Abstract
A 69-year-old woman who presented with severe renal dysfunction and diffuse alveolar hemorrhage was diagnosed with pulmonary–renal syndrome (PRS) based on the coexistence of serum myeloperoxidase (MPO)–antineutrophil cytoplasmic antibodies (ANCA) and anti-glomerular basement membrane (GBM) antibodies (Ab). Hemodialysis was started; plasma exchange and intravenous methylprednisolone pulse therapy were administered followed by oral prednisolone administration. Pulmonary hemorrhage decreased; however, renal dysfunction persisted. On maintenance hemodialysis and prednisolone therapy, MPO–ANCA and anti-GBM Ab became negative at 4 and 10 months, respectively; thereafter, they became positive again at 18 and 35 months, respectively. At 36 months, there was relapse of pulmonary hemorrhage. Plasma exchange and intravenous methylprednisolone pulse therapy were administered; pulmonary hemorrhage ceased, and both antibodies became negative. It is known that PRS cases that are double positive for ANCA and anti-GBM Ab occasionally relapse after remission, and, even though they are double positive at initial diagnosis, most relapses occur with reappearance or re-elevation of ANCA but with absence of anti-GBM-Ab. Therefore, this was a rare relapsing case that presented with double-positive serology. Further, our observation that the reappearance of ANCA preceded that of anti-GBM-Ab suggests that ANCA contribute to the reproduction of anti-GBM Ab.
Keywords: Anti-GBM antibody, MPO–ANCA, Pulmonary–renal syndrome, Rapidly progressive glomerulonephritis, Relapse
Introduction
Pulmonary–renal syndrome (PRS) is a life-threatening condition defined as rapidly progressive glomerulonephritis (RPGN) and lung hemorrhage. PRS is histologically characterized by necrotizing and crescentic glomerulonephritis and pulmonary capillaritis [1]. The two common causes of PRS are anti-glomerular basement membrane (GBM) disease and antineutrophil cytoplasmic antibody (ANCA)-associated disease (AAV), including microscopic polyangiitis, granulomatosis with polyangiitis, and eosinophilic granulomatosis with polyangiitis [1]. Anti-GBM disease and AAV have different etiologies; anti-GBM antibody (Ab) deposition on GBM is relevant to the pathogenesis of anti-GBM disease, while ANCA-induced neutrophil activation underlies the pathogenesis of AAV [2]. However, anti-GBM-Ab and ANCA co-exist more commonly than expected to occur by chance in PRS or RPGN patients. One-fifth to one-half of anti-GBM disease patients have ANCA, and up to 10% of AAV patients possess anti-GBM Ab [3–7]. Further, double-seropositive patients show clinical and histological features that are distinct from those observed in single-seropositive patients (with presence of either antibody) [3, 4, 7, 8]. The double-seropositive patients occasionally relapse, similar to AAV patients, while relapse is rare in the anti-GBM Ab single-positive patients [3, 9]. It is noteworthy that even though they are double-seropositive at initial diagnosis, most relapses occur with reappearance or re-elevation in ANCA but without presence of anti-GBM Ab [3, 9, 10]. Thus far, only one case has been reported to be double-seropositive at onset and double-seropositive again at the time of relapse [3]. However, for this case, the time course of each Ab titer before relapse was not described. Here, we present a case of a rare double-seropositive PRS patient who relapsed after sequential reappearance of MPO–ANCA and anti-GBM Ab.
Case report
A 69-year-old woman was admitted to a local hospital because of low-grade fever, general fatigue, and appetite loss that had persisted for 1 month. There was no history of previous admission or kidney disease. Serum creatinine level 1 year before the admission was 0.59 mg/dL as per her medical records. There was no remarkable familial history, drug history, or allergic history. She was a homemaker and a non-smoker. After admission, she developed oliguria, with a serum creatinine level of 16.2 mg/dL; hemodialysis was started. She had positive-serum MPO–ANCA (64.3 U/mL, normal range < 3.5 U/mL) and anti-GBM Ab (191 U/mL, normal range < 2.0 U/mL). Serum cryoglobulin was negative. At day 13, she presented with hemoptysis and was transferred to our hospital. At that time, her body temperature was 37.3 °C, and oxygen saturation level on room air remained at 98%. She had pale conjunctiva, fine crackles in her left lung base, and mild pitting edema in both lower legs. As shown in Table 1, laboratory tests revealed a low hemoglobin level (6.8 g/dL), a mild increase in the white blood cell count, low albumin level (2.2 g/dL), elevated serum creatinine level (8.45 mg/dL), and high C-reactive protein level (6.25 mg/dL). Urine tests showed proteinuria and hematuria. Chest computed tomography (CT) showed consolidation shadows in both the lungs, dominant on the dorsal inferior lobe of the left lung (Fig. 1a, b), indicating diffuse alveolar hemorrhage. She was diagnosed with PRS, based on renal dysfunction, diffuse alveolar hemorrhage, and the coexistence of MPO–ANCA and anti-GBM Ab.
Table 1.
Laboratory data at initial admission
| Urinalysis | Biochemical tests | Serological tests | |||
|---|---|---|---|---|---|
| Protein | (3+) | TP | 5.5 g/dL | CRP | 6.25 mg/dL |
| Sugar | (1+) | Alb | 2.2 g/dL | ANA | 40 folds |
| RBC | > 100/HPF | T-Bil | 0.2 mg/dL | CH50 | 47.2 U/mL |
| WBC | 20–29/HPF | AST | 8 IU/L | C3c | 120 mg/dL |
| ALT | 9 IU/L | C4 | 42.9 mg/dL | ||
| Peripheral blood | LDH | 125 IU/L | IgA | 198 IU/mL | |
| RBC | 217 × 104/uL | ALP | 182 IU/L | IgE | 139 IU/mL |
| Hb | 6.8 g/dL | yGTP | 14 IU/L | PR-3 ANCA | < 10 U/mL |
| Ht | 19.9% | BUN | 46 mg/dL | MPO–ANCA | 52 U/mL |
| WBC | 9400/uL | Cr | 8.45 mg/dL | Anti-GBM Ab | 190 U/mL |
| Neu. | 77.7% | Na | 138 mEq/L | β-D-glucan | 18.0 pg/mL |
| Lymph. | 9.6% | K | 3.6 mEq/L | T-SPOT.TB | Negative |
| Mono. | 9.9% | Cl | 102 mEq/L | Anti-MAC Ab | Negative |
| Eos. | 2.4% | Ca | 7.9 mg/dL | Procalcitonin | 0.65 ng/mL |
| Baso. | 0.4% | P | 3.9 mg/dL | KL-6 | 157 U/mL |
| Platelet | 31.2 × 104/uL | ||||
Fig. 1.
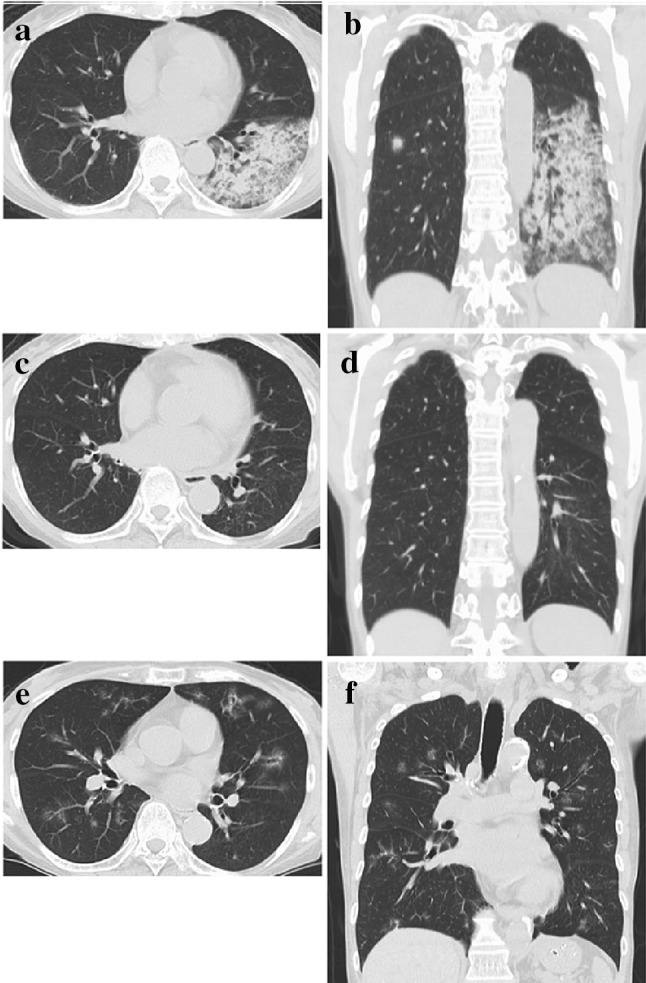
Chest CT images. a, b Images on the first admission. c, d Images at 12 months after the onset (remission phase). e, f Images on the second admission for relapse
As shown in Fig. 2, hemodialysis was continued, and plasma exchange was started immediately. Methylprednisolone pulse therapy was administered at 1000 mg/day for 3 days followed by oral prednisolone therapy. Hemoptysis reduced within 2 weeks; however, renal function was not restored. The levels of both, anti-GBM Ab and MPO–ANCA, decreased promptly. Two months thereafter, she was discharged on maintenance hemodialysis with low-dose prednisolone therapy. Levels of MPO–ANCA and anti-GBM Ab further decreased to reach the normal range at 4 and 10 months, respectively, after disease onset. The repeated CT performed at 12 months showed almost complete alleviation of pulmonary hemorrhage (Fig. 1c, d).
Fig. 2.
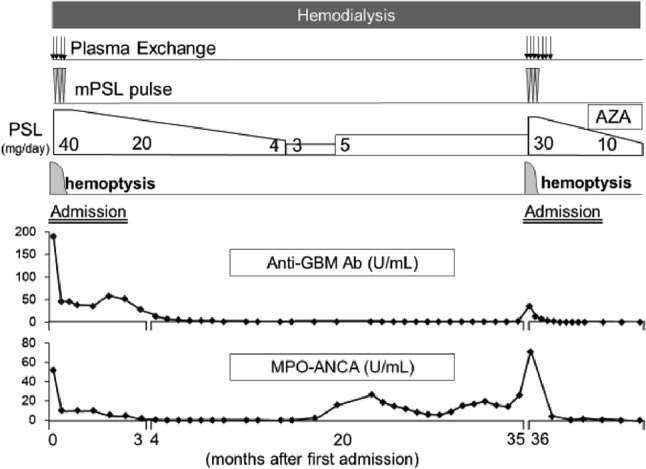
Clinical course after admission. mPSL methylprednisolone, PSL prednisolone, AZA azathioprine
However, MPO–ANCA turned positive again at 18 months and remained positive without clinical exacerbation or elevation in the CRP levels. The prednisolone dose was slightly increased from 3 to 5 mg/day based on the rise in the MPO–ANCA. At 35 months, anti-GBM Ab also became positive (5.7 U/mL). At 36 months, she suddenly developed hemoptysis with a rapid increase in the anti-GBM and MPO–ANCA levels (35 U/mL and 69.9 U/mL, respectively), and a mild elevation in the CRP levels (0.4 mg/dL) (Fig. 2). She was admitted with a diagnosis of a relapse.
At re-admission, chest CT identified multiple ground-glass opacities in the whole lung fields (Fig. 1e, f), and bronchoscopy revealed blood in the airways, indicating alveolar hemorrhage. Relapse was diagnosed; plasma exchange and methylprednisolone pulse therapy at 1000 mg/day for 3 days were re-started. Pulmonary hemorrhage reduced; the MPO–ANCA and anti-GBM Ab levels decreased to within the normal range. She was discharged 1 month thereafter, with low-dose prednisolone and azathioprine as maintenance therapy; she was in remission at 2 years after the relapse (Fig. 2).
Discussion
Clinical features of double-seropositive PRS or RPGN patients have been documented in previous studies (Table 2) [3–5, 7, 9, 11–13]; the age at the onset ranged from 55 to 69 years, commensurate with those in MPO–ANCA single-positive patients and higher than those for anti-GBM Ab single-positive patients. The serum creatinine levels at presentation ranged from 3.5 to 10.3 mg/dL, comparable to those in anti-GBM Ab single-positive patients and higher than those in ANCA single-positive patients. Pulmonary hemorrhage, which is associated with high mortality [3, 5, 7], was present in 38–77% of the patients, similar to the percentage in anti-GBM Ab single-positive patients and higher than that in ANCA single-positive patients. Renal survival rate ranged from 0 to 53% at 1 year, slightly worse than that in anti-GBM Ab single-positive patients and considerably worse than that in ANCA single-positive patients. These data indicate that the clinical presentation at onset and short-term outcomes in the double-seropositive patients are similar to those in anti-GBM patients, except for the older age.
Table 2.
Representative case series on PRS or RPGN with double-positive ANCA and anti-GBM Ab, and single-positive ANCA and/or single-positive anti-GBM-Ab
| Authors [references #] | Serotype | Cases, n | Age | Male gender (%) | Lung hemorrhage (%) | Serum creatinine (mg/dL) | Renal survival at 1 year (%) | Patient survival at 1 year (%) | Relapse rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| McAdoo et al. [3] | DP | 37 | 64 | 38 | 38 | 3.5 | 53 | 83 | 22 |
| SP-ANCA | 568 | 62 | 54 | 23 | 2.1 | 88 | 90 | 37 | |
| SP-GBM Ab | 41 | 58 | 46 | 40 | 3.1 | 44 | 87 | 0 | |
| Alchi et al. [9] | DP | 9 | 69 | 44 | 44 | NA | 16 | 88 | 22 |
| SP-GBM Ab | 34 | 52 | 44 | 38 | NA | 0 | |||
| Srivastava et al. [13] | DP | 9 | 59 | 33 | 56a | 9.1 | 0 | 100 | 11 |
| SP-ANCA | 18 | 57 | 56 | 39a | 3.1 | 89 | 94 | NA | |
| SP-GBM Ab | 6 | 52 | 33 | 50a | 9.6 | 33 | 100 | NA | |
| Cui et al. [11] | DP | 39 | 60 | 56 | 41 | 9.6 | 25 | 49 | NA |
| SP-GBM Ab | 137 | 37 | 73 | 47 | 9.5 | 73 | 80 | NA | |
| Zhao et al. [7] | DP | 61 | 55 | 54 | 77 | 10.1 | 15 | 38 | NA |
| Rutgers et al. [4] | DPb | 10 | 64 | 80 | NA | 10.3 | 10 | 79 | NA |
| SP-ANCAb | 98 | 63 | 67 | NA | 5.0 | 64 | 75 | NA | |
| SP-GBM | 13 | 52 | 39 | NA | 9.6 | 15 | 100 | NA | |
| Levy et al. [5] | DP | 27 | 66 | 48 | 41 | 7.2 | 26 | 52 | 0 |
| Levy et al. [12] | SP-GBM Ab | 71 | 40 | 56 | 62 | 3.6 | 53 | 77 | 3 |
PRS pulmonary renal syndrome, RPGN rapidly progressive glomerulonephritis, DP double positive for ANCA and anti-GBM antibody, SP single positive
aSeveral respiratory symptoms in addition to lung hemorrhage, i.e., pleurisy, nodules, cavities and infiltrates are included
bIndividuals with positive PR3-ANCA are excluded from the study because of small numbers
However, double-seropositive patients reportedly have different characteristics from those with anti-GBM disease with respect to the long-term aspects. The clinical course of anti-GBM disease is generally monophasic and a recurrence of either clinical conditions or autoantibody production is extremely rare following the disappearance of anti-GBM Ab [14]. A recent large multicenter cohort study reported relapse rates of 22% and 37% for double-seropositive patients and ANCA single-positive patients, respectively, while no relapse was observed in anti-GBM Ab single-positive patients; thus, frequency of relapse is more similar to that in ANCA single-positive patients rather than that in anti-GBM Ab single-positive patients [3]. It is noteworthy that ANCA were positive but anti-GBM Ab were negative at relapse in seven of the eight relapsing double-seropositive patients in the above-mentioned multicenter study, and only one patient became double-seropositive again at relapse [3]. To our knowledge, our patient represents the second case of double-seropositive PRS or RPGN wherein not only ANCA, but also anti-GBM Ab became positive at relapse. We note that another course of double-positive serology in relapsing RPGN cases was reported, in that ANCA were present but anti-GBM-Ab were absent at initial diagnosis, while both antibodies were present at the relapse [15, 16].
The mechanisms of the association between ANCA and anti-GBM Ab in double-seropositive patients are not well understood [3, 8]. Serrratrice et al. reported the case of a patient with anti-GBM disease who initially experienced AAV and suddenly developed anti-GBM disease 3 years thereafter [15]. They hypothesized that ANCA-associated damage to the GBM uncovers “hidden antigens” from the GBM and induces the formation of anti-GBM Ab [15]. It is noteworthy that patients with double-positive serology were shown to have anti-GBM Ab not only against α3(IV)NC, a well-known target of anti-GBM disease, but also against other components in GBM, suggesting that ANCA-associated damage to GBM uncovers a wide spectrum of GBM components [17]. In our case, both, MPO–ANCA and anti-GBM Ab were already positive at presentation and the sequence of production of these antibodies before disease onset is unknown. However, after remission, careful monitoring of both the antibodies demonstrated that reappearance of ANCA preceded that of anti-GBM Ab by 17 months, and there was no clinical exacerbation in the meanwhile. Thereafter, the patient experienced lung hemorrhage with a rapid rise in the level of both antibodies. In our case, we hypothesize that the reproduced ANCA alone were insufficient to induce lung hemorrhage. However, the combination of ANCA and additional causal factors, such as viral infection, evoked subclinical inflammation in the lungs and disrupted the lung basement membrane, triggering reproduction of anti-GBM Ab. Thereafter, synergistic effects of ANCA and anti-GBM Ab may have led to lung hemorrhage.
In our case, we did not perform kidney biopsy. Thus, we did not observe deposition of anti-GBM antibody, and could not rule out other possible causes of RPGN. We acknowledge that this is a limitation of our report.
To conclude, we present a rare case of PRS that relapsed with sequential elevation of ANCA and anti-GBM Ab. Our case is unique in that both Ab levels were serially measured after the remission, and the sequential reappearances of these antibodies were identified before the relapse. Our observation may provide insights into the pathogenesis of PRS with double-positive serology.
Acknowledgements
This case report was presented in the 18th international vasculitis and ANCA Workshop (Tokyo, Japan, 2017).
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
Informed consent
Informed consent was obtained from the patient for publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Niles JL, Bottinger EP, Saurina GR, Kelly KJ, Pan G, Collins AB, et al. The syndrome of lung hemorrhage and nephritis is usually an ANCA-associated condition. Arch Intern Med. 1996;156(4):440–445. doi: 10.1001/archinte.1996.00440040118013. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 3.McAdoo SP, Tanna A, Hruskova Z, Holm L, Weiner M, Arulkumaran N, et al. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92(3):693–702. doi: 10.1016/j.kint.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutgers A, Slot M, van Paassen P, van Breda Vriesman P, Heeringa P, Tervaert JW. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis. 2005;46(2):253–262. doi: 10.1053/j.ajkd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66(4):1535–1540. doi: 10.1111/j.1523-1755.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 6.Short AK, Esnault VL, Lockwood CM. Anti-neutrophil cytoplasm antibodies and anti-glomerular basement membrane antibodies: two coexisting distinct autoreactivities detectable in patients with rapidly progressive glomerulonephritis. Am J Kidney Dis. 1995;26(3):439–445. doi: 10.1016/0272-6386(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Yang R, Cui Z, Chen M, Zhao MH, Wang HY. Characteristics and outcome of Chinese patients with both antineutrophil cytoplasmic antibody and antiglomerular basement membrane antibodies. Nephron Clin Pract. 2007;107(2):c56–62. doi: 10.1159/000107803. [DOI] [PubMed] [Google Scholar]
- 8.Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63(3):1164–1177. doi: 10.1046/j.1523-1755.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- 9.Alchi B, Griffiths M, Sivalingam M, Jayne D, Farrington K. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transpl. 2015;30(5):814–821. doi: 10.1093/ndt/gfu399. [DOI] [PubMed] [Google Scholar]
- 10.Zoysa DE, Jr., Taylor D, Thein H, Yehia M. Incidence and features of dual anti-GBM-positive and ANCA-positive patients. Nephrology (Carlton). 2011;16(8):725–9. doi: 10.1111/j.1440-1797.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- 11.Cui Z, Zhao J, Jia XY, Zhu SN, Zhao MH. Clinical features and outcomes of anti-glomerular basement membrane disease in older patients. Am J Kidney Dis. 2011;57(4):575–582. doi: 10.1053/j.ajkd.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Levy JB, Turner AN, Rees AJ, Pusey CD. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134(11):1033–1042. doi: 10.7326/0003-4819-134-11-200106050-00009. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava A, Rao GK, Segal PE, Shah M, Geetha D. Characteristics and outcome of crescentic glomerulonephritis in patients with both antineutrophil cytoplasmic antibody and anti-glomerular basement membrane antibody. Clin Rheumatol. 2013;32(9):1317–1322. doi: 10.1007/s10067-013-2268-5. [DOI] [PubMed] [Google Scholar]
- 14.Levy JB, Lachmann RH, Pusey CD. Recurrent goodpasture's disease. Am J Kidney Dis. 1996;27(4):573–578. doi: 10.1016/S0272-6386(96)90169-9. [DOI] [PubMed] [Google Scholar]
- 15.Serratrice J, Chiche L, Dussol B, Granel B, Daniel L, Jego-Desplat S, et al. Sequential development of perinuclear ANCA-associated vasculitis and anti-glomerular basement membrane glomerulonephritis. Am J Kidney Dis. 2004;43(3):e26–30. doi: 10.1053/j.ajkd.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher JL, Sinha S, Reeve R, Kalra PA. Importance of checking anti-glomerular basement membrane antibody status in patients with anti-neutrophil cytoplasmic antibody-positive vasculitis. Postgrad Med J. 2008;84(990):220–222. doi: 10.1136/pgmj.2007.062752. [DOI] [PubMed] [Google Scholar]
- 17.Yang R, Hellmark T, Zhao J, Cui Z, Segelmark M, Zhao MH, et al. Antigen and epitope specificity of anti-glomerular basement membrane antibodies in patients with Goodpasture disease with or without anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2007;18(4):1338–1343. doi: 10.1681/ASN.2006111210. [DOI] [PubMed] [Google Scholar]


