Abstract
Background
Patient-specific instrumentation (PSI) systems for total shoulder arthroplasty (TSA) can improve glenoid component placement, but may involve considerable expense and production delays. The purpose of this study was to evaluate a novel technique for in-house production of 3-dimensionally printed, patient-specific glenoid guides. We hypothesized that our PSI guide would improve the accuracy of glenoid guide pin placement compared with a standard TSA guide.
Methods
We randomized 20 cadaveric shoulders to receive pin placement via the PSI guide (n = 10, study group) or standard TSA guide (n = 10, control group). PSI guides were designed to fit each glenoid based on 3-dimensional scapular models constructed from computed tomography scans. A presurgical plan was created for the guide pin trajectory in neutral version and inclination based on individual scapular anatomy. After pin placement, 3-dimensional models from repeated computed tomography scans were superimposed to calculate deviation from the presurgical plan for each specimen.
Results
Inclination deviation was significantly lower in the PSI group than in the standard guide group (1.5° ± 1.6° vs. 6.4° ± 5.0°, P = .009). The glenoid entry site exhibited significantly less deviation in the PSI group (0.8 ± 0.6 mm vs. 2.1 ± 1.2 mm, P = .008). The average production cost and time for the PSI guides were $29.95 and 4 hours 40 minutes per guide, respectively.
Conclusions
The PSI guide significantly improved the accuracy of glenoid pin placement compared with the standard TSA guide. Our PSI guides can be produced in-house, inexpensively, and with substantially reduced time compared with commercially available guides.
Keywords: Patient-specific instrumentation, shoulder arthroplasty, shoulder replacement, 3D printing, patient specific, glenoid, guide
Achieving accurate glenoid component implantation remains the most difficult technical challenge in total shoulder arthroplasty (TSA), particularly in patients with advanced glenoid wear.5, 6 In recent years, there has been increased interest regarding patient-specific instrumentation (PSI) as a means of improving the accuracy and precision of glenoid prosthesis implantation. The motivation to improve the accuracy of glenoid component placement stems from research indicating that poorly placed glenoid components in retroversion greater than 10° to 15° experience significantly increased stresses at the bone-implant interface, as well as increased rates of osteolysis.10, 16, 20 Therefore, it is believed that more accurate glenoid implantation facilitated by patient-specific 3-dimensional (3D) preoperative planning and instruments manufactured according to the individual patient's anatomy may reduce the rate of glenoid loosening, potentially leading to improved implant survivorship. Although no long-term clinical data are currently available, several cadaveric and clinical studies have demonstrated that PSI can significantly and reliably reduce errors in glenoid prosthesis implantation during TSA.13, 15, 20, 30
In general, most PSI systems currently available for TSA function similarly: Clinical imaging modalities such as computed tomography (CT) are used to create computerized 3D scapular models.20, 34 On the basis of these 3D models, preoperative plans are created for angular and directional positioning of the central glenoid guide pin (subsequently used to direct glenoid reaming) and/or the glenoid prosthetic component itself.6, 9, 19, 34 Patient-specific devices are constructed according to these plans and used intraoperatively to accurately place the central glenoid guide pin.6, 13, 15 The positioning of the glenoid guide pin and/or glenoid prosthesis is then verified and compared with the preoperative plan via postoperative imaging and 3D modeling.15, 20
Commercial manufacturers currently market various PSI systems for use in TSA, several of which have been used in previous studies.6, 13, 15, 23, 30 Although their performances have generally been shown to be reliable, outsourcing PSI device production to an external manufacturer may not be a practical or accessible option for all surgeons. The additional expenses involved with outsourcing may be a considerable barrier for many, and production delays of up to 6 weeks per device may deter surgeons from investing substantial resources in such PSI systems.32 For PSI technology to realistically be considered for widespread use in TSA, it needs to consistently outperform the current surgical standard of care while allowing for efficient and economical modes of production.
The purpose of our study was to evaluate the accuracy and precision of glenoid guide pin placement using a novel, in-house produced, 3-dimensionally printed patient-specific glenoid guide in a cadaveric model. We hypothesized that our patient-specific guides would significantly decrease angulation and positional errors of glenoid guide pin placement compared with standard instrumentation in a cadaveric model. Furthermore, we hypothesized that these devices could be produced with significantly reduced costs and time compared with commercially available PSI systems.
Methods
Cadaveric specimens
We obtained 20 fresh-frozen male cadaveric shoulder specimens (including 4 bilateral specimens from 2 donors) for this study. The specimens were randomized via an electronic random number generator to either the PSI group (n = 10) or standard instrumentation control group (n = 10). The mean age ± standard deviation of the specimens in the PSI and control groups was 56.9 ± 5.9 years and 57.2 ± 6.5 years, respectively (P = .916). The average body mass index was 24.9 ± 3.5 and 28.6 ± 6.9 in the PSI and control groups, respectively (P = .148). The PSI group comprised 5 left and 5 right shoulders, whereas the control group comprised 4 left and 6 right shoulders (P = .653).
Preoperative glenoid evaluation
Each cadaveric specimen underwent a CT scan in the supine position on a Brightspeed scanner (General Electric, Waukesha, WI, USA) according to the following parameters: 0.625-mm slice thickness, 120 kV, 260 milliampere-seconds, and image matrix of 512 × 512. The field of view was adapted with a maximum of 180 mm, and the pixel size was calculated at 0.32 mm. The 2-dimensional DICOM (Digital Imaging and Communications in Medicine) CT image files were imported into Mimics Medical software (version 20.0; Materialise NV, Leuven, Belgium) to create 3D models of each scapular specimen by segmentation. The 3D model reconstructions were first reviewed to exclude the presence of a glenoid fracture or obvious presence of previous disease. The native glenoid morphology was then characterized by measuring angles of glenoid version and inclination according to methods previously described by Bryce et al4 and Hoenecke et al17 because quantification of these angles has been shown to be more accurate on 3D reconstructions vs. 2-dimensional image slices.12, 26 The glenoid was also categorized preoperatively according to the Walch classification system.33 The preoperative quantification of native glenoid morphology can be found in Table I. There were no statistically significant differences between the PSI and standard instrumentation groups in mean native glenoid version (–5.5° ± 2.64° and –4.0° ± 3.5°, respectively; P = .292) or inclination (7.5° ± 3.0° and 9.2° ± 5.4°, respectively; P = .411).
Table I.
Preoperative comparison of specimen demographic characteristics
| Demographic characteristic | PSI guide | Standard TSA guide | P value |
|---|---|---|---|
| Age, yr | 56.9 ± 5.9 | 57.2 ± 6.6 | .916 |
| BMI, kg/m2 | 24.9 ± 3.5 | 28.6 ± 6.9 | .148 |
| Laterality | 5 R and 5 L | 4 R and 6 L | .653 |
| Version, ° | –5.6 ± 2.6 | –4.1 ± 3.5 | .292 |
| Inclination, ° | 7.5 ± 3.0 | 9.2 ± 5.4 | .411 |
| Walch glenoid classification,33 % | |||
| Type A1 | 40.0 | 50.0 | .653 |
| Type A2 | 10.0 | 20.0 | .531 |
| Type B1 | 50.0 | 30.0 | .361 |
PSI, patient-specific instrumentation; TSA, total shoulder arthroplasty; BMI, body mass index; R, right; L, left.
The level of statistical significance was defined as P < .05. Negative values indicate retroversion or inferior inclination.
Surgical planning
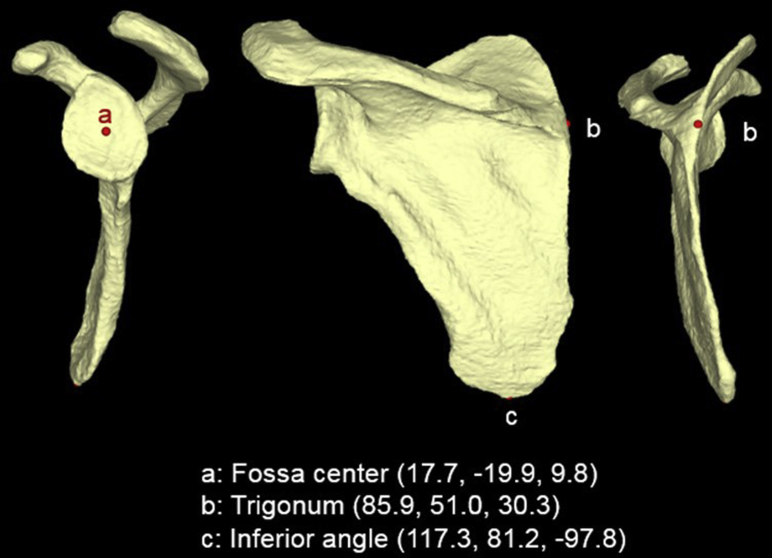
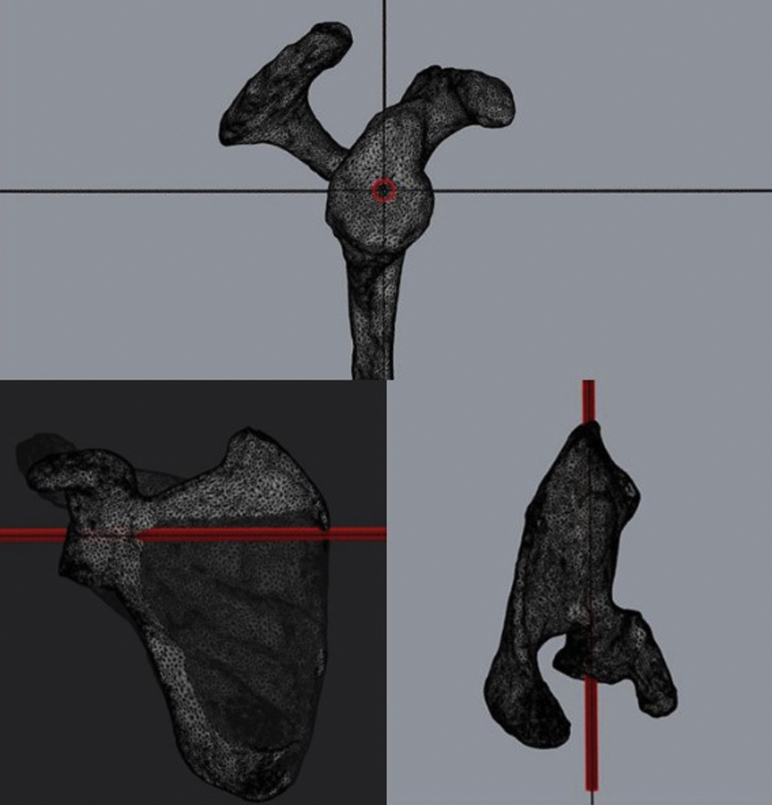
The 3D scapular models previously created in Mimics Medical software (version 20.0) were exported in stereolithography (STL) file format as input data for a custom-written Visual C++ program (Microsoft Foundation Class; Microsoft, Redmond, WA, USA) to begin surgical planning. Within the customized software program, an algorithm was used to automatically calculate the exact coordinates for 3 points needed to establish a coronal scapular plane for each specimen, as previously described by Bryce et al.4 The scapular plane was defined by the following 3 points: (1) most distal point of the inferior angle of the scapula; (2) glenoid fossa center; and (3) scapula trigonum, or intersection of the medial border of the scapula with the scapular spine (Fig. 1).4 The glenoid fossa center was determined by the following procedures: A glenoid surface polygon model was created by automatically selecting polygons facing the lateral direction. Normal vectors of the polygons comprising the glenoid surface model were averaged, and a mean normal vector of the overall glenoid surface was determined. A centroid of the glenoid surface was calculated and projected onto the glenoid surface in the direction of the mean normal vector.21 The projected point was defined as the glenoid fossa center. This process was repeated for the other 2 scapular plane points. After determination of all 3 scapular plane coordinates, the 3D scapular model STL file was imported into Rhinoceros 3D (Robert McNeel & Associates, Seattle, WA, USA), commercially available computer-aided design application software, to begin PSI guide construction. By use of the previously established coordinates, the scapular plane was created for the 3D scapular model within Rhinoceros 3D. An axial plane was then constructed orthogonal to the coronal scapular plane, with the planes intersecting at the level of the established center of the glenoid. With these 2 planes used as a reference, a pin trajectory of 0° of version and 0° of inclination that passed through the exact center of the glenoid was established for each PSI guide (Fig. 2). This central pin trajectory of 0° of version and 0° of inclination has been used in previous cadaveric and clinical studies examining the impact of PSI devices on anatomic TSA.15, 19, 22, 31
Figure 1.
Representative example of 3 coordinates determined for coronal scapular plane based on 3-dimensional computed tomography scan reconstruction.4
Figure 2.
Model of predetermined glenoid guide pin trajectory of 0° of version and 0° of inclination through calculated center glenoid location. Red is a cylinder that simulates placement of the central guide pin.
PSI device construction
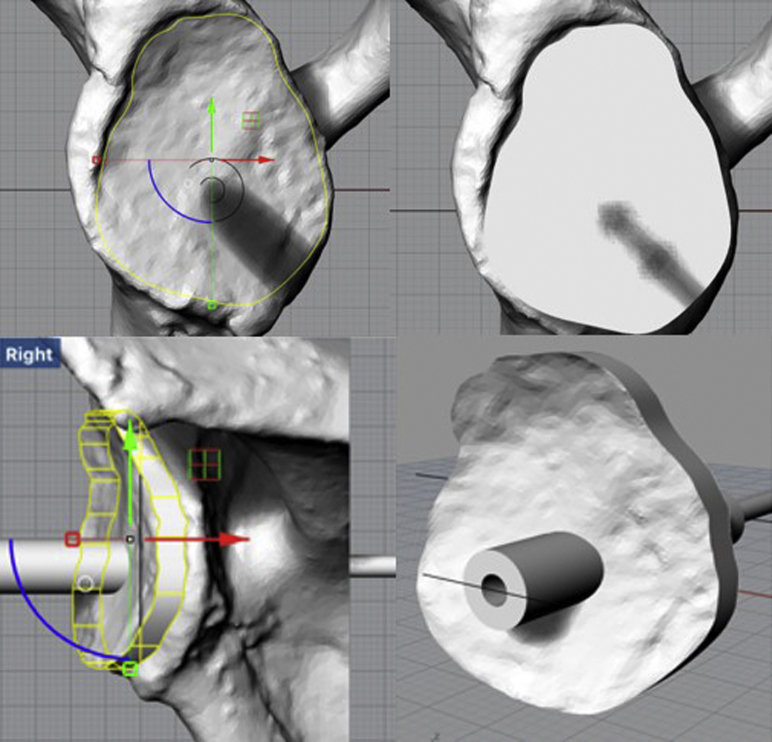
Once the appropriate trajectory was established, the PSI device was constructed according to the individual scapular anatomy for each case. The overall design of our novel PSI guide involved a perfect reflection of the glenoid face morphology fitted with a tunnel to place the central glenoid guide pin in the predetermined trajectory (Fig. 3). The perfect reflection allowed for an exact fit between the PSI guide and the face of the glenoid according to the individual anatomy and morphology of each specimen. The tactile feedback from this perfect fit indicated proper positioning on the glenoid, and an arrow pointing in the cranial direction printed on each PSI guide served as an additional measure to aid proper orientation. Commercially available PSI devices serve the same general purpose—to direct the placement of the central glenoid pin—although particular methods of alignment and fixation may differ slightly.6, 13, 30 Once the PSI guide was finalized, it was exported in STL file format to be 3-dimensionally printed. All guides were printed on the Formlabs Form 2 desktop 3D printer (Formlabs, Somerville, MA, USA), which uses stereolithography additive manufacturing technology to convert digital STL files into 3D objects. Formlabs Dental SG Resin, a class 1 biocompatible resin (EN-ISO 10993-1:2009/AC:2010, United States Pharmacopeia class VI), designed to be sterilized by autoclave, was used to print all PSI devices. Appropriate post-processing of all devices was carried out according to the manufacturer's instructions.
Figure 3.
Design of patient-specific instrumentation device to fit glenoid face. The glenoid guide is designed to perfectly reflect each glenoid's morphology. Green arrow is y axis and red arrow is x axis in two-dimensional space. Yellow shape displays the outline of the patient-specific instrumentation guide. Blue displays a portion of a circle drawn using the origin of x and y axes as the center. The completed hollow cylinder (bottom right) directs placement of the central guide pin in the desired trajectory.
Guide pin implantation
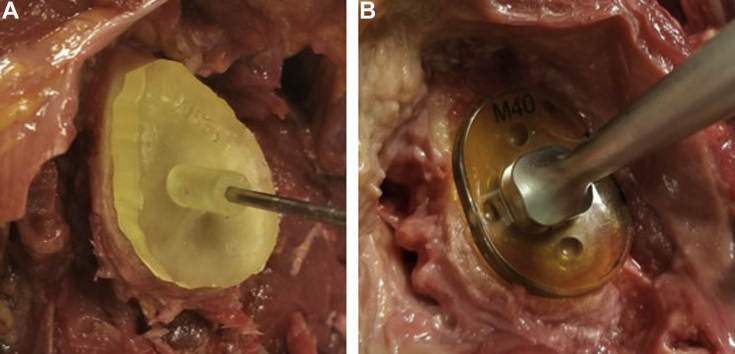
Initially, a deltopectoral approach was performed on cadaveric specimens with a subscapularis peel. Capsular release was carried out. The humerus was removed to allow equivalent access to the glenoid across all specimens. In all cadaveric shoulders, the labral and cartilaginous soft tissues were débrided down to the bony glenoid surface. The remainder of the shoulder musculature remained intact to simulate the conditions of a deltopectoral approach as well as possible. Each PSI device was then placed on the glenoid face of the corresponding scapular specimens in the PSI group (n = 10). The perfect reflection of the glenoid face on the PSI device allowed it to be quickly and properly positioned in the correct orientation (Fig. 4). A board-certified, fellowship-trained orthopedic surgeon verified correct device positioning and subsequently drilled a 2.5-mm-diameter Kirschner wire guide pin in a bicortical manner through the glenoid in a single attempt. Manual palpation confirmed that the guide pin exited the glenoid vault.
Figure 4.
Patient-specific device and standard total shoulder arthroplasty guide in use. (A) Patient-specific device with placement of central glenoid guide pin. (B) Standard total shoulder arthroplasty guide in process of placing central glenoid guide pin.
For the control group (n = 10), the exact same methodology was used to prepare each specimen and place the central glenoid pin. However, instead of a PSI device, a standard TSA drill guide (Wright Medical, Memphis, TN, USA) was used for pin placement (Fig. 4). The structure of the standard TSA guide included a straight drill path that exited a slightly convex glenoid plate designed to lie on the face of the glenoid. The standard TSA guide was designed so that the position on the glenoid face and angular orientation could be adjusted manually according to surgeon preference. Three standard guide options were available for use, depending on the morphology of each glenoid (small, medium, and large), which were chosen based on surgeon judgment. The standard goal pin trajectory of 0° of version and 0° of inclination through the center of the glenoid was used for all control-group specimens. With this in mind, the surgeon manually adjusted both the position and angular orientation of the standard TSA guide, as needed, with the aim of achieving as close to the goal guide pin trajectory as possible. Once satisfied with the position and orientation of the standard TSA guide, the surgeon drilled a 2.5-mm-diameter Kirschner wire guide pin in a bicortical manner through the glenoid in a single attempt. Manual palpation confirmed that the guide pin exited the glenoid vault.
Evaluation of accuracy
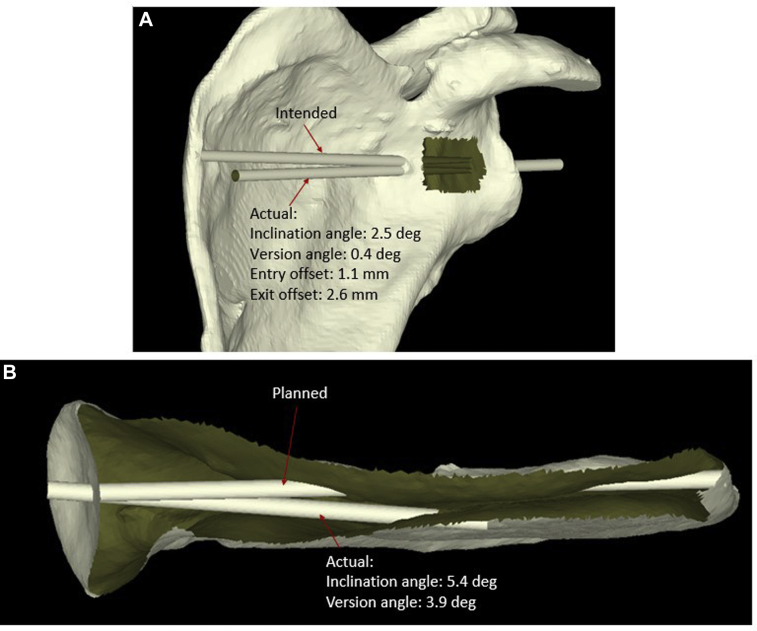
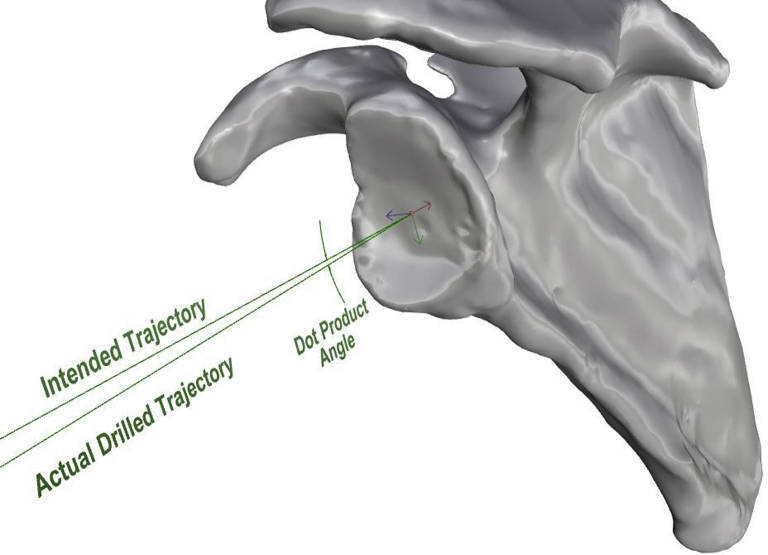
Postoperative CT scans were performed for each specimen by the same imaging protocol, and the DICOM files from each CT scan were used to create postoperative 3D scapular models as previously described. The STL files for each specimen were then uploaded into the Visual C++ custom program for evaluation of pin placement accuracy. The postoperative 3D scapular model was superimposed on the preoperative scapular model using the same 3 previously established coordinates for the coronal scapular plane. After completion of this process, a blinded observer with no knowledge of specimen allocation quantified deviation of the bicortical drill path from the predetermined trajectory for each specimen. The following 4 measurements were performed: (1) deviation of the pin version angle from 0°, (2) deviation of the pin inclination angle from 0°, (3) pin entry point positional offset on the glenoid face center (in millimeters), and (4) pin exit point positional offset on the glenoid vault (in millimeters) (Fig. 5). These measurements have been previously described in studies using PSI systems in TSA.6, 13, 19, 34 The vectorial dot product (in degrees) was also calculated to express the angular relationship between the version and inclination vectors3, 7, 18 (Fig. 6).
Figure 5.
Calculation of deviation in version, inclination, and offset of entry and exit points from presurgical plan. (A) View of planned vs. actual trajectory of central glenoid guide pin and calculation of deviation in version angle, inclination angle, entry point location, and exit site location. (B) Axial view of planned vs. actual glenoid guide pin trajectory. deg, degrees.
Figure 6.
Visual illustration of angle between version and inclination angle vectors calculated using dot product. Red, blue, and green arrows are the directions of the x, y, and z axes (respectively) that originate from the calculated center of the glenoid face in three-dimensional space.
Statistical analyses
Sample size calculations were performed using G*Power software (version 3.0.10; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany).11 Power analysis for a 2-sample independent Student t test (1:1 allocation ratio of PSI to standard instrumentation) was carried out a priori using published data comparing version angle deviation with PSI guides vs. standard instrumentation to calculate the effect size.19 With an α value of .05 and β of 85.0% for the difference in mean degrees in version deviation, a necessary sample size of 20 was calculated to detect a statistically significant difference.
All subsequent statistical analyses were performed with Stata/IC software (version 15; StataCorp, College Station, TX, USA). Two-tailed independent Student t tests were used to compare preoperative evaluation of native glenoid mean version and mean inclination, as well as age and body mass index, between the PSI group and standard instrumentation control group. The Fisher exact test was used to compare categorical variables of laterality and Walch glenoid classification. Two-tailed independent Student t tests were used to compare differences in postoperative version angle, inclination angle, dot product angle, entry point offset, and exit point offset between the PSI group and standard instrumentation control group.
Results
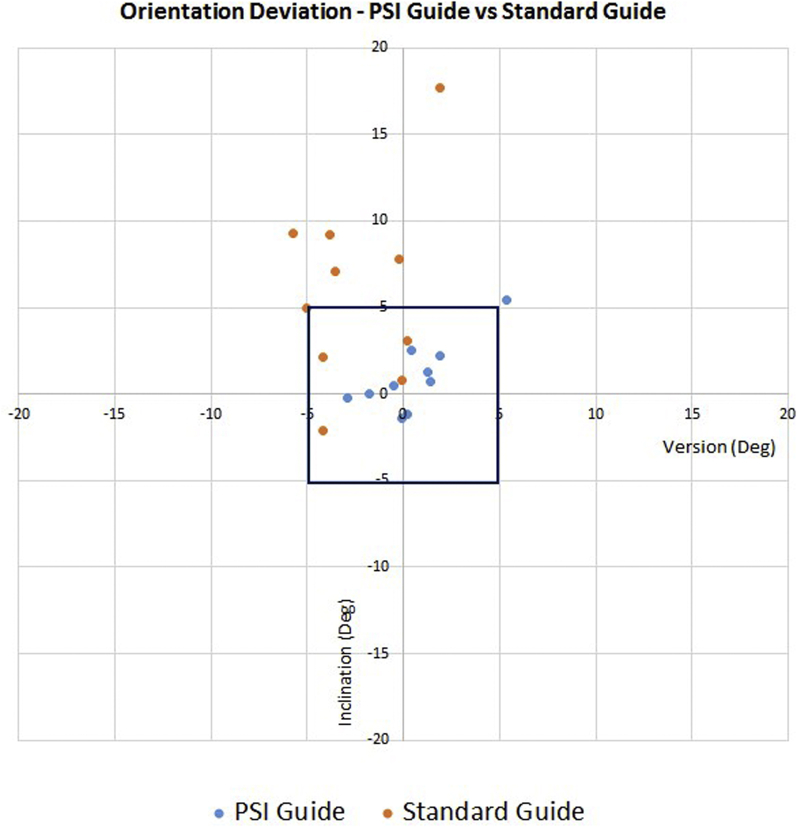
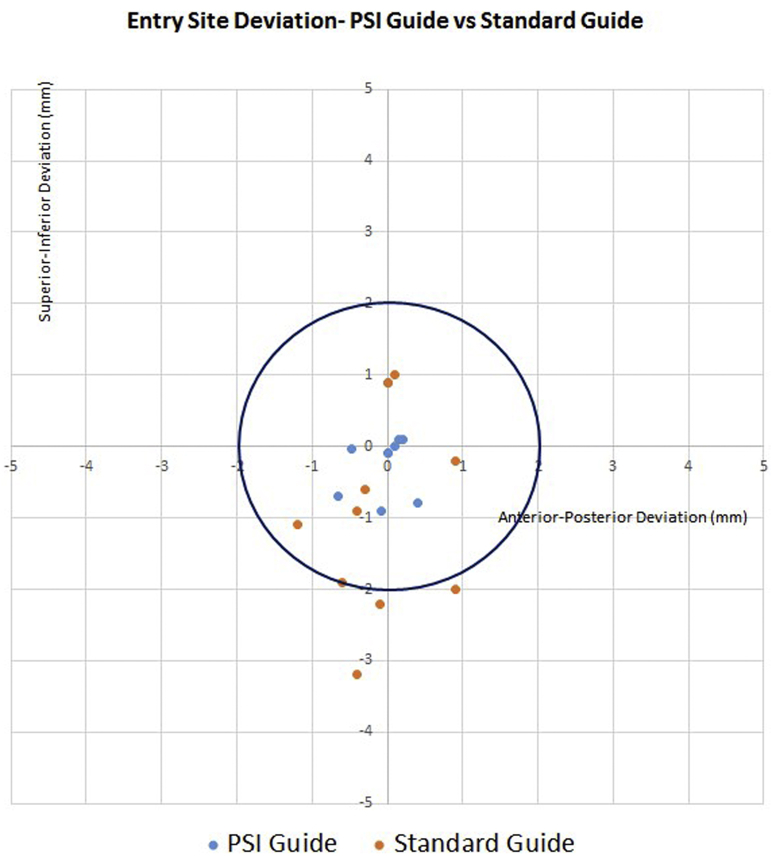
Guide pin version deviation did not significantly differ between the PSI group and standard instrumentation control group (1.6° ± 1.6° vs. 2.9° ± 2.1°, P = .141). Guide pin inclination deviation was significantly lower in the PSI group than in the control group (1.5° ± 1.6° vs. 6.4° ± 5.0°, P = .009) (Fig. 7). Similarly, the dot product angle between vectors indicating deviation from the predetermined trajectory was significantly lower in the PSI group than in the control group (2.4° ± 1.7° vs. 7.5° ± 4.8°, P = .005). The glenoid entry site exhibited less positional offset from the presurgical plan in the PSI group than in the control group (0.8 ± 0.6 mm vs. 2.1 ± 1.2 mm, P = .008) (Fig. 8). Glenoid exit site deviation did not differ significantly in the PSI group compared with the control group (3.6 ± 6.1 mm vs. 4.8 ± 3.0 mm, P = .613). Deviations in version angle, inclination angle, dot product angle, entry site location, and exit site location for all specimens are shown in Table II.
Figure 7.
Orientation deviation for patient-specific instrumentation (PSI) guide vs. standard instrumentation guide. The square represents the orientation within ±5° of version and inclination angle. Deg, degrees.
Figure 8.
Entry site deviation for patient-specific instrumentation (PSI) guide vs. standard guide. The circle represents the entry site location within a 2-mm radius of the calculated glenoid center in the anterior-posterior and superior-inferior directions.
Table II.
Postoperative deviation from presurgical plan
| Specimen No. | Version deviation, ° | Inclination deviation, °∗ | Dot product angle, °∗ | Entry site deviation, mm∗ | Exit site deviation, mm |
|---|---|---|---|---|---|
| Patient-specific instrumentation guide | |||||
| 1 | 1.8 | 0 | 1.8 | 0.3 | 1.6 |
| 2 | 0.1 | 1.4 | 1.4 | 0.1 | 0.9 |
| 3 | 0.2 | 1.2 | 1.2 | 0.1 | 0.8 |
| 4 | 1.9 | 2.2 | 2.9 | 0.7 | 2.1 |
| 5 | 1.3 | 1.3 | 1.9 | 1.2 | 1.8 |
| 6 | 2.9 | 0.2 | 2.9 | 0.9 | 3.8 |
| 7 | 1.4 | 0.7 | 1.6 | 0.2 | 0.6 |
| 8 | 0.4 | 2.5 | 2.5 | 1.3 | 2.6 |
| 9 | 0.5 | 0.5 | 0.7 | 1.5 | 1.5 |
| 10 | 5.4 | 5.4 | 6.6 | 1.6 | 20.7 |
| Standard instrumentation guide | |||||
| 11 | 4.2 | 2.1 | 4.7 | 3.4 | 2.2 |
| 12 | 0.2 | 3.1 | 3.1 | 1.5 | 0.7 |
| 13 | 0.2 | 7.8 | 7.8 | 1.4 | 6.2 |
| 14 | 3.8 | 9.2 | 10 | 1.7 | 7.6 |
| 15 | 5 | 5 | 7.1 | 0.9 | 6.3 |
| 16 | 4.2 | 2.1 | 4.7 | 4.1 | 5.7 |
| 17 | 0.1 | 0.8 | 0.8 | 1.0 | 1.5 |
| 18 | 1.9 | 17.7 | 17.8 | 2.5 | 10.1 |
| 19 | 5.7 | 9.3 | 10.9 | 3.3 | 3.1 |
| 20 | 3.5 | 7.1 | 7.9 | 0.7 | 4.1 |
No significant difference between the patient-specific instrumentation and standard instrumentation groups was found for version angle (P = .141) or exit site (P = .613) deviation.
Significant differences were noted between the patient-specific instrumentation group and standard instrumentation group for mean deviation in inclination angle (P = .009), dot product angle (P = .005), and entry site deviation (P = .008).
The average time necessary to create the presurgical plan based on our automated algorithm and individual scapular anatomy was 32 minutes. The average time needed to design the PSI guide for a perfect fit on the glenoid face in the planned trajectory was 43 minutes. The average time needed for 3D printing and post-processing of each constructed PSI guide was 3 hours 25 minutes. The average total time needed to create each PSI guide from the beginning of surgical planning to post-processing was 4 hours 40 minutes. The 3D printer package used in this study had a list price of $3350. One liter of resin was purchased at a price of $316. The average volume of resin used per guide was 8.17 mL. Given the prices of the printer and resin, along with the average resin volume used per guide, 122 PSI devices could be produced from 1 liter of resin at an average calculated cost of $29.95 per device with this technique, excluding labor costs.
Discussion
This study demonstrates that our novel, in-house produced, 3-dimensionally printed patient-specific glenoid guide can significantly improve the accuracy of central glenoid guide pin placement compared with a standard TSA drill guide in a cadaveric model. In addition, the low-cost, reproducible design and production of this guide in under 5 hours offer the potential to decrease costs and lead times involved with PSI systems for TSA.
Using the 3-dimensionally printed PSI device to drill the central glenoid guide pin resulted in significantly reduced errors in inclination angle and entry site location compared with standard instrumentation. Although no significant difference was detected in guide pin version angle, quantification of both version and inclination angle vectors via the dot product also demonstrated that there was a significant reduction in orientation error in the PSI group vs. the control specimens. Our results are consistent with the majority of reported findings regarding PSI in TSA in the available literature. In 18 cadaveric scapular specimens, Walch et al34 reported that use of a patient-specific device produced a mean version angle error of 1.64° ± 1.01°, a mean inclination angle error of 1.42° ± 1.37°, and an overall 3D orientation error of 2.39° ± 1.16° for placement of the central glenoid guide pin. In addition, their average entry site pin position error was 1.05 ± 0.31 mm. Our errors for pin version, inclination, and entry site location with the PSI device were similar in magnitude; however, the investigation by Walch et al lacked a standard instrumentation control group for comparison. In another study, Iannotti et al19 found that the accuracy of central glenoid guide pin positioning in 9 arthritic scapular bone models significantly improved with use of 3D preoperative planning and a PSI device compared with 3D preoperative planning with standard instrumentation or compared with standard instrumentation alone. They reported that the combination of 3D preoperative planning and the use of a patient-specific device significantly improved pin positioning by an order of 3.7° ± 0.9° in version, 8.1° ± 1.2° in inclination, and 1.2 ± 0.2 mm in location compared with 3D planning and standard instrumentation. In a study with 18 cadaveric shoulder specimens, Levy et al23 used 3D preoperative planning and a patient-specific guide to drill a bicortical pathway through the glenoid for the baseplate of a reverse total shoulder prosthesis. Their analysis produced a mean version error of 2.6° ± 1.7°, a mean inferior tilt error of 1.2° ± 1.2°, and a mean entry site offset error of 1.2 ± 0.7 mm for drill path position. Although this investigation also lacked a standard instrumentation comparison, positioning error values after use of their PSI device were similar to our findings.
Additional studies have attempted to analyze the impact that various approaches to PSI technology may have on shoulder arthroplasty in both in vivo and in vitro models. In general, available evidence indicates that patient-specific devices improve the implantation accuracy of either the central glenoid guide pin or glenoid prosthesis based on some form of predetermined surgical plan.9, 13, 15, 20, 30 However, most published articles used patient-specific devices that were outsourced to an external manufacturer.2, 6, 8, 13, 14, 15, 22, 23, 27, 28, 30, 31, 34 Outsourcing production of patient-specific devices to external manufacturers may involve considerable expenses that could potentially exclude all but the highest-volume, highest-earning practices from benefiting from this technology. Outsourcing even portions of the planning or production may not reduce costs because manufacturers often offer their services in all-or-none packages that require purchase of the entire planning–to–device delivery process.25 In addition, production delays involved with construction and delivery of the PSI devices can be significant. Gauci et al13 reported that surgical planning needed to be initiated at a minimum of 10 working days (2 weeks) before surgery to accommodate production and delivery delays. Meanwhile, Trouilloud et al32 stated that a minimum of 5 weeks was necessary for the manufacturer to plan, construct, and deliver the PSI device used in their study. These significant delays may result in significant lead times or delays in operative scheduling, which not all surgeons or patients may be willing to accommodate.
The novel PSI device used in our study could potentially help overcome some of these obstacles to widespread accessibility of patient-specific technology in shoulder arthroplasty. The results of this preliminary validation study indicate that this PSI device performs as well as some of the other commercially available PSI devices when comparing the same outcome metrics.23, 34 Other studies have further evaluated implantation of the glenoid prosthesis, but the PSI devices used in those studies served the same purpose as ours (guiding the placement of the central glenoid guide pin prior to reaming) and similar reductions in positioning errors were reported.6, 13, 14, 30 In addition, even when start-up costs for equipment essential to manufacturing are incorporated, over 100 devices could be produced at less than $30 per device using our technique. These initial costs will be absorbed over time as more guides are produced with this technique, decreasing the cost of production even further. The devices could also theoretically be made the day before surgery is scheduled to take place because the total production time (from preoperative 3D planning to device post-processing) took less than 5 hours. Although a custom-made software program was used to define the 3 coordinates of the coronal scapular plane, other methods exist for describing these same standard anatomic landmarks that other surgeons potentially interested in adapting our technique can replicate.4, 14, 20 The design of our patient-specific device also requires less extensive dissection and preparation of glenohumeral structures than other designs that require exposure of the coracoid base and acromion to achieve adequate fixation, which may facilitate completion of the case.6, 9
Limitations
Some limitations exist in this study. To provide equivalent access to the glenoid across all specimens, the humerus was removed prior to guide pin placement. Efforts were taken to keep the remaining soft-tissue envelope similar to that which would be found in TSA. Although this may not perfectly simulate TSA operative conditions, our intent was to perform a controlled evaluation in a cadaveric model before it can be tested a clinical environment because this device has not previously been described in the literature. Previous studies have evaluated patient-specific systems in TSA by using Sawbones (Vashon, WA, USA) or polymer scapular models, as well as cadaveric scapular specimens without any associated soft tissue.1, 9, 19, 24, 34 In addition, we were unable to specifically request and obtain cadaveric specimens with glenohumeral osteoarthritis. The glenoids of patients actually undergoing TSA for glenohumeral arthritis are likely to exhibit greater glenoid deformity than the cadaveric specimens tested in our study, which were all Walch type A or B1. Although our results may not extrapolate to more significant glenoid deformity such as type B2 and C glenoids, our PSI device theoretically has even greater promise to improve glenoid guide pin accuracy in these more challenging deformities, given that it significantly improved accuracy compared with the standard guide in a setting without the added difficulty of severe arthritic wear.
Our power analysis determined that a sample size of 20 specimens was necessary for the purposes of this investigation, which is greater than that in the majority of available cadaveric or clinical studies investigating the impact of PSI devices on TSA.2, 9, 22, 23, 24, 27, 28, 29, 32, 34 Although it is impossible to represent the entire spectrum of TSA patients with this sample, the addition of a standard instrumentation control adds further validity that was absent in several previous investigations.2, 13, 23, 27, 32, 34 In general, evidence regarding the clinical impact of PSI systems in TSA is lacking. Currently, there are no available data demonstrating significant improvements in patient-reported outcomes or implant longevity after using PSI technology in TSA. In addition, no previous studies have evaluated the cost-effectiveness of PSI systems in TSA, which limits our ability to make comparisons with our technique. Some interesting factors when considering cost-effectiveness may include labor costs and time required to train staff in the proper operation of software and equipment. In addition, any extra costs associated with obtaining preoperative CT imaging with specified 3D protocols would be clinically relevant. However, these considerations are beyond the scope of our analysis. Despite these limitations, our findings of low costs and significantly reduced production time compared with available devices help address a significant need in the literature and add to the limited data available. It is clear that further investigation is warranted into all the aforementioned areas, as well as the potential impact of PSI technology on intraoperative time, perioperative complications, and long-term clinical outcomes, before PSI devices can be routinely recommended over standard instrumentation.
Conclusion
This study demonstrates a novel technique for in-house production of 3-dimensionally printed, patient-specific devices for TSA central glenoid guide pin placement. These patient-specific guides improved the accuracy of glenoid pin placement based on 3D CT measurements compared with standard TSA guides in a cadaveric model. Our patient-specific glenoid guides can be produced on demand, in-house, inexpensively, and with significantly reduced time compared with commercially available guides. Future studies will be required to validate these findings in clinical applications and determine whether improved glenoid placement impacts glenoid implant longevity or clinical outcomes.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
No particular grants or any outside funding sources were utilized during the execution of this study. All necessary funding was provided by the general research fund within the Division of Sports Medicine, Midwest Orthopaedics at Rush, Rush University Medical Center, Chicago, IL, USA.
Footnotes
This basic science study was exempt from review by the institutional review board.
References
- 1.Berhouet J., Gulotta L.V., Dines D.M., Craig E., Warren R.F., Choi D. Preoperative planning for accurate glenoid component positioning in reverse shoulder arthroplasty. Orthop Traumatol Surg Res. 2017;103:407–413. doi: 10.1016/j.otsr.2016.12.019. [DOI] [PubMed] [Google Scholar]; Berhouet J, Gulotta LV, Dines DM, Craig E, Warren RF, Choi D, et al. Preoperative planning for accurate glenoid component positioning in reverse shoulder arthroplasty. Orthop Traumatol Surg Res 2017;103:407-413. https://doi.org/10.1016/j.otsr.2016.12.019 [DOI] [PubMed]
- 2.Berhouet J., Rol M., Spiry C., Slimane M., Chevalier C., Favard L. Shoulder patient-specific guide: first experience in 10 patients indicates room for improvement. Orthop Traumatol Surg Res. 2018;104:45–51. doi: 10.1016/j.otsr.2017.11.005. [DOI] [PubMed] [Google Scholar]; Berhouet J, Rol M, Spiry C, Slimane M, Chevalier C, Favard L. Shoulder patient-specific guide: first experience in 10 patients indicates room for improvement. Orthop Traumatol Surg Res 2018;104:45-51. https://doi.org/10.1016/j.otsr.2017.11.005 [DOI] [PubMed]
- 3.Bourke A.K., van de Ven P., Gamble M., O'Connor R., Murphy K., Bogan E. Evaluation of waist-mounted tri-axial accelerometer based fall-detection algorithms during scripted and continuous unscripted activities. J Biomech. 2010;43:3051–3057. doi: 10.1016/j.jbiomech.2010.07.005. [DOI] [PubMed] [Google Scholar]; Bourke AK, van de Ven P, Gamble M, O'Connor R, Murphy K, Bogan E, et al. Evaluation of waist-mounted tri-axial accelerometer based fall-detection algorithms during scripted and continuous unscripted activities. J Biomech 2010;43:3051-3057. https://doi.org/10.1016/j.jbiomech.2010.07.005 [DOI] [PubMed]
- 4.Bryce C.D., Davison A.C., Lewis G.S., Wang L., Flemming D.J., Armstrong A.D. Two-dimensional glenoid version measurements vary with coronal and sagittal scapular rotation. J Bone Joint Surg Am. 2010;92:692–699. doi: 10.2106/jbjs.i.00177. [DOI] [PubMed] [Google Scholar]; Bryce CD, Davison AC, Lewis GS, Wang L, Flemming DJ, Armstrong AD. Two-dimensional glenoid version measurements vary with coronal and sagittal scapular rotation. J Bone Joint Surg Am 2010;92:692-699. https://doi.org/10.2106/jbjs.i.00177 [DOI] [PubMed]
- 5.Chin P.Y., Sperling J.W., Cofield R.H., Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15:19–22. doi: 10.1016/j.jse.2005.05.005. [DOI] [PubMed] [Google Scholar]; Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg 2006;15:19-22. https://doi.org/10.1016/j.jse.2005.05.005 [DOI] [PubMed]
- 6.Dallalana R.J., McMahon R.A., East B., Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10:59–66. doi: 10.4103/0973-6042.180717. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg 2016;10:59-66. https://doi.org/10.4103/0973-6042.180717 [DOI] [PMC free article] [PubMed]
- 7.de Vries W.H., Veeger H.E., Cutti A.G., Baten C., van der Helm F.C. Functionally interpretable local coordinate systems for the upper extremity using inertial & magnetic measurement systems. J Biomech. 2010;43:1983–1988. doi: 10.1016/j.jbiomech.2010.03.007. [DOI] [PubMed] [Google Scholar]; de Vries WH, Veeger HE, Cutti AG, Baten C, van der Helm FC. Functionally interpretable local coordinate systems for the upper extremity using inertial & magnetic measurement systems. J Biomech 2010;43:1983-1988. https://doi.org/10.1016/j.jbiomech.2010.03.007 [DOI] [PubMed]
- 8.Elliott R.S., Dallalana R.J. Patient-specific instrument-assisted structural glenoid bone grafting in reverse shoulder arthroplasty. Tech Shoulder Elbow Surg. 2017;18:145–150. doi: 10.1097/BTE.0000000000000123. [DOI] [Google Scholar]; Elliott RS, Dallalana RJ. Patient-specific Instrument-assisted Structural Glenoid Bone Grafting in Reverse Shoulder Arthroplasty. Techniques in Shoulder & Elbow Surgery 2017;18:145-150.
- 9.Eraly K., Stoffelen D., Vander Sloten J., Jonkers I., Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25:837–845. doi: 10.1016/j.jse.2015.09.034. [DOI] [PubMed] [Google Scholar]; Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg 2016;25:837-845. https://doi.org/10.1016/j.jse.2015.09.034 [DOI] [PubMed]
- 10.Farron A., Terrier A., Buchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006;15:521–526. doi: 10.1016/j.jse.2005.10.003. [DOI] [PubMed] [Google Scholar]; Farron A, Terrier A, Buchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg 2006;15:521-526. https://doi.org/10.1016/j.jse.2005.10.003 [DOI] [PubMed]
- 11.Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]; Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175-191. [DOI] [PubMed]
- 12.Friedman R.J., Hawthorne K.B., Genez B.M. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74:1032–1037. [PubMed] [Google Scholar]; Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am 1992;74:1032-1037. [PubMed]
- 13.Gauci M.O., Boileau P., Baba M., Chaoui J., Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B:1080–1085. doi: 10.1302/0301-620x.98b8.37257. [DOI] [PubMed] [Google Scholar]; Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J 2016;98-B:1080-1085. https://doi.org/10.1302/0301-620x.98b8.37257 [DOI] [PubMed]
- 14.Hendel M.D., Bryan J.A., Barsoum W.K., Rodriguez E.J., Brems J.J., Evans P.J. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94:2167–2175. doi: 10.2106/jbjs.k.01209. [DOI] [PubMed] [Google Scholar]; Hendel MD, Bryan JA, Barsoum WK, Rodriguez EJ, Brems JJ, Evans PJ, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg Am 2012;94:2167-2175. https://doi.org/10.2106/jbjs.k.01209 [DOI] [PubMed]
- 15.Heylen S., Van Haver A., Vuylsteke K., Declercq G., Verborgt O. Patient-specific instrument guidance of glenoid component implantation reduces inclination variability in total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:186–192. doi: 10.1016/j.jse.2015.07.024. [DOI] [PubMed] [Google Scholar]; Heylen S, Van Haver A, Vuylsteke K, Declercq G, Verborgt O. Patient-specific instrument guidance of glenoid component implantation reduces inclination variability in total and reverse shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:186-192. https://doi.org/10.1016/j.jse.2015.07.024 [DOI] [PubMed]
- 16.Ho J.C., Sabesan V.J., Iannotti J.P. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95:e82. doi: 10.2106/jbjs.l.00336. [DOI] [PubMed] [Google Scholar]; Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am 2013;95:e82. https://doi.org/10.2106/jbjs.l.00336 [DOI] [PubMed]
- 17.Hoenecke H.R., Jr., Hermida J.C., Flores-Hernandez C., D'Lima D.D. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:166–171. doi: 10.1016/j.jse.2009.08.009. [DOI] [PubMed] [Google Scholar]; Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:166-171. https://doi.org/10.1016/j.jse.2009.08.009 [DOI] [PubMed]
- 18.Howarth S.J. Comparison of 2 methods of measuring spine angular kinematics during dynamic flexion movements: skin-mounted markers compared with markers affixed to rigid bodies. J Manipulative Physiol Ther. 2014;37:688–695. doi: 10.1016/j.jmpt.2014.10.006. [DOI] [PubMed] [Google Scholar]; Howarth SJ. Comparison of 2 methods of measuring spine angular kinematics during dynamic flexion movements: skin-mounted markers compared with markers affixed to rigid bodies. J Manipulative Physiol Ther 2014;37:688-695. https://doi.org/10.1016/j.jmpt.2014.10.006 [DOI] [PubMed]
- 19.Iannotti J., Baker J., Rodriguez E., Brems J., Ricchetti E., Mesiha M. Three-dimensional preoperative planning software and a novel information transfer technology improve glenoid component positioning. J Bone Joint Surg Am. 2014;96:e71. doi: 10.2106/jbjs.l.01346. [DOI] [PubMed] [Google Scholar]; Iannotti J, Baker J, Rodriguez E, Brems J, Ricchetti E, Mesiha M, et al. Three-dimensional preoperative planning software and a novel information transfer technology improve glenoid component positioning. J Bone Joint Surg Am 2014;96:e71. https://doi.org/10.2106/jbjs.l.01346 [DOI] [PubMed]
- 20.Iannotti J.P., Weiner S., Rodriguez E., Subhas N., Patterson T.E., Jun B.J. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg Am. 2015;97:651–658. doi: 10.2106/jbjs.n.00493. [DOI] [PubMed] [Google Scholar]; Iannotti JP, Weiner S, Rodriguez E, Subhas N, Patterson TE, Jun BJ, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg Am 2015;97:651-658. https://doi.org/10.2106/jbjs.n.00493 [DOI] [PubMed]
- 21.Kishimoto M., Akeda K., Sudo A., Espinoza Orias A.A., Inoue N. In vivo measurement of vertebral endplate surface area along the whole-spine. J Orthop Res. 2016;34:1418–1430. doi: 10.1002/jor.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kishimoto M, Akeda K, Sudo A, Espinoza Orias AA, Inoue N. In vivo measurement of vertebral endplate surface area along the whole-spine. J Orthop Res 2016;34:1418-1430. https://doi.org/10.1002/jor.23354 [DOI] [PMC free article] [PubMed]
- 22.Lau S.C., Keith P.P.A. Patient-specific instrumentation for total shoulder arthroplasty: not as accurate as it would seem. J Shoulder Elbow Surg. 2018;27:90–95. doi: 10.1016/j.jse.2017.07.004. [DOI] [PubMed] [Google Scholar]; Lau SC, Keith PPA. Patient-specific instrumentation for total shoulder arthroplasty: not as accurate as it would seem. J Shoulder Elbow Surg 2018;27:90-95. https://doi.org/10.1016/j.jse.2017.07.004 [DOI] [PubMed]
- 23.Levy J.C., Everding N.G., Frankle M.A., Keppler L.J. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1563–1567. doi: 10.1016/j.jse.2014.01.051. [DOI] [PubMed] [Google Scholar]; Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1563-1567. https://doi.org/10.1016/j.jse.2014.01.051 [DOI] [PubMed]
- 24.Lewis G.S., Stevens N.M., Armstrong A.D. Testing of a novel pin array guide for accurate three-dimensional glenoid component positioning. J Shoulder Elbow Surg. 2015;24:1939–1947. doi: 10.1016/j.jse.2015.06.022. [DOI] [PubMed] [Google Scholar]; Lewis GS, Stevens NM, Armstrong AD. Testing of a novel pin array guide for accurate three-dimensional glenoid component positioning. J Shoulder Elbow Surg 2015;24:1939-1947. https://doi.org/10.1016/j.jse.2015.06.022 [DOI] [PubMed]
- 25.Materialise N.V. Materialise Academy; Leuven, Belgium: 2018. Patient-specific guides and implants. [Google Scholar]; MaterialiseNV. Patient-Specific Guides and Implants. In. Leuven, Belgium: Materialise Academy; 2018.
- 26.Moineau G., Levigne C., Boileau P., Young A., Walch G. Three-dimensional measurement method of arthritic glenoid cavity morphology: feasibility and reproducibility. Orthop Traumatol Surg Res. 2012;98:S139–S145. doi: 10.1016/j.otsr.2012.06.007. [DOI] [PubMed] [Google Scholar]; Moineau G, Levigne C, Boileau P, Young A, Walch G. Three-dimensional measurement method of arthritic glenoid cavity morphology: feasibility and reproducibility. Orthop Traumatol Surg Res 2012;98:S139-S145. https://doi.org/10.1016/j.otsr.2012.06.007 [DOI] [PubMed]
- 27.Pietrzak W.S. Biomet Orthopaedics; Warsaw, IN: 2013. Shoulder alignment obtained with the signature glenoid guide system: a cadaver study; pp. 1–3. http://www.zimmerbiomet.com/content/dam/zimmer-biomet/medical-professionals/shoulder/signature-glenoid-technology/shoulder-alignment-obtained-with-signature-glenoid-guide-system-cadaver-study.pdf. [Google Scholar]; Pietrzak WS. Shoulder Alignment Obtained with the Signature Glenoid Guide System: A Cadaver Study. In. Warsaw, IN: Biomet Orthopaedics; 2013, p. 1-3.
- 28.Subramanya S., Herald J. Reverse shoulder arthroplasty with patient-specific glenoid implant positioning guides. Tech Shoulder Elbow Surg. 2014;15:122–129. doi: 10.1097/BTE.0000000000000035. [DOI] [Google Scholar]; Subramanya S, Herald J. Reverse shoulder arthroplasty with patient-specific glenoid implant positioning guides. Techniques in Shoulder & Elbow Surgery 2014;15:122-129.
- 29.Suero E.M., Citak M., Lo D., Krych A.J., Craig E.V., Pearle A.D. Use of a custom alignment guide to improve glenoid component position in total shoulder arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:2860–2866. doi: 10.1007/s00167-012-2177-1. [DOI] [PubMed] [Google Scholar]; Suero EM, Citak M, Lo D, Krych AJ, Craig EV, Pearle AD. Use of a custom alignment guide to improve glenoid component position in total shoulder arthroplasty. Knee Surg Sports Traumatol Arthrosc 2013;21:2860-2866. https://doi.org/10.1007/s00167-012-2177-1 [DOI] [PubMed]
- 30.Throckmorton T.W., Gulotta L.V., Bonnarens F.O., Wright S.A., Hartzell J.L., Rozzi W.B. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: a multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24:965–971. doi: 10.1016/j.jse.2014.10.013. [DOI] [PubMed] [Google Scholar]; Throckmorton TW, Gulotta LV, Bonnarens FO, Wright SA, Hartzell JL, Rozzi WB, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: a multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg 2015;24:965-971. https://doi.org/10.1016/j.jse.2014.10.013 [DOI] [PubMed]
- 31.Throckmorton T.W., Vogt W., Wasmaier J., Hurst J.M., Frostick S., Sperling J.W. Patient-specific targeting guides for glenoid component placement in shoulder arthroplasty: technique and initial clinical experience. Tech Shoulder Elbow Surg. 2014;15:103–108. doi: 10.1097/BTE.0000000000000029. [DOI] [Google Scholar]; Throckmorton TW, Vogt W, Wasmaier J, Hurst JM, Frostick S, Sperling JW. Patient-specific targeting guides for glenoid component placement in shoulder arthroplasty: technique and initial clinical experience. Techniques in Shoulder & Elbow Surgery 2014;15:103-108.
- 32.Trouilloud P., Gonzalvez M., Martz P., Charles H., Handelberg F., Nyffeler R.W. Duocentric(R) reversed shoulder prosthesis and Personal Fit(R) templates: innovative strategies to optimize prosthesis positioning and prevent scapular notching. Eur J Orthop Surg Traumatol. 2014;24:483–495. doi: 10.1007/s00590-013-1213-2. [DOI] [PubMed] [Google Scholar]; Trouilloud P, Gonzalvez M, Martz P, Charles H, Handelberg F, Nyffeler RW, et al. Duocentric(R) reversed shoulder prosthesis and Personal Fit(R) templates: innovative strategies to optimize prosthesis positioning and prevent scapular notching. Eur J Orthop Surg Traumatol 2014;24:483-495. https://doi.org/10.1007/s00590-013-1213-2 [DOI] [PubMed]
- 33.Walch G., Badet R., Boulahia A., Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756–760. doi: 10.1016/s0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]; Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 1999;14:756-760. [DOI] [PubMed]
- 34.Walch G., Vezeridis P.S., Boileau P., Deransart P., Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24:302–309. doi: 10.1016/j.jse.2014.05.029. [DOI] [PubMed] [Google Scholar]; Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg 2015;24:302-309. https://doi.org/10.1016/j.jse.2014.05.029 [DOI] [PubMed]