Abstract
Phase II data suggest a benefit to autotransplantation for aggressive T-NHL in first remission; randomized trials have yet to validate this. We performed a retrospective analysis of aggressive T-NHL patients in the intergroup randomized consolidative autotransplant trial (SWOG 9704). Of the 370 enrolled, 40 had T-NHL: 12 were not randomized due to ineligibility (n=1), choice (n=2), or progression (n=9), leaving 13 randomized to control and 15 to ASCT. Two ASCT patients refused transplant and one failed mobilization. The 5-year landmark PFS/OS estimates for ASCT vs control groups were 40% vs 38% (p=0.56) and 40% vs 45% (p=0.98), respectively. No difference was seen based on IPI, or histologic subtype. Only 1/7 receiving BCNU-based therapy survived versus 4/5 receiving TBI. Aggressive T-NHL autotransplanted in first remission did not appear to benefit from consolidative ASCT. This and the 30% who dropped out pre-randomization mostly to progression, suggests that improved induction regimens be developed.
Background
Aggressive T-cell non-Hodgkin’s lymphoma (T-NHL) is a heterogeneous group of diseases with variable prognosis depending on subtype, disease stage and International Prognostic Index (IPI) score at diagnosis, that comprise approximately 10% of all diffuse aggressive NHL.. With the exception of ALK-positive anaplastic large T-cell lymphoma (ALCL) and localized peripheral T-cell lymphoma (PTCL), its prognosis is particularly poor with a 5-year overall survival combined of approximately 30% when treated with conventional chemotherapy alone (1,2). Thus the initial therapy for these rare lymphomas remains suboptimal as well as controversial, as most studies are limited by small numbers of patients, retrospective analyses and the inclusion of ALK-positive ALCL. This poor outcome has formed the basis of the interest in consolidative therapy for responders, to improve outcome in the form of autologous stem cell transplant (ASCT) (3- 11).
ASCT has long been known to improve both progression-free survival and overall survival among patients with diffuse aggressive non-Hodgkin’s lymphoma in second remission [12]. The use of consolidative ASCT in first remission for high-grade aggressive B- and T-cell NHL, defined as high-intermediate or high age-adjusted IPI disease, has also been controversial with most studies performed in B-cell NHL, before the routine use of rituximab containing regimens, with some but not all showing a potential benefit in progression free survival (PFS) but not overall survival (OP) [13]. While data from phase II trials have suggested a value of early ASCT for T-NHL [3-9], these studies are limited by the small number of patients treated, short follow up and/or inclusion of patients with ALK+ ALCL. Nevertheless largely due to the poor prognosis of patients with this group of diseases, and based solely on Phase II data, current national treatment guidelines do recommend consolidation with high-dose chemotherapy followed by ASCT for all transplant-eligible T-NHL patients in first remission. Indeed, no randomized studies exist demonstrating the benefit of early ASCT for T-NHL, especially for those with adverse risk factors (adverse histology, stage, completeness of initial remission).
We recently reported on an international, intergroup trial which re-visited the strategy of consolidative autotransplants for high risk patients with diffuse aggressive NHL of both B- and T-cell types (SWOG-led S9704 trial) [14]. After receiving 5 cycles of induction chemotherapy with either CHOP or R-CHOP, responding patients were randomized to either 3 more cycles of chemotherapy versus 1 additional cycle followed by an ASCT using either a carmustine or total body irradiation (TBI) based preparative regimen. While the transplant arm had again a superior progression-free survival (PFS) compared to the chemotherapy only arm, no overall survival (OS) advantage was noted. Importantly, no differential treatment effect for those with T-NHL patients as compared to B-NHL in the initial analysis of this study was found. However, a post hoc analysis of the entire trial did find a survival advantage for those with high IPI disease.
Based on the fact that aggressive poor risk T-NHL patients were included in S9704, and the pathology was centrally reviewed for diagnosis and inclusion, we sought to determine in the only randomized data to date, the value of a first remission consolidative transplant in this population of poor risk T-NHL patients.
Methods
Study Design, Patients and Oversight
SWOG S9704 trial [14] was a SWOG-led randomized intergroup trial conducted at 40 sites and included Eastern Cooperative Oncology Group (ECOG), Cancer and Leukemia Group B (CALGB), and the Canadian NCIC Clinical Trials Group. Eligible patients were 15-65 years old with biopsy-proven diffuse aggressive NHL (either B or T cell phenotype) with high-intermediate or high age adjusted international prognostic index (IPI). Patients with central nervous system involvement at diagnosis were not eligible for this trial. Central pathologic was performed to confirm diagnosis and eligibility based on 2008 WHO criteria. Untreated patients and those who received only 1 prior cycle of chemotherapy were allowed to enroll. All patients received 5 cycles of CHOP chemotherapy (with or without rituximab, depending on phenotype); responders were subsequently randomized to either 3 more cycles of CHOP (control arm) or 1 more cycle of CHOP followed by ASCT (transplantation arm). Enrollment began on August 15, 1999 and concluded on Dec 15, 2007 when the enrollment goal was met.
Of the induction-eligible 370 patients, 40 had an aggressive T-NHL phenotype and were subject to this subgroup analysis. Individual patient files were re-reviewed and those randomized after the first 5 cycles of CHOP were further analyzed for stage, IPI group, histology (centrally reviewed), and response to induction and consolidation. Furthermore, survival outcomes were updated.
The SWOG S9704 trial was designed by the leadership of the lymphoma committees of the U.S. and Canadian Cooperative Groups and was approved by the National Cancer Institute. The data were gathered and analyzed by the SWOG Statistical Center. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines and was approved by the local institutional review boards at each participating site. All patients provided a written informed consent prior to enrollment.
Treatment Plan
Enrolled patients were treated with 5 cycles of CHOP/CHOP-R administered every 3 weeks; those who achieved at least a partial response were eligible for randomization to either the control arm in which patients received 3 more cycles of CHOP/CHOP-R or the transplantation arm in which patients received 1 more cycle of CHOP/CHOP-R followed by consolidation with autologous stem cell transplantation [Figure-1]. Patients in the transplantation arm received either carmustine-based or total body irradiation (TBI)-based preparative regimen. Carmustine-based regimen consisted of high-dose carmustine (300 mg per square meter of body surface area) on day −6 with high-dose etoposide (60 mg per kilogram of ideal body weight) on day −4 and high-dose cyclophosphamide (100 mg per kilogram of ideal body weight) given on day −2. TBI-based preparative regimen consisted of a total of 12 Gy of radiation in eight 1.5-Gy fractions given twice daily on days −8 through −5, along with the same doses and schedule of etoposide and cyclophosphamide. All patients 60 years of age or older received the carmstine-based regimen; all patients younger than 60 years of age at a given institution either underwent TBI or received carmustine-based regimen, in accordance with institutional preference. Stem cells were infused on day 0; the site investigators determined decisions about supportive care.
Figure - 1:
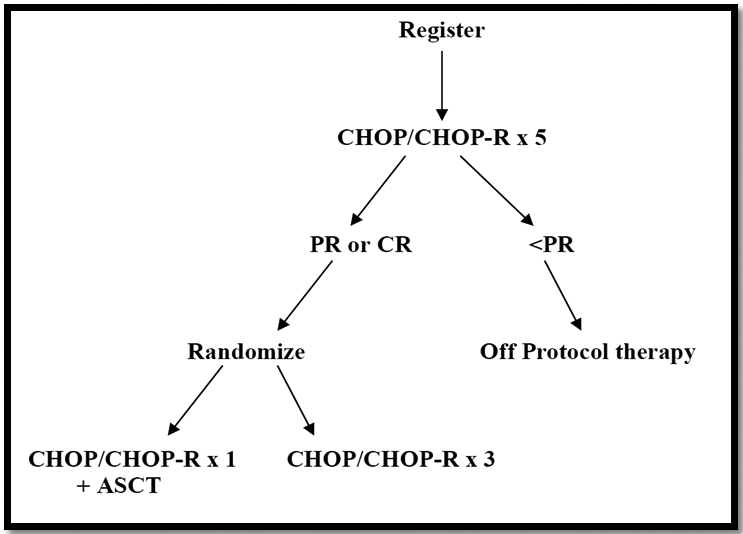
Treatment Plan
Disease evaluation post-treatment consisted of quarterly physical exam and computed tomographic scanning over a period of 2 years starting at day 60 after completion of assigned treatment. Additional therapy (including radiation) was only permitted in cases of biopsy-proven residual or progressive disease.
Statistical Analysis
The objectives of this subgroup analysis is to evaluate prospective early ASCT in age-adjusted high or high-intermediate risk T-NHL patients, perform a subset survival analysis for this aggressive poor risk subgroup, identify characteristics of patients with T-NHL enrolled in S9704 and the outcomes of those randomized and those who had early failure of treatment, and finally evaluate the prognostic factors for optimal outcomes in this group. The landmark progression-free survival (PFS) and landmark overall survival (OS) were analyzed in this subgroup. PFS was defined as the time from the date of randomization until the first observation of progressive disease or death due to any cause. Patients last known to be alive and progression-free were censored at the data of last contact. OS was defined as the time from the date of randomization until the date of death due to any cause. Patients last known to be alive were censored at the date of last contact. PFS and OS estimates with 95% confidence intervals were calculated using Kaplan-Meier method. PFS and OS were compared using 2-sided log-rank test at alpha level of 0.05. All eligible, randomized T-NHL patients were included in the analysis regardless of whether they actually received the treatment to which they were randomly assigned (intent-to-treat analysis).
Results
Patients Registration & Randomization
A total of 397 patients were registered for SWOG S9704 trial, of which 370 met eligibility criteria (Figure #1). Of the induction-eligible patients, 40 had T-NHL phenotype and were subject to this subgroup analysis (Figure #2). Of these, 28 patients (70%) were randomized after induction therapy; a similar ratio to the entire trial (68%). Twelve were not randomized due to either ineligibility (n= 1), patient choice (n= 2), or early disease progression (n= 9). Of the eligible 11 patients who did not continue, 9 were high-intermediate IPI and 8 had peripheral T cell (PTCL-NOS). The majority of patients (78%) who were excluded due to disease progression experienced that after cycle 3: 3 patients progressed after cycle 3, 1 after cycle 4 and 3 after cycle 5, however 2 patients progressed after only 1 cycle of treatment. Of the twenty-eight randomized T-NHL patients, 13 were randomized to the control arm and 15 to the transplantation arm, however, 3 did not undergo transplant due to either patient refusal (n= 2) or failure of mobilization (n= 1). Preparative regimens for those who received autologous stem cell transplant (n= 12) were either carmustine-based (n= 7) or TBI-based (n= 5).
Figure - 2:
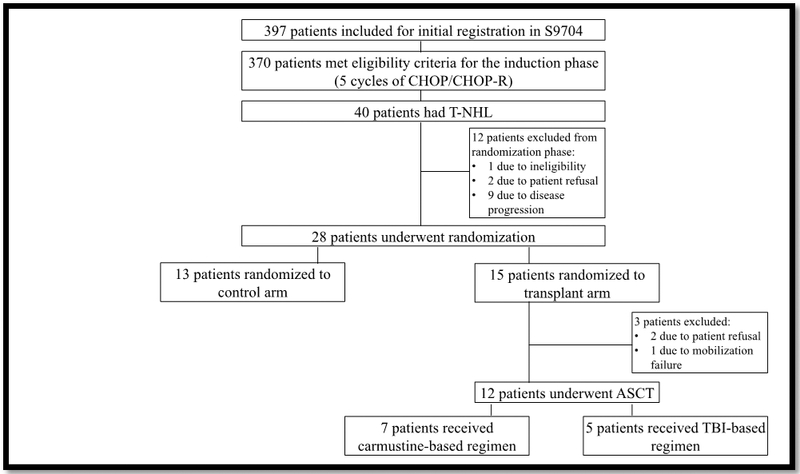
Patients Registration & Randomization
Patient Characteristics
The characteristics of the forty patients included in this subgroup analysis are summarized in Table-1. Median age for the randomized patients (n= 28) was 50 years. 19 of the 28 randomized patients were males (68%) and 9 were females (32%). Of these 28 patients, 21 patients (75%) had B-symptoms, and 14 (50%) had stage IV disease at diagnosis. Eighteen (64%) and 10 patients (36%) respectively were in the high-intermediate and high age-adjusted IPI risk group, respectively. The histologic subtypes treated included 11 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 7 with angioimmunoblastic large cell lymphoma and 10 with anaplastic large cell NHL (ALCL). Of the ALCL patients, only 3 were ALK positive.
Table – 1:
Patient Characteristics
| Randomized (n=28) |
Non-randomized (n=12) |
|
|---|---|---|
| Age | ||
| • Median | 50 years | 43 years |
| • Range | 26-65 | 34-61 |
| Gender- no.(%) | ||
| • Males | 19 (68%) | 6 (50%) |
| • Females | 9 (32%) | 6 (50%) |
| Histologic Subtype– no.(%) | ||
| • PTCL-NOS | 11 (39%) | 9 (75%) |
| • ALCL | 10 (36%) | 3 (25%) |
| • Angioimmunoblastic T-cell lymphoma | 7 (25%) | 0 (0%) |
| Age-adjusted IPI risk group | ||
| • High-intermediate risk | 18 (64%) | - |
| • High risk | 10 (36%) | - |
| B-symptoms at diagnosis- no.(%) | 21 (75%) | 8 (67%) |
| Stage IV disease at diagnosis- no.(%) | 14 (50%) | 9 (75%) |
Outcomes
The median length of follow-up among randomized patients last known alive is 7.8 years after randomization (range 2.9-12.4 years). Progression-free survival (PFS) and overall survival (OS) estimates were based on the intent-to-treat population. The 5-year PFS for the transplant group was 40% (95% CI: 16.5%, 62.8%) compared to 38% (95%CI: 14.1%, 62.8%) for the chemotherapy-only arm [2-sided log rank p-value = 0.56, Figure - 3]. 5-year overall survival estimates were 40% (95% CI: 16.5%, 62.8%) vs 45% (95%CI: 17.7%, 69.0%) for the transplant group and the control group, respectively [2-sided log rank p-value = 0.98, Figure - 4]. Furthermore, no difference noted in outcome based on IPI group, histology or stage of disease. Interestingly, of the seven patients who received carmustine-based preparative regimen prior to ASCT only one is a long-term survivor compared to four out of five receiving the TBI-based regimen. A detailed description of outcome and potential risk factors is shown in Table 2.
Figure - 3 :
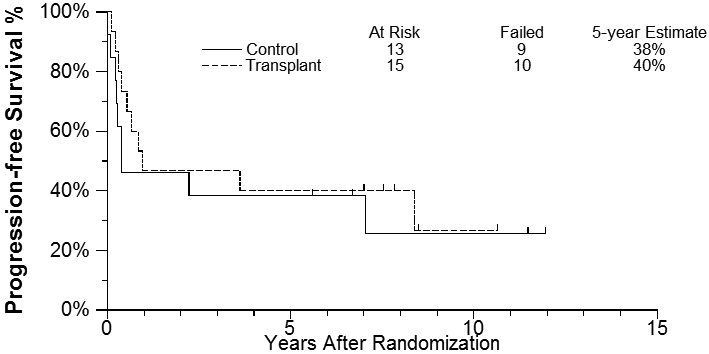
Progression-Free Survival Estimates
Figure - 4 :
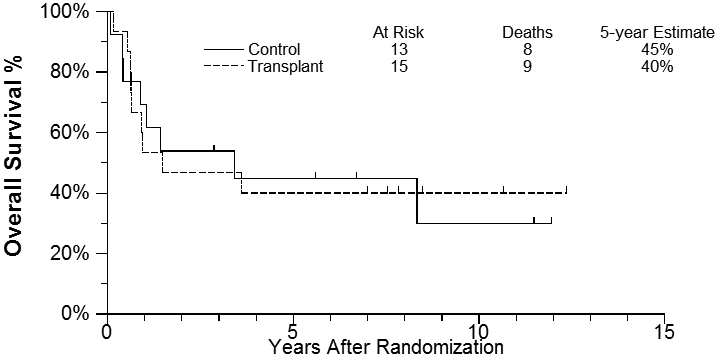
Overall Survival Estimates
Table 2:
Outcome and Risk Factors of Randomized Patients to Transplant
| # | Status | Arm | Survival (days) |
Age | Bulky | Stage | Symptoms | IPI2 | Sex | LDH | BM Involved |
Extranodal | Histology | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | A | Refused | 2954 | 29 | No | III | B | HI | M | NL | N | N | ALCL, ALK(+)3 | |
| 2. | A | BCV | 2639 | 61 | No | III | A | HI | F | High | N | Y | PTCL4 | |
| 3. | A | TBI | 4624 | 54 | No | III | B | H | F | High | Y | Y | ANGIO5 | |
| 4. | A | TBI | 2863 | 27 | Yes | IV | B | H | M | High | N | Y | PTCL | |
| 5. | A | TBI | 3976 | 46 | No | IV | A | HI | M | N | N | Y | ALCL, ALK(−) | |
| 6. | A | TBI | 3173 | 58 | No | III | A | HI | M | High | N | N | ALCL,ALK(+) | |
| 7. | D | Refused | 311 | 48 | No | IV | B | HI | M | High | Y | Y | PTCL | UNK |
| 8. | D | MF1 | 282 | 60 | No | III | B | HI | M | High | N | N | ALCL,ALK(−) | SEPSIS6 |
| 9. | D | BCV | 1408 | 51 | No | III | A | HI | M | High | N | N | ALCL,ALK(−) | UNK |
| 10. | D | BCV | 156 | 26 | No | IV | B | HI | M | NL | N | Y | ALCL,ALK(+) | NHL |
| 11. | D | BCV | 438 | 51 | No | IV | B | H | M | High | N | Y | ALCL,ALK(−) | NHL |
| 12. | D | BCV | 310 | 37 | No | IV | B | HI | M | High | Y | Y | PTCL | SHOCK6 |
| 13. | D | BCV | 427 | 59 | No | III | A | HI | F | High | N | N | PTCL | UNK |
| 14. | D | BCV | 641 | 65 | No | III | A | HI | M | High | N | N | ANGIO | NHL |
| 15. | D | TBI | 319 | 59 | Yes | IV | B | HI | M | High | N | Y | PTCL | NHL |
Mobilization Failure
HI:Hiqh Intermediate; H: High
ALCL (Anaplastic, ALK (+) or (−))
Peripheral T Cell Lymphoma
Angioimmunoblastic
Related to treatment for relapse
As ALK positive ALCL patients have a favorable prognosis, we further analyzed the outcomes of the randomized ALCL patients. Of the 7 ALK negative/unknown patients, 4 were randomized to transplant; one collected insufficient stem cells to proceed and relapsed at 4 months. Of the remaining 6, 3 were randomized to transplant and survived 9 months, 3.5 years and 10.5+ years, while those randomized to CHOP alone survived 7 years, 6.5+ and 8+ years. Of the 3 ALK positive patients, all were randomized to transplant;: 1 refused and is alive 7+ years, 1 died at 1 month of progressive disease and one is alive at 8.25+ years.
Discussion
With the exception of ALK-positive ALCL and localized T-cell lymphoma, the prognosis of diffuse aggressive T-NHL is particularly poor and largely determined by subtype, disease stage and International Prognostic Index (IPI) score at diagnosis [3,15-17]. Left untreated, the estimated survival of PTCL is measured in months with a 5-year overall survival after conventional chemotherapy alone of 20 percent and 6 percent for the patients with high intermediate (i.e, 3) or high (i.e, 4 to 5) IPI scores, respectively. The grim outcomes of these patients after chemotherapy generated the interest in consolidative approaches in the form of ASCT. However, after 15+ years of trial data, the heterogeneity of the different subtypes and relatively low-incidence of PTCL still make defining a “standard-of-care” a challenge [15-17].
Over the past decade, consolidation with ASCT for aggressive T-NHL in first remission was investigated by several prospective phase II trials [3-9,18] . While this has become an accepted standard of care for T-NHL given its poor prognosis when treated with chemotherapy alone, the favorable results were frequently limited by small sample size and/or short follow up, and do not account for patient selection bias. Ours is the first study evaluating ASCT in aggressive T-NHL in the context of a randomized prospective trial design. PFS and OS estimates in our study were based on the intent-to-treat population randomized only after a first remission was documented. Despite treating very high risk patients with high intermediate or high IPI disease, the 5-year PFS for the ASCT group was 40% compared to 38% for the chemotherapy-only arm (P value = 0.56, Figure - 3], and the 5-year overall survival estimates were only 40% vs 45% for the transplant group and the control group, respectively [P value = 0.98, Figure - 4]. While these data should be considered somewhat speculative in view of the small numbers randomized, 40 such high risk patients were entered onto the trial and the results stand as the largest randomized analysis of these high risk patients with no obvious benefit for those patients randomized.. In addition, the ASCT arm outcomes are nearly identical to outcomes reported recently by Wilhelm et al in a large single arm prospective study of ASCT in first remission [9]. They had a similar early dropout rate before the planned ASCT due to early progression and reported a nearly identical PFS and overall survival of 39% and 44% at 5 years. While they conclude that ASCT should be part of upfront therapy, our CHOP × 8 control arm showing a similar outcome would argue against this conclusion.
Whether ASCT is of value for certain T-NHL histologies is undetermined by our data. According to the International T-cell Lymphoma Project, survival of the different subtypes of PTCL is largely determined by histology, ranging from a 5-year OS of 90% in ALK-positive ALCL to less than 10% in hepatosplenic T-cell lymphoma [15,19]. Similar to other studies evaluating ASCT in T-NHL, our subgroup analysis is limited by the small sample size. However, with the exception of Corradini trial that included ALK-positive ALCL[5], all other ASCT trials did not show significant outcome differences between T-NHL subtypes [4, 6-9]. Indeed our analysis of the ALK negative patients adds to this conclusion.
Interestingly, in our analysis we found that TBI-based regimens were associated with improved survival (4/5) when compared to carmustine-based chemotherapy-only (1/7) preparative regimens. Historically, as is the case with all NHL, preparative treatment regimens are either carmustine or TBI-based. Most of the previous trials evaluating upfront ASCT in PTCL typically used chemotherapy-only preparative regimens [5-7, 19], with the only exception of the study by Wilhelm et al. where a fraction of patients received TBI/Cyclophosphamide [9]. Whether TBI-based therapy is optimal can not be determined by out data and thus remains to be determined. The transplant regimen was factored into the large CIBMTR analysis of ASCT vs allografting for both early and relapsed T-NHL and not found to be prognostic [11], however whether this regimen is superior for just first remission patients receiving ASCT alone is unknown..
While we found no difference in PFS or OS in this subgroup analysis of T-NHL patients randomized or not to autotransplant if responsive, whether in clinical PR or CR, after 5 cycles of CHOP there are limitations to this analysis, especially the small sample size and that this analysis is a subgroup retrospective analysis. This and the fact that one third of patients in our study progressed before randomization, has led our ongoing focus for T-NHL to focus on developing better induction therapies. While some have suggested that CHOP plus etoposide (CHOEP) [2,18], or other chemotherapy induction regimens [4-9] may be superior to CHOP, there are no controlled data. Of note, the CHOEP regimen’s benefit was initially noted to be mainly in younger patients, those with ALK-positive ALCL and a normal lactate dehydrogenase at diagnosis [2,20, 21]. Based on this we suspect that our results might not have been better with CHOEP as tested by the Nordic group trial which performed an ASCT in a similar poor prognosis group as we had with this induction regimen demonstrating a 5-year PFS of 44%, similar to our CHOP-treated patients [18] .
As an alternative to multiagent chemotherapy induction regimens, a recent National Cancer Institute Clinical Trials Planning Meeting in Lymphoma concluded that with several new targeted agents approved for T-NHL in the relapsed setting, the best approach going forward would be to either incorporate these in combination as novel doublets, combine them with a standard CHOP regimen, or utilize a personalized medicine approach based on recently described molecular or biomarkers, e.g. brentuximab vedotin for CD30+ subsets [16]. Such trials are currently being designed. Minimal residual disease (MRD) measurements, when available should be incorporated into the efficacy of these approaches, and if MRD positivity in first remission is a marker for relapse, a re-exploration of up front autologous or even allogeneic transplantation should be considered.
In conclusion, while the available data is still unclear as to the role of upfront ASCT in patients with aggressive T-NHL, both Phase II and our retrospective analysis of Phase III subset data demonstrate that the majority of T-NHL patients still die of their disease. Criteria are needed to better identify patients who would benefit best from this approach, and should take into consideration the different histologies, and molecular signatures of this diverse group of tumors.
Acknowledgements:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180888, CA180819, CA180820, CA180821, CA180863, CCSRI #021039; legacy grants CA46282, CA04919, CA11083, CA58658, CA46368, CA13612; and in part by Bristol-Myers Squibb. The content is solely the responsibility of the authors and does not necessarily represent the official views of either the National Institutes of Health or Bristol-Myers Squibb. The contributions of Dr. Raymond R Tubbs to this work are recognized posthumously and gratefully acknowledged by his co-authors.
REFERENCES
- 1.Vose J, Armitage J, Weisenburger D, International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124–30. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Trümper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–25. [DOI] [PubMed] [Google Scholar]
- 3.Sonnen R, Schmidt W-P, Müller-Hermelink HK, Schmitz N. The International Prognostic Index determines the outcome of patients with nodal mature T-cell ymphomas. Br J Haematol. 2005;129:366–72. [DOI] [PubMed] [Google Scholar]
- 4.Nademanee A, Palmer JM, Popplewell L, et al. High -dose therapy and autologous hematopoietic cell transplantation in peripheral T cell lymphoma (PTCL): Analysis of prognostic factors. Biol Blood Marrow Transplant 2011;17:1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corradini P, Tarella C, Zallio F, Dodero A, Zanni M, Valagussa P, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–8. [DOI] [PubMed] [Google Scholar]
- 6.Mercadal S, Briones J, Xicoy B, Pedro C, Escoda L, Estany C, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol 2008;19:958–63. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez J, Conde E, Gutiérrez A, Arranz R, León A, Marín J, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from The Gel-Tamo Study Group. Eur J Haematol. 2007;79:32–8. [DOI] [PubMed] [Google Scholar]
- 8.Reimer P, Rüdiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–13. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm M, Smetak M, Reimer P, Geissinger E, Ruediger T, Metzner B, et al. First-line therapy of peripheral T-cell lymphoma: extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016. July 29;6:e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jantunen E, D’Amore F. Stem cell transplantation for peripheral T-cell lymphomas. Leuk Lymphoma 2004;45:441–446. [DOI] [PubMed] [Google Scholar]
- 11.Smith S, Burns LJ, van Beisen K, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol 2013; 31:3100–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5. [DOI] [PubMed] [Google Scholar]
- 13.Stiff P What is the role of autologous transplant for lymphoma in the current era. Hematology Am Soc Hematol Educ Program 2015;2015:74–81. [DOI] [PubMed] [Google Scholar]
- 14.Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vose J, Armitage J, Weisenburger D,. International peripheral T-Cell and natural killer/T-cell lymphoma study: Pathology and clinical outcomes. J Clin Oncol 2008; 26:4124–4130. [DOI] [PubMed] [Google Scholar]
- 16.Casulo C, O’Connor O, Shustov A, et al. T-cell lymphoma: Recent advances in characterization and new opportunities for treatment. J Natl Cancer Inst 2017; 109;djw248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood. 2014;123:2636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.d’Amore F, Relander T, Lauritzen GF et al. Up -front autologous stem-cell transplantation in peripheral T-Cell lymphoma:NLG-T-01. J Clin Oncol 2012. 30:3093–3099. [DOI] [PubMed] [Google Scholar]
- 19.Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK-anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–504. [DOI] [PubMed] [Google Scholar]
- 20.Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124:1570–7. [DOI] [PubMed] [Google Scholar]
- 21.Lunning MA, Horwitz S. Treatment of peripheral T-cell lymphoma: are we data driven or driving the data? Curr Treat Options Oncol. 2013;14:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


