5,870 mL in the usual care group (P = .39) (Figure 2).
Hemodynamic, Laboratory, Radiographic, and Clinical Outcomes
Conservative fluid management did not overtly affect patients’ hemodynamics, respiratory function, duration of mechanical ventilation or vasopressor receipt, development of acute kidney injury, or receipt of renal replacement therapy (Table 2, Table E4–7). Only two patients (13.3%) in each study group experienced a plasma sodium concentration greater than 145 mmol/L in the first 14 days (P > .99). The conservative fluid management and usual care groups did not differ significantly with regard to in-hospital mortality (30.0% vs 26.7%, respectively; P > .99) or number of ICU-free days to day 14 (11 vs 9, respectively; P > .99) (Table 2).
Table 2.
Clinical Outcomes
| Outcome | Usual Care (n = 15) |
Conservative (n = 15) |
P Value |
|---|---|---|---|
| Clinical Outcomes | |||
| In-hospital mortality, No. (%) | |||
| Before ICU discharge | 2 (13.3) | 2 (13.3) | >.99 |
| Before hospital discharge | 4 (26.7) | 3 (30.0) | >.99 |
| Support-free days to study day 14 | |||
| ICU-free days , median [IQR] | 9 [0 – 12] | 11 [0 – 12] | >.99 |
| Ventilator-free days, median [IQR] | 13 [0 – 14] | 12 [0 −14] | .60 |
| Vasopressor-free days, median [IQR] | 13 [0 – 14] | 12 [0 – 14] | .60 |
| Renal replacement therapy-free days, median [IQR] | 14 [0 – 14] | 14 [14 – 14] | .36 |
| Renal Outcomes | |||
| Highest stage of acute kidney injury*, No. (%) | .28 | ||
| None | 5 (33.3) | 6 (42.9) | |
| Stage I | 0 (0.0) | 2 (14.3) | |
| Stage II | 4 (26.7) | 3 (21.4) | |
| Stage III | 6 (40.0) | 3 (21.4) | |
| Plasma creatinine, mg/dL | |||
| Highest after enrollment, median [IQR] | 2.3 [1.1 – 3.1] | 1.3 [.8 – 2.1] | .16 |
| Change from baseline to highest value, median [IQR] | 1.4 [0.1 – 2.2] | 0.4 [0.0 – 1.3] | .18 |
| Change from enrollment to highest value, median [IQR] | 0.0 [−0.1 – 1.4] | -.03 [−0.1 – 0.7] | .95 |
| Receipt of renal replacement therapy, No. (%) | 1 (6.7) | 1 (6.7) | >.99 |
ICU-free, ventilator-free, vasopressor-free, and renal replacement therapy free days refer to days alive and free from the specified therapy in the first 14 days after enrollment. ICU = intensive care unit, IQR = interquartile range
Acute kidney injury is defined according to Kidney Disease Improving Global Outcomes (KDIGO) criteria25.
DISCUSSION
In this pilot trial, a conservative fluid management protocol did not decrease mean daily fluid balance by the pre-specified threshold (500 mL per day) among patients with post-resuscitation sepsis. Despite achieving fluid output greater than input on every study day in the conservative fluid management arm, lower than anticipated IV fluid administration in usual care impeded separation between groups. These findings have important implications for the design and conduct of future studies of fluid management in sepsis.
Although numerous studies have evaluated fluid administration during sepsis resuscitation3–6,28,29, only three trials inform fluid management after resuscitation: the Fluid and Catheter Treatment Trial (FACTT)26 and two recent pilot studies of fluid restriction after sepsis resuscitation8,9. FACTT compared liberal versus conservative fluid management among 1,000 ventilated ARDS patients, 70% of whom had underlying infection26. In the liberal arm, cumulative fluid input exceeded output by 7 liters at 7 days compared to equal input and output in the conservative arm, resulting in a difference of 2 ventilator-free days and 2 ICU-free days between groups. In contrast, both recent pilot trials targeting fluid restriction after sepsis resuscitation achieved a numerical difference between groups in cumulative fluid balance by 5 days of around 1 liter8,9, with no differences in clinical outcomes.
Why did our trial fail to achieve the pre-specified separation in fluid balance between groups? One explanation is that fluid management in usual care was significantly different than anticipated. Unlike prior observational studies that reported fluid balances of five or more liters positive in the week after sepsis resuscitation11,19, patients in the usual care arm of our trial averaged less than 1 liter net positive in the 7 days after enrollment. Several factors may explain this unexpectedly low fluid balance. Inclusion of patients without shock may have selected sepsis patients who received less IV fluid. Beginning the trial 12 hours after ICU admission may have missed the period of greatest fluid administration. Current usual care may have shifted toward less fluid administration compared to historical cohorts.
Alternatively, our pre-specified threshold for separation in fluid balance between groups may have been unnecessarily large. When designing the trial, we felt that the difference in fluid balance would need to be at least 500 mL a day (half the daily difference in FACTT26) to exert a meaningful effect on organ edema and clinical outcomes. Recent data, however, suggest that even relatively small volumes of IV fluid may impact clinical outcomes6,7,29,30. When designing future trials of conservative fluid management in sepsis, consideration should be given to targeting more modest differences in absolute measures of fluid balance between groups.
In addition to the above, our study is limited by conduct at a single center, small sample size, and lack of blinding. The conservative fluid management protocol was developed by consensus – and may be subject to critique. Some experts would advocate for inclusion of dynamic measures of “fluid responsiveness” 31, but there are not yet data that guiding fluid administration by “fluid responsiveness” improves clinical outcomes. Our study attempted to control fluid management for 14 days, which was longer than prior trials and may be too long a time-period over which to effectively achieve separation between groups. Although we coordinated with the pharmacy to concentrate amenable medications, this did not result in a difference in medication volumes between groups. Additionally, targeting a 2.0 day absolute increase in ICU-free days in the phase III portion of the trial may have been overly ambitious for an intervention designed to decrease fluid balance by only 500 mL per day.
Our study also has several strengths. Despite most patients receiving vasopressors at enrollment, the conservative fluid management protocol achieved an average “net negative” fluid balance on each study day (without any overt harmful effects on clinical outcomes). The numerical difference between groups in cumulative fluid balance was greater than in any prior sepsis fluid management trial3–5,8,9,28. Strong compliance with fluid and diuretic administration instructions based on hourly blood pressure and urine output measurements demonstrates that adherence to an intensive study protocol is feasible.
Based on our findings, future trials of conservative fluid management in sepsis should: target patients likely to receive ongoing fluid administration; control fluid and diuretics in both study arms to prevent drift in usual care; and consider enrollment prior to ICU admission to control the period of highest fluid exposure.
CONCLUSIONS
In this pilot trial, a conservative fluid management protocol did not decrease mean daily fluid balance by ≥ 500 mL among patients with post-resuscitation sepsis. These findings may inform the design of future trials examining fluid management in sepsis.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the patients, nurses, nurse practitioners, residents, fellows, and attending physicians of the Vanderbilt Medical Intensive Care Unit for making this study possible.
SOURCES OF FUNDING
M.W.S. was supported by an National Heart, Lung, and Blood Institute (NHLBI) award (K23HL143053). Data collection utilized the Research Electronic Data Capture (REDCap) tool developed and maintained with Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH). W.H.S was supported in part by the National Institute of General Medical Sciences (K23GM110469). T.W.R. was supported in part by the NIH (R34HL105869). The funding institutions had no role in (1) conception, design, or conduct of the study, (2) collection, management, analysis, interpretation, or presentation of the data, or (3) preparation, review, or approval of the manuscript.
Footnotes
Subject Descriptor Number: 4.4 Clinical Trials in Critical Care Medicine
CONFLICTS OF INTEREST
All authors completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declared no potential conflicts of interest with the current work. W.H.S. reported serving on advisory boards for Venaxis, Inc., Ferring Pharmaceuticals, and Cempra Pharmaceuticals, and as a consultant for Abbott Point-of-Care. T.W.R. reported serving on an advisory board for Avisa Pharma, LLC and as the Director of Medical Affairs for Cumberland Pharmaceuticals, Inc.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45(3):486–552. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370(18):1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372(14):1301–11. [DOI] [PubMed] [Google Scholar]
- 5.ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371(16):1496–506. [DOI] [PubMed] [Google Scholar]
- 6.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011;364(26):2483–95. [DOI] [PubMed] [Google Scholar]
- 7.Andrews B, Semler MW, Muchemwa L, et al. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA 2017;318(13):1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med 2016;42(11):1695–705. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Kollef MH. Targeted Fluid Minimization Following Initial Resuscitation in Septic Shock: A Pilot Study. Chest 2015;148(6):1462–9. [DOI] [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41(2):580–637. [DOI] [PubMed] [Google Scholar]
- 11.Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011;39(2):259–65. [DOI] [PubMed] [Google Scholar]
- 12.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care Lond Engl 2013;17(5):R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest 2009;136(1):102–9. [DOI] [PubMed] [Google Scholar]
- 14.Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest 2000;117(6):1749–54. [DOI] [PubMed] [Google Scholar]
- 15.Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017; [DOI] [PubMed] [Google Scholar]
- 16.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012;367(20):1901–11. [DOI] [PubMed] [Google Scholar]
- 17.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012;367(2):124–34. [DOI] [PubMed] [Google Scholar]
- 18.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA J Am Med Assoc 2012;308(15):1566–72. [DOI] [PubMed] [Google Scholar]
- 19.Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care Lond Engl 2008;12(3):R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perner A, Gordon AC, Angus DC, et al. The intensive care medicine research agenda on septic shock. Intensive Care Med 2017;43(9):1294–305. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine; Chest 1992;101(6):1644–55. [DOI] [PubMed] [Google Scholar]
- 22.Grissom CK, Hirshberg EL, Dickerson JB, et al. Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome*. Crit Care Med 2015;43(2):288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang DT, Angus DC, Moss M, et al. Design and Rationale of the Reevaluation of Systemic Early Neuromuscular Blockade Trial for Acute Respiratory Distress Syndrome. Ann Am Thorac Soc 2017;14(1):124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA J Am Med Assoc 2012;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter 2012;2(Suppl):8. [Google Scholar]
- 26.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354(24):2564–75. [DOI] [PubMed] [Google Scholar]
- 27.McKown AC, McGuinn EM, Ware LB, et al. Preadmission Oral Corticosteroids Are Associated With Reduced Risk of Acute Respiratory Distress Syndrome in Critically Ill Adults With Sepsis. Crit Care Med 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345(19):1368–77. [DOI] [PubMed] [Google Scholar]
- 29.Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med 2014;42(11):2315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semler MW, Wanderer JP, Ehrenfeld JM, et al. Balanced Crystalloids versus Saline in the Intensive Care Unit. The SALT Randomized Trial. Am J Respir Crit Care Med 2017;195(10):1362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michard F, Teboul J-L. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002;121(6):2000–8. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


