Abstract
Exercise and health psychology have generated 2 sets of empirical studies guided by separate theory-driven axes. The first axis focuses on the causal relationship between chronic exercise and cognition and, more particularly, high-level cognitive functions such as executive functions (EFs). The second axis examines factors influencing the adherence process to physical activity (PA). Research conducted during the past decade shows that these 2 topics are closely linked, with EFs and effortful control playing a pivotal role in the bidirectional relationship linking PA and mental/brain health. The present article supports the idea that an individual engaged in the regular practice of effortful PA initiates a virtuous circle linking PA and effortful control in a bidirectional way. On the one hand, chronic exercise leads to an improvement of EFs and effortful control. On the other hand, gains in EFs and effortful control effectiveness lead to a reciprocal facilitation of the maintenance of PA over time. Some limitations and perspectives to this effort hypothesis are proposed in the last part of the article.
Keywords: Adherence, Effort, Executive functions, Physical activity, Salience network
Highlights
-
•
Practicing effortful exercises requires planning and inhibiting temptation to stop.
-
•
Regular practice of exercise leads to an enhancement of concentration and willpower.
-
•
A high capacity to concentrate facilitates the adherence to healthy behaviors.
-
•
A virtuous circle dynamically links exercise and the capacity to engage mental effort.
Graphical Abstract
1. Introduction
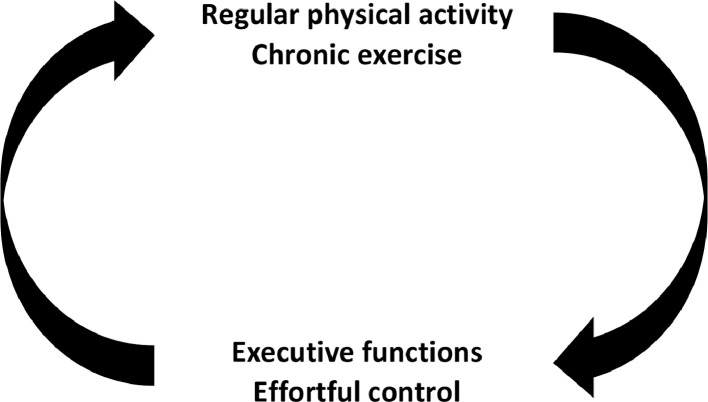
Starting a program of physical exercises after a long period of sedentariness or muscular deconditioning is often experienced as painful and difficult, physically as well as psychologically. However, the feelings of painfulness and drudgery gradually attenuate while individuals persist in regular exercise. Physical training generally leads to improvements in cardiovascular fitness and muscular strength, as well as psychological benefits that facilitate the adherence process. This article focuses on the virtuous circle linking exercise and cognition, which allows an individual to maintain the healthy behavior of exercising thanks to cognitive benefits (Fig. 1), despite the fact that costs and barriers associated with exercise are initially high and only decrease through practice. The physiological benefits of exercise are well-documented and well-recognized by physicians as well as scientists.1 In contrast, the cognitive benefits are less well-known and need to be promoted more widely. In this article, we explore the complete virtuous circle, first describing the positive effects of chronic exercise on cognitive performance and brain health and then showing the benefit of high capacity in effortful control and executive functions (EFs) on adherence to physical activity (PA) programs. As we discuss later, two causal relationships involved in the virtuous circle need more empirical evidence: (1) the causal relationship between chronic exercise and the connectivity of two large-scale neuronal networks underpinning effortful control and EFs, respectively, and (2) the causal relationship linking efficiency of effortful control and/or EFs to exercise programs adherence. Consequently, in the last section of this article, we suggest some experimental methodologies to address these questions in the future.
Fig. 1.
Schematic illustration of the bidirectional relationship between physical activity/chronic exercise and executive functions/effortful control. The right arrow illustrates the causal link between regular physical activity/chronic exercise and executive functions/effortful control; that is, regularly practicing exercise leads to an improvement of executive functions and effortful control. The left arrow illustrates the causal link between executive functions/effortful control and practicing regular physical activity/exercise; that is, a high efficiency in executive functions and effortful control leads to a better adherence to exercise.
Cross-sectional studies showing a positive relationship between level of PA and cognitive performance have an important limitation: they cannot establish a causal relationship between practicing PA and improvement in cognitive performance. However, the positive correlation observed between the level of PA and cognitive performance can be interpreted in two ways. The first interpretation converges with results from numerous intervention studies and considers that practicing PA regularly leads to an improvement of cognitive performance.2, 3, 4, 5 The second interpretation is less popular and has been subject to fewer investigations. It considers that people who have good cognitive functioning are inclined to practice PA more easily and more regularly. In fact, in the present article, we assume that both interpretations are valid and influence each other in a virtuous circle (Fig. 1).
Many intervention studies suggest that the positive effects of chronic exercise on cognition are preferentially observed in older adults and children.2, 3, 4, 5 These two populations are particularly targeted because specific components of cognition, such as EFs, are developing in children and declining in older adults and are, therefore, more sensitive to the effects of moderators that improve or impair EFs. The most popular hypothesis explaining the positive effect of chronic exercise on cognition, called the “neurotrophic hypothesis”, posits that, while exercising, the organism releases several neurotrophic molecules that stimulate hippocampal neurogenesis, brain angiogenesis, and the synthesis of monoamines.6 From the perspective of the neurotrophic hypothesis, the accumulation of exercise sessions leads to durable changes enhancing brain integrity and brain functioning and, consequently, cognitive health. However, alternative hypotheses have been proposed.7 This article focuses on one of these hypotheses, the so-called effort hypothesis. It considers that while practicing effortful physical exercises, individuals strengthen the connectivity of large-scale neuronal networks involved in effortful control and EFs. The benefit acquired in these two cognitive domains then leads to a facilitation of stopping unhealthy sedentary behaviors and adhering to healthy exercising. In Section 2, we define EFs, self-regulation, and effortful control and examine their roles in practicing PA.
2. EFs, self-regulation, effortful control, and PA
According to a well-known and frequently used taxonomy in cognitive psychology,8 at least three main and elementary components of EFs can be identified. They include (1) maintenance and updating of relevant information in working memory, (2) intentional inhibition of prepotent impulses, unwanted and intrusive thoughts, embarrassing emotions, or automatic responses, and (3) mental set shifting, also known as cognitive flexibility. Other high-level cognitive processes such as volition, planning, sustained and selective attention, and self-regulation have also been considered intrinsically linked to EFs.9, 10, 11 The concept of self-regulation, which is discussed in Section 4, refers to psychophysiological processes enabling an individual to guide his or her goal-directed activities over time and across changing circumstances.12 Self-regulation encompasses a large variety of regulatory activities from homeostatic adjustments automatically ensured by the hypothalamus to effortful self-control underpinned by the prefrontal cortices. EFs subserve and are a prerequisite of self-regulation.11 The regular practice of physical exercises according to exercise science recommendations requires the use of the three components of EFs described. First, the maintenance and updating of exercise-related goals in working memory are crucial for performance achievement. For instance, when running, walking, or cycling along a trail, individuals often set one or several intermediate goals throughout the whole route of the race in terms of maintaining an exercise intensity to reach waypoints before given times. Maintaining and updating these intermediate intensity-related goals in working memory through persistent activity is essential to having a good performance in line with the self-referenced goal for the whole race. Second, practicing exercise requires the intentional inhibition to resist the desire to stop exercise when the feeling of discomfort or fatigue is too high. Third, the regular practice of PA also requires planning the exercise session and remembering when to exercise at the planned time (prospective memory). EFs and self-regulation require mental effort as soon as the individual mobilizes them to cope with a stressful situation or carry out a demanding cognitive, social, or physical task. For instance, in all exercise performed at an uncomfortable intensity that needs to be maintained over time, mental effort is necessary to sustain the motor drive throughout the entire bout of exercise. From this perspective, cognitive-energetic models consider mental effort as a capacity-limited resource managed by the mechanism of effort.13,14 This mechanism allows the individual to maintain task performance under disturbance from stressors and to prevent the loss of task goals under circumstances, such as increased processing demands, exercise demands, and competition from other tasks. Underpinned by frontal and limbic structures that take part in a large-scale brain network called the “salience network (SN)” (described in Section 3), the mechanism of effort exerts active and effortful control over brain systems underlying EFs and self-regulation.15 In fact, effortful control is necessary for the activation and effectiveness of brain systems underlying EFs and self-regulation. As mentioned, this effortful control is capacity limited, but the effort hypothesis posits that this capacity can be improved through cognitive or physical training. Training programs aiming to improve effortful control must involve effortful cognitive processes such as EF or effortful physical exercises. According to homeostatic, reward, and other task-related feedback signals, the mechanism of effort makes decisions about maintaining an uncomfortable intensity, reducing it, stopping exercise, or shifting to another more comfortable activity.15
EFs are generally assessed through performance variations observed in cognitive tasks engaging these functions. The most commonly used tasks for this purpose include the n-back task and running-span task for updating working memory; the Stroop task, Eriksen flanker task, Simon task, go-no-go task, stop-signal task, and anti-saccade task for intentional inhibition; the trail-making test, Wisconsin card sorting test, and switching task for cognitive flexibility; and the Tower of London task for planning. Moreover, effortful control exerted by the mechanism of effort during the task (i.e., the mental effort invested in the task) can be assessed through several psychophysiological indices such as pupillary dilation, heart rate variability, or systolic pre-ejection period recorded throughout the duration of the task.16 Finally, self-regulation can be measured through behavioral indices such as adherence to exercise programs. In summary, effortful control and EFs are the cornerstones of the virtuous circle; they benefit from healthy behaviors such as regular exercise and play a crucial role in the adherence process allowing the maintenance of these healthy behaviors in the long term.
3. Causal relationship between chronic exercise and EFs and effortful control
A recent meta-analysis focusing on adults over 50 years of age that included 36 intervention studies showed a moderate effect size of 0.34 of chronic exercise on EFs.17 Another meta-analysis focusing on children from 4 to 18 years of age that included 36 intervention studies also showed small to moderate effects of chronic exercise on EFs.18 Given the data presented in these two meta-analyses, it can be concluded that there are strong empirical arguments for a real effect of chronic exercise on EFs and effortful control. However, to strengthen the rationale supporting the right arrow of Fig. 1, we now examine the effect of chronic exercise on brain networks and structures underpinning EFs and effortful control.
Correlational brain imaging studies conducted over the past 20 years provide strong arguments for three large-scale interconnected networks that underpin high-level cognitive functions.19 The default mode network (DMN) shows a task-negative activity profile. Its activation is associated with self-referenced thoughts, emotional processing, and autobiographical memory.20 The DMN mainly includes the orbital frontal cortex, the lateral temporal cortex, the inferior parietal lobe, the posterior cingulate/retrosplenial cortex, and the hippocampus/parahippocampal cortex. The central executive network (CEN), also described as the frontoparietal network, shows a task-positive activity profile because it is activated during a large variety of cognitive tasks that subserve EF.19 The CEN is mainly anchored in the dorsolateral prefrontal cortex, the ventrolateral prefrontal cortex, the dorsomedial prefrontal cortex, and the lateral parietal cortex.19, 21 Finally, the SN, alternatively called the cingulo-opercular network, also shows a task-positive activity profile. It underpins the mechanism of effort, making decisions about the direction and the intensity of the behavioral response in a specific situation (i.e., fight, flight, submission to a higher-ranking individual, resignation, computation of the amount of invested effort, etc.), and switching between the DMN and the CEN.15,16, 19, 22,23 The switching process activating the CEN and deactivating the DMN operates each time an individual engages in a cognitive task or an effortful exercise. Conversely, the switching process activating the DMN and deactivating the CEN operates each time an individual disengages his or her attention from the task and is distracted by self-referenced thoughts. The SN mainly consists of the orbital frontoinsular cortex, the dorsal anterior cingulate cortex (dACC), the anterior insula (AI), and the superior temporal gyrus.19, 21 We assume that the CEN and the SN benefit from chronic exercise and that these benefits in connectivity within and between these two networks improve the efficiency of EFs and facilitate adherence to exercise programs. The contribution of the DMN to the virtuous circle cannot be excluded (see Section 5). However, because it is not directly related to effortful control and EFs, we made the choice not to include it in the following analysis of the literature.
Several intervention studies using structural brain imaging showed a positive effect of chronic exercise on gray or white matter volume of brain structures underpinning EFs and the mechanism of effort.24, 25, 26, 27, 28 Colcombe et al.24 demonstrated that, among a group of community-dwelling older adults, a 6-month intervention of aerobic training 3 times per week led to an increase in gray matter volume of two main hubs of the SN, the dACC, and the left superior temporal lobe. Reiter et al.25 showed that, among a group of older adults with mild cognitive impairment, improvement in cardiorespiratory fitness induced by a 3-month walking intervention 4 times per week was positively correlated with increased cortical thickness in the left insula and left superior temporal gyrus, two important hubs of the SN. Ruscheweyh et al.26 showed that, among a group of community-dwelling older adults who participated in a 6-month Nordic walking or gymnastics program 3 times per week, total PA was positively associated with increases in local gray matter volume in the prefrontal and cingulate cortex, two areas involved in the CEN and the SN. Scheewe et al.27 showed that, among a group of patients with schizophrenia spectrum disorder, the cardiorespiratory fitness improvement induced by a 6-month intervention of aerobic and resistance exercises 2 times per week was significantly associated with thickening in the left hemisphere of the frontal, temporal, and cingulate cortex. Voss et al.28 showed that, among a group of community-dwelling older adults, greater aerobic fitness derived from a 12-month intervention of walking was associated with greater increases in white matter integrity in the frontal and temporal lobes and with greater improvements in short-term memory. Thus far, only two intervention studies using functional brain imaging have shown a positive effect of chronic exercise on connectivity within and between large-scale networks. Voss et al.29 showed that among a group of older adults, a 12-month intervention program of walking 3 times per week led to increased functional connectivity in the DMN and CEN associated with greater improvements in EF. Voss et al.30 showed that, among a group of community-dwelling older adults, connectivity between the right anterior lateral prefrontal cortex and the left hippocampus decreased significantly in a walking group compared with a control group in a 12-month intervention program.30 This literature review shows that exercise and health psychologists need more brain-imaging studies testing the causal link between chronic exercise and strengthening of connectivity within and between the CEN and the SN.
In this section, we presented behavioral and neurophysiological arguments for a causal link between chronic exercise and EFs as well as effortful control, represented by the right arrow of the virtuous circle (Fig. 1). An examination of the reciprocal relationship illustrated by the left arrow of Fig. 1 now follows.
4. Causal relationship between EFs and effortful control and adherence to exercise
The effort hypothesis posits that adherence to PA and other healthy behaviors depends on effortful control capacity and EF effectiveness, which are underpinned by the strength in connectivity within and between the CEN and the SN (see Section 3). For instance, as mentioned in Section 2, planning and intentional inhibition are two EFs that require mental effort and are very useful for practicing exercise sessions regularly. Consequently, it can be inferred that a low level of effortful control capacity and/or EF effectiveness would lead to adherence problems and, more generally, to behavioral change failures. Conversely, a high level or a gain in these cognitive functions would lead to increased adherence to healthy behaviors and a facilitation of behavioral change. As we will see later a low level in effortful control and/or EF effectiveness may come from dispositional factors (e.g., a low level of trait self-control, genetic polymorphisms detrimental for EFs) or from periods of temporary weakening, which may last from a few days to several years. The first part of this section focuses on problems of health behaviors associated with a dispositional low level of effortful control and EFs.
According to the projections of the World Health Organization (WHO) for 2030, five of the leading causes of death worldwide among the top 20 will be ischemic heart disease, stroke, chronic obstructive pulmonary disease, diabetes mellitus, and HIV/AIDS.31 Mortality is attributable to major health risk factors such as obesity, tobacco and drug use, sedentariness, and unprotected sex. For instance, the number of obese individuals is expected to increase by 65 million and 11 million in the United States and the United Kingdom, respectively, in the next 15 years. Worldwide, it is similarly expected that the total number of tobacco-related deaths will increase from 6.4 million in 2015 to 8.3 million in 2030. Likewise, in the next 15 years, serious deleterious health consequences and 2 million deaths per year can be attributed to a lack of PA. These projections have economic consequences for society and require a better understanding of how to help individuals change their unhealthy habits. All change starts with one new behavior choice, but the difficulty is not only in making a decision to change, but also in initiating and maintaining this change as long as possible.32 Behavioral change is a deliberate and voluntary process that requires cognitive resources to be successful. Effortful cognitive processes such as EFs are necessary for any individual to change his or her behavior and, particularly, to maintain a sufficient amount of PA per week to have health benefits.
A method to show that EFs are necessary to regulate behavior is to examine whether people engaged in behaviors such as psychoactive substance addiction (e.g., tobacco, alcohol, or cannabis), game or sex addiction, or compulsive behavior (e.g., snacking or kleptomania) also display a dysfunction in EFs. It could be hypothesized that a dysfunction in EFs would lead to a decrease in the ability to refrain from habitual unhealthy and/or anti-social behavior and then to a higher probability of occurrence of the deviant behaviors in these individuals. There is an extensive and growing body of literature on this topic supporting a link between EFs and the ability to refrain from unhealthy or antisocial behavior throughout the lifetime.33, 34 Overall, individuals with weaker EFs tend to display more deviant behaviors compared with their stronger EF counterparts. For instance, Goudriaan et al.33 showed that pathological gamblers and alcohol-dependent patients in their study were characterized by diminished executive functioning in inhibition, time estimation, cognitive flexibility, and planning tasks in comparison with control participants.33 Similarly, poor EFs have also been reported in youths who are at high risk for substance misuse,35 and EFs assessed at age 10–12 predict substance use disorder at age 19.36 It is also important to note that unhealthy behaviors such as smoking, drug use, and a sedentary lifestyle can have a detrimental effect on executive functioning,37 leading to a greater loss of control for concerned individuals and thus aggravating the situation. In other words, someone who starts and maintains an unhealthy behavior enters a vicious circle from which it is more and more difficult to escape.
The end of this section focuses on the maintenance of healthy behavior targeted by the present article: exercising. Martin Ginis and Bray38 pointed out that exercise adherence requires self-regulatory skills such as anticipating and developing plans to overcome exercise barriers, creating exercise plans and schedules, and managing exercise-related pain and discomfort. As mentioned in Section 2, all of these self-regulatory skills are directly related to EFs. For instance, the initiation and maintenance of a behavior such as practicing exercise several times a week requires the intentional inhibition to resist the desire to stop exercise when the feeling of discomfort or fatigue is too high;39 the impulse to stop is certainly more pronounced in sedentary adults and seniors who have maintained a sedentary lifestyle throughout their lives. Practicing exercise regularly also requires planning the exercise sessions in the coming week (i.e., goal setting), remembering when to exercise at the planned time (i.e., prospective memory), and accepting that positive effects of PA occur in the long term (i.e., delay of gratification), which are three higher cognitive functions directly related to EF.9, 40, 41 The predictive power of EF on PA engagement has been mainly demonstrated with cross-sectional studies.42, 43 To our knowledge, no intervention study has been conducted with the aim of demonstrating a causal link between an improvement in EF efficiency and adherence to exercise. We only found one intervention study showing the effects of an aerobic exercise training program on EFs, specifically inhibitory control, and the positive transfer of the gain in EFs to self-regulation in the dietary domain.44 However, a very interesting longitudinal study assessing adherence to exercise over 1 year after a 12-month resistance exercise training program showed that individuals with higher levels of EF are more likely to engage in the exercise routine because they have a higher capacity for overriding impulse rewards and shifting focus to delayed outcomes.45 In summary, EF and self-regulation are essential and very useful in the behavioral change process and the maintenance of a sufficient level of PA. Several articles have highlighted the bidirectionality of the relationship between PA and EF and/or self-regulation,46, 47, 48, 49 which has been formalized in Hall and Fong's temporal self-regulation theory for PA.50 This model highlights that the difficulty in maintaining long-term engagement in health behaviors is due to the short-term costs of the target behavior (e.g., fatigue or pain for exercising, privation for a diet regimen) and few long-term benefits. The relevance of this model is the association between health models such as the theory of planned behavior,51 which considers intention as a determinant of behavior and the role of EF and behavioral prepotency as moderators of the link between intention and behavior. Behavioral prepotency can be defined as behaviors that constitute a strong habit and that need to be changed, such a sedentary lifestyle, snacking, or smoking. Determining the role of these cognitive processes in behavioral change has provided an alternative and heuristic approach to explain success or failure to initiate and maintain such changes.11, 52
The difficulty in maintaining long-term engagement can also be related to momentary, acute, or chronic, but nondispositional, failure of self-regulation. On the one hand, people exercise less on days when their EFs and related self-regulatory skills are weakened, and this relationship has been shown to be stronger in individuals with low trait self-control.53 In the same way, participants exposed to an acute self-regulatory depletion manipulation generated lower levels of work during a 10-min bicycling task and planned to exert less effort during an upcoming exercise bout compared with control participants.38 On the other hand, long periods of self-regulation exhaustion can lead to maladaptive behaviors, such as relapses or absenteeism.54 If training is an effective technique to enhance EF and self-regulation, this technique could be disastrous when applied to individuals with chronic self-regulation exhaustion. When individuals cope with chronic self-regulation exhaustion, the training of self-regulatory skills has to be used with caution given the risk of aggravating the fatigue state. Other perspectives aiming to restore self-regulation resources can be proposed, such as combining progressive self-regulatory training with cognitive-behavioral therapies,55 recovery techniques,56 and/or habit formation.57 There is a body of work that suggests that cognitive-behavioral therapies focusing on self-regulation (called self-system therapy) can be used to target dysfunction in self-regulation by changing the availability, the importance, and the patterns of goal-directed behavior.58, 59 These therapies have recently been used in association with graded exercise therapy in people who have a persistent failure of self-regulation by acting on their motivational dysfunction rather than their cognitive dysfunction. Such therapies have yielded interesting results, particularly in relapse prevention.60 Other techniques that rely on the recovery of self-regulation resources can also be used. These recovery techniques have been studied mainly with sleep, which has an important impact on cognitive functioning and self-regulatory capacity. For instance, a set of cross-sectional studies has shown the usefulness of sleep hygiene in predicting psychological strain at work61 and the ability of individuals to engage in regular exercise.62 Another technique registered as a behavioral cognitive technique concerns habit formation.63 Gardner and Lally have extensively studied habit formation in the health domain and have shown that habit formation can be triggered automatically and can occur in the absence of effortful control.57, 64 According to Bargh,65 the repetition of a behavior in a specific context gives the potential to activate the behavior in the absence of cognitive effort, deliberation, or effortful control because alternative behaviors become less accessible in memory. In other words, because the context is associated with motivational reward, it becomes a signal showing an opportunity and an incentive to act. Lally et al.66 and Gardner57 reported that the formation of healthy habits renders healthy behaviors more resistant to lapses and relapses and facilitates the maintenance of new behavior after the intervention. In summary, the virtuous circle characterized by the relationship between chronic exercise and EFs and/or effortful control can become a vicious circle when people cease this healthy behavior and do not replace it by another effective training method or when techniques used to improve EFs are not adapted given the current weakness of self-regulatory skills. The accurate evaluation of failures in self-regulation is essential to target the most appropriate techniques. For instance, in the case of self-regulation exhaustion, it is appropriate to avoid techniques based on self-regulation training and engage in goal-directed-based techniques. In contrast, in the case of emotional dysregulation (e.g., systematic use of anxiety to cope with the anticipated consequences of a decision), the use of habit formation techniques must be preferred over any other technique.
5. Future research directions and conclusions
In this article, we have highlighted that there is currently a lack of empirical evidence for two causal relationships involved in the virtuous circle, while the link between chronic exercise and EF is fairly well-established using behavioral measures of EF. The first causal relationship that lacks empirical evidence is the relationship between chronic exercise and network connectivity within and between the CEN and the SN. As mentioned by Buckley et al.,67 it could be interesting to include the DMN in these analyses because it could be involved in contemplation periods during which people engage in introspection on their aspirations for exercise, planning exercise sessions, and evaluating exercise goals and discrepancies. The DMN could also be involved in the cognitive-behavioral therapies mentioned in Section 4, which used self-referenced processes. The second causal relationship that needs more empirical evidence is the relationship between improvement in EFs and adherence to exercise programs, which both require the mobilization of mental effort. The present section proposes two categories of interventions that would be useful to conduct in the next decade. They would allow a more careful examination of links necessary to establish the validity of the virtuous circle described in Fig. 1. The costs of such interventions are relatively high, but they are fundamental to testing the effort hypothesis. It is important to emphasize that, if the effort hypothesis is valid, several applications might follow in the domain of health and sport psychology to help patients stop unhealthy behaviors or maintain healthy behaviors, thus generating individual and societal benefits. The present article provides numerous arguments supporting the effort hypothesis but delineates two categories of interventions that would be necessary to more accurately test its validity.
The first category of intervention studies focuses on the effects of chronic exercise on large-scale neuronal networks involved in effortful control and EF. Based on Colcombe and Kramer's meta-analytic results5, 68 and on the literature presented in Section 3, we recommend randomized controlled trials (RCTs) with an intervention of a minimal duration of 6 months. In addition, at least 2 groups of participants should be planned: a treatment group participating in effortful physical exercise at least 3 times a week at the end of the intervention and a control group practicing effortless physical exercises with the same session schedule as the treatment group. Effortful physical exercises can be defined as any exercise requiring sustained attention or effort to maintain an uncomfortable intensity to reach the goal of the exercise. The duration of these exercises should be progressive and range between 15 and 45 min. The intensity of the exercises should be progressively increased on an individual basis. The progressivity in increasing exercise intensity should also be calibrated to obtain good compliance with the program (≥80%). The effortless physical exercises could use the same PA basis, for instance, walking or jogging, but practiced at a comfortable speed that requires very little attention or effort throughout each bout of exercise. Feelings of mental effort and pleasure during exercise should be measured throughout the program and in the two arms of the RCT. The completion of a battery of several cognitive tasks engaging EFs and, most particularly, intentional inhibition and planning and/or updating of working memory should be planned before the beginning and after the end of the intervention program. These two EFs are specifically targeted because they play an important role in the adherence process to exercise programs (see Section 2 for examples). Psychophysiological indices, such as pupillary dilation and heart rate variability, could be recorded throughout the cognitive tasks to examine the possible variations in effort engagement with time on task. For that reason, we recommend the use of continuous cognitive tasks with a minimal duration of 15 min. Finally, two resting-state functional magnetic resonance imaging scans at rest should be planned, one before the beginning and the other after the end of the intervention. Then, seed-based correlation analyses should be conducted to examine the chronic effect of exercise on functional connectivity within and between the DMN, the SN, and the CEN.
The second category of intervention focuses on the effects of cognitive training on PA adherence. The aim of the intervention would be to improve EFs and effortful control through cognitive training in a cohort of older adults and to observe the repercussions of this potential improvement on adherence to PA and other health-related behaviors in the current life of the participants. Why is it important to use cognitive training instead of physical training to improve EFs and effortful control in the validity test of the second causal relationship? Intervention studies that test the validity of the first causal relationship between chronic exercise and EFs generally use performance scores obtained in tasks engaging EFs and effortful control as the principal outcomes, i.e., tasks involving both CEN and SN, such as all tasks listed in Section 2. In addition, the effectiveness of the physical training program is generally validated through the improvement in physical fitness (e.g., increase in maximal oxygen consumption and/or increase in muscular strength). In contrast, in the validity test of the second causal relationship between EFs and adherence to exercise, the principal outcome of the RCT would be adherence to exercise and diet. In that case, it would not be suitable to use the same physical training program to improve the EFs and measure adherence to the physical training program as the main outcome. Indeed, it is problematic that the content of the physical training program is directly and intrinsically linked to adherence to this program. To clearly separate the cause and the consequence in the improvement in EF, it would be more pertinent to use cognitive training rather than physical training. However, a pertinent case of physical training planned to improve self-control strength leading to a transfer effect on a different physical exercise can be found in a study by Bray et al.69 These investigators examined the effect of a 2-week training program including maximal isometric handgrip squeezing exercises on performance in a cardiovascular endurance cycling performance carried out just before the beginning and after the end of the program. They showed that participants who completed the 2-week self-control training program improved their performance in the intensity-graded cycling exercise by increasing their time to failure in comparison with the control participants. Although it seems adequate to use a physical training program to improve effortful control and examine the consequence of this improvement on exercise adherence, we recommend using cognitive training to enhance far-transfer effects rather than near-transfer effects. Several narrative and meta-analytic reviews have highlighted the effectiveness of cognitive training and mental stimulation to improve EF.70, 71, 72 It should be noted that cognitive training programs have shown a greater effect size at improving EFs in comparison with physical training programs.73 These recent findings from process-specific EF training indicate that all the key domains of EF can be improved by cognitive training, not only in childhood and adolescence,71 but also in older adults.73 There is also evidence for transfer from one EF component to other components of EF (e.g., from task-switching training to working-memory abilities). The maintenance of the gains in executive functioning lasts several months. For instance, the benefit in working memory in old-old individuals after a verbal working-memory training program of 3 sessions completed within a 2-week timeframe persisted 8 months after the end of the intervention.74 However, the extent to which these improvements generalize and show positive transfers to everyday life activities remains strongly debated. Generally, psychologists have focused transfer analyses on cognitive tasks very similar to the trained task (i.e., near-transfer effects) and on dissimilar cognitive tasks (far-transfer effects), but never specifically on ecological tasks concerned with behavioral change. Based on these reviews, we recommend planning an RCT that includes two arms. In the first arm, the group of participants would take part in a cognitive training program with a volume of at least 10 h (e.g., two 1-h sessions per week for 5 weeks) and using a variety of effortful cognitive tasks engaging EF. In the second arm, the other group of participants would take part in a sham cognitive training program of the same duration and consisting of viewing neutral emotion movies and answering questions about the movies. In parallel with the intervention, the participants in the 2 groups would be instructed to follow the recommendations of the WHO concerning PA and diet, for example, practicing at least 150 min of moderate intensity exercise per week and eating at least 5 portions of fruits and vegetables per day. The adherence to these recommendations would be regularly collected from 1 week before the beginning of the intervention to at least 3 months after the end of the intervention. Concerning the measurement of PA adherence, the main outcome of the RCT, we recommend the use of two instruments: a weekly/daily PA notebook and actimetry.
Unfortunately, the virtuous circle is fragile, and, as mentioned in Section 4, it can be broken or even reversed when life accidents happen. In the case of circle reversal, it is more appropriate to talk about the initiation of a vicious circle that progressively leads the individual to a sedentary lifestyle, a weakening of physical and effort-based mental capacities, and sometimes health problems such as osteoporosis, obesity, or cardiovascular diseases.75,76 It seems that physical, brain, and psychological capacities function synergistically, mutually reinforcing one another in the case of the virtuous circle, and conversely making the mobilization of the other more difficult in the case of the vicious circle. From that perspective, Allan et al.48 proposed that the elements of the virtuous circle are linked by feedback loops. Our task in the future would be to clearly define environmental and individual factors that facilitate the rotation of the virtuous circle in the right direction and counteract the inverse rotation of the vicious circle. Finally, it is important to emphasize that the effort hypothesis presented in this article is not in opposition to the current and dominant neurotrophic hypothesis. Instead, it would be more appropriate to conceive that the mechanisms strengthening the large-scale networks underpinning effortful control and EF (the effort hypothesis) operate synergistically with the mechanisms enhancing brain plasticity (neurotrophic hypothesis).
Acknowledgments
We would like to thank Yu-Kai Chang and Jennifer Etnier for inviting us to submit a paper for this special topic, and Jennifer Etnier and Panteleimon Ekkekakis for their helpful comments on a previous version of this manuscript. The present review was supported by the French National Research Agency (ANR-12-MALZ-005-01).
Authors’ contributions
MA and NA elaborated the plan of the article, made the review of literature, and drafted the manuscript. Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
Both authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Bouchard C., Blair S.N., Haskell W.L. 3rd ed. Human Kinetics; Champaign, IL: 2012. Physical activity and health. ; 2012. [Google Scholar]
- 2.Hötting K., Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013;37:2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Karr J.E., Areshenkoff C.N., Rast P., Garcia-Barrera M.A. An empirical comparison of the therapeutic benefits of physical exercise and cognitive training on the executive functions of older adults: a meta-analysis of controlled trials. Neuropsychology. 2014;28:829–845. doi: 10.1037/neu0000101. [DOI] [PubMed] [Google Scholar]
- 4.Cerrillo-Urbina A.J., García-Hermoso A., Sánchez-López M., Pardo-Guijarro M.J., Santos Gómez J.L., Martínez-Vizcaíno V. The effects of physical exercise in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis of randomized control trials. Child Care Health Dev. 2015;41:779–788. doi: 10.1111/cch.12255. [DOI] [PubMed] [Google Scholar]
- 5.Kramer A.F., Colcombe S. Fitness effects on the cognitive function of older adults: a meta-analytic study-revisited. Perspect Psychol Sci. 2018;13:213–217. doi: 10.1177/1745691617707316. [DOI] [PubMed] [Google Scholar]
- 6.Stimpson N.J., Davison G., Javadi A.H. Joggin’ the noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci Biobehav Rev. 2018;88:177–186. doi: 10.1016/j.neubiorev.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Smiley-Oyen A.L., Lowry K.A., Francois S.J., Kohut M.L., Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascular fitness” hypotheses. Ann Behav Med. 2008;36:280–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 9.Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. 5th ed. Oxford University Press; New York, NY: 2012. Neuropsychological assessment. ; 2012. [Google Scholar]
- 10.Barkley R.A. Linkages between attention and executive functions. In: Lyon G.R., Krasnegor N.A., editors. Attention, memory, and executive function. Paul H. Brookes Publishing Co.; Baltimore, MD: 1996. pp. 307–325. [Google Scholar]
- 11.Hofmann W., Schmeichel B.J., Baddeley A.D. Executive functions and self-regulation. Trends Cogn Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Karoly P. Mechanisms of self-regulation: a systems view. Annu Rev Psychol. 1993;44:23–52. [Google Scholar]
- 13.Sanders A.F. Towards a model of stress and human performance. Acta Psychol (Amst) 1983;53:61–97. doi: 10.1016/0001-6918(83)90016-1. [DOI] [PubMed] [Google Scholar]
- 14.Hockey G.R. Compensatory control in the regulation of human performance under stress and high workload; a cognitive-energetical framework. Biol Psychol. 1997;45:73–93. doi: 10.1016/s0301-0511(96)05223-4. [DOI] [PubMed] [Google Scholar]
- 15.Shenhav A., Musslick S., Lieder F., Kool W., Griffiths T.L., Cohen J.D. Toward a rational and mechanistic account of mental effort. Annu Rev Neurosci. 2017;40:99–124. doi: 10.1146/annurev-neuro-072116-031526. [DOI] [PubMed] [Google Scholar]
- 16.Radulescu E., Nagai Y., Critchley H. Mental effort: brain and autonomic correlates in health and disease. In: Gendolla G.H.E., Tops M., Koole S.L., editors. Handbook of biobehavioral approaches to self-regulation. Springer; New York, NY: 2015. pp. 237–253. [Google Scholar]
- 17.Northey J.M., Cherbuin N., Pumpa K.L., Smee D.J., Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Bueno C., Pesce C., Cavero-Redondo I., Sanchez-Lopez M., Martínez-Hortelano J.A., Martínez-Vizcaino V. The effect of physical activity interventions on children's cognition and metacognition: a systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56:729–738. doi: 10.1016/j.jaac.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raichle M.E. The brain's default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 21.Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulden N., Khusnulina A., Davis N.J., Bracewell R.M., Bokde A.L., McNulty J.P. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 24.Colcombe S.J., Erickson K.I., Scalf P.E., Kim J.S., Prakash R., McAuley E. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 25.Reiter K., Nielson K.A., Smith T.J., Weiss L.R., Alfini A.J., Smith J.C. Improved cardiorespiratory fitness is associated with increased cortical thickness in mild cognitive impairment. J Int Neuropsychol Soc. 2015;21:757–767. doi: 10.1017/S135561771500079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruscheweyh R., Willemer C., Krüger K., Duning T., Warnecke T., Sommer J. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2011;32:1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Scheewe T.W., van Haren N.E., Sarkisyan G., Schnack H.G., Brouwer R.M., de Glint M. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomized controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 2013;23:675–685. doi: 10.1016/j.euroneuro.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Voss M.W., Erickson K.I., Prakash R.S., Chaddock L., Kim J.S., Alves H. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voss M.W., Prakash R.S., Erickson K.I., Basak C., Chaddock L., Kim J.S. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voss M.W., Heo S., Prakash R.S., Erickson K.I., Alves H., Chaddock L. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO) WHO; Geneva: 2018. Global health estimates summary tables: projection of deaths by cause, age and sex.www.who.int/healthinfo/global_burden_disease/projections/en/ Available at: 2018[accessed 14.08.2018] [Google Scholar]
- 32.Rothman A.J. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000;19:64–69. doi: 10.1037/0278-6133.19.suppl1.64. [DOI] [PubMed] [Google Scholar]
- 33.Goudriaan A.E., Oosterlaan J., de Beurs E., van den Brink W. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101:534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- 34.Hall P., Tran B., Lowe C., Vincent C., Mourtzakis M., Liu-Ambrose T. Expression of executive control in situational context: effects of facilitating versus restraining cues on snack food consumption. Health Psychol. 2015;34:539–546. doi: 10.1037/hea0000134. [DOI] [PubMed] [Google Scholar]
- 35.Tarter R.E., Mezzich A.C., Hsieh Y.C., Parks S.M. Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend. 1995;39:15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- 36.Tarter R.E., Kirisci L., Mezzich A., Cornelius J.R., Pajer K., Vanyukov M. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 37.Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Exp Clin Psychopharmacol. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- 38.Martin Ginis K.A., Bray S.R. Application of the limited strength model of self-regulation to understanding exercise effort, planning and adherence. Psychol Health. 2010;25:1147–1160. doi: 10.1080/08870440903111696. [DOI] [PubMed] [Google Scholar]
- 39.Audiffren M., André N. The strength model of self-control revisited: linking acute and chronic effects of exercise on executive functions. J Sport Health Sci. 2015;4:30–46. [Google Scholar]
- 40.Schnitzspahn K.M., Stahl C., Zeintl M., Kaller C.P., Kliegel M. The role of shifting, updating, and inhibition in prospective memory performance in young and older adults. Dev Psychol. 2013;49:1544–1553. doi: 10.1037/a0030579. [DOI] [PubMed] [Google Scholar]
- 41.Lee W.S., Carlson S.M. Knowing when to be “rational”: flexible economic decision making and executive function in preschool children. Child Dev. 2015;86:1434–1448. doi: 10.1111/cdev.12401. [DOI] [PubMed] [Google Scholar]
- 42.Giles G.E., Cantelon J.A., Eddy M.D., Brunyé T.T., Urry H.L., Mahoney C.R. Habitual exercise is associated with cognitive control and cognitive reappraisal success. Exp Brain Res. 2017;235:3785–3797. doi: 10.1007/s00221-017-5098-x. [DOI] [PubMed] [Google Scholar]
- 43.André N., Ferrand C., Albinet C., Audiffren M. Cognitive strategies and physical activity in older adults: a discriminant analysis. J Aging Res. 2018 doi: 10.1155/2018/8917535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowe C.J., Kolev D., Hall P.A. An exploration of exercise-induced cognitive enhancement and transfer effects to dietary self-control. Brain Cogn. 2016;110:102–111. doi: 10.1016/j.bandc.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Best J.R., Nagamatsu L.S., Liu-Ambrose T. Improvements to executive function during exercise training predict maintenance of physical activity over the following year. Front Hum Neurosci. 2014;8:353. doi: 10.3389/fnhum.2014.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raichlen D.A., Polk J.D. Linking brains and brawn: exercise and the evolution of human neurobiology. Proc Biol Sci. 2013;280 doi: 10.1098/rspb.2012.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daly M., McMinn D., Allan J.L. A bidirectional relationship between physical activity and executive function in older adults. Front Hum Neurosci. 2015;8:1044. doi: 10.3389/fnhum.2014.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allan J.L., McMinn D., Daly M. A bidirectional relationship between executive function and health behavior: evidence, implications, and future directions. Front Neurosci. 2016;10:386. doi: 10.3389/fnins.2016.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards M.K., Addoh O., Herod S.M., Rhodes R.E., Loprinzi P.D. A conceptual neurocognitive affect related model for the promotion of exercise among obese adults. Curr Obes Rep. 2017;6:86–92. doi: 10.1007/s13679-017-0244-0. [DOI] [PubMed] [Google Scholar]
- 50.Hall P.A., Fong G.T. Temporal self-regulation theory: a neurobiologically informed model for physical activity behavior. Front Hum Neurosci. 2015;9:117. doi: 10.3389/fnhum.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1992;50:179–221. [Google Scholar]
- 52.Hall P.A., Elias L.J., Fong G.T., Harrison A.H., Borowsky R., Sarty G.E. A social neuroscience perspective on physical activity. J Sport Exerc Psychol. 2008;30:432–449. doi: 10.1123/jsep.30.4.432. [DOI] [PubMed] [Google Scholar]
- 53.Schöndube A., Bertrams A., Sudeck G., Fuchs R. Self-control strength and physical exercise: an ecological momentary assessment study. Psychol Sport Exerc. 2017;29:19–26. [Google Scholar]
- 54.Muraven M., Tice D.M., Baumeister R.F. Self-control as limited resource: regulatory depletion patterns. J Pers Soc Psychol. 1998;74:774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- 55.Strauman T.J., Eddington K.M. Treatment of depression from a self-regulation perspective: basic concepts and applied strategies in self-system therapy. Cognit Ther and Res. 2017;41:1–15. doi: 10.1007/s10608-016-9801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fanning J., Porter G., Awick E.A., Ehlers D.K., Roberts S.A., Cooke G. Replacing sedentary time with sleep, light, or moderate-to vigorous physical activity: effects on self-regulation and executive functioning. J Behav Med. 2017;40:332–342. doi: 10.1007/s10865-016-9788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gardner B. A review and analysis of the use of ‘habit’ in understanding, predicting and influencing health-related behavior. Health Psychol Rev. 2015;9:277–295. doi: 10.1080/17437199.2013.876238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vieth A.Z., Strauman T.J., Kolden G.G., Woods T.E., Michels J.L., Klein M.H. Self-system therapy (SST): a theory based psychotherapy for depression. Clin Psychol Sci Pract. 2003;10:245–268. [Google Scholar]
- 59.Strauman T.J., Vieth A.Z., Merrill K.A., Kolden G.G., Woods T.E., Klein M.H. Self-system therapy as an intervention for self-regulatory dysfunction in depression: a randomized comparison with cognitive therapy. J Consult Clin Psychol. 2006;74:367–376. doi: 10.1037/0022-006X.74.2.367. [DOI] [PubMed] [Google Scholar]
- 60.McBride R.L., Horsfield S., Sandler C.X., Cassar J., Casson S., Cvejic E. Cognitive remediation training improves performance in patients with chronic fatigue syndrome. Psychiatry Res. 2017;257:400–405. doi: 10.1016/j.psychres.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 61.Barber L., Grawitch M.J., Munz D.C. Are better sleepers more engaged workers? A self-regulatory approach to sleep hygiene and work engagement. Stress Health. 2013;29:307–316. doi: 10.1002/smi.2468. [DOI] [PubMed] [Google Scholar]
- 62.Dzierzewski J.M., Buman M.P., Giacobbi P.R., Roberts B.L., Aiken-Morgan A.T., Marsiske M. Exercise and sleep in community-dwelling older adults: evidence for a reciprocal relationship. J Sleep Res. 2014;23:61–68. doi: 10.1111/jsr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michie S., Richardson M., Johnston M., Abraham C., Francis J., Hardeman W. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 64.Lally P., Gardner B. Promoting habit formation. Health Psychol Rev. 2013;7(Suppl. 1):S137–S158. [Google Scholar]
- 65.Bargh J.A. The four horsemen of automaticity: awareness, intention, efficiency, and control in social cognition. In: Wyer R.S., Srull T.K., editors. Handbook of social cognition. Lawrence Erlbaum; Hillsdale, NJ: 1994. pp. 1–40. [Google Scholar]
- 66.Lally P., van Jaarsveld C.H.M., Potts H.W.W., Wardle J. How are habits formed: modelling habit formation in the real world. Eur J Soc Psychol. 2010;40:998–1009. [Google Scholar]
- 67.Buckley J., Cohen J.D., Kramer A.F., McAuley E., Mullen S.P. Cognitive control in the self-regulation of physical activity and sedentary behavior. Front Hum Neurosci. 2014;8:747. doi: 10.3389/fnhum.2014.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colcombe S., Kramer A.F. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 69.Bray S.R., Graham J.D., Saville P.D. Self-control training leads to enhanced cardiovascular exercise performance. J Sports Sci. 2015;33:534–543. doi: 10.1080/02640414.2014.949830. [DOI] [PubMed] [Google Scholar]
- 70.Reijnders J., van Heugten C., van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev. 2013;12:263–275. doi: 10.1016/j.arr.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Karbach J., Unger K. Executive control training from middle childhood to adolescence. Front Psychol. 2014;5:390. doi: 10.3389/fpsyg.2014.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly M.E., Loughrey D., Lawlor B.A., Robertson I.H., Walsh C., Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;15:28–43. doi: 10.1016/j.arr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Karr J.E., Areshenkoff C.N., Rast P., Garcia-Barrera M.A. An empirical comparison of the therapeutic benefits of physical exercise and cognitive training on the executive functions of older adults: a meta-analysis of controlled trials. Neuropsychology. 2014;28:829–845. doi: 10.1037/neu0000101. [DOI] [PubMed] [Google Scholar]
- 74.Borella E., Carretti B., Zanoni G., Zavagnin M., De Beni R. Working memory training in old age: an examination of transfer and maintenance effects. Arch Clin Neuropsychol. 2013;28:331–347. doi: 10.1093/arclin/act020. [DOI] [PubMed] [Google Scholar]
- 75.Bonaiuti D., Shea B., Iovine R., Negrini S., Robinson V., Kemper H.C. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database of Syst Rev. 2002;(3) doi: 10.1002/14651858.CD000333. [DOI] [PubMed] [Google Scholar]
- 76.Owen N., Sparling P.B., Healy G.N., Dunstan D.W., Matthews C.E. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc. 2010;85:1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]