Mature T and natural killer (NK)-cell neoplasms are heterogeneous non-Hodgkin lymphomas (NHL), accounting for 10% of all NHL in the United States. Compared to aggressive B-cell lymphomas, they have a much lower cure rate after anthracycline-based chemotherapy regimens[1]. In a retrospective analysis of T and NK-cell lymphomas treated between 2000 to 2010 with predominantly CHOP-like regimens, the 3-year progression free survival (PFS) and overall survival (OS) were 32% and 52%, respectively[1]. By comparison, a matched cohort of diffuse large B-cell lymphoma (DLBCL) treated with R-CHOP had a 5-year OS of 70%[2]. The disparity can be partially explained by the addition of the anti-CD20 monoclonal antibody, rituximab, to combination chemotherapy for DLBCL[3, 4]. Frontline therapy for most patients with peripheral T-cell lymphomas (PTCLs) is CHOP-like chemotherapy without a monoclonal antibody, but with exception of ALK+ anaplastic large cell lymphoma (ALCL), most patients will relapse. In a phase 2 trial of untreated patients with PTCL, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-EPOCH) had an overall response rate (ORR) of 78% with 61% achieving complete response (CR)[5]. The 2-year PFS of DA-EPOCH was only 53%, however. Active agents that can be safely be added to DA-EPOCH in PTCL offer the potential to improve the cure rate.
Alemtuzumab is a humanized monoclonal antibody targeting CD52, a cell surface glycoprotein expressed on both normal and malignant B- and T-cells, in addition to macrophages and monocytes[6]. When bound to cell surface CD52, alemtuzumab causes cellular lysis via activation of complement and direct cell-mediated cytotoxicity. The expression of CD52 in T-cell lymphoproliferative neoplasms is variable, with rates ranging from 35–92% in PTCL-NOS and 40–100% in angioimmunoblastic T-cell lymphoma[7, 8]. Given poor outcomes in T & NK cell neoplasms, we hypothesized that addition of alemtuzumab to DA-EPOCH in CD52+, aggressive T & NK cell neoplasms may improve the cure rate. We performed a single-center phase 1/2 study of alemtuzumab in combination with DA-EPOCH in patients with T & NK cell neoplasms.
Patients (age ≥17 years) were enrolled between September 2003 and December 2008, and data was locked in January 2017. Patients had untreated, aggressive T & NK cell lymphomas that expressed CD52. Patients with ALK-positive ALCL and T cell precursor disease were not eligible. Pathological diagnosis was determined by Laboratory of Pathology, NCI. Further eligibility criteria included normal organ function unless due to tumor involvement.
Alemtuzumab was administered in three escalating dose cohorts at intravenous doses of 30 mg, 60 mg, and 90 mg. A 3+3 dose-escalation design was used. Alemtuzumab was given day 1 of each cycle followed by dose-adjusted EPOCH on days 1–5 of a 21-day cycle [9]. Six to eight cycles of therapy were administered unless progressive disease was noted on interim assessment. For CNS disease treatment and high-risk CNS prophylaxis see Supplement 1. Prior to each cycle, CD4/CD8/NK cell counts and polymerase chain reaction test for cytomegalovirus were obtained. All patients received prophylactic antimicrobials to prevent pneumocystis jiroveci, herpes simplex virus, and fungal infections.
The primary objective was to assess the feasibility and safety of administering alemtuzumab in combination with DA-EPOCH and to determine a maximum tolerated dose (MTD) of alemtuzumab. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for AEs, version 3.0. Dose-limiting toxicity (DLT) for alemtuzumab was defined as grade 3 allergic toxicity, grade 3 non-hematologic toxicity lasting longer than 6 hours after the infusion or grade 4 non-hematologic toxicity (excluding dyspnea). Secondary objectives included assessments of best response, PFS, and OS. Efficacy was evaluated by response criteria via International Working Group Criteria[10]. Responses were assessed after the fourth and sixth cycles of therapy. At completion of therapy, patients had clinic visits and CT scans every 3 months for the first year, every 4 months for the second year, every 6 months for years 3–5, and annually thereafter.
Overall survival was determined from the on-study date until date of death or last follow-up. PFS was determined from the on-study date until date of progression, death without prior progression, or last follow-up. The probability of OS and PFS was determined by Kaplan-Meier method. The statistical significance between a pair of Kaplan-Meier curves was determined by a log-rank test. All p-values are two-tailed without adjustment for multiple comparisons.
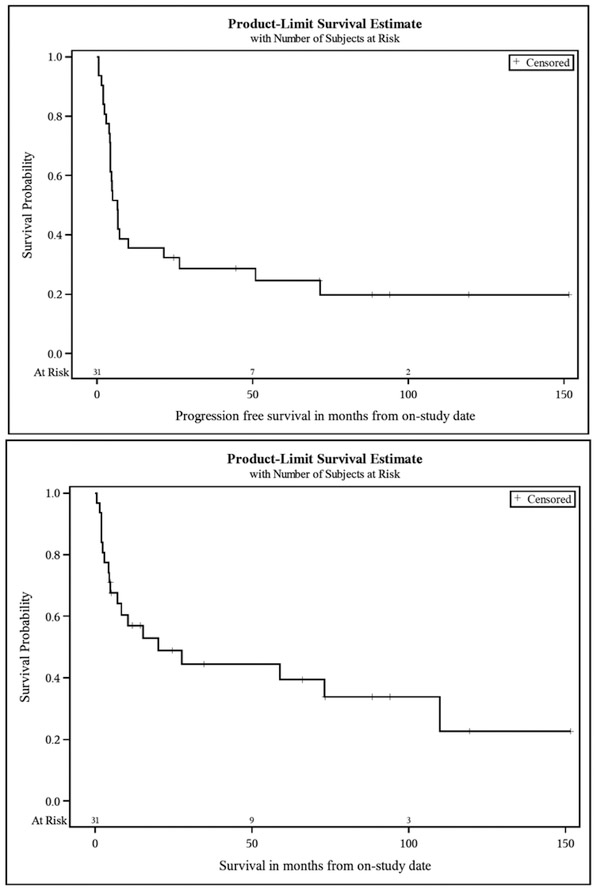
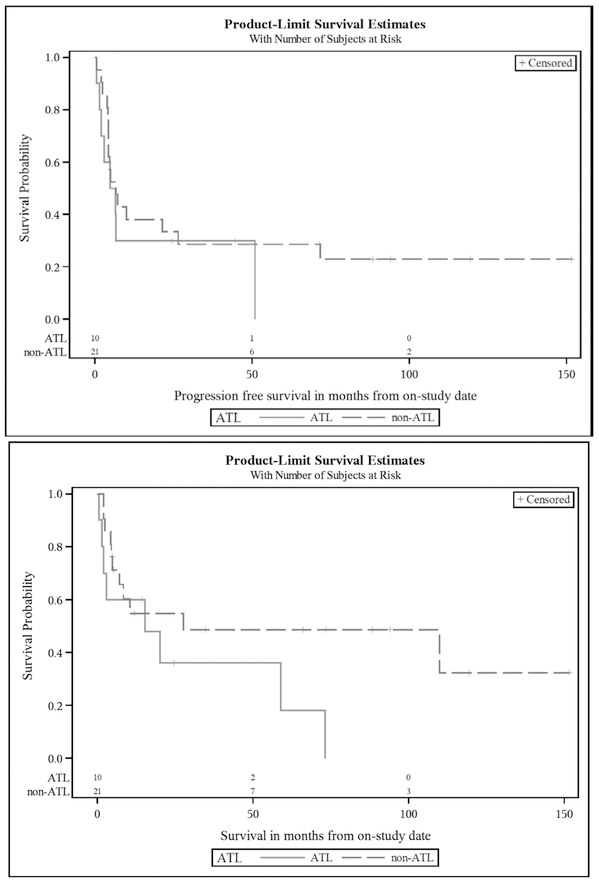
A total of 31 patients were enrolled (Table 1). In phase 1, four patients were treated with 30mg alemtuzumab and three patients each were treated with 60mg and 90mg, respectively. The remaining patients received 30mg, which was determined as the safe tolerated dose. One patient never received the study regimen due to rapidly progressive neurologic compromise from CNS involvement. Of 30 patients treated, 17 achieved a CR (56.6%), 8 achieved a PR (26.6%; 83.3% ORR), 2 had progressive disease (6.6%), and 1 had stable disease (3.3%); two patients were unevaluable secondary to death during treatment. With median potential follow-up of 10.9 years, the median PFS was 6.6 months and the median OS was 20.2 months (Figure 1). In addition to the two patients with progressive disease during therapy, there were 5 cases of treatment-related mortality and 16 subsequent relapses attributing to PFS rates. Subset analysis of ATL versus non-ATL histologies was not statistically significant but trended towards a more favorable OS with non-ATL histologies (Figure 2).
Table 1.
Clinical Characteristics and Response.
| Parameter | Value | Evaluable No. patients |
CR No. patients (%) |
PR No. patients (%) |
SD No. patients (%) |
PD No. patients (%) |
|---|---|---|---|---|---|---|
| Number of patients | 31 | 28 | 17 (60.7%) | 8 (28.5%) | 1 (3.5%) | 2 (7.1%) |
| Age, median y (range) | 49 (17-77) | |||||
| Gender (M / F) | 15M / 16F | |||||
| Ann Arbor stage no. patients (%) | ||||||
| Stage I-II | 3 (9.6%) | |||||
| Stage III-IV | 28 (90.3%) | |||||
| IPI score no. patients (%) | ||||||
| Low + Low-intermediate (0-2) | 11 (35.4%) | |||||
| High-intermediate +High (3-5) | 20 (64.5%) | |||||
| Histologic subtype no. patients(%) | ||||||
| PTCL-NOS | 11 (35.4%) | 10 | 7 (70%) | 3 (30%) | ||
| ATL | 10 (32.2%) | 8 | 5 (62.5%) | 2 (25%) | 1 (12.5%) | |
| AITL | 4 (12.9%) | 4 | 3 (75%) | 1 (25%) | ||
| CGDTCL | 2 (6.4%) | 2 | 1 (50%) | 1 (50%) | ||
| Hepatosplenic TCL | 2 (6.4%) | 2 | 1 (50%) | 1 (50%) | ||
| Other* | 2 (6.4%) | 2 | 2 (100%) |
Abbreviations: PTCL-NOS, peripheral T cell lymphoma not-otherwise-specified; ATL, adult T cell leukemia/lymphoma; AITL, angioimmunoblastic lymphoma; CGDTCL, cutaneous gamma delta T cell lymphoma
Other histologic subtypes included one case of ALK-negative anaplastic large cell lymphoma and NK/T cell lymphoma
Figure 1.
Kaplan–Meier Curves for Progression-free Survival and Overall Survival. The PFS probability (top panel) for treated patients was 35.5% (95% CI: 19.4 – 51.9%) at 12 months and 32.3% (95% CI: 16.9 – 48.6%) at 24 months, with median PFS 6.6 months (95% CI: 4.3 – 21.5 months). The OS probability (bottom panel) for patients treated with DA-EPOCH with alemtuzumab was 56.9% (95% CI: 37.5 – 72.4%) at 12 months and 48.8% (95% CI: 29.6 – 65.5%) at 24 months, with median OS 20.2 months (95% CI: 5.0 – 109.9 months).
Figure 2.
Kaplan-Meier Curves for Progression-free Survival and Overall Survival for ATL vs. non-ATL histologies. The median PFS (top panel) for ATL and non-ATL histologies was 5.7 months (95% CI: 0.5 – 51.0 months) and 6.8 months (95% CI: 4.3 – 26.4 months), respectively (p=0.41). The median OS (bottom panel) for ATL and non-ATL histologies was 15.3 months (95% CI: 0.5 – 73.2 months) and 27.6 months (95% CI: 5.0 months – undefined), respectively (p=0.11).
Thirty patients received at least one cycle of DA-EPOCH with alemtuzumab and were analyzed for safety. In phase 1, no protocol-defined DLTs were observed, but severe myelosuppression occurred in two patients in both the 60mg cohort and the 90mg cohort. Hence, the MTD was considered to be 30mg of alemtuzumab. The median number of cycles completed was 6 (range 1–7), but only 63% of patients completed the planned therapy. The most common AEs were infectious and hematologic (Supplement 1). Grade 3/4 AEs that occurred in greater than 10% of all cycles included anemia (33.1%), neutropenia (66.2%), febrile neutropenia (18.5%), lymphopenia (48.3%), thrombocytopenia (27.2%), and infection (12.6%). In 28 patients where CD4 counts were available during therapy, the median CD4 count of individual patient averages during therapy was 163 cells/mm3 (range 7 – 15383 cells/mm3).
Infectious complications represented the most common non-hematologic AEs, including 19 cases of grade 3/4 infections (12.6% of all cycles) and five treatment-related deaths. Two patients died of sepsis and multi-system organ failure. A third patient died on day 4 of cycle 3 from cardiac arrest. At post-mortem, there was no evidence of pulmonary embolism or myocardial infarction, suggesting arrhythmia. A fourth patient died during cycle 5 from pneumonia. The fifth patient died during cycle 6 from disseminated toxoplasmosis and gastrointestinal hemorrhage. This patient had been on inhaled pentamidine for pneumocystis prophylaxis due to sulfa allergy. Reactivation of CMV by PCR was seen in 16 (51%) patients, most of whom were successfully treated with valganciclovir.
We report results from a phase 1/2 study of DA-EPOCH with alemtuzumab for untreated, mature T and NK cell neoplasms. This combination resulted in an ORR of 83.3% with 56.6% of treated patients achieving CR. No protocol-defined DLTs were observed, but the regimen caused an unacceptably high rate of infections, including toxic deaths. Dose escalation of alemtuzumab above 30mg was not tolerated due to severe myelosuppression. Further, remissions were not durable and median PFS was only 6.6 months.
Alemtuzumab with CHOP for untreated PTCL has been reported previously with similar results. These studies differed in the treatment schedule of CHOP given (14 day cycles to 28 day cycles), though each used 30mg of alemtuzumab (1–3 times per cycle). The ORR ranged from 75–90% with CR of 60–71%. In two of the studies[11, 12], 2-year OS was 53–55% and 2-year EFS/FFS 27–48%; a third study had 1-year EFS and OS of 44% and 43%, respectively[13]. The infectious complications of each study were significant with CMV reactivation in 9–35% of patients and treatment-related mortality of 4–20%. The results of our study validate these findings with DA-EPOCH and do not demonstrate improve OS with the addition of alemtuzumab.
The frontline treatment of PTCL does not result in cure for the majority of patients. Anthracycline-based combination chemotherapy regimens are commonly used, and addition of etoposide may improve outcomes in younger patients[14]. Efforts to improve upon CHOP with the addition of bortezomib, denileukin diftitox, alemtuzumab, or everolimus have not improved survival, despite having higher response rates than CHOP. In CD30-expressing, aggressive T cell lymphomas, the use of brentuximab vedotin in combination with standard doses of CHOP (omitting vincristine) led to an ORR of 100% with 92% achieving CR, though 5-year PFS was 52%[15]. Given the high ORR but low PFS rates with frontline therapy of PTCL, expanding upon consolidation or maintenance therapies may be necessary to improve disease-free and overall survival.
In conclusion, we found that DA-EPOCH with alemtuzumab had an ORR of 83.3% in untreated patients with aggressive T and NK cell neoplasms. The MTD of alemtuzumab was determined to be 30 mg. However, the toxicity of the regimen is unacceptable and the remissions were not durable. The addition of alemtuzumab increased the risk of opportunistic infections expected with dose-adjusted EPOCH. Other targeted therapies with less myelosuppression should be prioritized in combination with anthracycline-based regimens to improve the cure rate in PTCL.
Supplementary Material
References
- 1.Coiffier B, Brousse N, Peuchmaur M, Berger F, Gisselbrecht C, Bryon PA, Diebold J. Peripheral T-cell lymphomas have a worse prognosis than B-cell lymphomas: a prospective study of 361 immunophenotyped patients treated with the LNH-84 regimen. The GELA (Groupe d’Etude des Lymphomes Agressives). Ann Oncol. 1990;1:45–50. [DOI] [PubMed] [Google Scholar]
- 2.Abramson JS, Feldman T, Kroll-Desrosiers AR, Muffly LS, Winer E, Flowers CR, Lansigan F, Nabhan C, Nastoupil LJ, Nath R, Goy A, Castillo JJ, Jagadeesh D, Woda B, Rosen ST, Smith SM, Evens AM. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Ann Oncol. 2014;25:2211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, Belhadj K, Bordessoule D, Ferme C, Tilly H. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell R, Jaeger U, Zinzani PL, Shpilberg O, Kvaloy S, de Nully Brown P, Stahel R, Milpied N, Lopez-Guillermo A, Poeschel V, Grass S, Loeffler M, Murawski N, MabThera International Trial G. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–22. [DOI] [PubMed] [Google Scholar]
- 5.Maeda Y, Nishimori H, Yoshida I, Hiramatsu Y, Uno M, Masaki Y, Sunami K, Masunari T, Nawa Y, Yamane H, Gomyo H, Takahashi T, Yano T, Matsuo K, Ohshima K, Nakamura S, Yoshino T, Tanimoto M. Dose-adjusted EPOCH chemotherapy for untreated peripheral T-cell lymphomas: a multicenter phase II trial of West-JHOG PTCL0707. Haematologica. 2017;102:2097–103. Epub 2017/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hale G The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy. 2001;3:137–43. Epub 2002/08/13. [DOI] [PubMed] [Google Scholar]
- 7.Jiang L, Yuan CM, Hubacheck J, Janik JE, Wilson W, Morris JC, Jasper GA, Stetler-Stevenson M. Variable CD52 expression in mature T cell and NK cell malignancies: implications for alemtuzumab therapy. Br J Haematol. 2009;145:173–9. Epub 2009/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodig SJ, Abramson JS, Pinkus GS, Treon SP, Dorfman DM, Dong HY, Shipp MA, Kutok JL. Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H). Clin Cancer Res. 2006;12:7174–9. [DOI] [PubMed] [Google Scholar]
- 9.Wilson WH, Grossbard ML, Pittaluga S, Cole D, Pearson D, Drbohlav N, Steinberg SM, Little RF, Janik J, Gutierrez M, Raffeld M, Staudt L, Cheson BD, Longo DL, Harris N, Jaffe ES, Chabner BA, Wittes R, Balis F. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99:2685–93. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. [DOI] [PubMed] [Google Scholar]
- 11.Gallamini A, Zaja F, Patti C, Billio A, Specchia MR, Tucci A, Levis A, Manna A, Secondo V, Rigacci L, Pinto A, Iannitto E, Zoli V, Torchio P, Pileri S, Tarella C. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–23. [DOI] [PubMed] [Google Scholar]
- 12.Kluin-Nelemans HC, van Marwijk Kooy M, Lugtenburg PJ, van Putten WL, Luten M, Oudejans J, van Imhoff GW. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol. 2011;22:1595–600. [DOI] [PubMed] [Google Scholar]
- 13.Kim JG, Sohn SK, Chae YS, Cho YY, Yang DH, Lee JJ, Kim HJ, Shin HJ, Chung JS, Cho GJ, Lee WS, Joo YD, Sohn CH, Oh SJ. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: a phase II study. Cancer Chemother Pharmacol. 2007;60:129–34. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A, Pfreundschuh M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–25. Epub 2010/07/28. [DOI] [PubMed] [Google Scholar]
- 15.Fanale MA, Horwitz SM, Forero-Torres A, Bartlett NL, Advani RH, Pro B, Chen RW, Davies A, Illidge T, Uttarwar M, Lee SY, Ren H, Kennedy DA, Shustov AR. Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood. 2018;131:2120–4. Epub 2018/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.