Abstract
Background
Successful healing of the subscapularis during anatomic total shoulder arthroplasty surgery is critical to optimize functional outcomes and avoid complications. The purpose of this study was to examine the upper and lower subscapularis nerve insertion in relation to the musculotendinous junction to estimate the risk of nerve injury. Our hypothesis was that arm position changes the risks to these nerves when exposing the anterior glenoid.
Methods
Twenty cadaveric shoulders were dissected, and the subscapular nerves were identified from the posterior cord of the brachial plexus to the muscle insertion. The nerve length from the origin to the muscle insertion and the distance to the myotendinous junction were measured in various shoulder positions including neutral, external, and internal rotation.
Results
The mean length of the upper subscapular nerve was 51.4 ± 12.8 mm; that of the lower subscapular nerve was 50.5 ± 14 mm. The mean distance from the insertion of the upper subscapular nerve to the myotendinous junction 53.0 ± 14.7 mm with external rotation, 38.5 ± 9.7 mm with neutral rotation, and 30.0 ± 9.2 mm with internal rotation. The mean distance from the lower subscapular nerve to the myotendinous junction was 44.5 ± 13.8 mm with external rotation, 31.9 ± 9.3 mm with neutral rotation, and 25.4 ± 8.8 mm with internal rotation. The internally rotated position placed these nerves closest to the glenohumeral joint.
Conclusion
The upper and lower subscapular nerves insert in the muscle belly close to the myotendinous junction, putting them at risk of iatrogenic injury. Care must be taken to avoid damage with retractor placement in the anterior glenoid neck as these nerves are at risk of compression or torsional injury.
Keywords: Subscapularis, shoulder arthroplasty, anatomy, cadaveric study, deltopectoral approach, innervation subscapularis
The subscapularis is a muscle originating from the anterior surface of the scapular body and inserting on the lesser tuberosity of the humerus. The superior portion is innervated by the upper subscapular nerve, and the inferior portion, by the lower subscapular nerve.11 These nerves most frequently arise from the posterior cord of the brachial plexus.7
The deltopectoral approach provides access to the glenohumeral joint during shoulder arthroplasty. Arm position and the relationship of the subscapularis are important during shoulder arthroplasty as, frequently, the arm is placed in positions to improve exposure to the glenoid for implant placement. This approach involves releasing the subscapularis tendon and anterior capsule to expose the glenoid. This release may be performed via a lesser tuberosity osteotomy, subscapularis peel, or subscapularis tenotomy. Regardless of the method, the subscapularis must be repaired to prevent anterior dislocation after anatomic total shoulder arthroplasty, as well as the use of some reverse total shoulder prosthesis designs.2, 6 In addition, without a functioning subscapularis, internal rotation strength has been shown to be significantly reduced.9 A systematic review comparing lesser tuberosity osteotomy, subscapularis peel, and tenotomy found no significant difference in healing rates, as well as increases in fatty infiltration, with a poor correlation between tendon integrity and functional testing.4
Despite adequate repair and tendon healing, several studies have shown that the function of the subscapularis remains abnormal.8, 12, 14 Although the mechanism is unknown, several theories have been proposed including poor tendon quality, preoperative muscular atrophy, overly aggressive therapy, and inappropriate implant size or version. One hypothesis that has not been thoroughly investigated is that the mechanism may be related to incidental compression of the subscapular nerves during retraction of the subscapularis during dissection and exposure of the anterior glenoid. Previous studies reported variability in the number as well as contribution of the subscapular nerves, and the authors did not address the issue as to how these findings may pertain to shoulder arthroplasty.3, 13, 21 In addition, exposure of the anterior glenoid during the Latarjet procedure, anterior open capsulorrhaphy, or other bone block procedures for the treatment of shoulder instability using a subscapularis split places these nerves at risk of iatrogenic injury.
The aim of this study was to describe the anatomy of the upper and lower subscapular nerves, the location of the nerve insertion into the subscapularis, and how nerve position changes with arm position in relation to the muscle-tendon junction. We hypothesized that retractors placed in the anterior glenoid neck as well as arm position during total shoulder arthroplasty would place the innervation to the subscapularis at risk of injury.
Materials and methods
The torsos and bilateral upper extremities of 10 fresh-frozen cadaveric specimens (20 shoulders) were used for dissection in this investigation. The cadavers consisted of 9 male specimens and 1 female specimen, with an average age of 70.6 years (range, 57-92 years). The average height of the specimens was 174.0 cm (range, 160.0-185.4 cm), and the average weight was 67.2 kg (range, 46.7-85.3 kg), with an average body mass index of 22.2 ± 3.9. The shoulders of the cadavers underwent no prior surgery and had no trauma.
An extended deltopectoral incision was used for exposure. The humeral insertion and clavicular origin of the pectoralis major were identified and transected, and the muscle was reflected medially and inferiorly. Similarly, the conjoined tendon was detached from the coracoid process and reflected distally. Last, the coracoid insertion of the pectoralis minor was transected and reflected inferomedially to expose the brachial plexus. The posterior cord was identified and traced to its bifurcation into the axillary and radial nerves. The posterior cord and its branches were dissected until both the upper and lower subscapular nerves were identified and traced to the insertion sites of the subscapularis muscle belly. The course of the axillary nerve was traced to the glenohumeral joint capsule, diving posteriorly at the inferior aspect of the glenoid. The subcutaneous tissue overlying the myotendinous junction of the subscapularis was removed until the entire muscle-tendon unit could be visualized. A suture was placed at the myotendinous junction, at a point measured to be the center of the tendon with respect to its height in the coronal plane.
A silk suture was used to obtain measurements in this study. When the anatomic landmark of interest was identified, a silk suture was laid over the cadaveric specimen's anatomy, pulled taut, and cut to match the length of the measurement. The length of each suture was measured using digital calipers, and the results were rounded to the nearest millimeter. A minimum of 2 observers confirmed each measurement. The width of the subscapularis was measured from its superior border to its inferior border at its insertion on the lesser tuberosity. The length of each nerve was measured from its origin off the brachial plexus to the point where it penetrated the muscle belly. The distance of each nerve was measured from the suture in the myotendinous junction to the nerve's insertion into the subscapularis. These measurements were taken with the arm in maximum external rotation, neutral rotation, and maximum internal rotation with the arm adducted at the side. Last, we noted the origin of each of these nerves off the brachial plexus. The change in position of each nerve to the musculotendinous junction was analyzed using an analysis of variance with statistical significance set at P < .05.
Results
The upper subscapular nerve originated from the posterior cord in all 20 shoulders. The lower subscapular nerve originated from the posterior cord in 17 shoulders and from the axillary nerve in 3. In all 20 shoulders, the upper subscapular nerve penetrated the subscapularis proximal to the lower subscapular nerve (Fig. 1). The mean width of the tendinous portion of the subscapularis footprint insertion on the lesser tuberosity was 24.4 ± 5.7 mm (range, 17-35 mm). We measured only the upper two-thirds tendinous portion of the subscapularis as there is significant variation in the lower one-third muscular portion along with its confluence with the capsule, making measurements difficult. The mean length of the upper subscapular nerve was 51.4 ± 12.8 mm (range, 31-89 mm), and that of the lower subscapular nerve was 50.5 ± 14 mm (range, 30-86 mm). The mean distance from the insertion of the upper subscapular nerve to the myotendinous junction with the arm adducted and externally rotated was 53.0 ± 14.7 mm (range, 32-85 mm). When the arm was adducted and rotated to neutral, the distance decreased to 38.5 ± 9.7 mm (range, 24-60 mm), and when adducted and internally rotated, this distance decreased to 30.0 ± 9.2 mm (range, 18-45 mm). All of these values were significant (P < .001 for each comparison). The mean distance from the lower subscapular nerve to the myotendinous junction was 44.5 ± 13.8 mm (range, 26-71 mm) when adducted and externally rotated and decreased to 31.9 ± 9.3 mm (range, 19-45 mm) in adduction and neutral rotation. This distance decreased to 25.4 ± 8.8 mm (range, 15-41 mm) when positioned in adduction and internal rotation. All of these values were significant (P < .001 for each comparison) (Tables I and II). Although we could not quantify the effects, the subscapularis was folded over itself as would occur during exposure of the glenoid, and both compression and torsion of the upper and particularly lower subscapular nerves were found (Fig. 2).
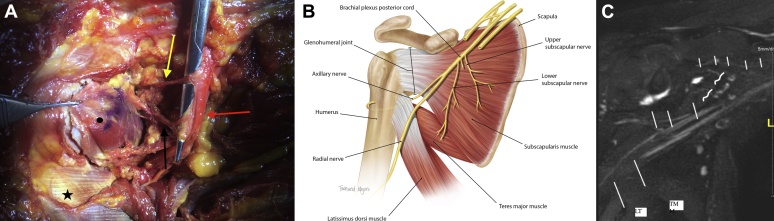
Figure 1.
(A) Photograph of cadaveric dissection: subscapularis muscle-tendon junction (•), latissimus dorsi tendon (★), posterior cord of brachial plexus ( ), upper subscapular nerve (
), upper subscapular nerve ( ), and lower subscapular nerve (
), and lower subscapular nerve ( ). The forceps are holding the subscapularis tendon after tenotomy, with the scissors elevating the upper and lower subscapular nerves. (B) Illustration of described anatomy. The inferior one-third muscular portion of the subscapularis as it inserts into the humerus has been excluded to better visualize the path of the axillary nerve as it travels posteriorly at the level of the glenohumeral joint capsule. (C) Magnetic resonance imaging of brachial plexus. Right shoulder magnetic resonance neurography illustrates the nerves in the regional area: suprascapular nerve (small —), upper and lower subscapular nerves (curved —), axillary nerve (medium —), radial nerve (large —). LT, latissimus dorsi tendon; TM, teres major.
). The forceps are holding the subscapularis tendon after tenotomy, with the scissors elevating the upper and lower subscapular nerves. (B) Illustration of described anatomy. The inferior one-third muscular portion of the subscapularis as it inserts into the humerus has been excluded to better visualize the path of the axillary nerve as it travels posteriorly at the level of the glenohumeral joint capsule. (C) Magnetic resonance imaging of brachial plexus. Right shoulder magnetic resonance neurography illustrates the nerves in the regional area: suprascapular nerve (small —), upper and lower subscapular nerves (curved —), axillary nerve (medium —), radial nerve (large —). LT, latissimus dorsi tendon; TM, teres major.
Table I.
Distance from insertion of nerve to MTJ as function of arm rotation
| Upper subscapular nerve to MTJ, mm | Lower subscapular nerve to MTJ, mm | |
|---|---|---|
| External rotation | 53.0 ± 14.7 | 44.5 ± 13.8 |
| Neutral | 38.5 ± 9.7 | 31.9 ± 9.3 |
| Internal rotation | 30.0 ± 9.2 | 25.4 ± 8.8 |
MTJ, myotendinous junction.
Significance was set at P < .05.
Table II.
Change in distance from nerve insertion to MTJ with arm rotation
| Comparison of length change with arm position |
||||
|---|---|---|---|---|
| Upper subscapular nerve to MTJ |
Lower subscapular nerve to MTJ |
|||
| Difference (95% CI), mm | P value | Difference (95% CI), mm | P value | |
| External rotation–neutral | 14.5 (9.08-19.9) | <.001 | 12.05 (3.88-20.22) | .002 |
| Internal rotation–neutral | 8.6 (4.77-12.4) | <.001 | 7.05 (–1.12 to 15.22) | .1 |
| External rotation–internal rotation | 23.1 (14.68-31.5) | <.001 | 19.1 (10.93-27.27) | <.001 |
MTJ, myotendinous junction; CI, confidence interval of difference.
Significance was set at P < .05.
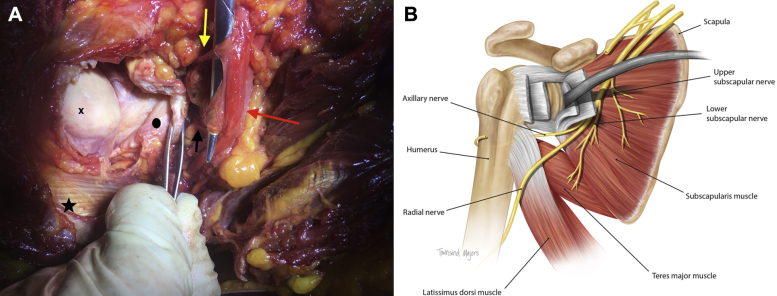
Figure 2.
(A) Photograph of cadaveric dissection showing how the retracted subscapularis tendon can cause compression and traction to the nerves. X indicates the humeral head; •, articular side of reflected subscapularis tendon; ★, latissimus dorsi tendon;  , posterior cord of the brachial plexus;
, posterior cord of the brachial plexus;  , upper subscapular nerve; and
, upper subscapular nerve; and  , lower subscapular nerve. The forceps are holding the subscapularis tendon after tenotomy, with the scissors elevating the upper and lower subscapular nerves. (B) Illustration of the described anatomy with the retractor placed in the anterior glenoid neck showing how this can place the nerves to the subscapularis at risk.
, lower subscapular nerve. The forceps are holding the subscapularis tendon after tenotomy, with the scissors elevating the upper and lower subscapular nerves. (B) Illustration of the described anatomy with the retractor placed in the anterior glenoid neck showing how this can place the nerves to the subscapularis at risk.
Discussion
The purpose of this investigation was to describe the location of the muscle insertion of the upper and lower subscapular nerves, the distance of the insertion site to the muscle-tendon junction, and how nerve position changes with arm position. Careful handling of the subscapularis during glenoid exposure and understanding the location of the muscle innervation are critical to avoid iatrogenic injury. The long course of the lower subscapularis nerve as it penetrates the inferior subscapularis muscle and goes on to innervate the teres major places this structure at particular risk of injury as it is under traction as well as torsion during exposure of the glenoid. We believe that this provides an explanation for a site of potential iatrogenic injury that can result in abnormal subscapularis function despite an intact repair.
The results of our study show that the upper subscapular nerve and the lower subscapular nerve insert into the muscle belly 38.5 ± 9.7 mm and 31.9 ± 9.3 mm, respectively, medial to the myotendinous junction with the arm in neutral rotation. Leschinger et al13 measured the upper subscapular nerve and lower subscapular nerve to insert 23.7 ± 6.1 mm and 24.3 ± 6.6 mm, respectively, medial to the musculotendinous junction. These findings differ from those in our examination. Leschinger et al used embalmed forequarter specimens, which can significantly alter anatomic relationships and nerve position. In contrast, our study used intact torsos with the brachial plexus undisrupted from its origin from the spine, preserving the nerve length, and our specimens were fresh frozen, thus preventing the risk of nerve contraction or a change in elasticity due to chemical processing. Two prior studies reported variability in the number of subscapular nerves and reported a middle subscapular nerve.13, 21 Both investigations used embalmed forequarter shoulder specimens. Although anatomic variability in the number of subscapular nerves is possible, we did not encounter a distinct middle subscapular nerve but did recognize a significant number of branching patterns as the main upper and lower subscapular nerves inserted into the muscle belly. Yung et al21 acknowledged this as a possibility in their description of the middle subscapular nerve branch and described this as a branch from the larger upper subscapular nerve. In addition, we do not know what role the use of an embalmed specimen plays; we can only hypothesize how much of the muscle may pull away and atrophy from the chemical processing, exposing more branching patterns that may not be visualized in a fresh specimen as these nerve branches lie deep to the muscle belly and are only exposed once muscle bulk is decreased.
Kasper et al10 reported the effect of external rotation on the lower subscapular nerve position as it relates to the rim of the glenoid and muscular insertion. Their study reported 35.2 mm in neutral rotation and 16.9 mm in maximum external rotation (P < .001). In contrast, our findings showed 38.5 ± 9.7 mm for the upper subscapular nerve and 31.9 ± 9.3 mm for the lower subscapular nerve insertion to the myotendinous junction in neutral rotation and 53.0 ± 14.7 mm for the upper subscapular nerve and 44.5 ± 13.8 mm for the lower subscapular nerve in external rotation (both comparisons statistically significant, P < .001).
Several studies have shown that a deficient subscapularis after anatomic total shoulder arthroplasty can lead to instability, pain, weakness, decreased range of motion, and ultimately, component failure.1, 9, 14, 16, 20 In addition, a biomechanical study by Terrier et al19 showed that a subscapularis-deficient shoulder results in increased force by the supraspinatus and middle deltoid, causing superior humeral head migration, resulting in eccentric glenoid loading, putting the glenoid component at risk of accelerated wear, loosening, and ultimately, failure.
Despite repair, the subscapularis frequently remains incompetent. Miller et al17 clinically evaluated 41 patients after total shoulder arthroplasty and found that 25 had an abnormal liftoff examination and 23 reported subjective weakness of internal rotation with difficulty tucking in their shirt. Gerber et al8 reported postoperative magnetic resonance imaging findings after shoulder arthroplasty and noted that 45% (15 of 34 shoulders) had increased fatty infiltration of the subscapularis muscle. The reasons for these findings are still largely unknown. Certainly, the ability of the tendon to heal may play a significant role in this persistent subscapularis insufficiency. Jackson et al9 reported that 7 of 15 shoulders showed failure of a repaired subscapularis at 6 months postoperatively. Other studies have also demonstrated high retear rates regardless of the technique used for subscapularis management during total shoulder arthroplasty.4, 12, 18 Furthermore, a subscapularis split during the Latarjet procedure or other bone and/or soft-tissue instability surgical procedure may place the upper and lower subscapular nerves at risk of direct injury given the location of insertion. We speculate that the results of our study provide some insight as to why there may still be subscapularis dysfunction with increased fatty infiltration despite an intact tendon. The innervation of the subscapularis may be irreversibly altered by the traction and compression sustained at the time of surgery.
Several studies have examined subscapularis healing rates with an intact tendon repair and concomitant increased muscle fatty infiltration.4, 8, 12 De Wilde et al5 retrospectively examined 36 patients after lesser tuberosity osteotomy, with a 100% healing rate and a 13% decrease in the cross-sectional area of the subscapularis muscle at an average of 18 months (range, 13-33 months) of follow-up. This finding suggests an alternative pathophysiology for the subscapularis abnormalities noted after anatomic total shoulder arthroplasty. One explanation for this observation is the possibility of an iatrogenic injury to one or both of the nerves innervating the subscapularis at the index surgical procedure. Perhaps the most likely point when the lower subscapular nerve would be injured is during retraction of the subscapularis to expose the glenoid. Often, a traction suture is placed into the tendon after release from the lesser tuberosity, and a retractor is placed to keep the tendon retracted medially. According to our results, the lower subscapular nerve penetrates the subscapularis anywhere from 15 to 71 mm from the musculotendinous junction depending on arm rotation. Often, the subscapularis tendon is released with the arm in internal rotation to better visualize the lesser tuberosity. This places both the upper (average, 30 mm) and lower (average, 25 mm) subscapular nerves at risk of being kinked or compressed during retraction. If the nerve remains kinked or compressed for a prolonged period, changes to the perineural circulation may occur. Furthermore, nerve compression results in edema formation with subsequent slowing of axonal transport, leading to muscular dysfunction in the setting of an intact subscapularis repair.15 In addition, the course of the lower subscapularis nerve, as it travels through and past the subscapularis to innervate the teres major, is long, making it vulnerable to injury during retraction.
The findings of this study provide evidence that there is a significant risk of iatrogenic damage to the subscapular nerves and this may be responsible for the pathophysiology of subscapular insufficiency after anatomic total shoulder arthroplasty. In addition, just as the nerve supply to the subscapularis may be kinked and/or compressed, the vascular supply may also be at risk. Future studies to evaluate the precise location of the subscapular artery as it arises from the axillary artery and inserts into the subscapularis are needed before a conclusion can be made regarding its effect on postoperative subscapularis function. Damage to the blood supply of the subscapularis may cause difficulties in healing and may explain the high retear rate after repair. It would likely also cause an increase in fatty atrophy of the subscapularis muscle belly seen on postoperative imaging.
A strength of this study is the use of an intact fresh-frozen torso with bilateral shoulders and an undisrupted brachial plexus. This allows for a more accurate representation of the clinical anatomy with no nerve retraction from detachment of the forequarter and transection of the nerve origin from the spine. A weakness of this study is that the sample size was relatively small, with only 20 shoulders from 10 cadaveric specimens or intact torsos. Although this may not allow for a demonstration of anatomic variability, as with all samples from differing cadavers, the incidence of bilateral anatomic symmetry regarding nerve position, insertion, length, and origin is unknown. Another limitation to this cadaveric study is that it is unknown how these findings would be affected in the clinical scenario. Future studies are needed to validate these findings clinically using nerve conduction to allow for direct assessment of when these nerves are placed at risk of stretch or torsional injuries.
The position of the arm during shoulder arthroplasty changes multiple times throughout surgery to aid in exposure of both the humeral and glenoid sides of the joint. The results of this study show that nerve position is directly related to arm rotation. Performing capsular releases around the anterior glenoid neck and subscapularis can place the subscapularis nerves at risk of injury. On the basis of our results, we recommend keeping the arm in an adducted, externally rotated position and avoiding excessive medial dissection on the anterior surface of the subscapularis past the muscle-tendon junction to keep these structures at the maximum distance from the area of dissection, as well as to avoid injury. In addition, limiting the time the anterior glenoid neck retractor is in place and allowing the subscapularis tendon to frequently rest in a relaxed position can prevent compression and/or kinking of the nerves to the subscapularis. Future studies are needed to validate these findings in the clinical setting using intraoperative nerve monitoring.
Conclusion
Subscapularis insufficiency is a commonly noted sequela after anatomic total shoulder arthroplasty either from repair failure or due to functional abnormalities with muscle atrophy despite an intact repaired tendon. The consequences of subscapularis dysfunction and/or failure manifest as instability, pain, weakness, and restricted range of motion, placing the components at risk of loosening and failure. Although copious research has been performed on the occurrence of subscapularis insufficiency, there are minimal data evaluating the possibilities as to the etiology. The upper and lower subscapular nerves penetrate the subscapularis quite close to the musculotendinous junction and may be damaged during aggressive retraction or dissection during surgery. These nerves insert farther from the musculotendinous junction when the shoulder is externally rotated; therefore, consideration should be taken when positioning the arm during subscapularis dissection and capsular release.
Disclaimer
Michael Khazzam receives research support from and is a paid consultant for Tornier/Wright Medical. All the other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was not required.
References
- 1.Brems J.J. Complications of shoulder arthroplasty: infections, instability, and loosening. Instr Course Lect. 2002;51:29–39. [PubMed] [Google Scholar]
- 2.Chalmers P.N., Rahman Z., Romeo A.A., Nicholson G.P. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:737–744. doi: 10.1016/j.jse.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Checchia S.L., Doneaux P., Martins M.G., Meireles F.S. Subscapularis muscle enervation: the effect of arm position. J Shoulder Elbow Surg. 1996;5:214–218. doi: 10.1016/s1058-2746(05)80009-6. [DOI] [PubMed] [Google Scholar]
- 4.Choate W.S., Kwapisz A., Momaya A.M., Hawkins R.J., Tokish J.M. Outcomes for subscapularis management techniques in shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2018;27:363–370. doi: 10.1016/j.jse.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 5.De Wilde L.F., DeConinck T., De Neve F., Berghs B.M. Subscapularis release in shoulder replacement determines structural muscular changes. Clin Orthop Relat Res. 2012;470:2193–2201. doi: 10.1007/s11999-012-2291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards T.B., Williams M.D., Labriola J.E., Elkousy H.A., Gartsman G.M., O'Connor D.P. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Fazan V.P.S., Amadeu A.D.S., Caleffi A.L., Rodrigues Filho O.A. Brachial plexus variations in its formation and main branches. Acta Cir Bras. 2003;18:14–18. doi: 10.1590/S0102-86502003001200006. [DOI] [Google Scholar]
- 8.Gerber C., Yian E.H., Pfirrmann C.A., Zumstein M.A., Werner C.M. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87:1739–1745. doi: 10.2106/JBJS.D.02788. [DOI] [PubMed] [Google Scholar]
- 9.Jackson J.D., Cil A., Smith J., Steinmann S.P. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:1085–1090. doi: 10.1016/j.jse.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Kasper J.C., Itamura J.M., Tibone J.E., Levin S.L., Stevanovic M.V. Human cadaveric study of subscapularis muscle innervation and guidelines to prevent denervation. J Shoulder Elbow Surg. 2008;17:659–662. doi: 10.1016/j.jse.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Kato K. Innervation of the scapular muscles and its morphological significance in man. Anat Anz. 1989;168:155–168. [PubMed] [Google Scholar]
- 12.Lapner P., Sabri E., Rakhra K., Bell K., Athwal G. Healing rates and subscapularis fatty infiltration after lesser tuberosity osteotomy versus subscapularis peel for exposure during shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:396–402. doi: 10.1016/j.jse.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Leschinger T., Hackl M., Zeifang F., Martin Scaal M., Muller L.P., Wegmann K. Nerve supply of the subscapularis during anterior shoulder surgery: definition of a potential risk area. Arch Orthop Trauma Surg. 2017;137:135–140. doi: 10.1007/s00402-016-2585-7. [DOI] [PubMed] [Google Scholar]
- 14.Liem D., Kleeschulte K., Dedy N., Schulte T.L., Steinbeck J., Marquardt B. Subscapularis function after transosseous repair in shoulder arthroplasty: transosseous subscapularis repair in shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:1322–1327. doi: 10.1016/j.jse.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Lundborg G., Dahlin L.B. The pathophysiology of nerve compression. Hand Clin. 1992;8:215–227. [PubMed] [Google Scholar]
- 16.Miller B.S., Joseph T.A., Noonan T.J., Horan M.P., Hawkins R.J. Rupture of the subscapularis tendon after shoulder arthroplasty: diagnosis, treatment, and outcome. J Shoulder Elbow Surg. 2005;14:492–496. doi: 10.1016/j.jse.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Miller S.L., Hazrati Y., Klepps S., Chiang A., Flatow E.L. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12:29–34. doi: 10.1067/mse.2003.128195. [DOI] [PubMed] [Google Scholar]
- 18.Scalise J.J., Ciccone J., Iannotti J.P. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92:1627–1634. doi: 10.2106/JBJS.G.01461. [DOI] [PubMed] [Google Scholar]
- 19.Terrier A., Larrea X., Malfroy Camine V., Pioletti D.P., Farron A. Importance of the subscapularis muscle after total shoulder arthroplasty. Clin Biomech (Bristol, Avon) 2013;28:146–150. doi: 10.1016/j.clinbiomech.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Trappey G.J., IV, O'Connor D.P., Edwards T.B. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469:2505–2511. doi: 10.1007/s11999-010-1686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yung S.W., Lazarus M.D., Harryman D.T., II Practical guidelines to safe surgery about the subscapularis. J Shoulder Elbow Surg. 1996;5:467–470. doi: 10.1016/s1058-2746(96)80019-x. [DOI] [PubMed] [Google Scholar]