Abstract
Crystal-storing histiocytosis (CSH) is a rare disorder characterized by the accumulation of nonneoplastic histiocytes containing intracytoplasmic crystallized immunoglobulins. In most cases, there is an associated underlying lymphoplasmacytic neoplasm expressing Ig kappa light chain. About 131 cases of CSH have been identified. There is a localized and a generalized form of CSH and it can involve several sites including bone marrow, lungs, lymph nodes, liver, spleen, gastrointestinal tract, and kidney. Generalized CSH is less frequent and involves multiple organs and tends to have a worst prognosis than localized CSH. Around 20 cases of renal involvement in CSH have been reported so far. Paraprotein-induced crystalline nephropathy can be divided into two categories based on whether the crystals in the kidney are intracellular (including light chain proximal tubulopathy with crystals and CSH) or extracellular (including the crystalline variant of myeloma cast nephropathy and crystalglobulin-induced nephropathy). The former tends to present with slowly worsening kidney dysfunction and generally has a good prognosis, whereas the latter usually presents with rapidly progressive renal failure and is associated with poor renal outcome. We present a case of generalized CSH associated with extracellular crystalline nephropathy with a fulminant and fatal clinical course. Kappa light-chain crystals were found exclusively extracellularly within the tubular lumen, not within the tubular epithelial cells nor the histiocytes, and the massive presence of those precipitates led to the acute renal failure. Consequently, we review the renal involvement in CSH in the literature and discuss the unique mechanism of renal injury in this case.
Keywords: Crystal-storing histiocytosis, Crystalline nephropathy, Histiocytes, Kappa light chains, Kidney
Introduction
Crystal-storing histiocytosis (CSH) is a rare disorder characterized by the accumulation of nonneoplastic histiocytes containing intracytoplasmic refractile eosinophilic crystals, representing most often a lysosomal accumulation of crystallized immunoglobulin (Ig). About 131 cases of CSH have been described so far. In the majority of cases (76–90%), there is an associated underlying lymphoplasmacytic neoplasm expressing Ig kappa light chain (LC) without any association with a specific type of Ig heavy chain. In the remaining 10–34% of cases, CSH is associated with other conditions, including Fanconi syndrome, autoimmune disorders, reactive inflammatory conditions, metabolic disorders, and drugs (e.g. clofazimine) [1]. CSH can involve several sites including bone marrow (BM), lungs, kidney, lymph nodes, liver, spleen, and gastrointestinal tract. There is a localized form of CSH (58–82% of cases) that is confined to a single organ. Generalized CSH (18–42% of cases) involves multiple organ sites and tends to have a worst prognosis than those with localized CSH [1, 2]. The intracytoplasmic crystals are composed of Ig light and/or heavy chain fragments enriched in Ig variable regions [3]. Paraprotein-induced crystalline nephropathy can be divided into two categories based on whether the crystals in the kidney are intracellular (including light chain proximal tubulopathy with crystals and CSH) or extracellular (including the crystalline variant of myeloma cast nephropathy and crystalglobulin-induced nephropathy). The latter usually presents with rapidly progressive renal failure and is associated with poor renal outcome [4].
We present a case of generalized CSH associated with extracellular crystalline nephropathy with a fulminant and fatal clinical course. Consequently, we review the renal involvement in CSH in the literature and discuss the unique mechanism of renal injury in this case.
Case report
A 65-year-old male had a history of myelodysplastic syndrome (MDS) with multilineage dysplasia, lacking excess blasts or cytogenetic abnormalities diagnosed at age 50. The patient was classified as low-risk according to both the Revised International Prognostic Scoring System and the World Health Organization Classification-Based Prognosis Scoring System and was treated with periodical transfusion of red cell concentrates (RCC). Bone marrow (BM) biopsy performed 10 months before admission showed 78% of ring sideroblasts and 2% of blasts, without cytogenetic abnormalities. Six weeks before admission, the patient was treated with transurethral resection of bladder tumor, confirming a noninvasive papillary urothelial tumor (pTa).
The patient presented to the emergency room with a 24-h history of vomitting, diarrhea, left flank pain, and shivers without fever. Physical examination revealed BP 95/68 mmHg, HR 99 bpm, temperature 36.9 °C, pale conjunctivae, and a subcentimetric paraumbilical subcutaneous abscess. Blood tests showed hemoglobin 6.7 g/dL, leukocytes 43,000/µL (neutrophils 30,000/µL, lymphocytes 11,200/µL), platelets 47,000/µL, INR 1.24, fibrinogen 290 mg/dL, lactate dehydrogenase (LDH) 802 U/L, creatinine 1.95 mg/dL, estimated glomerular filtration rate (eGFR) 35 mL/min/1.73 m2, calcium 8 mg/dL, C-reactive protein 0.1 mg/dL, and the remainder was unremarkable. Vitamin B12 and folic acid levels were within normal range. Urine test showed 10–20 erythrocytes/high-powered field (hpf), 10–20 leucocytes/hpf, negative nitrite, proteins 100 mg/dL. Peripheral blood (PB) smear demonstrated marked neutrophilia and dysplastic features that were compatible with MDS. Antibiotic treatment with cefepime was initiated and the abscess was drained.
One week after admission, the patient presented a poor evolution with persistent leukocytosis with neutrophilia, refractory anemia despite daily transfusion of 3 RCC, and progressive thrombocytopenia with a nadir of 15,000 platelets/µL. Blood tests also demonstrated high ferritin levels with a maximum level of 83,503 µg/L (ferritin levels were 1200 µg/L 1 month before admission), increased LDH with a maximum level of 2151 U/L, decreased fibrinogen with a nadir of 220 mg/dL, and elevated triglyceride levels (391 mg/dL). Plasmatic haptoglobin levels were decreased (< 10 mg/dL) and the direct and indirect Coombs tests were negative. Adamts13 activity was normal. Hemophagocytic lymphohistiocytosis (HLH) was suspected. A BM biopsy revealed hypercellular marrow and showed plenty of CD68 + histiocytes with intracytoplasmic erythrocytes and iron deposits. The erythroid cell line was deeply decreased and 7% of blasts were detected without other abnormalities. The BM evaluation was consistent with the diagnosis of HLH and MDS. Consequently, infectious causes were ruled out: the subcutaneous abscess had been solved; blood, urine, and stool cultures were sterile, as well as serologic tests for bacterial, viral, fungal, and parasitic infections; interferon-gamma release assays were negative. In addition, autoimmune and tumoral causes were also ruled out: a thoracic, abdominal and pelvic computed tomography (CT) did not show tumors, osteolytic lesions, or lymph node enlargement, and showed hepatosplenomegaly and abundant ascites; tumoral biomarkers, auto-antibodies, rheumatoid factor, and cold globulins were negative. Lymphocytic genotype was normal in PB. Complement C3 levels were within normal range and complement C4 was decreased (19.7 mg/dL). Plasmatic Ig G and Ig A were decreased (343 mg/dL and 46 mg/dL, respectively) with Ig M levels within normal range. Serum kappa free LCs were slightly elevated (3.32 mg/dL, normal range 0.3–1.9 mg/dL), and the kappa/lambda ratio on PB was normal.
Six days later, renal function worsened presenting oligoanuria. Urinalysis showed macroscopic non-glomerular hematuria and the urine albumin/creatinine ratio was 700 mg/g. Twenty-four hours later, renal function drastically worsened with anuria and creatinine levels of 7.6 mg/dL and eFGR < 15 mL/min/1.73 m2. Empirical treatment with dexamethasone (10 mg/m2/day) and a single dose of 500 mg of intravenous cyclophosphamide was started and leukocytosis returned to normal levels. However, anemia and thrombocytopenia remained unchanged, and renal function did not improve, leading to hemodialysis 2 days later. One week later the patient died from a gastrointestinal bleeding. A post-mortem pathologic study was performed.
Autopsy findings
Autopsy of the patient revealed an enlarged spleen (1500 g) and liver (2910 g). No enlarged lymph nodes were found.
Light microscopy revealed marked hepatic sinusoidal dilatation and presence of extramedullary hematopoiesis, with focal periportal lymphoid infiltrate without fibrosis. Hepatic macrophages showed iron overload. BM was markedly hyperplastic secondary to erythroid and granulocytic lines. Lymphoid or plasma cell infiltration was not observed. Hemophagocytic phenomenon was not observed. Splenic hematopoiesis was also found. A diffuse infiltration of histiocytes with large cytoplasm and intracytoplasmic crystalloid inclusions was observed into the BM, liver, lymph nodes, and splenic insterstitium (Fig. 1). Renal parenchyma was notably autolytic, with mild glomerular mesangial expansion and marked interstitial edema without inflammatory infiltration. Numerous large needle-shaped or rhomboid-like crystals were found in the tubular lumen. Crystals showed negative birefringence with polarized light (Fig. 2). Urinary bladder did not show evidence of malignancy.
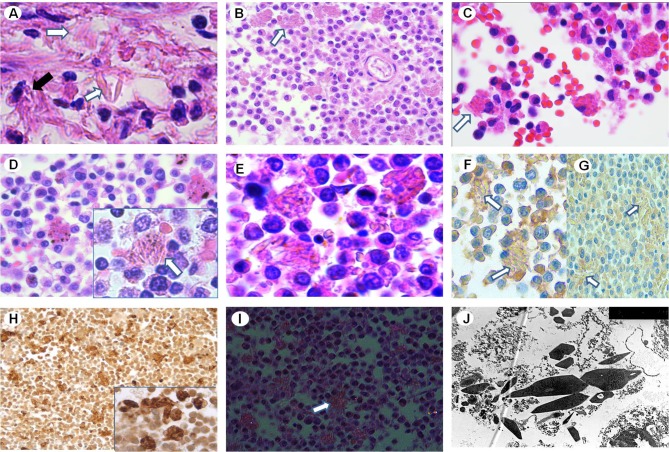
Fig. 1.
a Liver’s histiocytes showing intracytoplasmic crystalloid inclusions (black arrow) and extracellular crystals (white arrows) within periportal space (H–E staining × 10 000). Interstitial diffuse infiltration of histiocytes with intracytoplasmic crystalloid inclusions (arrows) in splenic parenchyma (b), BM (c) and lymph node (d) (b–d H–E staining × 4000). e Higher magnification of histiocytes in splenic parenchyma (H–E staining × 6000). Immunohistochemistry shows negative reactivity of the histiocytes (arrows) for kappa (f) and lambda (g) light chains (f, g × 4000). h Positive reactivity for CD68 in histiocytes of BM (× 4000). i Crystals (arrow) show negative birefringence under polarized light (× 2000). j Ultrastructural examination of the spleen shows intracytoplasmic electron dense hexagonal-like crystals, without periodic organization of the substructure (× 25,000)
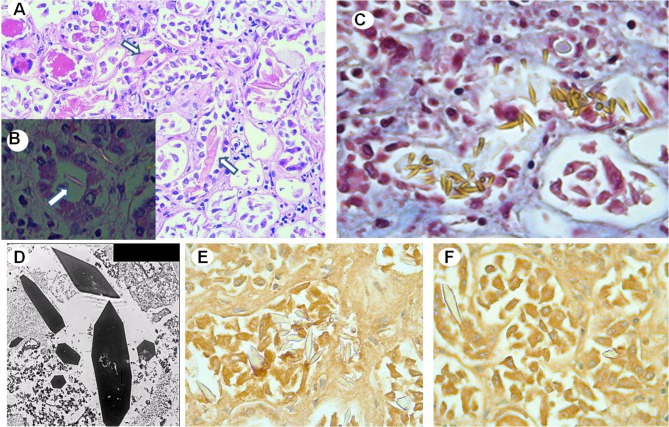
Fig. 2.
a Renal tissue shows intratubular large needle-shaped or rhomboid-like crystals (arrows) (H–E staining × 2000). b Intratubular crystals (arrow) show negative birefringence under polarized light (× 4000). c Crystal stain strongly yellow with the Masson’s trichrome stain. d Transmission electron microscopy microphotograph showing geometrically shaped crystals without periodic organization within the tubular lumen (× 25,000). Immunohistochemistry shows negative reactivity of crystals for kappa (e) and lambda (f) light chains (× 4000)
Previous BM biopsies were reassessed. BM biopsy performed 10 months before did not show histiocytes with intracytoplasmic crystalline deposit. However, crystalline material inclusions in the histiocytes were identified after reviewing the BM biopsy performed during hospitalization, that was erroneously identified as HLH.
Immunohistochemistry
Immunohistochemistry was performed in crystal-storing histiocytes and intratubular crystals of kidney. Immunohistochemistry and immunofluorescence microscopy for light and heavy chains were performed from paraffin sections and from paraffin and frozen sections, respectively. The histiocytes with intracytoplasmic crystals were positive for CD68 (Fig. 1). Immunohistochemically, the crystals were negative for Ig and LC (Fig. 1). Direct immunofluorescence tests performed from frozen sections were negative for heavy chains, complement, and LC. Immunofluorescence performed on formalin-fixed, paraffin-embedded, pronase-treated sections was negative. Congo red was also negative. Hemophagocytic phenomenon was not observed.
Electron microscopy
Ultrastructural examination revealed abundant intracytoplasmic electron dense rhomboid-like or hexagonal-like crystals within histiocytes of the liver, spleen, BM, and lymph nodes. Crystals of kidney tubular lumen were also massive and were electron dense, without periodic organization of the substructure (Figs. 1, 2).
Protein analysis
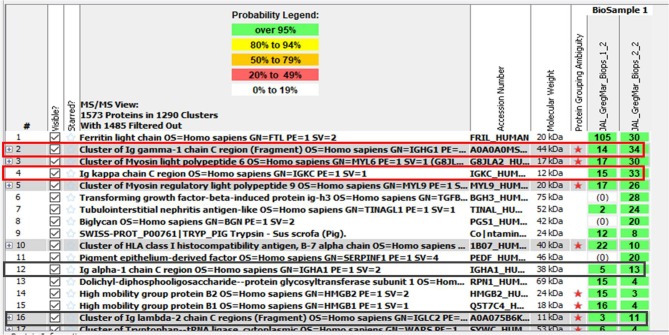
Mass spectrometric proteomic analysis was performed in spleen and kidney tissue. It was not possible to perform microdissection of crystal component. Both samples demonstrated fragments of Ig heavy chain constant region (IgG) and fragments of kappa LC constant region (Fig. 3).
Fig. 3.
Mass spectrometric proteomic analysis listing the proteins identified in the spleen and kidney tissue. Biops 1–2 represent peptides detected in the spleen. Biops 2–2 represent peptides detected in then kidney. Fragments of IgG heavy chain constant region and fragments of kappa light chain constant region were detected in both tissues
The final diagnosis was generalized crystal-storing histiocytosis in the BM, liver, and lymph nodes, associated with extracellular kappa light chains crystalline nephropathy.
Discussion
CSH is a rare disorder characterized by the intra-lysosomal accumulation of crystals composed of Ig light and/or heavy chain fragments enriched in Ig variable regions within histiocytes [1, 3].
Epidemiology
A PubMed search of the English literature, from 1987 to 2017, identified 23 cases of generalized-CSH out of a total of 131 CSH cases [1]. The average age at diagnosis was 54–60 years, with a nearly equal male:female distribution.
Etiology
76–90% of cases are associated with disorders that express monoclonal immunoglobulins, such as lymphoproliferative or plasma cell disorders. In most cases, the crystalline material within the histiocytes is kappa LC monoclonal protein without a consistent affiliation with any specific heavy chain. In the remaining 10–24% of cases, CSH is associated with other conditions, including clofazimine, systemic mastocytosis, rheumatoid arthritis, plasma cell granuloma or other conditions. 1.2% of cases are not associated with any other diseases. In some cases, the crystalline material is not an immunoglobulin, as it occurs with clofazimine-induced CSH, Charcot–Leyden crystal-associated CSH, and CSH associated with hereditary cystinosis [1, 2].
Pathophysiology
The exact mechanism for crystal formation in CSH has not been fully elucitaded and may involve multiple factors, including simple overproduction, abnormal secretion or impaired excretion of immunoglobulins. But some authors have reported minimal or no serum or urine paraprotein levels [5–8], as in our case. Thus, more likely than simple overproduction it seems that stored paraproteins have sequence abnormalities at specific sites that promote crystallization or adversely affect intralysosomal degradation or both [9]. Apparently, crystal formation is more related to kappa LCs rather than to a specific heavy chain [2] and this suggests that the crystal formation is determined by the type of LC molecule that might be abnormal. That hypothesis was supported by the molecular detection of unusual amino acid substitutions in the variable region of the stored kappa LC [9]. Those sequence alterations may impart changes in the Ig configuration that can potentially lead to protein crystallization and resistance or altered lysosomal degradation within the histiocytes, thereby leading to crystal accumulation. Alternatively, patients may have inherited or acquired histiocyte processing defects that result in Ig crystal formation [3]. CSH occurring in some patients with proximal renal tubular dysfunction (Fanconi syndrome) does add credence to the hypothesis that in some instances CSH may be due to decreased excretion of Ig [2].
Pathology and differential diagnosis
Some cases have been detected primarily in extramedullary sites; however, the involvement of the BM has been described in 22% and 70% of localized and generalized cases, respectively [1]. Because of the rarity of the disease, the accumulation of crystal-storing histiocytes in the BM often presents diagnostic difficulties [9]. Conditions mimicking CSH include hemophagocytic lymphohistiocytic syndrome, storage diseases such as Gaucher’s, mycobacterial and fungal infections, xanthogranuloma, Langerhans histiocytosis, fibrous histiocytoma, rhabdomyoma, granular cell tumor, oncocytic neoplasms, and other conditions [3]. Similarly, pathologists should be aware that an underlying plasma cell neoplasm or B-cell lymphoma could be masked by the histiocytic infiltrates [1]. Macrophages in CSH very often present with membrane-bound electron-dense rhomboid, hexagonal, or more needle-like crystal profiles in the cytoplasm. At higher magnification these crystals often show a periodic organization of the crystalline substructure [9]. Lymphoplasmacytic infiltrates are seen accompanying the histiocytic infiltrate. These histiocytes are strongly positive for CD68 and CD163 and are negative for desmin, muscle-specific actin, myoglobin, S100, and CD1a. The crystals stain positive with phosphotungstic acid hematoxylin and are variably positive with the periodic acid-Schiff (PAS) stain. They are usually not birefringent and nonpolarizable. The crystals may be monoclonal, polyclonal, or may not stain with immunohistochemical markers for immunoglobulins. Ultrastructurally, the crystals are membrane-bound dense, elongated, rectangular, or rhomboid crystals within histiocytic cytoplasm. On immunohistochemical analysis, the crystals are typically monoclonal but in some instances may be polyclonal or even fail to stain. Failure of the immunoglobulin crystals to stain may be due to the following: suboptimal tissue fixation, antigen masking resulting from the crystalline structure of the protein, altered molecular configuration of the protein with decreased antigenicity, or the fact that the crystals are truly not of immunoglobulin origin and represent one of the other CSH variants [2]. In a literature review that found 80 cases of CSH, the immunoprofile was often not indicated. The specific type of heavy chain was mentioned in only 37 cases, and of these, 14 were IgM, 10 IgG, 6 IgA and 7 polyclonal. The LC component was documented in 51 cases, and of these, 33 were kappa, 8 lambda, and 10 polyclonal [2].
Localized and generalized forms
Crystals can be formed in either histiocytes in soft tissues or parenchymal cells. The most frequently affected organs are BM, lung/pleura, kidney, lymph node, gastrointestinal tract, head, and neck. The initial clinical presentation depends on the site of crystal formation and is, therefore, varied. Some patients present with soft tissue masses in which not only histiocytes, but also fibroblastic cells, contain crystals [1, 10]. There is a localized form of CSH (58–82% of cases) that is confined to a single organ. Generalized CSH involves multiple organ sites and is characterized by being less frequent (18–42% of cases), involving BM more frequently and having a worst prognosis [1, 2, 9].
Renal involvement
In the present case, the identification of kappa LCs within histiocytes led us to consider the association of an underlying plasma cell disorder such as multiple myeloma (MM), one of the conditions most typically associated with CSH. However, the patient did not show hypercalcemia, osteolytic lesions, clonal plasma cells in BM biopsy nor a serum M-spike. On the other hand, the patient presented a kappa LC deposit in the lumen of renal tubule, resembling what happens in MM. MM can cause renal injury through paraprotein or nonparaprotein mechanisms [4, 11–13]. Paraprotein-induced crystalline nephropathy can be divided into two categories based on whether the crystals in the kidney are intracellular (including the light chain proximal tubulopathy [LCPT] with crystals or “light chain Fanconi syndrome” and CSH) or extracellular (including the crystalline variant of myeloma cast nephropathy (MCN) and crystalglobulin-induced nephropathy). The former tends to present with slowly worsening kidney dysfunction and generally has a good prognosis, whereas the latter usually presents with rapidly progressive renal failure and associates poor renal outcome [4, 11–13]. About 20 cases have been reported of renal involvement in CSH up to the date [1]. In LCPT and CSH, these reabsorbed LCs are typically of kappa type and possess innate physicochemical properties that resist proteolysis and promote selfaggregation and crystal formation [11]. Our patient exhibited autolytic renal parenchyma, with mild glomerular mesangial expansion in the glomerulus and marked interstitial edema without inflammatory infiltration. Kappa LC crystalline inclusions were found exclusively extracellularly within the lumen of renal tubule, not within the tubular epithelial cells nor the histiocytes, and the massive presence of those precipitates could lead to the acute renal failure. However, the exact cause of the renal failure in our patient is difficult to determine. In addition, the overproduction mechanism that would explain the massive presence of kappa LC crystalline inclusions within the lumen of renal tubule was not present: in the setting of a sudden acute renal failure, the patient did not previously show serum M-spike and the free LC concentrations were not high enough to overwhelm the reabsorption capacity of proximal tubules, as usually happens in MCN.
Treatment and prognosis
Our patient’s prognosis was marked by the presence of anemia and thrombocytopenia due to both CSH and MDS and the presence of acute renal failure. The kidney is the third most frequent organ affected in CSH. However, the prognosis of CSH with renal involvement remains unclear [1, 11]. Treatment and prognosis of patients with CSH vary according to the associated disease [2]. Generalized forms are often associated with a rapid, fatal clinical course [5]. Interestingly, many myeloma patients with CSH have reported longer survival than the median survival for myeloma in general. A likely explanation for this difference is diagnosis at an earlier stage of disease in CSH-myeloma patients, due to the associated renal failure, organomegaly or keratopathy. In the absence of renal failure, the additional finding of CSH does not appear to be associated with a poorer prognosis for myeloma patients [12, 13].
In conclusion, in the setting of CSH, the kidney may be involved with crystal deposition in the epithelial cells of the glomerulus and tubules, in histiocytes or plasma cells in the interstitium or in the mesangium. This is the first case of generalized CSH with exclusively extracellular kappa LC crystalline depositions within tubular lumen.
Acknowledgements
We are grateful to the patient and his family because they allowed us to perform the post-mortem study.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Informed consent
Written informed consent was obtained from the patient’s family for publication of this study.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fang H, Chiu A, Reichard KK. Crystal-storing histiocytosis in bone marrow: a clinicopathologic study of eight cases and review of the literature. Am J Clin Pathol. 2018;149:148–163. doi: 10.1093/ajcp/aqx150. [DOI] [PubMed] [Google Scholar]
- 2.Dogan S, Barnes L, Cruz-Vetrano WP. Crystal-storing histiocytosis: report of a case, review of the literature (80 cases) and a proposed classification. Head Neck Pathol. 2012;6:111–120. doi: 10.1007/s12105-011-0326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanagal-Shamanna R, Xu-Monette ZY, Miranda RN, Dogan A, Zou D, Luthra R, Weber DM, O’Malley DP, Jorgensen JL, Khoury JD, Bueso-Ramos CE, Orlowski RZ, Medeiros LJ, Young KH. Crystal-storing histiocytosis: a clinicopathological study of 13 cases. Histopathology. 2016;68:482–491. doi: 10.1111/his.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta V, El Ters M, Kashani K, Leung N, Nasr SH. Crystalglobulin-induced nephropathy. J Am Soc Nephrol. 2015;26:525–529. doi: 10.1681/ASN.2014050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones D, Bhatia VK, Krausz T, Pinkus GS. Crystal-storing histiocytosis: a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol. 1999;30:1441–1448. doi: 10.1016/S0046-8177(99)90166-1. [DOI] [PubMed] [Google Scholar]
- 6.Kapadia SB, Enzinger FM, Heffner DK, Hyams VJ, Frizzera G. Crystal-storing histiocytosis associated with lymphoplasmacytic neoplasms. Report of three cases mimicking adult rhabdomyoma. Am J Surg Pathol. 1993;17:461–467. doi: 10.1097/00000478-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Padmalatha C, Warner TF, Hafez GR. Pseudo-Gaucher cell in IgMk plasmacytoid lymphoma. Am J Surg Pathol. 1981;5:501–505. doi: 10.1097/00000478-198107000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Thorson P, Hess JL. Transformation of monocytoid B-cell lymphoma to large cell lymphoma associated with crystal-storing histiocytes. Arch Pathol Lab Med. 2000;124:460–462. doi: 10.5858/2000-124-0460-TOMBCL. [DOI] [PubMed] [Google Scholar]
- 9.Lebeau A, et al. Generalized crystal-storing histiocytosis associated with monoclonal gammopathy: molecular analysis of a disorder with rapid clinical course and review of the literature. Blood. 2002;100:1817–1827. [PubMed] [Google Scholar]
- 10.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108:2520–2530. doi: 10.1182/blood-2006-03-001164. [DOI] [PubMed] [Google Scholar]
- 11.Wu CK, Yang AH, Lai HC, Lin BS. Combined proximal tubulopathy, crystal-storing histiocytosis, and cast nephropathy in a patient with light chain multiple myeloma. BMC Nephrol. 2017;18:170. doi: 10.1186/s12882-017-0584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitman SD, Wang J, Serros ER, Zuppan C. A 70-year-old woman with acute renal failure. Crystal-storing histiocytosis. Arch Pathol Lab Med. 2006;130:1077–1078. doi: 10.5858/2006-130-1077-AYWWAR. [DOI] [PubMed] [Google Scholar]
- 13.Sethi S, Cuiffo BP, Pinkus GS, Rennke HG. Crystal-storing histiocytosis involving the kidney in a low-grade B-cell lymphoproliferative disorder. Am J Kidney Dis. 2002;39:183–188. doi: 10.1053/ajkd.2002.29914. [DOI] [PubMed] [Google Scholar]