Abstract
Introduction Pregnant women with polycystic ovarian syndrome (PCOS) have high risk of pregnancy loss. Pathophysiological mechanisms appear to be associated with obesity, hormonal factors, or blood clotting disorders. Our aim is to perform a systematic review and meta-analysis on the relationship between coagulation disorders and risk of recurrent miscarriage (RM) in patients with PCOS and to identify coagulation biomarkers for this condition.
Material and Methods PubMed and MEDLINE databases were searched for publications in English language. The search terms used included “RM”, “polycystic ovary syndrome”, “coagulation disorders”, and “thrombophilia”. Odds ratios (ORs) and 95% confidence intervals (CIs) for miscarriage in different RM groups (with and without PCOS).
Results A total of 575 publications including the search terms were identified. Six studies were included for qualitative analysis, and five were included for quantitative analysis (meta-analysis). We found no association between RM and inherited thrombophilias in patients with PCOS: (1) Factor V Leiden (OR, 0.74; 95% CI, 0.38 – 1.45; p = 0.38); (2) C677T methylenetetrahydrofolate reductase polymorphism (MTHFR) (OR, 1.01; 95% CI, 0.64 – 1.59; p = 0.97); and (3) A1297C MTHFR polymorphism (OR, 1.08; 95% CI, 0.62 – 1.89; p = 0.77). Other potential biomarkers were identified, with emphasis on plasminogen activator inhibitor type 1.
Conclusion Data available in the current literature revealed that there was no association between RM and inherited thrombophilias in patients with PCOS. RM patients with PCOS have a high risk of thromboembolic events.
Key words: abortion, biomarker, disturbed ovarian function, polymorphism
Zusammenfassung
Einleitung Schwangere Frauen mit polyzystischem Ovarsyndrom (PCOS) haben ein hohes Fehlgeburtsrisiko. Es scheint eine Assoziation zwischen den pathophysiologischen Mechanismen einerseits und Adipositas, hormonellen Faktoren und Koagulationsstörungen andererseits zu geben. Ziel dieser Studie war es, eine systematische Übersicht und eine Metaanalyse über die Beziehungen zwischen Gerinnungsstörungen und dem Risiko wiederholter Fehlgeburten (RM) bei Patientinnen mit PCOS durchzuführen und die Koagulationsbiomarker für diesen Zustand zu identifizieren.
Material und Methode Die Datenbanken von PubMed und MEDLINE wurden nach englischsprachigen Publikationen zu diesem Thema durchsucht. Die hierfür verwendeten Suchbegriffe waren „RM“, „polycystic ovary syndrome“ (polyzystisches Ovarsyndrom), „coagulation disorders“ (Koagulationsstörungen) sowie „thrombophilia“ (Thrombophilie). Die Odds Ratios (ORs) und 95%-Konfidenzintervalle (KIs) für Fehlgeburten wurden für die verschiedenen RM-Gruppen (mit und ohne PCOS) berechnet.
Ergebnisse Insgesamt wurden 575 Artikel mit diesen Suchbegriffen identifiziert. Sechs Studien wurden für die qualitative Analyse und 5 für die quantitative Analyse (Metaanalyse) herangezogen. Wir fanden keine Beziehung zwischen RM und einer vererbten Thrombophilie in Patientinnen mit PCOS: (1) Faktor-V-Leiden (OR, 0,74; 95%-KI, 0,38 – 1,45; p = 0,38); (2) C677T-Methylen-Tetrahydrofolat-Reduktase-Polymorphismus (MTHFR) (OR, 1,01; 95%-KI, 0,64 – 1,59; p = 0,97), und (3) A1297C-MTHFR-Polymorphismus (OR, 1,08; 95%-KI, 0,62 – 1,89; p = 0,77). Es wurden andere potenzielle Biomarker ausgemacht mit einem Schwerpunkt auf den Plasminogen-Aktivator-Hemmer Typ 1.
Schlussfolgerung Die aus der aktuellen Literatur entnommenen Daten zeigten, dass es keine Assoziation zwischen RM und einer vererbten Thrombophilie bei Patientinnen mit PCOS gibt. RM-Patientinnen mit PCOS haben ein höheres Risiko für thromboembolische Ereignisse.
Schlüsselwörter: Fehlgeburt, Biomarker, gestörte Ovarfunktion, Polymorphismus
Introduction
Polycystic ovarian syndrome (PCOS), also known as polycystic ovarian disease and Stein-Leventhal syndrome, was first described in 1935 by Irving Stein and Michael Leventhal in an article published in the American Journal of Obstetrics and Gynecology entitled “Amenorrhea associated with polycystic ovaries”. In the original publication, the authors described a series of cases in which seven patients had enlarged ovaries (diagnosed by transabdominal pneumonography) associated with menstrual changes, infertility, pain, or hyperandrogenism. The patients also presented with obesity (three patients), hirsutism (five patients), and acne (one patient) 1 , 2 . In 1939, the gestational outcomes of these seven patients were published: five patients became pregnant after surgery, one patient had a male factor present, and one was lost during follow-up after 11 months 3 .
PCOS is the most common endocrine disorder in women of reproductive age. Its prevalence is variable, depending on the ethnic group and diagnostic criteria, and occurs in around 5 to 16% of women in reproductive phase of life 4 . Prevalence is higher among the following groups:
infertile and oligomenorrhagic women;
obese with or without insulin resistance;
history of type 1, type 2, or gestational diabetes;
history of premature adrenarche;
first-degree relatives with PCOS; and
PCOS is not only related to gynecological disorders (infertility and menstrual irregularity), as initially described by Stein and Leventhal, but is also associated with other pathologies such as cardiovascular disease, metabolic syndrome, obesity, type 1 and 2 diabetes mellitus, gestational diabetes, nonalcoholic steatohepatitis, obstructive sleep apnea, endometrial cancer, anxiety, depression, and eating disorders 6 , 7 , 8 . The relationship between PCOS and obstetric complications, neonatal outcome, and adult life after gestation in a mother with PCOS is not well understood 6 , 7 .
Recurrent miscarriage (RM) is defined as two or more pregnancy losses before 20 weeks of gestation 9 . The incidence of RM ranges from 0.5 to 2.3%, and may vary due to different definitions and different methodologies used in the statistical calculation. However, an increase in the incidence of recurrent miscarriage has been observed 10 . Genetic alterations, uterine anatomical changes, hormonal disorders, and antiphospholipid syndrome (APS) are responsible for around half of the cases of RM, and the other half still remains without a clear diagnosis 11 . While the association between PCOS and RM is described in the literature, the mechanisms involved are not well defined 12 , 13 , 14 . PCOS carriers have metabolic, endocrine (insulin resistance, dyslipidemia, obesity, chronic inflammation), and coagulation disorders that may be related to pregnancy loss 6 , 15 , 16 , 17 .
Since the late 1970s, when the relationship between APS and pregnancy loss was first described, studies have evaluated the relationship between blood coagulation and gestational pathologies. It is believed that thromboembolic events at the site of implantation and the placental bed provide the basis for the pathophysiology of pregnancy losses and other obstetric complications, such as preeclampsia, fetal growth restriction, fetal death, and preterm delivery 18 , 19 . The relationship between APS and RM is well established in the literature 20 . However, the relationship between hereditary thrombophilias and other thrombophilic disorders with RM remains unclear 21 .
The aim of this systematic review and meta-analysis was to identify biomarkers of coagulation disorders in women with RM and history of PCOS, highlighting the possible pathophysiological mechanisms involved and the possible interventions that may reduce the risk of gestational loss in these patients.
Methods
This systematic review was performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 22 . PubMed and MEDLINE databases were searched for publications available up to and including October 2018. The search terms used were “RM”, “polycystic ovary syndrome”, “coagulation disorders”, and “thrombophilia”.
Data extraction was performed by two reviewers. The title and abstracts of the articles identified in the initial search were independently evaluated according to the following inclusion criteria: type of study, population (women with a history of recurrent miscarriage and PCOS), risk factor assessment (coagulation disorder), and primary outcome (miscarriage). Articles were limited to studies using humans and published in English. Studies investigating physiopathological mechanisms were also evaluated and discussed.
Available data were imported into Review Manager, version 5.3.5 (The Cochrane Collaboration), for quantitative analysis.
Results
Identified and selected studies
Five hundred seventy five publications related to the search terms were identified. All animal studies and systematic review were excluded, and included only six studies that evaluated biomarkers of coagulation disorders in patients with RM and history PCOS ( Fig. 1 ). These six studies evaluated a total of 392 patients with RM and PCOS. Details of the studies included in the statistical analysis and discussion are described in Table 1 . No prospective or intervention studies were identified. The six studies selected were observational case-control studies. The studies were conducted in different countries, including Iran (three studies), the United States (one study), Germany (one study), and Poland (one study), and were published in the period from 2003 to 2016 23 , 24 , 25 , 26 , 27 , 28 .
Fig. 1.
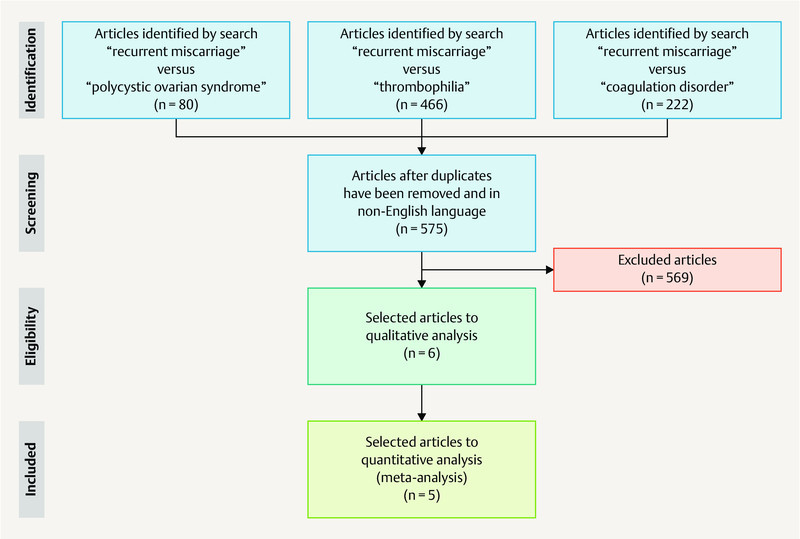
Flowchart of the search showing preferred reporting items used for the Systematic Reviews and Meta-Analyses (PRISMA).
Table 1 Studies on coagulations disorders among women with polycystic ovarian syndrome (PCOS) with a history of recurrent miscarriage (RM).
| Author, year (country) | Study type | Groups | Biomarkers | Comments |
|---|---|---|---|---|
| RM was defined as a history of three or more spontaneous pregnancy losses at less than 20 weeks of gestation 23 , 24 , 25 , 26 . RM was defined as the antecedent of two or more pregnancy losses at less than 20 weeks. 27 , 28 Rotterdam criteria for PCOS diagnosis. 24 , 25 , 26 , 27 , 28 National Institutes of Health (NIH) criteria for PCOS diagnosis 23 . | ||||
| Glueck et al., 2003 23 (United States of America) | Case-control | PCOS-RM (33 Caucasian women) RM without PCOS (16 Caucasian women) Controls (116 healthy Caucasian women) |
G1691A Factor V Leiden G20210A prothrombin C677T MTHFR PAI-1 (4 G/5 G) gene polymorphism Platelet glycoprotein PL A1A2 gene mutations Homocysteine Proteins C, S, free S, antithrombin Anticardiolipin antibodies IgG and IgM Dilute Russelʼs viper venom time Activated partial thromboplastin time Factor VIII Factor XI Lipoprotein (a) PAI-Fx |
The thrombophilic G1691A Factor V Leiden mutation is associated with RM in women with and without PCOS; hypofibrinolysis (high PAI-Fx) is also associated with RM in women with PCOS. |
| Idali et al., 2012 24 (Iran) | Case-control | 177 RM (38 women with RM-PCOS, 33 with ovarian PCOS-RM, and 106 RM without PCOS, 100 female controls) | A1298C MTHFR C677T MTHFR PAI-1 (4 G/5 G) gene polymorphisms |
MTHFR A1298C and PAI-1 4 G/5 G mutations were more prevalent in Iranian women suffering from RM with and without PCOS. |
| Moini et al., 2012 25 (Iran) | Case-control | 92 women with RM-PCOS 92 women with RM without PCOS. |
G1691A Factor V Leiden Homocysteine Proteins C, S, free S, antithrombin Anticardiolipin antibodies IgG and IgM |
There was a trend toward higher prevalence of protein S deficiency in RM-PCOS women (p = 0.05). |
| Kazerooni et al., 2013 26 (Iran) | Case-control | 60 patients with RM-PCOS 60 patients with PCOS and without RM 60 patients with RM and without PCOS 60 healthy individuals |
Testosterone Fasting insulin Homocysteine Plasminogen activator inhibitor activity (PAI-Fx) Protein C, Protein S, antithrombin Activated protein C ratio (APCR) Factor V Leiden Prothrombin G20210A MTHFR gene mutations |
Hyperinsulinemia, hyperandrogenemia, hypofibrinolysis, and hyperhomocysteinemia, as well as APCR and factor V Leiden mutations, are associated with RM in patients with PCOS. |
| Rogenhofer et al., 2013 27 (Germany) | Case-control | 100 PCOS patients (27 no recorded pregnancies; 73 RM), 500 fertile women and 533 random population Controls |
M2/ANXA5 Polymorphism | M2/ANXA5 seems an independent RM risk factor in PCOS patients that progressively correlates with the number of first trimester pregnancies. |
| Szafarowska et al., 2016 28 (Poland) | Case-control | 76 PCOS women (63 RM and 13 infertility) 56 non-PCOS women (40 RM and 16 infertility) |
A1298C MTHFR C677T MTHFR |
MTHFR mutation was not associated with PCOS in the Polish population. There was correlation between the MTHFR A1298C mutation and RM in the non-PCOS group. |
The majority of studies were selected cases of RM using a history of three or more spontaneous pregnancy losses at less than 20 weeks of gestation 23 , 24 , 25 , 26 . Only the two most recent studies considered RM as the antecedent of two or more pregnancy losses at less than 20 weeks 27 , 28 . Most studies used the Rotterdam criteria for PCOS diagnosis 24 , 25 , 26 , 27 , 28 , and only one study 23 used the National Institutes of Health (NIH) criteria.
Coagulation biomarkers
The first study to evaluate the relationship between coagulation disorders in patients with RM and history of PCOS was published by Glueck el al. 23 . They observed a higher prevalence of Factor V Leiden (FVL) in patients diagnosed with RM and PCOS compared with the normal control group, but this was not more prevalent compared with the RM group without PCOS. The authors also observed a reduction in the fibrinolytic activity of patients with RM and PCOS, through high activity of the plasminogen activated inhibitor (PAI-Fx) compared with the RM group without PCOS (p = 0.0363) and the normal control group (p = 0.0039). Age and body mass index among the RM groups (with or without PCOS) were similar, showing that fibrinolysis changes were independent of obesity 23 .
Idali et al. 24 observed a higher prevalence of hereditary thrombophilias (A1298C methylenetetrahydrofolate reductase polymorphism [MTHFR A1298C] and PAI-1 4 G/5 G) in patients with a history of RM (with or without PCOS) compared with controls. However, the prevalence of these thrombophilias was similar compared with patients with RM with and without PCOS.
Moini et al. 25 compared the prevalence of the following thrombophilias in groups of RM patients with and without PCOS: protein C deficiency, protein S deficiency, antithrombin deficiency, FVL, hyperhomocysteinemia, and APS. They identified at least one clotting disorder in 70.7% (65/92) of women with RM and PCOS compared with 47.8% (44/92) of patients with RM without PCOS (p = 0.002). Protein C deficiency was the only thrombophilia that showed a higher prevalence in patients with RM and PCOS (21.7 vs. 10.9%, p = 0.04).
Kazerooni et al. 26 identified high levels of total testosterone, hyperhomocysteinemia, insulin resistance, increased PAI activity, and resistance to activated protein C in women with RM and PCOS compared with women with RM without PCOS and the control group. Among the evaluated thrombophilias, the authors observed a relationship between FVL and RM, independent of the group (with or without PCOS). There was no significant difference between the groups regarding levels of protein C, protein S, and antithrombin.
The relationships between MTHFR polymorphisms (A1298C and C677T) and annexin A5 (M2/ANXA5) and RM in PCOS patients were evaluated by Szafarowska et al. 28 and Rogenhofer et al. 27 . MTHFR polymorphisms appeared to be unrelated to pregnancy loss in the group of PCOS patients, whereas M2/ANXA5 appeared to be an independent risk factor for pregnancy loss in PCOS patients.
Among the six studies that evaluated coagulation disorder biomarkers in patients with RM diagnosed with PCOS, it was only possible to use genetic biomarker (hereditary thrombophilias) data from five studies for statistical analysis. Rogenhofer et al. 27 were not included in the statistic analysis because it was the only study that evaluated M2/ANXA5. No statistical significance was found in relation to FVL ( Fig. 2 a ) 23 , 25 , 26 and MTHFR polymorphisms (A1298C and C677T) ( Fig. 2 b and c ) in RM patients with PCOS compared with patients with RM without PCOS 24 , 26 , 28 .
Fig. 2.
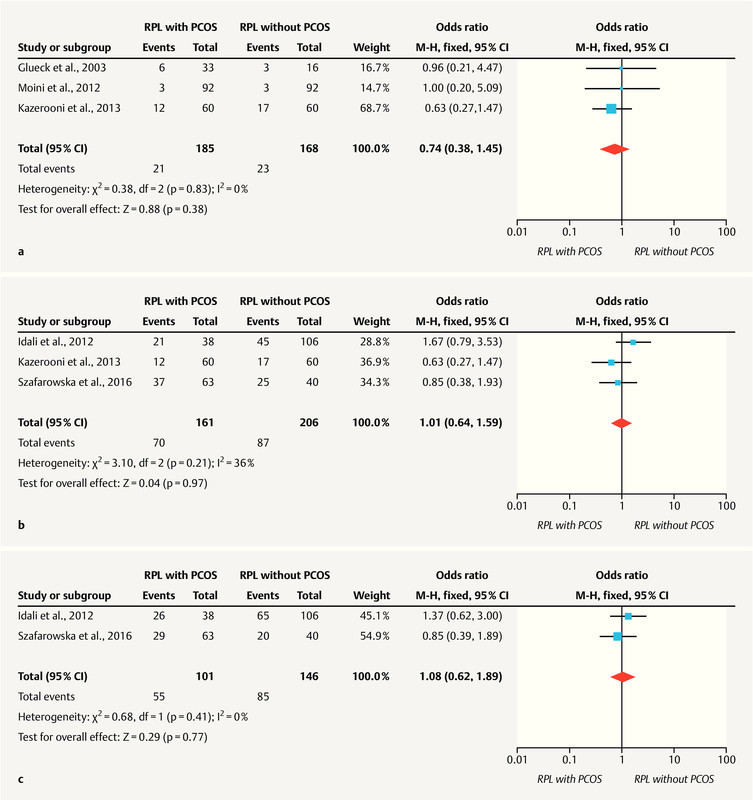
a Factor V Leiden meta-analysis. Forest plot for RM with PCOS versus RM without PCOS: recurrent miscarriage. b C677T MTHFR meta-analysis. Forest plot of RM with PCOS versus RM without PCOS: recurrent miscarriage. c A1298C MTHFR meta-analysis. Forest plot of RM with PCOS versus RM without PCOS: recurrent miscarriage.
Discussion
PCOS is an endocrine condition initially described as a gynecological disorder associated with menstrual irregularity due to chronic anovulation and enlarged ovaries with the presence of multiple small cysts. PCOS has attracted much interest from researchers due to its reproductive and metabolic repercussions (glucose intolerance, obesity, diabetes, hypertension, metabolic syndrome, and cancer). Studies have shown that women with PCOS are at increased risk of changes to blood clotting via mechanisms that remain poorly understood 2 , 15 , 17 . It is possible that the metabolic alterations observed in these patients are involved in the pathophysiology of coagulation disorders, including obesity, chronic inflammatory process, excess androgen hormones, dyslipidemia, and insulin resistance 15 .
Over the last decades, studies have described anomalies in blood coagulation in patients with PCOS, demonstrating an imbalance between the thrombogenic and antithrombogenic components of the coagulation system. Higher platelet number, higher mean platelet volume, platelet activation, higher levels of von Willebrand factor, elevated PAI-1, elevated asymmetric dimethyl- l -arginine, elevated levels of d -higher tissue factor, faster thrombin generation, protein C deficiency, and lower levels of plasminogen activity are some of the changes seen in coagulation components in non-pregnant patients diagnosed with PCOS 15 .
Physiologically, gestation is a prothrombotic state, which becomes more pronounced closer to term. Conditions other than pregnancy may also increase the risk of thromboembolic events in the gestational period. Shan et al. studied pregnant women with or without PCOS during the first trimester of pregnancy and observed that pregnant women with a previous diagnosis of PCOS had a higher procoagulant state due to a higher concentration of activated clotting factors VIII and activated X 29 .
The possible relationship between thromboembolic events and RM, such as those observed in the pathophysiology of APS, has prompted studies investigating the relationship between thrombophilic (inherited or acquired) state and pregnancy loss. The current evidence does not allow the inclusion of inherited thrombophilias in the list of etiologies of RM, although several authors have shown a higher prevalence of some hereditary thrombophilias in patients with a history of RM. Recently, studies have demonstrated a higher prevalence of PAI-1 4 G/5 G mutations, MTHFR polymorphisms (A1298C and C677T), factor VII polymorphisms, FVL, and prothrombin allelic polymorphisms (A20210G) among populations of women with a history of RM 18 , 30 , 31 , 32 .
Currently, the international guidelines from reproductive medicine societies who guide the management of RM couples only recommend APS investigation, and discourage screening of inherited thrombophilias or other biomarkers of coagulation disorders 33 , 34 , 35 , 36 . The existence of a possible association between inherited thrombophilias and RM has led to intervention studies using heparin (non-fractionated and low molecular weight) associated or not associated with platelet anti-aggregating agents. However, these studies have not been able to reduce the risk of pregnancy loss in these patients 37 . Also, the current international guidelines for management of patients with a history of RM do not outline specific recommendations for women diagnosed with PCOS (RM and PCOS) 33 , 34 , 35 , 36 .
Some authors argue that there is a higher prevalence of inherited thrombophilias in patients with RM and PCOS. They believe that the lack of evidence for a relationship between RM and inherited thrombophilias is due to studies that include all women with RM. Selecting one specific group of these patients may lead to identification of strong evidence of this relationship. However, despite a small number of studies and patients, the present meta-analysis of the reviewed literature corroborates the evidence that there is no relation between inherited thrombophilias and all patients with RM, even in this specific group of patients with PCOS.
Data available in the current literature on women with no established obstetric history suggest a strong relationship of PCOS with increased platelet aggregation and reduced plasma fibrinolytic activity. Elevated platelet numbers and PAI-1 levels, and alteration of other coagulation inhibitors represent some biomarkers in patients with PCOS 15 .
Among the reviewed studies, PAI is a biomarker that requires more attention. PAI is a factor of the fibrinolytic system that plays relevant roles in hemostatic balance, tissue remodeling, angiogenesis, and reproduction 38 . Gris et al. described high levels of PAI-1 in RM patients compared with healthy pregnant women 39 . Studies of women with PCOS with no established obstetric history revealed a relationship between insulin resistance and increased activity of PAI, corroborating the findings of Kazerooni et al. in a group of patients with RM and PCOS 26 , 40 , 41 .
In women with PCOS, PAI was also associated with a greater inflammatory process that is also involved in the etiology of RM 41 . Koiou et al. 40 believe that the highest activity of PAI in PCOS patients is dependent on patient body weight, which is elevated in patients with PCOS and obesity. However, Shan et al. 29 and Glueck et al. 23 reported that elevated PAI activity occurs regardless of the patientʼs body weight.
Recently, reproductive medicine associations published guidelines for the management of PCOS patients. The basic recommendation for these patients includes diet control and physical activity 6 . Furthermore, they showed that metformin improves gestational outcomes in these patients 6 . Some intervention studies in patients with PCOS with no established obstetric history have shown a beneficial effect of metformin use on coagulation biomarkes 42 , 43 . Burchall et al. 42 observed a decrease in PAI activity in a group of patients with PCOS treated with metformin.
Other PCOS therapeutic options have shown a beneficial effect on gestational outcomes in PCOS patients. The use of liraglutide, a long-acting glucagon-like peptide-1 (GLP-1) analog, in patients with PCOS revealed improved results in cycles of in vitro fertilization and coagulation disorders 43 , 44 , 45 . PCOS patients undergoing bariatric surgery typically present better metabolic control and improved inflammatory status 46 , 47 .
The limitation of this systematic review and meta-analysis is the reduced number of previous publications about this topic. Due to few published studies it was not possible to analyze different biomarkers in this population. To our surprise, this is the first review showing an association between PCOS, recurrent miscarriage, and coagulation disorders. The strength of this present study was to identify possible coagulation biomarkers in recurrent miscarriage patients with PCOS.
The present systematic review reveals that patients with RM and PCOS deserve a differential approach during the evaluation and follow-up during the perigestational period. These patients have a high thromboembolic risk compared with other RM patients. Currently, the small number of available studies did not observe the relationship between inherited thrombophilias and RM patients with PCOS; however, it is not yet possible to rule out this association completely. Procoagulant anomalies are due to PCOS metabolic aberrations. Clinical studies are required to investigate possible biomarkers of clotting disorders in patients with RM and PCOS. It is also important to propose intervention studies with drugs to reduce insulin resistance, such as metformin, GLP-1 analogs, sodium-glucose cotransporter-2 inhibitors, and dipeptidyl peptidase-4 inhibitors, either associated or not associated with anticoagulant therapies.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Stein I F, Leventhal M L. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. [Google Scholar]
- 2.Azziz R, Adashi E Y. Stein and Leventhal: 80 years on. Am J Obstet Gynecol. 2016;214:2470–2.47E13. doi: 10.1016/j.ajog.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Stein I F, Cohen M R. Surgical treatment of bilateral polycystic ovaries–Amenorrhea and sterility. Am J Obstet Gynecol. 1939;38:465–480. [Google Scholar]
- 4.Bozdag G, Mumusoglu S, Zengin D. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-Analysis. Hum Reprod. 2016;31:2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 5.Azziz R.Epidemiology and genetics of the polycystic ovary syndrome in adults – UpToDateOnline:https://www.uptodate.com/contents/epidemiology-and-genetics-of-the-polycystic-ovary-syndrome-in-adultslast access: 06.11.2018
- 6.Teede H J, Misso M L, Costello M F. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooney L G, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril. 2018;110:794–809. doi: 10.1016/j.fertnstert.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Anagnostis P, Tarlatzis B C, Kauffman R P. Polycystic ovarian syndrome (PCOS): Long-term metabolic consequences. Metabolism. 2018;86:33–43. doi: 10.1016/j.metabol.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Zegers-Hochschild F, Adamson G D, Dyer S. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108:393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Rasmark Roepke E, Matthiesen L, Rylance R. Is the incidence of recurrent pregnancy loss increasing? A retrospective register-based study in Sweden. Acta Obstet Gynecol Scand. 2017;96:1365–1372. doi: 10.1111/aogs.13210. [DOI] [PubMed] [Google Scholar]
- 11.El Hachem H, Crepaux V, May-Panloup P. Recurrent pregnancy loss: current perspectives. Int J Womens Health. 2017;9:331–345. doi: 10.2147/IJWH.S100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty P, Goswami S K, Rajani S. Recurrent Pregnancy Loss in Polycystic Ovary Syndrome: Role of Hyperhomocysteinemia and Insulin Resistance. PLoS One. 2013;8:e64446. doi: 10.1371/journal.pone.0064446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matjila M J, Hoffman A, van der Spuy Z M. Medical conditions associated with recurrent miscarriage–Is BMI the tip of the iceberg? Eur J Obstet Gynecol Reprod Biol. 2017;214:91–96. doi: 10.1016/j.ejogrb.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Tziomalos K, Dinas K. Obesity and Outcome of Assisted Reproduction in Patients With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne) 2018;9:149. doi: 10.3389/fendo.2018.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Targher G, Zoppini G, Bonora E. Hemostatic and fibrinolytic abnormalities in polycystic ovary syndrome. Semin Thromb Hemost. 2014;40:600–618. doi: 10.1055/s-0034-1384512. [DOI] [PubMed] [Google Scholar]
- 16.Manneras-Holm L, Baghaei F, Holm G. Coagulation and fibrinolytic disturbances in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:1068–1076. doi: 10.1210/jc.2010-2279. [DOI] [PubMed] [Google Scholar]
- 17.Burchall G F, Piva T J, Linden M D. Comprehensive Assessment of the Hemostatic System in Polycystic Ovarian Syndrome. Semin Thromb Hemost. 2015;42:55–62. doi: 10.1055/s-0035-1564837. [DOI] [PubMed] [Google Scholar]
- 18.Wolski H, Barlik M, Drews K. Contribution of inherited thrombophilia to recurrent miscarriage in the Polish population. Ginekol Pol. 2017;88:385–392. doi: 10.5603/GP.a2017.0072. [DOI] [PubMed] [Google Scholar]
- 19.Karadağ C, Yoldemir T, Karadağ S D. Obstetric outcomes of recurrent pregnancy loss patients diagnosed wıth inherited thrombophilia. Ir J Med Sci. 2017;186:707–713. doi: 10.1007/s11845-017-1569-0. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber K, Sciascia S, de Groot P G. Antiphospholipid syndrome. Nat Rev Dis Prim. 2018;4:17103. doi: 10.1038/nrdp.2017.103. [DOI] [PubMed] [Google Scholar]
- 21.Arachchillage D, Makris M. Inherited Thrombophilia and Pregnancy Complications: Should We Test? Semin Thromb Hemost. 2018 doi: 10.1055/s-0038-1657782. [DOI] [PubMed] [Google Scholar]
- 22.PRISMA Group . Moher D, Liberati A, Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glueck C J, Wang P, Bornovali S. Polycystic ovary syndrome, the G1691A Factor V Leiden mutation, and plasminogen activator inhibitor activity: Associations with recurrent pregnancy loss. Metabolism. 2003;52:1627–1632. doi: 10.1016/j.metabol.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Idali F, Zareii S, Mohammad-Zadeh A. Plasminogen Activator Inhibitor 1 and Methylenetetrahydrofolate Reductase Gene mutations in Iranian Women with Polycystic Ovary Syndrome. Am J Reprod Immunol. 2012;68:400–407. doi: 10.1111/aji.12002. [DOI] [PubMed] [Google Scholar]
- 25.Moini A, Tadayon S, Tehranian A. Association of thrombophilia and polycystic ovarian syndrome in women with history of recurrent pregnancy loss. Gynecol Endocrinol. 2012;28:590–593. doi: 10.3109/09513590.2011.650754. [DOI] [PubMed] [Google Scholar]
- 26.Kazerooni T, Ghaffarpasand F, Asadi N. Correlation between thrombophilia and recurrent pregnancy loss in patients with polycystic ovary syndrome: A comparative study. J Chinese Med Assoc. 2013;76:282–288. doi: 10.1016/j.jcma.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Rogenhofer N, Engels L, Bogdanova N. Independent association of the M2/ANXA5 haplotype with recurrent pregnancy loss (RPL) in PCOS patients. Metabolism. 2013;62:1057–1060. doi: 10.1016/j.metabol.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Szafarowska M, Segiet A, Jerzak M M. Methylenotetrahydrololate reductase A1298C and C677T polymorphisms and adverse pregnancy outcome in women with PCOS. Neuro Endocrinl Lett. 2016;37:141–146. [PubMed] [Google Scholar]
- 29.Shan Y, Wang A, Sun Y. Coagulation and fibrinolytic indices during the first trimester of pregnancy in women with polycystic ovary syndrome: A preliminary study. Reprod Sci. 2013;20:1390–1397. doi: 10.1177/1933719113485293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Liu Y, Zhang R. Meta-analysis of the association between plasminogen activator inhibitor-1 4G/5G polymorphism and recurrent pregnancy loss. Med Sci Monit. 2015;21:1051–1056. doi: 10.12659/MSM.892898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turki R F, Assidi M, Banni H A. Associations of recurrent miscarriages with chromosomal abnormalities, thrombophilia allelic polymorphisms and/or consanguinity in Saudi Arabia. BMC Med Genet. 2016;17 (S1):69. doi: 10.1186/s12881-016-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlik M, Seremak-Mrozikiewicz A, Drews K. Correlation between factor VII and PAI-1 genetic variants and recurrent miscarriage. Ginekol Pol. 2016;87:504–509. doi: 10.5603/GP.2016.0034. [DOI] [PubMed] [Google Scholar]
- 33.Davenport W B, Kutteh W H. Inherited Thrombophilias and Adverse Pregnancy Outcomes. Obstet Gynecol Clin North Am. 2014;41:133–144. doi: 10.1016/j.ogc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Toth B, Würfel W, Bohlmann M. Recurrent Miscarriage: Diagnostic and Therapeutic Procedures. Guideline of the DGGG (S1-Level, AWMF Registry No. 015/050, December 2013) Geburtsh Frauenheilk. 2015;75:1117–1129. doi: 10.1055/s-0035-1558299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser J, Branch D W. Recurrent Pregnancy Loss. Clin Obstet Gynecol. 2016;59:464–473. doi: 10.1097/GRF.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 36.Huchon C, Deffieux X, Beucher G. Pregnancy loss: French clinical practice guidelines. Eur J Obstet Gynecol Reprod Biol. 2016;201:18–26. doi: 10.1016/j.ejogrb.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 37.de Jong P G, Goddijn M, Middeldorp S. Antithrombotic therapy for pregnancy loss. Hum Reprod Update. 2013;19:656–673. doi: 10.1093/humupd/dmt019. [DOI] [PubMed] [Google Scholar]
- 38.Ye Y, Vattai A, Zhang X. Role of Plasminogen Activator Inhibitor Type 1 in Pathologies of Female Reproductive Diseases. Int J Mol Sci. 2017;18:1651. doi: 10.3390/ijms18081651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gris J C, Neveu S, Mares P. Plasma fibrinolytic activators and their inhibitors in women suffering from early recurrent abortion of unknown etiology. J Lab Clin Med. 1993;122:606–615. [PubMed] [Google Scholar]
- 40.Koiou E, Tziomalos K, Dinas K. Plasma plasminogen activator inhibitor-1 levels in the different phenotypes of the polycystic ovary syndrome. Endocr J. 2012;59:21–29. doi: 10.1507/endocrj.ej11-0023. [DOI] [PubMed] [Google Scholar]
- 41.Ye Y, Vattai A, Zhang X. Role of Plasminogen Activator Inhibitor Type 1 in Pathologies of Female Reproductive Diseases. Int J Mol Sci. 2017;18:1651. doi: 10.3390/ijms18081651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burchall G, Piva T, Ranasinha S. Differential Effects on Haemostatic Markers by Metformin and the Contraceptive Pill: A Randomized Comparative Trial in PCOS. Thromb Haemost. 2017;117:2053–2062. doi: 10.1160/TH17-04-0248. [DOI] [PubMed] [Google Scholar]
- 43.Kahal H, Aburima A, Ungvari T. The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocr Disord. 2015;15:14. doi: 10.1186/s12902-015-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzotzas T, Karras S, Katsiki N. Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists in the Treatment of Obese Women with Polycystic Ovary Syndrome. Curr Vasc Pharmacol. 2017;15:218–229. doi: 10.2174/1570161114666161221115324. [DOI] [PubMed] [Google Scholar]
- 45.Salamun V, Jensterle M, Janez A. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur J Endocrinol. 2018;179:1–11. doi: 10.1530/EJE-18-0175. [DOI] [PubMed] [Google Scholar]
- 46.Skubleny D, Switzer N J, Gill R S. The Impact of Bariatric Surgery on Polycystic Ovary Syndrome: a Systematic Review and Meta-analysis. Obes Surg. 2016;26:169–176. doi: 10.1007/s11695-015-1902-5. [DOI] [PubMed] [Google Scholar]
- 47.Escobar-Morreale H F. Surgical management of metabolic dysfunction in PCOS. Steroids. 2012;77:312–316. doi: 10.1016/j.steroids.2011.12.004. [DOI] [PubMed] [Google Scholar]


