Abstract
The fertility success rates of clinical and laboratory-assisted reproductive techniques (ART) remain low, despite major advances. The aim of this study was to conduct a systematic literature review and assess whether the intrauterine administration of human chorionic gonadotropin (hCG) before embryo transfer (ET) improved the clinical outcomes of sub-fertile women undergoing assisted reproduction. The electronic databases PUBMED, EMBASE and Web of Science were systematically searched for randomized controlled trials (RCTs) published from inception to June 2018. The trial data were independently extracted and analyzed using risk ratios (RRs) and 95% confidence intervals (CIs) according to a random- or fixed-effect model (as appropriate), and a meta-analysis was conducted using Review Manager 5.2 software. The meta-analysis included 3241 patients from 12 RCTs, and the combined results demonstrated that intrauterine hCG injection significantly improved the rates of clinical (RR = 1.33; 95% CI: 1.12 – 1.58) and ongoing pregnancy (RR = 1.87; 95% CI: 1.54 – 2.27), compared with controls. However, intrauterine hCG injection had no significant effect on the implantation rate (RR = 1.30; 95% CI: 0.89 – 1.90), abortion rate (RR = 1.06; 95% CI: 0.78 – 1.44), ectopic pregnancy rate (RR = 0.77; 95% CI: 0.17 – 3.42) or live birth rate (RR = 0.99; 95% CI: 0.60 – 1.63). In a subgroup analysis, the intrauterine injection of > 500 IU hCG led to a significant increase in the implantation rate (RR = 1.64; 95% CI: 1.04 – 2.61) relative to controls. Furthermore, the subgroup of women with cleavage-stage ETs who received an intracavity injection of hCG (IC-hCG) exhibited increases in the implantation, clinical pregnancy and ongoing pregnancy rates, compared to women with cleavage-stage ETs and no IC-hCG. The current evidence indicates that intrauterine hCG administration before ET provides an advantage in terms of the clinical pregnancy and ongoing pregnancy rates.
Key words: human chorionic gonadotropin, intrauterine injection, embryo transfer, meta-analysis
Zusammenfassung
Trotz wichtiger Fortschritte bleiben die Erfolgsraten der klinischen und laborassistierten Reproduktionstechniken (ART) niedrig. Ziel dieser Studie war es, eine systematische Analyse der Literatur durchzuführen, um herauszufinden, ob die intrauterine Gabe von humanem Choriongonadotropin (hCG) vor dem Embryotransfer (ET) das klinische Ergebnis bei subfertilen Frauen verbessert, die sich einer assistierten Reproduktion unterziehen. Die elektronischen Datenbanken PUBMED, EMBASE und Web of Science wurden systematisch nach randomisierten, vor Juni 2018 veröffentlichten, kontrollierten Studien durchsucht. Bei der Analyse wurde Modelle mit zufälligen bzw. festen Effekten verwendet. Die Studiendaten wurden individuell analysiert. Das relative Risiko (RR) und das 95%ige Konfidenzintervall (KI) wurden kalkuliert. Die Metaanalyse wurde mithilfe der Review Manager 5.2 Software durchgeführt. Die Metaanalyse umfasste 3241 Patientinnen aus 12 randomisierten kontrollierten Studien. Die kombinierten Ergebnisse zeigen, dass eine intrauterine hCG-Injektion die klinischen Schwangerschaftsrate (RR = 1,33; 95%-KI 1,12 – 1,58) sowie die Rate der weiterführenden Schwangerschaften (RR = 1,87; 95%-KI 1,54 – 2,27) signifikant verbesserte, verglichen mit der Kontrollgruppe. Dagegen hatte eine intrauterine hCG-Injektion keine signifikanten Auswirkungen auf die Implantationsrate (RR = 1,30; 95%-KI: 0,89 – 1,90), die Fehlgeburtenrate (RR = 1,06; 95%-KI 0,78 – 1,44), die Rate ektoper Schwangerschaften (RR = 0,77; 95%-KI 0,17 – 3,42) sowie die Lebendgeburtenrate (RR = 0,99; 95%-KI 0,60 – 1,63). Bei einer Untergruppenanalyse stellte sich heraus, dass eine intrauterine Injektion von > 500 IU hCG zu einer signifikant höheren Implantationsrate führte (RR = 1,64; 95%-KI 1,04 – 2,61), verglichen mit der Kontrollgruppe. Ferner stellte sich heraus, dass die Implantationsrate, die klinische Schwangerschaftsrate und die weiterführende Schwangerschaftsrate höher waren bei einer Untergruppe von Frauen, die ein Embryo in der Teilungsphase transferiert bekamen und eine intrakavitäre hCG-Injektion (IC-hCG) erhielten, verglichen mit Frauen, die ebenfalls ein Embryo in der Teilungsphase transferiert bekamen und keine IC-hCG erhielten. Nach der derzeitigen Beweislage scheint es, dass eine intrauterine hCG-Gabe vor dem ET Vorteile hinsichtlich der klinischen Schwangerschaftsrate und der weiterführenden Schwangerschaftsrate aufweist.
Schlüsselwörter: humanes Choriongonadotropin, intrauterine Injektion, Embryotransfer, Metaanalyse
Introduction
Infertility is defined as the inability of a couple to conceive spontaneously after at least 12 months of regular sexual intercourse without contraception. An estimated 15% of couples in developed countries are affected by infertility 1 . Despite major advances, clinical and laboratory-assisted reproductive techniques (ART) continue to yield low fertility success rates due to their dependence on multiple hormone and cytokine pathways 2 , 3 , 4 . For example, embryo implantation, a very critical process during ART, is regulated by various factors such as embryo quality, endometrial receptivity and embryo–endometrium synchronization.
Human chorionic gonadotropin (hCG), a unique heterodimeric placental glycoprotein hormone with biological functions in the endometrium and corpus luteum, is among the most important factors affecting implantation 5 , 6 . Before implantation, hCG directly promotes the expression of angiogenic factors such as vascular endothelial growth factor (VEGF) and stimulates the growth of maternal blood vessels by binding to endometrial receptors 7 . A recent animal model study indicated that the systemic administration of hCG at the time of embryo transfer (ET) improved the subsequent pregnancy rate 8 . Another study demonstrated that the intrauterine injection of hCG before ET improved the implantation and pregnancy rates during in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) cycles 3 . Still, the reported effects of hCG intrauterine injection before embryo transfer during assisted reproduction have not been completely consistent 9 , 10 , 11 , 12 , 13 , 14 .
The most recent systematic review of the effects of intrauterine HCG injection on IVF outcomes was published in 2016 15 . The authors of that review identified eight relevant studies and demonstrated that patients in the intrauterine hCG injection group had a significantly higher clinical pregnancy rate when compared with the control group (risk ratio [RR] = 1.18, 95% confidence interval [CI]: 1.00 – 1.39, i.e., no difference from a 1.39-fold increased effect). However, the authors included two oral abstracts in their analysis of the clinical pregnancy rate and therefore did not provide a reliable meta-analysis of this topic. The present meta-analysis therefore aimed to search the literature and identify the results of randomized controlled trials (RCTs) that compared the IVF/ISCI outcomes of subjects who received intrauterine hCG injection before embryo transfer with those of controls.
Materials and Methods
Literature search strategy
Two authors (THP and HSF) independently and systematically searched the electronic databases PUBMED, EMBASE and Web of Science for published studies from inception to June 2018. The following core search terms were used: “hCG”, “rhCG”, “recombinant hCG”, “human chorionic gonadotrophin”, “assisted reproductive techniques”, “IVF”, “in vitro fertilization”, “ICSI”, “intracytoplasmic sperm injections”, “embryo transfer”, “intrauterine injection”, “intrauterine HCG”, “implantation”, “RCT” and “randomized controlled trial”. The search was limited to articles published in English. The reference lists from the identified articles were also screened manually.
Inclusion and exclusion criteria
Studies that met the following criteria were included:
a RCT design;
an intervention involving intrauterine hCG injection vs. no injection or placebo;
inclusion of sub-fertile women undergoing IVF/ICSI;
inclusion of sub-fertile women undergoing embryo transfer and
at least 1 of the following outcomes: implantation rate, clinical pregnancy rate, abortion rate, ongoing pregnancy rate and ectopic pregnancy rate.
The following exclusion criteria were also applied:
studies without original data, such as case reports, abstracts, reviews and letters;
studies without a RCT design, such as cohort studies, case-control studies and retrospective studies;
an inability to extract data from the literature and
animal experiments.
Data extraction and quality assessment
Two investigators (YQ and CY) independently extracted the following outcome-related data from the eligible studies: first authorʼs name, year of publication, country, study size and main results. Additionally, two authors (THP and WCL) assessed the quality of each study using the Cochrane Collaboration tool 16 , which screened the studies for the following methodological features: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Disagreements and uncertainty were resolved by discussion with a third author (JL) until consensus was achieved.
Statistical analysis
Statistical analyses were conducted using Review Manager 5.2 (Cochrane Collaboration). Heterogeneity across studies was assessed using I 2 values and standard χ 2 tests. An I 2 ≥ 50% indicated significant heterogeneity, and a random-effects model was applied for the subsequent meta-analysis. Otherwise, a fixed-effects model was applied. Data are presented as risk ratios (RRs) with 95% confidence intervals (CIs). Publication bias was evaluated using a funnel plot.
Results
Study selection and quality assessment
The details of the literature search strategy are presented in Fig. 1 . The search strategy initially identified 658 articles. After screening the titles and abstracts and applying the inclusion/exclusion criteria, 636 articles were excluded. Of the 22 articles that met the initial selection criteria, 10 were excluded after a careful reading of the full text. Finally, this meta-analysis included 12 RCTs 3 , 9 , 10 , 11 , 12 , 13 , 14 , 17 , 18 , 19 , 20 , 21 involving 3241 patients. The characteristics of each included study are listed in Table 1 . The review authorsʼ judgments regarding each risk-of-bias item for each study are presented in Table 2 .
Fig. 1.
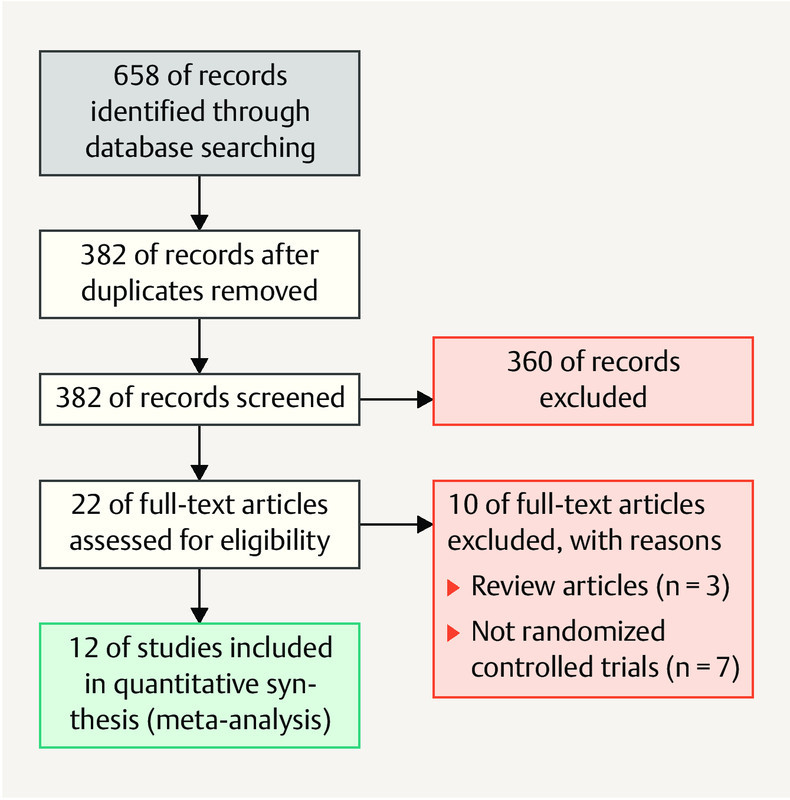
Flow diagram of the study selection process for the meta-analysis.
Table 1 Characteristics of the studies included in the meta-analysis.
| Author (year) | Country | Embryo stage | Country | Patients (n) | Outcomes included in the meta-analysis | |
|---|---|---|---|---|---|---|
| hCG | Control | |||||
| Mansour (2011) | Egypt | Cleavage | Egypt | 500 IU: 107 | 105/93 | Clinical pregnancy rate |
| Hong (2014) | USA | Blastocyst fresh or frozen | USA | 500 IU: 148 | 152 | Implantation rate, clinical pregnancy rate, ongoing pregnancy rate, abortion rate |
| Santibañez (2014) | Mexico | Day 3 fresh or frozen | Mexico | 500 IU: 101 | 109 | Clinical pregnancy rate |
| Zarei (2014) | Iran | Day 3 | Iran | 250 µg (equivalent to 6500 IU): 84 | 98 | Implantation rate, clinical pregnancy rate, abortion rate, ongoing pregnancy rate, ectopic pregnancy rate |
| Aaleyasin (2015) | Iran | Day 2 – 3 | Iran | 500 IU: 240 | 243 | Clinical pregnancy rate, Implantation rate, abortion rate, live birth rate |
| Wirleitner (2015) | Austria | Day 5 blastocyst | Austria | 500 IU: 599 | 587 | Implantation rate, clinical pregnancy rate, abortion rate, live birth rate |
| Dehghani (2016) | Iran | Day 2 – 3 | Iran | 500 IU: 53 1000 IU: 53 |
51 | Clinical pregnancy rate, abortion rate |
| Hossini (2016) | Iran | Cleavage or blastocyst, frozen | Iran | 500 IU: 50 | 50 | Implantation rate, clinical pregnancy rate, ongoing pregnancy rate |
| Huang (2016) | China | Day 3 frozen | China | 1000 IU: 62 | 50 | Clinical pregnancy rate, abortion rate, ongoing pregnancy rate |
| Navali (2016) | Iran | Day 3 fresh | Iran | 500 IU: 71 | 67 | Clinical pregnancy rate, ongoing pregnancy rate, abortion rate, ectopic pregnancy rate |
| Mostajeran (2017) | Iran | Blastocyst | Iran | 700 IU: 46 | 48 | Clinical pregnancy rate |
| Hafezi (2018) | India | Cleavage frozen | India | 500 IU: 60 | 60 | Clinical pregnancy live, abortion rate, live birth rate |
Table 2 Quality assessment of the included studies.
| Author (year) | Random Sequence Generation | Allocation Concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Mansour (2011) | Yes | Yes | Unclear | Unclear | No | Unclear | Unclear |
| Hong (2014) | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Santibañez (2014) | Yes | Unclear | Yes | Yes | Yes | Unclear | Unclear |
| Zarei (2014) | Yes | Unclear | Yes | Yes | No | Unclear | Unclear |
| Aaleyasin (2015) | Yes | No | No | No | Yes | Yes | Yes |
| Wirleitner (2015) | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes |
| Dehghani Firouzabadi (2016) | Yes | Yes | Unclear | Unclear | Yes | Unclear | Unclear |
| Hossini (2016) | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Huang (2016) | Yes | Unclear | Yes | Unclear | Yes | Unclear | Unclear |
| Navali (2016) | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Mostajeran (2017) | Yes | Unclear | Yes | Unclear | Yes | Unclear | Unclear |
| Hafezi (2018) | Yes | Unclear | Yes | Unclear | No | Unclear | Unclear |
Implantation rate
Only four studies 14 , 17 , 18 , 20 evaluated the implantation rate. As significant heterogeneity was observed among the included studies (I 2 = 92%, p < 0.00001), a random effect model was applied. Here, a meta-analysis found no evidence of a difference in implantation rates between the hCG and control groups (RR = 1.30; 95% CI: 0.89 – 1.90) ( Fig. 2 a ).
Fig. 2.
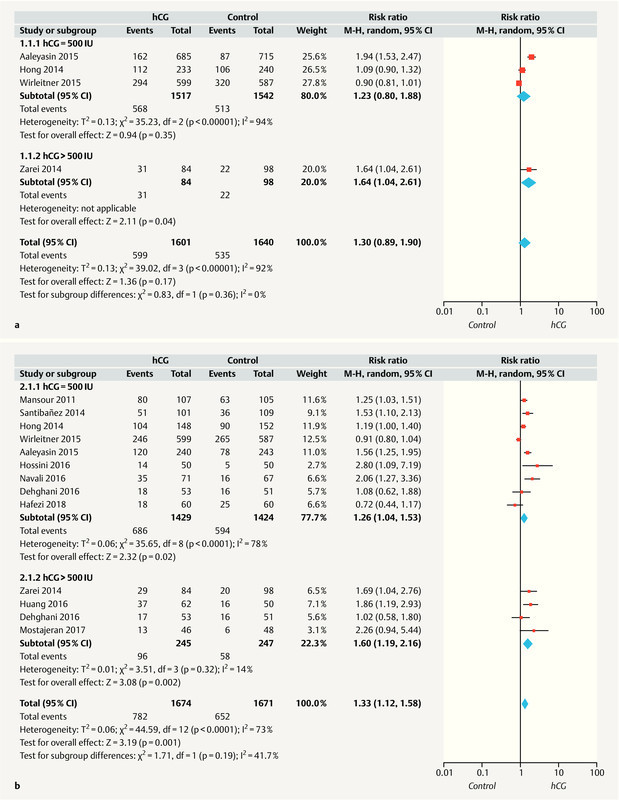
Forest plots of randomized controlled trials (RCTs) comparing patients who received intrauterine hCG administration vs. no hCG. a Implantation rate; b clinical pregnancy rate. CI = confidence interval.
Next, a subgroup analysis was performed to determine whether the hCG dose (500 vs. > 500 IU) or ET stage (cleavage vs. blastocyst) would affect the implantation rate. Although we observed no significant difference in the implantation rates when the subgroup receiving 500 IU intrauterine hCG was compared with the control group (RR = 1.23; 95% CI: 0.80 – 1.88; 3 studies), evidence indicated a significantly increase in the implantation rate among subjects who received > 500 IU intrauterine hCG (RR = 1.64; 95% CI: 1.04 – 2.61; 1 study). Furthermore, the subgroup of women who received cleavage-stage ETs with intra-cavity hCG (IC-hCG) exhibited a greater increase in the implantation rate, compared to those who received cleavage-stage ETs without IC-hCG (RR = 1.88; 95% CI: 1.52 – 2.32; two studies). However, a data synthesis revealed no significant difference in the implantation rates of women who received blastocyst-stage ETs with IC-hCG, compared to those who received blastocyst-stage ETs without IC-hCG (RR = 0.97; 95% CI: 0.81 – 1.17; two studies) ( Table 3 ).
Table 3 Effect of ET stage (cleavage vs. blastocyst stage) on the results of the meta-analysis.
| Subgroup title | No. of studies | No. of participants | Effect size (RR) | p-value |
|---|---|---|---|---|
| Implantation rate | 4 | 3242 | 1.30 [0.89, 1.90] | < 0.00001 |
|
2 | 1582 | 1.88 [1.52, 2.32] | 0.53 |
|
2 | 1659 | 0.97 [0.81, 1.17] | 0.1 |
| Clinical pregnancy rate | 11 | 3245 | 1.30 [1.09, 1.55] | 0.14 |
|
8 | 1665 | 1.38 [1.16, 1.65] | 0.52 |
|
3 | 1580 | 1.11 [0.83, 1.48] | 0.09 |
| Abortion rate | 9 | 2729 | 1.06 [0.78, 1.44] | 0.69 |
|
7 | 1243 | 1.16 [0.73, 1.86] | 0.79 |
|
2 | 1486 | 0.99 [0.66, 1.49] | 0.98 |
| Ongoing pregnancy rate | 4 | 915 | 1.81 [1.48, 2.21] | < 0.00 001 |
|
4 | 915 | 1.81 [1.48, 2.21] | < 0.00 001 |
| Live birth rate | 3 | 1789 | 0.99 [0.60, 1.63] | < 0.00 001 |
|
2 | 603 | 0.99 [0.34, 2.86] | 0.0005 |
|
1 | 1186 | 0.93 [0.80, 1.07] | 0.29 |
Clinical pregnancy rate
As shown in Fig. 2 b , all included studies 3 , 9 – 14 , 17 , 18 , 19 , 20 , 21 reported the clinical pregnancy rate, and significant heterogeneity was observed among the studies (I 2 = 73%, p < 0.0001). A random effects model analysis of the pooled data revealed a statistically significant increase in the clinical pregnancy rate in the hCG group, compared with the control group (RR = 1.33; 95% CI: 1.12 – 1.58).
A subgroup analysis was conducted to determine whether the hCG dose (500 vs. > 500 IU) or ET stage (cleavage vs. blastocyst) would affect the clinical pregnancy rate. Notably, the RRs for the subgroups receiving 500 and > 500 IU hCG were 1.26 (95% CI: 1.04 – 1.53; 9 studies) and 1.60 (95% CI: 1.19 – 2.16; 4 studies), respectively, relative to the controls. Furthermore, among women receiving cleavage-stage ETs, the clinical pregnancy rate was higher for those with IC-hCG, compared to those without IC-hCG (RR = 1.30; 95% CI: 1.09 – 1.55; eight studies). However, the data synthesis of women receiving blastocyst-stage ETs revealed no significant difference in the clinical pregnancy rate between those with and without IC-hCG (RR = 1.11; 95% CI: 0.83 – 1.48; three studies) ( Table 3 ).
Abortion rate
Eight included studies 10 , 11 , 13 , 14 , 17 , 18 , 20 , 21 reported the abortion rate, and no heterogeneity was observed among these studies (I 2 = 0%, p = 0.68). A meta-analysis identified no statistically significant difference in the abortion rates of the ICG and control groups (fixed-effect model; RR = 1.06; 95% CI: 0.78 – 1.44) ( Fig. 3 a ).
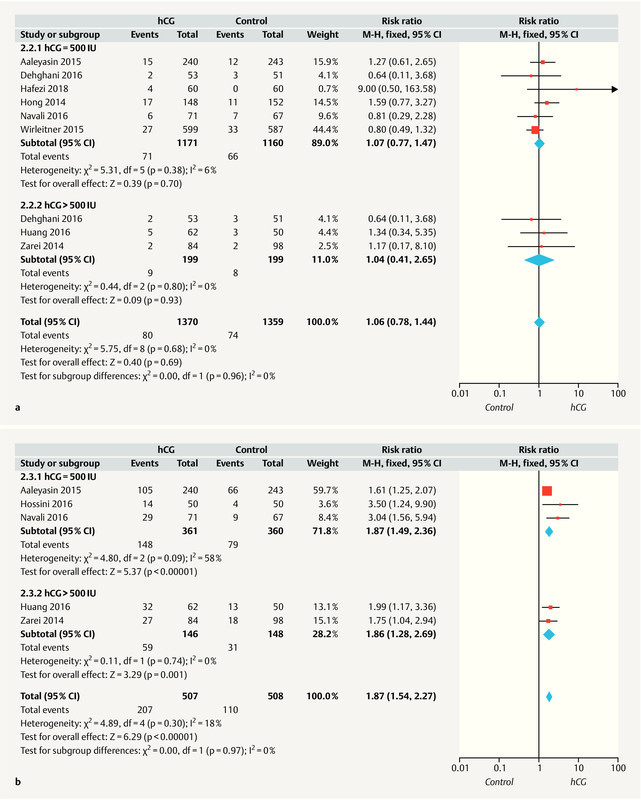
Fig. 3.
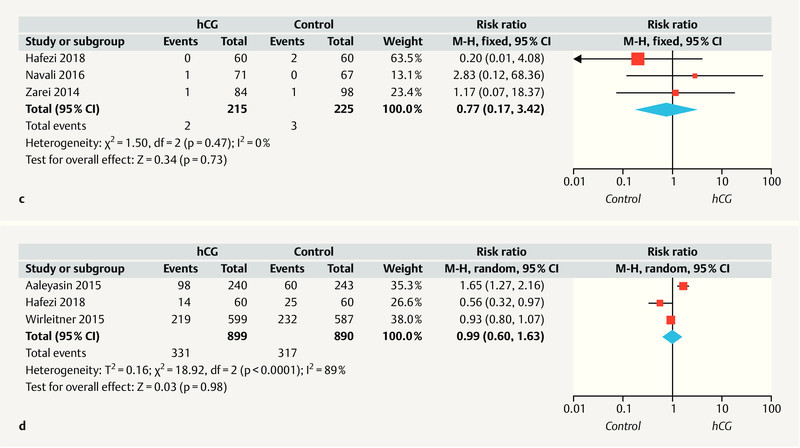
Forest plots of randomized controlled trials (RCTs) comparing patients who received intrauterine hCG administration vs. no hCG. a Abortion rate; b ongoing pregnancy rate; c ectopic pregnancy rate; d live birth rate. CI = confidence interval.
A subgroup analysis was conducted to determine whether the hCG dose (500 vs. > 500 IU) or ET stage (cleavage vs. blastocyst) would affect the abortion rate. Compared to the control group, women who received 500 and > 500 IU hCG had RRs of 1.07 (95% CI: 0.77 – 1.47; six studies) and 1.04 (95% CI: 0.41 – 2.65; three studies), respectively. Furthermore, a data synthesis revealed no significant differences in the abortion rates between women with and without IC-hCG in both the subgroups receiving cleavage-stage and blastocyst-stage ETs (RR = 1.16; 95% CI: 0.73 – 1.86 and RR = 0.99; 95% CI: 0.66 – 1.49; respectively) ( Table 3 ).
Ongoing pregnancy rate
As shown in Fig. 3 b , five of the included studies 11 , 12 , 17 , 18 , 21 reported the ongoing pregnancy rate, and a low level of heterogeneity was detected among these studies (I 2 = 18%, p = 0.30). A fixed effects model analysis of the pooled data revealed a significantly higher ongoing pregnancy rate in the hCG group, compared with the control group (RR = 1.87; 95% CI: 1.54 – 2.27).
Next, a subgroup analysis was conducted to determine whether the hCG dose (500 vs. > 500 IU) or ET stage (cleavage vs. blastocyst) would affect the ongoing pregnancy rate. Compared to the control group, the RRs for women receiving 500 and > 500 IU hCG were 1.87 (95% CI: 1.49 – 2.36; three studies) and 1.86 (95% CI: 1.28 – 2.69; two studies), respectively. Additionally, among women receiving cleavage-stage ETs, those with IC-hCG had a higher ongoing pregnancy rate, compared to those without IC-hCG (RR = 1.8; 95% CI: 1.48 – 2.21; 4 studies) ( Table 3 ).
Ectopic pregnancy rate
As shown in Fig. 3 c , three studies 10 , 18 , 21 reported the ectopic pregnancy rate, and no heterogeneity was detected (I 2 = 0%, p = 0.47). The fixed effects model analysis of pooled data showed no evidence of a significant difference in the ectopic pregnancy rate between the ICG and control groups (RR = 0.77; 95% CI: 0.17 – 3.42).
Live birth rate
As shown in Fig. 3 d , three studies 10 , 14 , 17 reported the live birth rate, and significant heterogeneity was observed (I 2 = 89%, p < 0.0001). A random effects model analysis of the pooled data found no significant difference in the live birth rate between the ICG and control groups (RR = 0.99; 95% CI: 0.60 – 1.63).
Next, a subgroup analysis was conducted to determine whether the ET stage (cleavage vs. blastocyst) would affect the live birth rate. However, the data synthesis revealed no significant difference in the live birth rate between women with and without IC-hCG in both the subgroups receiving cleavage-stage or blastocyst-stage ETs (RR = 0.99; 95% CI: 0.34 – 2.86; two studies and RR = 0.93; 95% CI: 0.80 – 1.07; respectively) ( Table 3 ).
Publication bias
A funnel plot was used to qualitatively evaluate publication bias among the studies comparing the clinical pregnancy rates of the hCG (≥ 500 IU) and control groups. The partially symmetrical funnel plot presented in Fig. 4 indicates no potential publication bias in the included studies.
Fig. 4.
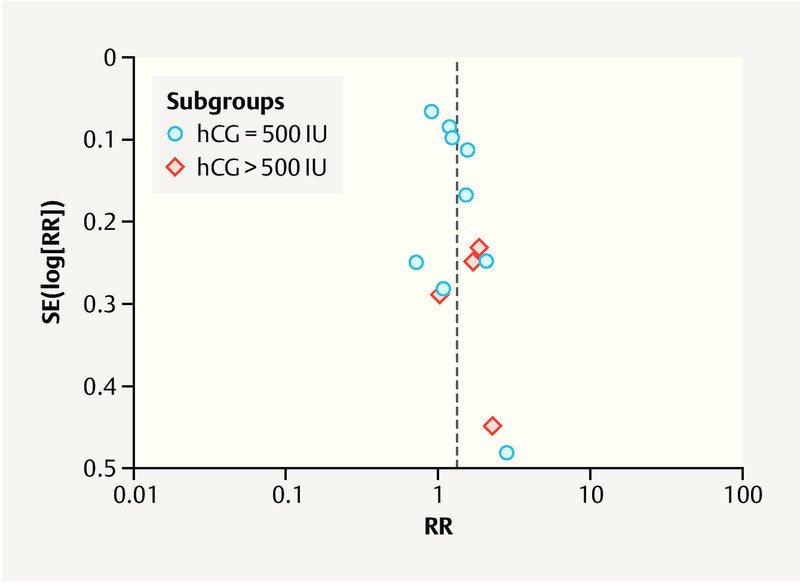
Funnel plot used to detect publication bias. RR = risk ratio.
Discussion
HCG rescues the maternal corpus luteum in early pregnancy via a classical endocrine mechanism 22 , and several studies have identified this hormone as a key factor during preparation for implantation and regulation of the uterine environment 23 , 24 , 25 , 26 . Recently, Ye et al. 27 reviewed pooled data from women who received intrauterine hCG injections at a very wide range of doses but did not evaluate publication bias in that meta-analysis. Ye and colleagues reported that intrauterine hCG injection significantly increased the rates of biochemical, clinical and ongoing pregnancy, compared with the control 27 . However, another meta-analysis 15 reported that intrauterine hCG administration had an ambiguous effect on the clinical pregnancy rate. However, that analysis included two oral abstracts, and the 95% CI of the applied RR included the value of 1 (i.e., no evidence of an effect).
In this meta-analysis, we specifically evaluated the effects of different intrauterine hCG doses prior to ET on various outcomes of ART. Our findings are important because this was the first study to separately analyze the effects of different doses of hCG (500 vs. > 500 IU). Furthermore, our analysis retrieved six additional relevant studies published during the 2 years since the last meta-analysis 15 . Our results regarding the clinical and ongoing pregnancy rates, as well as the implantation rate, were consistent with the previous meta-analysis 15 and partially consistent with the previous by Ye and colleagues 27 . Furthermore, our analysis of the effects of embryonic stage on the outcomes revealed that women who received cleavage-stage ETs with IC-hCG exhibited increases in the implantation, clinical pregnancy and ongoing pregnancy rates, compared to their counterparts without IC-hCG.
HCG is secreted by the embryo during early development, and the level of this hormone in the culture media correlates positively with the grade of the developing embryo, as well as with the number of blastomeres 28 . Furthermore, hCG plays well-established and important roles in promoting angiogenesis and regulating the inflammatory response during embryo implantation. Therefore, the intrauterine administration of HCG before an ET can overcome decreases in endometrial receptivity induced by ART treatments 29 , 30 , 31 . Therefore, the intrauterine injection of hCG appears to increase the chance of implantation and to significantly increase the clinical and ongoing pregnancy rates.
This study had several strengths worth noting. First, this meta-analysis was based on rigorous methodology because all identified studies were RCTs. Second, the studies included in this meta-analysis were of a relatively satisfactory level of quality and fulfilled our predefined inclusion criteria. Third, a large number of individuals were pooled from various trials, which significantly enhanced the statistical power of the meta-analysis. Fourth, no obvious publication bias was identified, indicating that the results of this meta-analysis are unbiased.
Despite these important findings, however, our study also had some potential limitations. First, our analysis included only 12 RCTs, some of which included rather small numbers of participants. These factors might have affected the validity and reliability of the conclusions. Second, studies were retrieved only from online and English-language databases. Accordingly, several relevant studies may have been missed, and language bias may have been introduced. Third, although all included studies were RCTs, some did not report specific methods of randomization, such as allocation concealment and blinding, which may have led to publication and reporting biases. Fourth, the type of hCG was not consistent across studies, which might have affected the results of the meta-analysis. Finally, obvious heterogeneity was detected for several outcomes. This heterogeneity may be attributable to inter-study variations such as differences in inclusion and exclusion criteria, sample sizes and patient demographics (e.g., race, age, body mass index).
Conclusion
Our results indicate that the intrauterine injection of hCG before ET led to improved clinical pregnancy and ongoing pregnancy rates. However, well-designed, large multi-center RCTs are warranted to provide further evidence.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 81402125 and no. 83662509).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.te Velde E R, Eijkemans R, Habbema H D. Variation in couple fecundity and time to pregnancy, an essential concept in human reproduction. Lancet. 2000;355:1928–1929. doi: 10.1016/s0140-6736(00)02320-5. [DOI] [PubMed] [Google Scholar]
- 2.Skakkebaek N E, Jorgensen N, Main K M. Is human fecundity declining? Int J Androl. 2006;29:2–11. doi: 10.1111/j.1365-2605.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 3.Mansour R, Tawab N, Kamal O. Intrauterine injection of human chorionic gonadotropin before embryo transfer significantly improves the implantation and pregnancy rates in in vitro fertilization/intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril. 2011;96:1370–13740. doi: 10.1016/j.fertnstert.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 4.de Mouzon J, Lancaster P, Nygren K G. World collaborative report on Assisted Reproductive Technology, 2002. Hum Reprod. 2009;24:2310–2320. doi: 10.1093/humrep/dep098. [DOI] [PubMed] [Google Scholar]
- 5.Kane N, Kelly R, Saunders P T. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology. 2009;150:2882–2888. doi: 10.1210/en.2008-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan H, Versnel M A, Cheung W Y. Chorionic gonadotropin can enhance innate immunity by stimulating macrophage function. J Leukoc Biol. 2007;82:926–933. doi: 10.1189/jlb.0207092. [DOI] [PubMed] [Google Scholar]
- 7.Fritz M A, Speroff L. 8th ed. USA: Lippincot Williams and Wilkins; 2011. Clinical gynecologic Endocrinology and Infertility. [Google Scholar]
- 8.Wallace L D, Breiner C A, Breiner R A. Administration of human chorionic gonadotropin at embryo transfer induced ovulation of a first wave dominant follicle, and increased progesterone and transfer pregnancy rates. Theriogenology. 2011;75:1506–1515. doi: 10.1016/j.theriogenology.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Mostajeran F, Godazandeh F, Ahmadi S M. Effect of intrauterine injection of human chorionic gonadotropin before embryo transfer on pregnancy rate: A prospective randomized study. J Res Med Sci. 2017;22:6. doi: 10.4103/1735-1995.199096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafezi M, Madani T, Arabipoor A. The effect of intrauterine human chorionic gonadotropin flushing on live birth rate after vitrified-warmed embryo transfer in programmed cycles: a randomized clinical trial. Arch Gynecol Obstet. 2018;297:1571–1576. doi: 10.1007/s00404-018-4752-2. [DOI] [PubMed] [Google Scholar]
- 11.Huang P, Wei L, Li X. A study of intrauterine infusion of human chorionic gonadotropin (hCG) before frozen-thawed embryo transfer after two or more implantation failures. Gynecol Endocrinol. 2017;33:67–69. doi: 10.1080/09513590.2016.1207164. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini R S, Farzad L, Abdollahi S. Effect of intrauterine injection of human chorionic gonadotropin before frozen-thawed embryo transfer on implantation and clinical pregnancy rate: A randomized controlled trial. International Journal of Womenʼs Health and Reproduction Sciences. 2016;4:200–203. [Google Scholar]
- 13.Dehghani Firouzabadi R, Janati S, Razi M H. The effect of intrauterine human chorionic gonadotropin injection before embryo transfer on the implantation and pregnancy rate in infertile patients: A randomized clinical trial. Int J Reprod Biomed (Yazd) 2016;14:657–664. [PMC free article] [PubMed] [Google Scholar]
- 14.Wirleitner B, Schuff M, Vanderzwalmen P. Intrauterine administration of human chorionic gonadotropin does not improve pregnancy and life birth rates independently of blastocyst quality: a randomised prospective study. Reprod Biol Endocrinol. 2015;13:70. doi: 10.1186/s12958-015-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman A, Pundir J, Elsherbini M. The effect of intrauterine HCG injection on IVF outcome: a systematic review and meta-analysis. Reprod Biomed Online. 2016;33:350–359. doi: 10.1016/j.rbmo.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J P, Altman D G, Gotzsche P C. The Cochrane Collaborationʼs tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaleyasin A, Aghahosseini M, Rashidi M. In vitro fertilization outcome following embryo transfer with or without preinstillation of human chorionic gonadotropin into the uterine cavity: a randomized controlled trial. Gynecol Obstet Invest. 2015;79:201–205. doi: 10.1159/000363235. [DOI] [PubMed] [Google Scholar]
- 18.Zarei A, Parsanezhad M E, Younesi M. Intrauterine administration of recombinant human chorionic gonadotropin before embryo transfer on outcome of in vitro fertilization/intracytoplasmic sperm injection: A randomized clinical trial. Iran J Reprod Med. 2014;12:1–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Santibanez A, Garcia J, Pashkova O. Effect of intrauterine injection of human chorionic gonadotropin before embryo transfer on clinical pregnancy rates from in vitro fertilisation cycles: a prospective study. Reprod Biol Endocrinol. 2014;12:9. doi: 10.1186/1477-7827-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong K H, Forman E J, Werner M D. Endometrial infusion of human chorionic gonadotropin at the time of blastocyst embryo transfer does not impact clinical outcomes: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2014;102:1591–159500. doi: 10.1016/j.fertnstert.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Navali N, Gassemzadeh A, Farzadi L. Intrauterine administration of hCG immediately after oocyte retrieval and the outcome of ICSI: a randomized controlled trial. Hum Reprod. 2016;31:2520–2526. doi: 10.1093/humrep/dew236. [DOI] [PubMed] [Google Scholar]
- 22.Fluhr H, Krenzer S, Deperschmidt M. Human chorionic gonadotropin inhibits insulin-like growth factor-binding protein-1 and prolactin in decidualized human endometrial stromal cells. Fertil Steril. 2006;86:236–238. doi: 10.1016/j.fertnstert.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Edwards R G. Chorionic gonadotrophin, genes and embryonic signals regulating the implantation window. Reprod Biomed Online. 2007;14:538–540. doi: 10.1016/s1472-6483(10)60904-2. [DOI] [PubMed] [Google Scholar]
- 24.Paiva P, Hannan N J, Hincks C. Human chorionic gonadotrophin regulates FGF2 and other cytokines produced by human endometrial epithelial cells, providing a mechanism for enhancing endometrial receptivity. Hum Reprod. 2011;26:1153–1162. doi: 10.1093/humrep/der027. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava A, Sengupta J, Kriplani A. Profiles of cytokines secreted by isolated human endometrial cells under the influence of chorionic gonadotropin during the window of embryo implantation. Reprod Biol Endocrinol. 2013;11:116. doi: 10.1186/1477-7827-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugihara K, Kabir-Salmani M, Byrne J. Induction of trophinin in human endometrial surface epithelia by CGbeta and IL-1beta. FEBS Lett. 2008;582:197–202. doi: 10.1016/j.febslet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Ye H, Hu J, He W. The efficacy of intrauterine injection of human chorionic gonadotropin before embryo transfer in assisted reproductive cycles: Meta-analysis. J Int Med Res. 2015;43:738–746. doi: 10.1177/0300060515592903. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Zhang R, Han D.[Association of human chorionic gonadotropin level in embryo culture media with early embryo development] Nan Fang Yi Ke Da Xue Xue Bao 2014341039–1041.1047 [PubMed] [Google Scholar]
- 29.Lee T K, Kim D I, Song Y L. Differential inhibition of Scutellaria barbata D. Don (Lamiaceae) on HCG-promoted proliferation of cultured uterine leiomyomal and myometrial smooth muscle cells. Immunopharmacol Immunotoxicol. 2004;26:329–342. doi: 10.1081/iph-200026841. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher A, Brachwitz N, Sohr S. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182:5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee P, Fazleabas A T. Endometrial responses to embryonic signals in the primate. Int J Dev Biol. 2010;54:295–302. doi: 10.1387/ijdb.082829pb. [DOI] [PMC free article] [PubMed] [Google Scholar]


