Abstract
McArdle disease (glycogen storage disease type V) is a rare hereditary metabolic myopathy. It can be overlooked clinically because it often presents as chronic asymptomatic hypercreatine phosphokinasemia (hyperCKemia). However, vigorous exercise or infections can trigger severe rhabdomyolysis. We present the case of a patient with long-term idiopathic hyperCKemia who, after contracting an upper respiratory tract infection, developed severe rhabdomyolysis and acute kidney injury. Upon hemodialysis, his renal function recovered and CK levels fell to below baseline, and maintenance therapy with vitamin B6 was also started. A molecular diagnosis of McArdle disease was subsequently made. Whole-exome sequencing revealed homozygous c1538delG (p.Asp511Thr fs*28) mutations in the PYGM gene, which was a novel mutation. Therefore, when investigating idiopathic hyperCKemia, glycogen storage disorders should also be considered.
Keywords: Acute kidney injury, Glycogen storage disease type V, Hypercreatine phosphokinase, McArdle disease, Rhabdomyolysis
Introduction
Patients with chronic asymptomatic hypercreatine phosphokinase (CK)emia do not present with severe symptoms and tend to be overlooked. In these cases of chronic or intermittent muscle destruction (presenting as hyperCKemia), patients usually present with few symptoms and no renal failure [1]. However, acute kidney injury (AKI) is a potential complication of severe rhabdomyolysis, regardless of the cause of rhabdomyolysis [1]. Additionally, if AKI occurs as a result of rhabdomyolysis, the prognosis of patients with hyperCKemia is substantially worse. Causes of rhabdomyolysis include common pathological conditions such as drug-induced hyperCKemia, but also genetic defects [1, 2], such as McArdle disease, which is also known as glycogen storage disease type V [2]. Our experience of treating a patient with McArdle disease and rhabdomyolysis is described below.
Case report
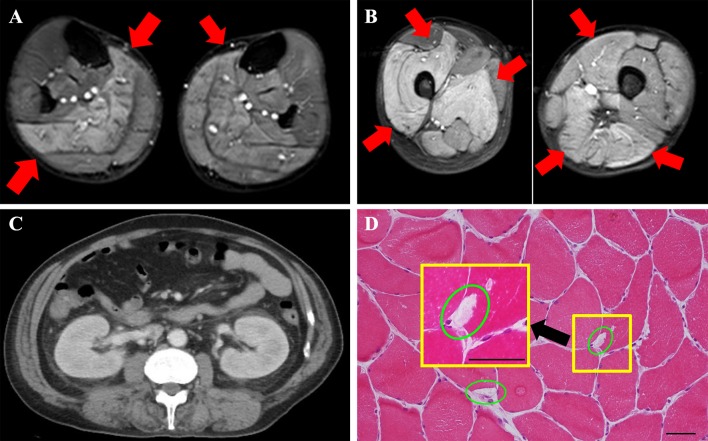
The patient was a 57-year-old man and his family history was unremarkable. He underwent further evaluation for hyperCKemia 7 years previously, but the cause remained unidentified. Based on this background, the cause of hyperCKemia was hypothesized to be drug-induced myopathy caused by antihypertensive medication (he was a hypertensive patient), and his prescription was adjusted. His CK concentration was between 2000 and 10,000 U/L while he was under observation, and he was unaware of any subjective symptoms. He displayed symptoms of an upper respiratory airway infection and elevated fever the day before he visited our hospital. However, the causative organism of this infection was not identified. After this, his movement gradually weakened in the extremities and he was finally unable to move, whereupon he was admitted to our hospital. The findings of blood examination at admission were marked hyperCKemia (298,940 U/L), hypermyoglobinemia (115,520 ng/mL), hyperpotassemia (7.5 mEq/L), and AKI (creatinine 3.19 mg/dL), and he was hospitalized for treatment (Table 1). When he was hospitalized, he was in an anuric state. There were no abnormal findings upon brain magnetic resonance imaging (MRI) examination. The cause of his inability to move was hypothesized to be rhabdomyolysis based on the remarkably high CKemia levels. MRI [Short-Tau Inversion Recovery (STIR)] imaging showed diffuse high signals in his bilateral thighs, which suggested myolysis (Fig. 1a, b red arrow). His kidney was enlarged (Fig. 1c, computed tomography examination), and it was hypothesized that AKI was a secondary condition resulting from myolysis. The day after admission, his CK level rose to 436,400 U/L and hemodialysis (HD) was started for AKI (creatinine, 6.72 mg/dL) and hyperpotassemia (6.3 mEq/L) resulting from rhabdomyolysis (Fig. 2). The patient underwent a total of seven HD treatments, and his myoglobin and CK improved to within the normal range. Ultimately, his urine volume was sufficiently secured and he was able to withdraw from HD therapy.
Table 1.
Laboratory results on admission
| Parameters | Value | Reference range |
|---|---|---|
| (Urine) | ||
| pH | 5.5 | 5.0–6.5 |
| Occult blood | 3+ | Negative |
| Red blood cell (/HPF) | 1–4 | < 5 |
| Granular casts (/WF) | 20–29 | Negative |
| Urine protein/creatinine ratio (g/g) | 3.14 | < 0.15 |
| Myoglobin (ng/mL) | 12,014 | < 10 |
| (Blood gas analysis, room air) | ||
| pH | 7.37 | 7.36–7.44 |
| PaCO2 | 24.0 | 36–44 |
| HCO3− | 13.5 | 22–26 |
| (Blood) | ||
| Leukocyte count (/µL) | 6100 | 4500–9000 |
| Hemoglobin (g/dL) | 18.6 | 13.6–17.0 |
| Platelet count (×104/µL) | 16.9 | 14–36 |
| Urea nitrogen (mg/dL) | 37.7 | 8.0–22.0 |
| Creatinine (mg/dL) | 3.19 | 0.60–1.10 |
| eGFR (mL/min/1.73 m2) | 17.1 | < 90 |
| Uric acid (mg/dL) | 5.2 | 3.6–7.0 |
| Albumin (g/dL) | 2.8 | 4.0–5.0 |
| Aspartate transaminase | 874 | < 35 |
| Alanine transaminase | 156 | < 40 |
| Lactate dehydrogenase (U/L) | 5371 | 119–229 |
| Creatine phosphokinase (U/L) | 298,940 | 38–196 |
| Myoglobin (ng/mL) | 115,520 | < 70 |
| Sodium (mEq/L) | 125 | 138–146 |
| Potassium (mEq/L) | 7.5 | 3.6–4.9 |
| Chloride (mEq/L) | 89 | 99–109 |
| Corrected serum calcium (mg/dL) | 6.9 | 8.6–10.4 |
| Phosphate (mg/dL) | 6.3 | 2.5–4.7 |
| C-reactive protein (mg/dL) | 11.61 | < 0.30 |
| Activity of muscle glycogen phosphorylase (µmol/min/g muscle tissue) | 2.7 | 58.9 ± 17.5 (mean ± SD) |
Fig. 1.
Findings upon radiological imaging and muscle biopsy. a Left upper extremity findings on MRI Short-Tau Inversion Recovery (STIR). MRI STIR imaging showed diffuse high signals in the bilateral thighs, which suggested myolysis (red arrow). b Left lower extremity findings on MRI STIR. MRI STIR imaging showed diffuse high signals in the bilateral thighs, which suggested myolysis (red arrow). c Computed tomography (CT) findings in the kidney. Swelling of the kidney was observed, but atrophy was not observed. No post-renal failure findings were observed. d Muscle biopsy tissue (hematoxylin and eosin stain). The scale bars are 100 µm (larger square) and 20 µm (smaller square). Muscle biopsy tissue showed almost normal findings. Vacuolated muscle fibers, a finding that suggests rhabdomyolysis, were present, although they were rare
Fig. 2.
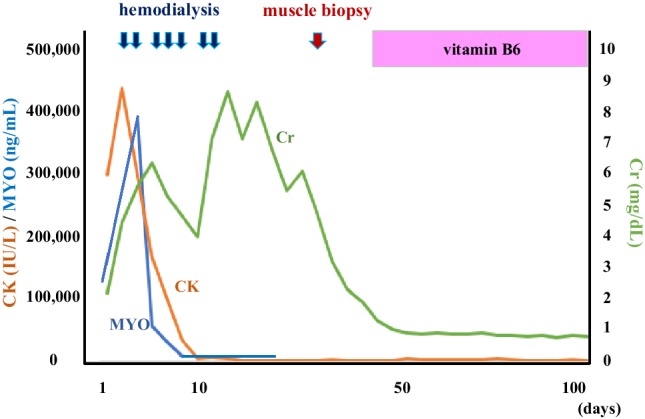
Clinical course. CK creatine phosphokinase, Cr creatinine, MYO myoglobin
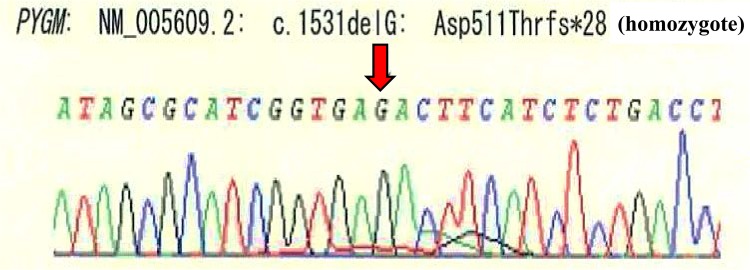
Because the cause of sustained muscle injury remained unknown, we considered the presence of a genetic disorder and performed a muscle biopsy on day 30 after admission. We performed a muscle biopsy from the muscles of the upper arm that showed abnormal findings on MRI. His muscle tissue magnitude discordance was mild and lymphocyte infiltration was not found in the tissue. In the muscle biopsy tissue, regenerating fiber necrosis was unclear. In the collected muscle tissue, vacuolated muscle fiber was found, which suggests that rhabdomyolysis was present, although it was slight (Fig. 1d green circle). We recalled glycogen storage diseases are diseases that are likely to cause this remarkable hyperCKemia. For this reason, simultaneous screening tests of filter paper blood centered on glycogen storage disease (especially muscle glycogenoses) were performed. This examination showed a remarkable lack of the enzyme. Because a definitive diagnosis of glycogenogenic disease, which is a differentiating disease for myolysis, is made based on enzyme activity of muscle glycogen phosphorylase using biopsy muscle tissue [3], we measured the patient’s enzyme activity. The activity of muscle glycogen phosphorylase was markedly decreased at 2.7 µmol/min/g muscle tissue [control muscle glycogen phosphorylase activity: 58.9 ± 17.5 (mean ± SD)]. Next, we performed gene analysis for mutations in the PYGM gene, which encodes the muscle-specific isoform of muscle glycogen phosphorylase. Whole-exome sequencing revealed homozygous c1538delG (p.Asp511Thr fs*28) mutations in the PYGM gene (Fig. 3). The mutation in the PYGM gene was a novel mutation. Based on the reduction in enzyme activity and the pattern of genetic mutation, it was confirmed that McArdle disease was the cause of his myolysis.
Fig. 3.
Gene analysis. Defect of G (guanine) ·frameshift mutation (red arrow)
After the final diagnosis, the patient was given 90 mg/day oral vitamin (vit) B6 supplements (pyridoxine hydrochloride) as a treatment based on previous treatment reports [4]. Treatment with vit B12 was started to maintain the renal function that had recovered following HD. Renal function fully recovered following this treatment and the CK level improved to approximately 1000–2000 U/L. He was discharged on day 110 after admission.
There was no family medical history of consanguinity where AKI occurred or where muscle weakness was conspicuous. However, his paternal grandparents and parents had consanguineous marriages (Fig. 3).
Discussion
Our patient had been followed for 7 years without identification of the cause of muscle injury (presenting as hyperCKemia). When he was in mortal danger from AKI and hyperpotassemia caused by rhabdomyolysis, the cause was finally diagnosed as McArdle disease. When differentiating between idiopathic muscle injury that presents as hyperCKemia, inborn errors of metabolism including glycogen storage diseases should be considered. In this case, a definite diagnosis of McArdle disease was confirmed by enzyme activity measurement and gene analysis of muscle biopsy tissue. The patient’s renal dysfunction resulting from rhabdomyolysis ultimately resolved fully after HD. The patient’s CK concentration was reduced after receiving oral vit B6 therapy and he was subsequently discharged from the hospital. He had a novel mutation, with no published report of this variant in the past. Therefore, we considered the variant in the PYGM gene to be a novel mutation because the p.Asp511Thr frameshift in the PYGM protein caused by other variants was already reported and in silico analysis also evaluated this variant as likely pathogenic.
Among glycogenogenic diseases, congenital metabolic disorders exhibiting muscle symptoms are called myogenic glycogen storage diseases. There are 11 hereditary disorders of glycogen metabolism that affect the muscle [5]. Brian McArdle reported the first patient in 1951, who presented with exercise-induced myalgia [6]. McArdle disease (glycogen storage disease type V) is a rare hereditary metabolic myopathy that is caused by a deficiency in muscle glycogen phosphorylase. This disease has an autosomal recessive mode of inheritance [7, 8]. Muscle glycogen phosphorylase exists in the muscle cell in association with vit B6, and most vit B6 in the body (85%) is pooled in skeletal muscle. The activity of muscle glycogen phosphorylase in patients with McArdle disease is below 10% of normal. Thus, secondary vit B6 deficiency is caused by this disease. The vit B6 content in muscle biopsy specimens from McArdle disease patients is decreased markedly [6]. Vit B6 is a co-factor for numerous enzymes that are involved in amino acid metabolism and neurotransmitter biosynthesis. Muscle glycogen phosphorylase also requires vit B6 as a cofactor. Therefore, vit B6 has been used as a therapeutic agent for McArdle disease [7]. In previous reports of patients with McArdle disease, muscle fatigue significantly decreased after several weeks of vit B6 supplementation [7]. Therefore, it is thought that vit B6 therapy for McArdle disease is important for asymptomatic hyperCKemia in the chronic phase. However, there is no report that vit B6 was effective for treating acute rhabdomyolysis. This is presumably because McArdle disease is rare and it infrequently causes rhabdomyolysis. Furthermore, it is possible that the diagnosis may not be in place in the acute phase when rhabdomyolysis develops.
The relationship between rhabdomyolysis and infection in our patient was considered based on a review on pathological conditions in metabolic myopathies, as described below [9]. The energy required for muscle contraction is supplied by adenosine triphosphate (ATP) and the glycolytic system is required for ATP production in muscle. However, in McArdle disease, there is a deficiency in muscle glycogen phosphorylase, which causes impaired glycogen degradation and results in decreased ATP production and tricarboxylic acid (TCA) cycle inhibition. Thus, there is insufficient ATP for skeletal muscle contraction, resulting in exercise intolerance, painful muscle spasms during exercise, and CK elevation. Rhabdomyolysis is a symptom of exercise intolerance caused by ATP depletion in McArdle disease. Therefore, McArdle disease shows muscle symptoms caused by ATP depletion in the muscle. The symptoms of muscle results from substrate use disorder for ATP production. Although muscle injury is frequent in McArdle disease, massive rhabdomyolysis with severe AKI has rarely been reported [10]. However, in the absence of muscle glycogen phosphorylase activity, a history of vigorous physical exercise or infections can trigger more severe rhabdomyolysis [11, 12], as in our patient. Our patient showed constant mild muscle injury, although it was asymptomatic. However, he had an upper respiratory airway infection and a fever for a few days before being admitted to our hospital. We speculate that, in our patient, infection and fever were involved as factors that promoted depletion of ATP. We hypothesized that this infection caused his severe muscle symptoms and induced rhabdomyolysis.
His family history was unclear because, to the best of the patient’s knowledge, there were no family members whose symptoms had become serious before. Therefore, we speculated that they may have only been carriers of the mutation, because they had a full life span. However, his paternal grandparents and parents had consanguineous marriages. Because compound heterozygous PYGM mutations are relatively common [13], this consanguineous marriage may explain why this novel homozygous mutation occurred. Additionally, his muscle biopsy tissue showed almost normal findings. Vacuolated muscle fibers, a finding that suggests rhabdomyolysis, were confirmed, although it was rare. This suggests the following: first, it was conceivable that the collected tissues were not from injured areas, and second, that the muscle organization did not exhibit any abnormality because the biopsy was taken too early. In severe rhabdomyolysis, at least several weeks should pass after the clinical event before performing a muscle biopsy because the muscle biopsy findings will be uninformative at an early stage, when the tissue may show no specific findings other than necrosis [1]. There was a previous report that vit B6 status and muscle performance may be linked in McArdle disease and that there is the potential for enhancement of muscle performance with vit B6 supplementation [7]. Another study suggests that the ingestion of sucrose before exercise can markedly improve exercise tolerance in patients with McArdle disease [14]. However, effective treatment methods for this disease have yet to be clarified. The patient benefited from HD treatment with a progressive and complete recovery of his renal function after seven HD treatments. Additionally, his CK remained low after taking vit B6 orally.
Rhabdomyolysis is diagnosed based on increased muscle cell content in the blood, including myoglobin, creatine kinase, potassium, and various sarcoplasmic proteins [1]. The risk of AKI in rhabdomyolysis is usually low when CK levels at admission are less than 15,000–20,000 U/L [15]. However, in this case, the patient had AKI induced by rhabdomyolysis because his peak CK concentration was 436,400 U/L.
The primary feature of managing rhabdomyolysis-induced AKI is the initial, aggressive replenishment of body fluids. Patients often require approximately 10 L of fluid per day, although the infusion dose is adjusted based on the severity of rhabdomyolysis [1]. There was no indication for administering sodium bicarbonate, which results in alkaline urine [1]. There was also no indication to use therapeutic strategies involving alkalinization, mannitol, or diuretics, which are more empirical approaches [1]. The techniques and devices used for classic dialytic techniques do not remove myoglobin effectively because of the size of the protein [16], and it is recommended that dialysis therapy should be a renal indication (treatment of refractory hyperkalemia, acidosis, or volume overload) [1]. However, a previous report showed that using a super-high-flux membrane for continuous hemofiltration achieved much greater myoglobin clearance compared with conventional hemofiltration [17]. However, this device is unfortunately not available for use in Japan.
Conclusion
Herein, we investigated idiopathic hyperCKemia, and congenital metabolic disorders, such as McArdle disease, that should be kept in mind. Additionally, when a patient presents with rhabdomyolysis, it is necessary to perform a large-volume fluid infusion or dialysis as a renal indication. Finally, this case showed a novel compound heterozygous mutation in PYGM in a patient with McArdle disease.
Acknowledgements
We thank Takashi Kurashige (Department of Neurology, National Hospital Organization Kure Medical Center) for performing the muscle biopsy on our patient. We thank Dr. Ichizo Nishino (National Institute of Neuroscience, National Center of Neurology and Psychiatry) for evaluating enzymatic activities. We thank Simon Teteris, PhD, from the Edanz Group (http://www.edanzediting.com/ac), for editing the English text of a draft of this manuscript.
Author contributions
All authors treated the patient and made decisions about the patient’s examinations and therapies. AS and SH reviewed previous publications and drafted the manuscript. TM supervised the manuscript. AS, SH, TA, YY, and TI were the patient’s principal physicians and treated him as an inpatient and outpatient. HI, AM, and SY drafted the diagnostic direction from the perspective of a neurologist, which led to a definite diagnosis. All authors contributed to the interpretation of the etiology and discussion. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Human and animal rights
This article does not contain studies with human or animal participants.
Informed consent
Written consent to publish this information was obtained from the patient.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayaka Satoh, Email: a-satoh@umin.ac.jp.
Shuma Hirashio, Email: shumah@hiroshima-u.ac.jp.
Takahiro Arima, Email: arima1120takahiro@yahoo.co.jp.
Yumi Yamada, Email: yamaday0uu@gmail.com.
Taisuke Irifuku, Email: t.irifuku@gmail.com.
Haruka Ishibashi, Email: harutan@mail.goo.ne.jp.
Atsuko Motoda, Email: amoto@hiroshima-u.ac.jp.
Yoshimasa Sueda, Email: sueda_yoshimasa@hiro-hosp.jp.
Takao Masaki, Phone: +81-82-257-1506, Email: masakit@hiroshima-u.ac.jp.
References
- 1.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico A, Bertini E. Metabolic neuropathies and myopathies. Handb Clin Neurol. 2013;113:1437–1455. doi: 10.1016/B978-0-444-59565-2.00013-7. [DOI] [PubMed] [Google Scholar]
- 3.Burke J, Hwang P, Anderson L, Lebo R, Gorin F, Fletterick R. Intron/exon structure of the human gene for the muscle isozyme of glycogen phosphorylase. Proteins. 1987;2:77–187. doi: 10.1002/prot.340020303. [DOI] [PubMed] [Google Scholar]
- 4.Izumi R, Suzuki N, Kato K, Warita H, Tateyama M, Nakashima I, Itoyama Y. A case of McArdle disease: efficacy of vitamin B6 on fatigability and impaired glycogenolysis. Intern Med. 2010;49:1623–1625. doi: 10.2169/internalmedicine.49.3525. [DOI] [PubMed] [Google Scholar]
- 5.DiMauro S, Lamperti C. Muscle glycogenoses. Muscle Nerve. 2001;24:984–999. doi: 10.1002/mus.1103. [DOI] [PubMed] [Google Scholar]
- 6.McArdle B. Myopathy due to a defect in muscle glycogen breakdown. Clin Sci. 1951;1:13–35. [PubMed] [Google Scholar]
- 7.Phoenix J, Hopkins P, Bartram C, Beynon RJ, Quinlivan RC, Edwards RH. Effect of vitamin B6 supplementation in McArdle’s disease: a strategic case study. Neuromuscul Disord. 1998;8:210–212. doi: 10.1016/S0960-8966(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 8.Lebo RV, Gorin F, Fletterick RJ, Kao FT, Cheung MC, Bruce BD, Kan YW. High-resolution chromosome sorting and DNA spot-blot analysis assign McArdle’s syndrome in chromosome 11. Science. 1984;225:57–59. doi: 10.1126/science.6587566. [DOI] [PubMed] [Google Scholar]
- 9.DiMauro S, Garone C, Naini A. Metabolic myopathies. Curr Rheumatol Rep. 2010;12:386–393. doi: 10.1007/s11926-010-0119-9. [DOI] [PubMed] [Google Scholar]
- 10.Fikri-Benbrahim O, Cazalla-Cadenas F, Vadillo-Bemejo A. From acute renal failure to the diagnosis of McArdle’s disease. Nefrologia. 2013;33:605–606. doi: 10.3265/Nefrologia.pre2013.Feb.11885. [DOI] [PubMed] [Google Scholar]
- 11.Loupy A, Pouchot J, Hertig A, Bonnard G, Bouvard E, Rondeau E. Massive rhabdomyolysis revealing a McArdle disease. Rev Med Interne. 2007;28:501–503. doi: 10.1016/j.revmed.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Kranck S, Wacker P, Milliet N, Brünisholz M. One rare case report of acute renal insufficiency in rhabdomyolysis. Rev Med Suisse Romande. 2002;122:539–541. [PubMed] [Google Scholar]
- 13.Park HJ, Shin HY, Cho YN, Kim SM, Choi YC. The significance of clinical and laboratory features in the diagnosis of glycogen storage disease type v: a case report. J Korean Med Sci. 2014;29:1021–1024. doi: 10.3346/jkms.2014.29.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vissing J, Haller RG. The effect of oral sucrose on exercise tolerance in patients with McArdle’s disease. N Engl J Med. 2003;349:2503–2509. doi: 10.1056/NEJMoa031836. [DOI] [PubMed] [Google Scholar]
- 15.Hatamizadeh P, Najafi I, Vanholder R, Rashid-Farokhi F, Sanadgol H, Seyrafian S, Mooraki A, Atabak S, Samimagham H, Pourfarziani V, Broumand B, Van Biesen W, Lameire N. Epidemiologic aspects of the Bam earthquake in Iran: the nephrologic perspective. Am J Kidney Dis. 2006;47:428–438. doi: 10.1053/j.ajkd.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Ronco C. Extracorporeal therapies in acute rhabdomyolysis and myoglobin clearance. Crit Care. 2005;2:141–142. doi: 10.1186/cc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naka T, Jones D, Baldwin I, Fealy N, Bates S, Goehl H, Morgera S, Neumayer HH, Bellomo R. Myoglobin clearance by super high-flux hemofiltration in a case of severe rhabdomyolysis: a case report. Crit Care. 2005;9:R90–R95. doi: 10.1186/cc3034. [DOI] [PMC free article] [PubMed] [Google Scholar]