Abstract
Obstructive sleep apnea (OSA) is associated with coronary artery disease (CAD) and with an increased risk for myocardial infarction, stroke or death due to cardiovascular disease. Optical frequency-domain imaging (OFDI) is a useful modality for evaluating the characteristics of atherosclerotic plaque. The purpose of the study was to use OFDI to investigate the association of OSA with coronary plaque characteristics in patients undergoing percutaneous coronary intervention (PCI). We retrospectively analyzed OFDI data for coronary artery plaques from 15 patients with OSA and 35 non–OSA patients treated between October 2015 and October 2018. Plaque morphology was evaluated for 70 lesions, including 21 from patients with OSA and 49 from non–OSA patients. Compared with the non–OSA group, patients with OSA had significantly higher prevalences of thinned cap fibroatheroma (TCFA) (67% vs. 35%, P = 0.014) and microchannels (86% vs. 55%, P = 0.014); a significantly higher mean lipid index (1392 ± 982 vs. 817 ± 699, P = 0.021), macrophage grade (8.4 ± 6.4 vs. 4.8 ± 4.5, P = 0.030), and maximum number of microchannels (1.5 ± 1.0 vs. 0.7 ± 0.7, P = 0.001); and a significantly lower mean minimum fibrous cap thickness (69.4 ± 28.7 vs. 96.1 ± 51.8 μm, P = 0.008). This OFDI analysis suggests that OSA is associated with unstable plaque characteristics in patients with CAD. More intensive medical management for stabilization of coronary atherosclerotic plaque is required in patients with OSA.
Electronic supplementary material
The online version of this article (10.1007/s00380-019-01363-8) contains supplementary material, which is available to authorized users.
Keywords: Obstructive sleep apnea, Plaque instability, Optical frequency domain imaging
Introduction
Obstructive sleep apnea (OSA) is a common disorder with an estimated prevalence of 10–50% in men and 3–24% in women [1–4]. OSA is an independent risk factor for coronary artery disease (CAD), and up to 70% of patients with CAD have undiagnosed OSA [5]. Several studies have shown that OSA is associated with an increased risk for cardiovascular diseases such as myocardial infarction, stroke or death from cardiovascular disease [6–9].
Optical coherence tomography (OCT) and optical frequency domain imaging (OFDI) are intravascular imaging modalities that use reflection of near-infrared light to create images. These methods give images with high resolution of 10–20 µm, which is 10 times higher than that of intravascular ultrasound (IVUS). Recent OCT and OFDI studies have reported plaque rupture, lipid-rich plaques, thinned cap fibroatheroma (TCFA), cholesterol crystals, macrophage accumulation, and microchannels as characteristics of unstable plaque [4, 10–19].
Computed tomography (CT) in patients with OSA has revealed larger coronary plaque burdens and a larger lipid core compared to patients without OSA [20, 21]. IVUS has shown that patients with OSA have a larger coronary atherosclerotic plaque volume than patients without OSA [22]. OCT and OFDI are superior to these modalities due to their higher resolution in visualizing in-depth microstructure. In the current study, the association of OSA with features of plaque stability was examined using OFDI in patients undergoing percutaneous coronary intervention (PCI), as the first such study of this association.
Methods
Sample population
Data were analyzed retrospectively for 50 consecutive patients (37 men, 13 women) aged > 30 years who underwent OFDI-guided PCI at Hokkaido Cardiovascular Hospital between October 2015 and October 2018. For each participant, polysomnography (PSG) monitoring was performed within 6 months of OFDI-guided PCI. PSG monitoring was conducted in a standardized fashion. Overnight PSG was performed using an SAS-3200 (Nihon Kohden, Tokyo, Japan). A total of 21 lesions were examined in 15 patients with OSA based on an apnea hypopnea index (AHI) ≥ 15, and 49 lesions were examined in 35 patients without OSA (AHI < 15). The mean ages in the OSA and non–OSA groups were 71.8 ± 10.4 and 69.7 ± 8.9 years, respectively, with no significant difference between the groups. Patients presenting with left main CAD and cardiogenic shock were already excluded from 50 consecutive patients who underwent OFDI-guided PCI. Patients who accepted continuous positive airway pressure therapy (CPAP) as a treatment for OSA were also excluded. The study was approved by the Ethics Committee of Hokkaido Cardiovascular Hospital and was performed in compliance with the Declaration of Helsinki and ethical principles for medical research involving human subjects. All patients gave written informed consent.
Coronary angiography
Coronary angiograms were analyzed by offline quantitative coronary angiography (GE ver. 5.10.1, Pie Medical Imaging BV. Maastricht, The Netherlands). Reference diameter, minimum lumen diameter, diameter stenosis, and lesion length were measured.
OFDI method and analysis
An OFDI imaging catheter (FastView™, Terumo Corp., Tokyo, Japan) was advanced using a 0.014-inch guidewire with the help of a 6- or 7-Fr guiding catheter, and the imaging core was placed at a site distal to the lesion. Before PCI, OFDI was performed with continuous flush of contrast media at a rate of 4 mL/s, and the OFDI wire was pulled back at a rate of 20–40 mm/s. OFDI was generally performed without dilation by a balloon catheter, but the lesion was dilated with a small-sized balloon if the OFDI catheter could not pass through the lesion because of severe stenosis. For patients with acute coronary syndrome (ACS) without spontaneous recanalization, aspiration thrombectomy was performed prior to OFDI. The plaque morphology of culprit lesions was studied. Other lesions in the same epicardial artery with > 50% stenosis in angiography were also included in OFDI analysis. After identifying the most stenotic cross-section, the 5-mm proximal and distal cross-sections (total length: 10 mm) were examined. Cross-sectional images were then analyzed every 1 mm and evaluated for the presence of plaque rupture, plaque erosion, luminal thrombus, and categorized into lipid-rich plaque, TCFA, or a lesion with macrophage infiltration, cholesterol crystals, microchannels or calcification. OFDI analysis was conducted by two independent investigators (T.K. and R.K.) who were blinded to the clinical course of each patient. When there was discordance between the investigators, a consensus reading was obtained.
Definitions of OFDI findings
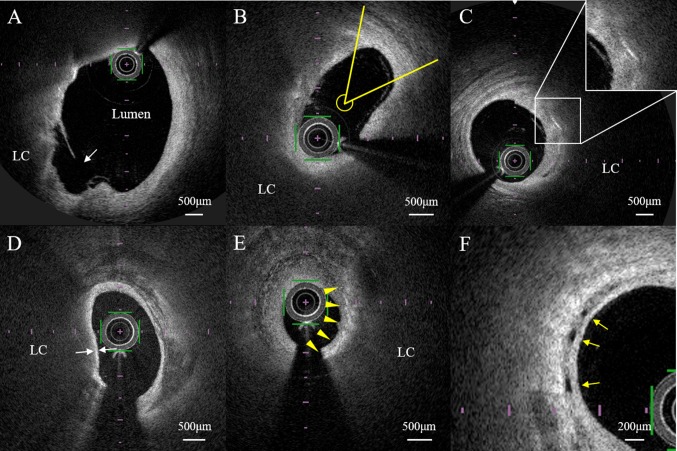
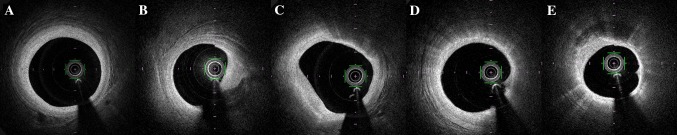
OFDI analysis revealed the presence of plaque rupture, plaque erosion, intraluminal thrombus, lipid-rich plaque, TCFA, macrophage accumulation, microvessels, and cholesterol crystals in the plaque. Plaque rupture was defined as the presence of fibrous cap discontinuity leading to communication between necrotic tissue and the lumen (Fig. 1a) [23, 24]. Plaque erosion was defined as a lesion without fibrous cap disruption and the presence of thrombus [25]. A thrombus was defined as a well-delineated mass with a high signal attached to the luminal surface or floating within the lumen [24]. Lipid-rich plaques were defined as lesions with a lipid arc > 180° (Fig. 1b) [24]. The lipid arc was measured within a lipid-rich plaque, and the maximum value was recorded (Fig. 1b). Lipid-core length was defined as the length of plaque with > 90° of lipid and was measured on the longitudinal view. The lipid index is the maximum lipid arc multiplied by the lipid-core length [26]. A cholesterol crystal was defined as a thin, linear region of high signal intensity within the lipid plaque, without backscattering (Fig. 1c) [24]. TCFA was defined as a fibrous cap thickness (FCT) < 65 μm, where FCT is the minimum thickness of a signal-rich layer from the coronary artery lumen to the inner border of the underlying lipid in the culprit lesion (Fig. 1d) [24]. Macrophage accumulation was defined as increased signal intensity within the fibrous cap, accompanied by heterogeneous backward shadows (Fig. 1e), and macrophages were semiquantified based on axial and circumferential distribution, as follows: grade 0, no macrophages; grade 1, localized macrophage accumulation; grade 2, clustered accumulation in < 1 quadrant; grade 3, clustered accumulation in ≥ 1 and < 3 quadrants; and grade 4, clustered accumulation in ≥ 3 quadrants (Fig. 2) [24, 27]. The range for the macrophage score was 0–40, based on summation of the 0–4 grades across all slices. Microchannels were defined as small vesicular or tubular structures with diameters of 50 to 300 μm within the intima (Fig. 1f) [24]. The number of microchannels was also counted at the cross-section with the highest number of microchannels [19]. Calcification was defined as well-delineated and low backscattered heterogeneous regions [24]. Spotty calcium deposits were defined as those with length < 4 mm and maximal arc < 90°, and deposits not meeting these criteria were classified as large calcium deposits [28, 29]. Microcalcification was defined as a small microcalcification with a maximal calcium angle < 22.5° and a maximal calcification length < 1 mm [30].
Fig. 1.
Representative plaque images from optical frequency domain imaging (OFDI). a Ruptured plaque (white arrow) with fibrous cap discontinuity leading to communication between the lipid core (LC) and lumen. b Measurement of the lipid arc for a lipid-rich plaque. The maximum lipid arc was measured within a lipid-rich plaque (yellow lines). c A cholesterol crystal was defined as a thin, linear region of high signal intensity within the lipid plaque, without backscattering. d Measurement of fibrous cap thickness. The thickness was measured at the thinnest point three times, and the average was taken (white arrows). e Macrophage accumulation was defined as increased signal intensity within the fibrous cap, accompanied by heterogeneous backward shadows (arrowheads). f Microchannels were defined as small vesicular or tubular structures with diameters of 50–300 μm within the intima
Fig. 2.
Semiquantification of macrophage accumulation in optical frequency domain imaging (OFDI). Representative cross–sectional OFDI images with the following grades: a grade 0, no macrophages; b grade 1, localized accumulation; c grade 2, clustered accumulation in < 1 quadrant; d grade 3, clustered accumulation in ≥ 1 and < 3 quadrants; and e grade 4, clustered accumulation in ≥ 3 quadrants
Statistical analysis
Continuous variables are shown as means ± standard deviation (SD) or median (interquartile range) and categorical variables as counts and percentages. The normality of distributions was assessed by the Kolmogorov–Smirnov test. Between-group differences were examined by Pearson chi-square or Fisher exact test for categorical variables and Student t test or Mann–Whitney U test for continuous variables, as appropriate. P < 0.05 was considered to be significant. All data were analyzed with SPSS 25.0 (IBM Corp., Armonk, NY).
Results
Clinical characteristics
The clinical characteristics of the OSA and non–OSA groups are compared in Table 1. The mean AHI was significantly higher in the OSA group (30.9 ± 12.6 vs. 7.2 ± 3.8, P < 0.001). The mean LDL-C did not differ significantly between the groups (107 ± 29 vs. 104 ± 45 mg/dl, P = 0.732). All other characteristics, including medications and concomitant diseases, were similar in the two groups. All patients who had statin therapy prior to PCI were treated with strong statin (atorvastatin 10 mg/day, rosuvastatin 2.5–5 mg/day or pitavastatin 2 mg/day).
Table 1.
Baseline characteristics in patients with and without obstructive sleep apnea
| Item | Non-OSA (n = 35) | OSA (n = 15) | P-value |
|---|---|---|---|
| Age (years) | 69.7 ± 8.9 | 71.8 ± 10.4 | 0.480 |
| Male | 27 (77) | 10 (67) | 0.493 |
| Body mass index | 23.6 ± 3.2 | 23.6 ± 3.3 | 0.986 |
| Diabetes mellitus | 11 (31) | 4 (27) | 0.736 |
| Hypertension | 23 (66) | 12 (80) | 0.502 |
| Dyslipidemia | 29 (83) | 12 (80) | 0.810 |
| Chronic kidney disease | 7 (20) | 4 (27) | 0.713 |
| Current smoker | 13 (37) | 4 (27) | 0.474 |
| Apnea hypopnea index | 7.2 ± 3.8 | 30.9 ± 12.6 | < 0.001 |
| Family history of coronary artery disease | 4 (11) | 3 (20) | 0.415 |
| History of PCI or CABG | 15 (43) | 5 (33) | 0.529 |
| History of myocardial infarction | 11 (31) | 1 (7) | 0.079 |
| History of TIA or cerebral infarction | 3 (9) | 0 | 0.545 |
| History of peripheral artery disease | 0 | 1 (7) | 0.300 |
| Prior statin use | 11 (31) | 6 (40) | 0.558 |
| Duration <3 months | 0 | 0 | – |
| 3–12 months | 3 (9) | 2 (13) | 0.607 |
| ≥12 months | 8 (23) | 4 (27) | 0.942 |
| Prior aspirin use | 15 (43) | 7 (47) | 0.804 |
| Prior clopidogrel use | 11 (31) | 2 (13) | 0.294 |
| Prior ACEI or ARB | 9 (26) | 6 (40) | 0.333 |
| Prior calcium channel blocker use | 12 (34) | 4 (27) | 0.746 |
| Prior beta blocker use | 10 (29) | 4 (27) | 0.891 |
| Prior eicosapentaenoic acid | 4 (11) | 1 (7) | 0.607 |
| Prior ezetimibe | 4 (11) | 0 | 0.302 |
| Hemoglobin (g/dl) | 13.5±1.5 | 13.3 ± 2.0 | 0.800 |
| HbA1c (%) | 6.4 ± 1.4 | 6.2 ± 1.1 | 0.662 |
| Glucose (mg/dl) | 140 ± 78 | 144 ± 64 | 0.882 |
| LDL-C (mg/dl) | 104 ± 45 | 107 ± 29 | 0.732 |
| Triglyceride (mg/dl) | 115 (86) | 172 (136) | 0.564 |
| HDL-C (mg/dl) | 49 ± 12 | 50 ± 14 | 0.936 |
| LDL-C to HDL-C ratio | 2.2 ± 1.1 | 2.3 ± 0.9 | 0.660 |
| Acute coronary syndrome | 18 (51) | 10 (67) | 0.320 |
| ST-elevation myocardial infarction | 9 (26) | 6 (40) | 0.333 |
| Non ST-elevation myocardial infarction | 9 (26) | 4 (27) | 0.944 |
Values are mean ± SD or number (%) of observations
PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, TIA transient ischemic attack, ACEangiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker
Angiographic findings
Plaque location and angiographic data are shown for the two groups in Table 2. The OSA group had a significantly higher stenosis diameter (87.7% ± 14.6% vs. 79.7% ± 15.4%, P = 0.044). There were no significant differences in plaque location or in other angiographic data between the two groups.
Table 2.
Angiographic characteristics in patients with and without obstructive sleep apnea
| Item | Non-OSA (n = 49) | OSA (n = 21) | P-value |
|---|---|---|---|
| Plaque location | |||
| Left anterior descending artery, n (%) | 19 (39) | 10 (48) | 0.491 |
| Left circumflex artery, n (%) | 13 (27) | 4 (19) | 0.503 |
| Right coronary artery, n (%) | 17 (35) | 7 (33) | 0.912 |
| Minimum lesion diameter, mm | 1.21±0.57 | 0.92±0.59 | 0.060 |
| Reference diameter, mm | 2.90±0.57 | 2.93±0.42 | 0.789 |
| Lesion length, mm | 15.2±6.2 | 16.8±7.4 | 0.372 |
| Diameter stenosis, % | 79.7±15.4 | 87.7±14.6 | 0.044 |
Plaque characteristics assessed by OFDI
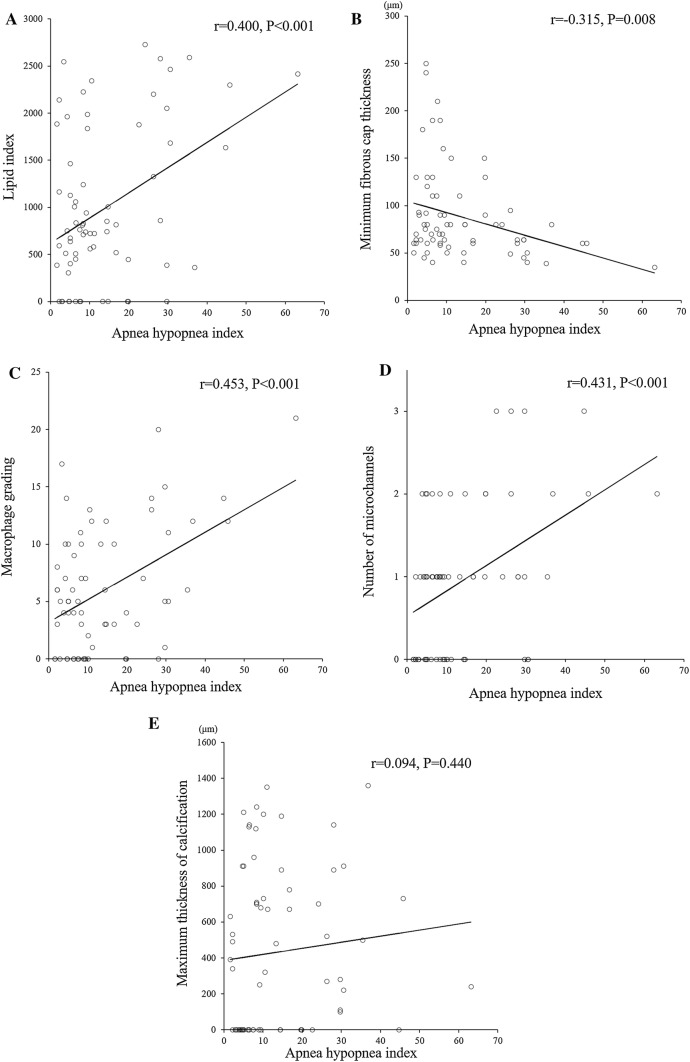
The results of qualitative and semi-quantitative analysis of OFDI characteristics of the coronary plaques are shown in Table 3. Since the scores of macrophage grading and maximum number of microchannels were not distributed normally, Mann–Whitney U-test was used to compare differences between the two groups. Other continuous variables regarding OFDI characteristics were normally distributed. The OSA group had significantly higher prevalences of TCFA (67% vs. 35%, P = 0.014) and microchannels (86% vs. 55%, P = 0.014), a significantly higher mean lipid index (1392 ± 982 vs. 817 ± 699, P = 0.021), macrophage grade (8.4 ± 6.4 vs. 4.8 ± 4.5, P = 0.030), and maximum number of microchannels (1.5 ± 1.0 vs. 0.7 ± 0.7, P = 0.001), and a significantly lower mean minimum FCT (69.4 ± 28.7 vs. 96.1 ± 51.8 μm, P = 0.008) compared to the non–OSA group. AHI was positively correlated with the plaque characteristics of lipid index (r = 0.400, P < 0.001, Fig. 3a), macrophage grade (r = 0.453, P < 0.001, Fig. 3c), and number of microchannels (r = 0.431, P < 0.001, Fig. 3d), inversely correlated with the minimum FCT (r = − 0.315, P = 0.008; Fig. 3b), and not significantly correlated with the maximum thickness of calcification (r = 0.094, P = 0.440; Fig. 3e).
Table 3.
OFDI characteristics in patients with and without obstructive sleep apnea
| Item | Non-OSA (n = 49) | OSA (n = 21) | P-value |
|---|---|---|---|
| Number of subjects | 35 | 15 | |
| Number of plaques | 49 | 21 | |
| Plaques/subjects | 1.4±0.5 | 1.4±0.6 | 0.872 |
| Plaque rupture | 6 (12) | 4 (19) | 0.473 |
| Plaque erosion | 6 (12) | 3 (14) | 0.815 |
| Luminal thrombus | 12 (24) | 8 (38) | 0.248 |
| Lipid-rich plaque | 18 (37) | 10 (48) | 0.394 |
| Maximum lipid arc (degrees) | 155 ± 71 | 184 ± 90 | 0.161 |
| Lipid length (mm) | 4.5 ± 3.1 | 6.3 ± 3.5 | 0.028 |
| Lipid index | 817 ± 699 | 1392 ± 982 | 0.021 |
| Thin capped fibroatheroma (TCFA) | 17 (35) | 14 (67) | 0.014 |
| Minimum fibrous cap thickness (μm) | 96.1±51.8 | 69.4±28.7 | 0.008 |
| Macrophage infiltration | 34 (69) | 18 (86) | 0.152 |
| Macrophage grading | 4.8±4.5 | 8.4±6.4 | 0.030 |
| Cholesterol crystals | 18 (37) | 10 (48) | 0.394 |
| Microchannels | 27 (55) | 18 (86) | 0.014 |
| Maximum number of microchannels | 0.7±0.7 | 1.5±1.0 | 0.001 |
| Calcification | 26 (53) | 16 (76) | 0.070 |
| Large calcification | 16 (33) | 7 (33) | 0.956 |
| Spotty calcification | 13 (27) | 7 (33) | 0.564 |
| Maximum thickness of calcification | 426±468 | 449±414 | 0.516 |
| Microcalcification | 5 (10) | 6 (29) | 0.075 |
Fig. 3.
Relationships of apnea hypopnea index (AHI) with plaque characteristics. AHI was significantly correlated with a lipid index (r = 0.400, P < 0.001) b minimum fibrous cap thickness (r = − 0.315, P = 0.008) c macrophage grade (r = 0.453, P < 0.001), and d number of microchannels (r = 0.431, P < 0.001), but not with e maximum thickness of calcification (r = 0.094, P = 0.440)
Risk factors for higher lipid index, TCFA, macrophage invasion, and microchannels
Multiple logistic regression analyses were performed to identify risk factors for higher lipid index, TCFA, macrophage invasion, and microchannels. Because history of PCI or CABG, history of myocardial infarction, prior aspirin use and prior clopidogrel use were correlated with prior statin use, they were excluded from the multiple variable analysis. We similarly excluded LDL-cholesterol (LDL-C) when we entered LDL-C to HDL-cholesterol (HDL-C) ratio in the multiple variable model. In multiple variable analyses, AHI, prior statin use, and glucose concentration were independently associated with a higher lipid index (Table 4); AHI and LDL-cholesterol (LDL-C) to HDL-cholesterol (HDL-C) ratio were associated with TCFA (Table 5); AHI and prior statin use were associated with greater macrophage grading (Table 6); and AHI, hemoglobin level and HDL-C were associated with greater microchannels (Table 7).
Table 4.
Logistic regression analysis of lipid index
| Analysis | ||||
|---|---|---|---|---|
| Single | Multiple | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years) | 0.40 (0.13–1.22) | 0.106 | ||
| Male | 1.00 (0.34–2.92) | 1.000 | ||
| Body mass index | 2.67 (0.92–7.77) | 0.072 | ||
| Diabetes mellitus | 1.00 (0.35–2.82) | 1.000 | ||
| Hypertension | 1.93 (0.70–5.32) | 0.206 | ||
| Dyslipidemia | 0.56 (0.16–1.93) | 0.360 | ||
| Chronic kidney disease | 1.93 (0.62–6.07) | 0.259 | ||
| Current smoker | 1.30 (0.47–3.59) | 0.607 | ||
| Obstructive sleep apnea | 2.67 (0.92–7.77) | 0.072 | ||
| Apnea hypopnea index | 6.30 (1.61–24.7) | 0.008 | 8.08 (1.70–38.5) | 0.009 |
| Family history of coronary artery disease | 2.67 (0.63–11.3) | 0.183 | ||
| History of PCI or CABG | 0.43 (0.16–1.15) | 0.092 | ||
| History of myocardial infarction | 0.25 (0.08–0.80) | 0.020 | ||
| Prior statin use | 0.20 (0.07–0.59) | 0.004 | 0.18 (0.05–0.62) | 0.007 |
| Prior aspirin use | 0.44 (0.17–1.16) | 0.096 | ||
| Prior clopidogrel use | 0.35 (0.12–1.07) | 0.065 | ||
| Prior ACEI or ARB | 0.42 (0.14–1.24) | 0.117 | ||
| Prior calcium channel blocker use | 0.66 (0.24–1.86) | 0.435 | ||
| Prior beta blocker use | 0.77 (0.28–2.11) | 0.607 | ||
| Prior eicosapentaenoic acid | 0.36 (0.07–2.02) | 0.247 | ||
| Prior ezetimibe | 0.23 (0.02–2.15) | 0.197 | ||
| Hemoglobin (g/dl) | 2.36 (0.81–6.93) | 0.117 | ||
| HbA1c (%) | 2.67 (0.63–11.3) | 0.183 | ||
| Glucose (mg/dl) | 3.63 (1.20–10.9) | 0.022 | 6.22 (1.62–23.8) | 0.008 |
| LDL-C (mg/dl) | 2.01 (0.77–5.22) | 0.152 | ||
| Triglyceride (mg/dl) | 1.83 (0.61–5.47) | 0.277 | ||
| HDL-C (mg/dl) | 0.60 (0.19–1.91) | 0.385 | ||
| LDL-C to HDL-C ratio | 2.67 (0.92–7.77) | 0.072 | ||
| Acute coronary syndrome | 2.65 (0.99–7.11) | 0.054 | ||
| ST-elevation myocardial infarction | 2.18 (0.79–5.96) | 0.134 | ||
| Non ST-elevation myocardial infarction | 1.35 (0.46–3.96) | 0.585 | ||
OR odds ratio, CI confidence interval, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, TIA transient ischemic attack, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker
Table 5.
Logistic regression analysis of TCFA
| Analysis | ||||
|---|---|---|---|---|
| Single | Multiple | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years) | 2.21 (0.83–5.89) | 0.113 | ||
| Male | 1.35 (0.45–4.02) | 0.593 | ||
| Body mass index | 0.51 (0.20–1.34) | 0.174 | ||
| Diabetes mellitus | 0.43 (0.14–1.29) | 0.133 | ||
| Hypertension | 1.37 (0.50–3.78) | 0.544 | ||
| Dyslipidemia | 0.62 (0.19–2.09) | 0.444 | ||
| Chronic kidney disease | 1.87 (0.61–5.77) | 0.276 | ||
| Current smoker | 1.07 (0.39–2.96) | 0.894 | ||
| Obstructive sleep apnea | 3.77 (1.28–11.1) | 0.016 | ||
| Apnea hypopnea index | 11.7 (2.37–57.6) | 0.003 | 7.55 (1.35–42.4) | 0.022 |
| Family history of coronary artery disease | 0.49 (0.12–2.08) | 0.333 | ||
| History of PCI or CABG | 0.39 (0.14–1.05) | 0.063 | ||
| History of myocardial infarction | 0.48 (0.16–1.46) | 0.196 | ||
| Prior statin use | 0.20 (0.06–0.64) | 0.006 | 0.46 (0.12–1.68) | 0.238 |
| Prior aspirin use | 0.87 (0.34–2.23) | 0.767 | ||
| Prior clopidogrel use | 0.66 (0.22–1.94) | 0.446 | ||
| Prior ACEI or ARB | 0.78 (0.27–2.24) | 0.648 | ||
| Prior calcium channel blocker use | 0.92 (0.33–2.58) | 0.875 | ||
| Prior beta blocker use | 0.82 (0.29–2.27) | 0.700 | ||
| Prior eicosapentaenoic acid | 0.47 (0.09–2.60) | 0.386 | ||
| Prior ezetimibe | 0.29 (0.03–2.75) | 0.282 | ||
| Hemoglobin (g/dl) | 0.39 (0.10–1.49) | 0.169 | ||
| HbA1c (%) | 0.39 (0.14–1.05) | 0.063 | ||
| Glucose (mg/dl) | 0.40 (0.13–1.19) | 0.100 | ||
| LDL-C (mg/dl) | 3.02 (1.09–8.40) | 0.033 | ||
| Triglyceride (mg/dl) | 0.38 (0.14–1.01) | 0.053 | ||
| HDL-C (mg/dl) | 0.38 (0.14–1.01) | 0.053 | ||
| LDL-C to HDL-C ratio | 7.11 (2.09–24.2) | 0.002 | 4.53 (1.15–17.8) | 0.030 |
| Acute coronary syndrome | 1.41 (0.53–3.71) | 0.492 | ||
| ST-elevation myocardial infarction | 3.13 (1.12–8.71) | 0.029 | 1.97 (0.57–6.77) | 0.284 |
| Non ST-elevation myocardial infarction | 0.39 (0.12–1.23) | 0.108 | ||
OR odds ratio, CI confidence interval, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, TIA transient ischemic attack, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker
Table 6.
Logistic regression analysis of macrophage infiltration
| Analysis | ||||
|---|---|---|---|---|
| Single | Multiple | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years) | 3.23 (0.93–11.3) | 0.066 | ||
| Male | 1.26 (0.43–3.69) | 0.672 | ||
| Body mass index | 0.48 (0.15–1.53) | 0.215 | ||
| Diabetes mellitus | 1.04 (0.37–2.95) | 0.940 | ||
| Hypertension | 1.14 (0.42–3.09) | 0.804 | ||
| Dyslipidemia | 0.46 (0.13–1.67) | 0.237 | ||
| Chronic kidney disease | 1.11 (0.36–3.41) | 0.858 | ||
| Current smoker | 1.75 (0.62–4.94) | 0.290 | ||
| Obstructive sleep apnea | 2.08 (0.72–6.05) | 0.177 | ||
| Apnea hypopnea index | 7.80(1.61–37.9) | 0.011 | 5.97 (1.10–32.5) | 0.039 |
| Family history of coronary artery disease | 2.18 (0.52–9.25) | 0.289 | ||
| History of PCI or CABG | 0.32 (0.12–0.85) | 0.023 | ||
| History of myocardial infarction | 0.38 (0.13–1.12) | 0.078 | ||
| Prior statin use | 0.20 (0.07–0.58) | 0.003 | 0.23 (0.07–0.73) | 0.012 |
| Prior aspirin use | 0.19 (0.07–0.52) | 0.001 | ||
| Prior clopidogrel use | 0.27 (0.09–0.84) | 0.024 | ||
| Prior ACEI or ARB | 0.79 (0.28–2.22) | 0.649 | ||
| Prior calcium channel blocker use | 0.68 (0.24–1.90) | 0.465 | ||
| Prior beta blocker use | 0.45 (0.16–1.27) | 0.132 | ||
| Prior eicosapentaenoic acid | 0.60 (0.12–2.91) | 0.526 | ||
| Prior ezetimibe | 1.29 (0.20–8.21) | 0.791 | ||
| Hemoglobin (g/dl) | 2.25 (0.78–6.49) | 0.133 | ||
| HbA1c (%) | 3.39 (0.65–17.6) | 0.147 | ||
| Glucose (mg/dl) | 3.00 (1.08–8.34) | 0.035 | 2.95 (0.93–9.39) | 0.066 |
| LDL-C (mg/dl) | 2.32 (0.77–6.95) | 0.133 | ||
| Triglyceride (mg/dl) | 0.41 (0.15–1.08) | 0.071 | ||
| HDL-C (mg/dl) | 0.60 (0.22–1.64) | 0.317 | ||
| LDL-C to HDL-C ratio | 1.93 (0.69–5.44) | 0.212 | ||
| Acute coronary syndrome | 1.33 (0.51–3.48) | 0.557 | ||
| ST-elevation myocardial infarction | 0.77 (0.29–2.07) | 0.603 | ||
| Non ST-elevation myocardial infarction | 2.00 (0.65–6.13) | 0.225 | ||
OR odds ratio, CI confidence interval, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, TIA transient ischemic attack, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker
Table 7.
Logistic regression analysis of microchannels
| Analysis | ||||
|---|---|---|---|---|
| Single | Multiple | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years) | 4.19 (0.50–35.1 | 0.187 | ||
| Male | 0.26 (0.08–0.85) | 0.025 | 0.33 (0.06–1.69) | 0.182 |
| Body mass index | 2.13 (0.61–7.43) | 0.234 | ||
| Diabetes mellitus | 1.52 (0.47–4.88) | 0.482 | ||
| Hypertension | 1.23 (0.38–4.05) | 0.728 | ||
| Dyslipidemia | 0.67 (0.18–2.52) | 0.547 | ||
| Chronic kidney disease | 0.66 (0.16–2.66) | 0.558 | ||
| Current smoker | 0.38 (0.10–1.51) | 0.170 | ||
| Obstructive sleep apnea | 5.46 (1.69–17.6) | 0.005 | ||
| Apnea hypopnea index | 8.04 (2.36–27.3) | 0.001 | 9.44 (1.99–44.9) | 0.005 |
| Family history of coronary artery disease | 2.41 (0.59–9.84) | 0.220 | ||
| History of PCI or CABG | 0.99 (0.33–2.99) | 0.981 | ||
| History of myocardial infarction | 0.50 (0.13–1.97) | 0.318 | ||
| Prior statin use | 1.48 (0.48–4.57) | 0.493 | ||
| Prior aspirin use | 0.53 (0.17–1.63) | 0.525 | ||
| Prior clopidogrel use | 0.28 (0.06–1.38) | 0.118 | ||
| Prior ACEI or ARB | 1.06 (0.32–3.51) | 0.930 | ||
| Prior calcium channel blocker use | 0.42 (0.11–1.64) | 0.211 | ||
| Prior beta blocker use | 0.38 (0.10–1.51) | 0.170 | ||
| Prior eicosapentaenoic acid | 1.28 (0.23–7.29) | 0.781 | ||
| Prior ezetimibe | 0.77 (0.08–7.36) | 0.817 | ||
| Hemoglobin (g/dl) | 0.12 (0.04–0.42) | 0.001 | 0.18 (0.04–0.79) | 0.022 |
| HbA1c (%) | 1.86 (0.62–5.59) | 0.271 | ||
| Glucose (mg/dl) | 6.32 (0.77–51.9) | 0.086 | ||
| LDL-C (mg/dl) | 1.88 (0.62–5.73) | 0.266 | ||
| Triglyceride (mg/dl) | 0.58 (0.19–1.75) | 0.334 | ||
| HDL-C (mg/dl) | 6.72 (1.77–25.5) | 0.005 | 5.31 (1.03–27.3) | 0.046 |
| LDL-C to HDL-C ratio | 0.33 (0.09–1.13) | 0.078 | ||
| Acute coronary syndrome | 0.68 (0.23–2.05) | 0.496 | ||
| ST-elevation myocardial infarction | 0.33 (0.08–1.28) | 0.108 | ||
| Non ST-elevation myocardial infarction | 1.86 (0.57–6.09) | 0.303 | ||
OR odds ratio, CI confidence interval, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, TIA transient ischemic attack, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker
Correlations among microchannels, macrophage grade, and FCT
There were significant correlations among microchannels, macrophages and FCT. Macrophage grading was positively correlated with the number of microchannels (r = 0.383, P = 0.001; Supplemental Fig. 1), and FCT was inversely correlated with the macrophage grade (r = − 0.415, P < 0.001; Supplemental Fig. 2).
Observer variabilities
OFDI images were analyzed by two independent observers. The inter-observer reliabilities and intra-observer reproducibilities measured by the Pearson coefficient were r = 0.90 and 0.91 for lipid index, r = 0.92 and 0.94 for minimum FCT, r = 0.95 and 0.93 for macrophage grading, and r = 0.92 and 0.95 for maximum number of microchannels, respectively.
Discussion
The main findings in this study were (1) that patients with OSA had a larger lipid burden, thinner fibrous cap, greater macrophage accumulation, and more microchannels compared to those without OSA; (2) lipid index, minimum FCT, macrophage accumulation and microchannels were positively or inversely correlated with AHI; and (3) in patients undergoing PCI, AHI, prior statin use and glucose concentration were predictors of lipid index; AHI and LDL-C to HDL-C ratio were predictors of TCFA; AHI and prior statin use were predictors of macrophage grading; and AHI, hemoglobin and HDL-C were predictors of greater microchannels. To the best of our knowledge, this study is the first in depth comparison of coronary artery plaques in patients with and without OSA, with analysis of correlations of AHI with characteristics of unstable plaque using OFDI in patients who underwent PCI. These observations improve understanding of the pathophysiology of OSA, and may have important implications for management of patients with OSA presenting with CAD.
Comparison with previous studies
Our results are concordant with OFDI data from previous studies, with microchannels, macrophage accumulation, and TCFA found in 37–60%, 30–74%, and 11–34% of patients who underwent PCI, respectively [31–33]. FCT measured by OCT was significantly lower in plaques with positive remodeling and in low-attenuation plaques on CT angiography, compared with two-feature–negative plaques (76 ± 24 vs. 192 ± 49 μm, P < 0.001) [34].
Lipid-rich plaque
A large lipid core is an important contributor to plaque rupture through mechanically increasing the tension of the fibrous cap covering the lipid core, leading to disruption [35]. In patients with OSA, intermittent hypoxia (IH) during sleep can increase oxidative stress, leading to oxidative modification of lipoproteins and other molecules [36–38]. These oxidized particles cause endothelial surface injury and promote accumulation of cholesterol in atherosclerotic plaque [39, 40]. CT studies have shown larger coronary plaque burdens and a larger lipid core in non–culprit lesions of patients with OSA compared to those in patients without OSA [20, 21], which is consistent with our data (Table 3, Fig. 3A).
TCFA and FCT
Previous reports have shown that a thin fibrous cap is one of the most important features of unstable plaque in coronary and carotid artery [41–43]. Since matrix metalloproteinases (MMPs) released by macrophages induce thinning of fibrous caps of atherosclerotic plaques through collagen breakdown [44], more macrophage accumulation in the fibrous cap might lead to a thinner fibrous cap (Supplemental Fig. 2). Therefore, more macrophage accumulation in the plaque might contribute to a higher prevalence of TCFA and a lower FCT in patients with OSA (Table 3, Fig. 3b).
Macrophage accumulation
In a murine model of OSA, IH during sleep caused recruitment of more macrophages to the aortic wall [45, 46], and IH during sleep for 6 weeks led to a significant increase in the percentage of macrophages in the aortic wall (6.4% ± 0.3% vs. 8.1% ± 0.3%, P = 0.003) [46]. Macrophages are thought to be extravasated from intraplaque microvessels into plaque tissue [47]. The number of microchannels in the plaque was weakly, but positively, correlated with the macrophage grade (Supplemental Fig. 1). Therefore, more intraplaque microvessels and direct invasion from the lumen of coronary arteries might contribute to greater macrophage accumulation in patients with OSA (Table 3, Fig. 3c).
Microchannels
Serum concentrations of vascular endothelial growth factor (VEGF) and endothelin-1, which have important roles in angiogenesis, are increased in patients with OSA [48, 49]. Oxidative stress due to hypoxia in these patients induces endothelial dysfunction, leading to endothelial-derived microparticle (MP) formation [50]. MPs are small plasma membrane vesicles with diameters of about 0.05–1.00 μm that are released by plasma membranes of cells such as leukocytes, platelets, endothelial cells, erythrocytes and smooth muscle cells in response to damage [51]. Ayers et al. showed that the concentrations of platelet- and leukocyte-derived MPs were elevated in patients with OSA [52], and Tual-Chalot et al. found that MPs from patients with OSA induced an increase of angiogenesis through VEGF- and endothelin-1-mediated pathways [49]. These mechanisms might explain the finding of more microchannels in patients with OSA and CAD (Table 3, Fig. 3d).
Unstable plaque in OSA and clinical perspectives
Several studies have shown that OSA might cause or accelerate atherosclerosis [53, 54]. In this study, multiple regression analysis showed that AHI is independently correlated with features of unstable plaque (Table 4, 5, 6, 7). These results support the hypothesis that OSA plays an important role in development of atherosclerotic plaque and plaque instability. However, in the Sleep Apnea Cardiovascular Endpoints (SAVE) trial, CPAP did not result in a lower incidence of the primary end point than usual care alone [55]. In this trial, the mean duration of CPAP use was only 3.3 h per night, which might not be sufficient for preventing cardiovascular events. After propensity-score matching, the incidence of a cerebrovascular event was significantly lower in 561 patients who used CPAP for more than 4 h per night, compared to that in the usual-care group (hazard ratio, 0.52, P = 0.02) [55]. Furthermore, there is evidence that treatment of OSA with CPAP improves endothelial function and reduces the risk for cardiovascular events [56], and a randomized controlled trial showed that CPAP therapy resulted in a significant reduction of carotid intima-media thickness [57]. Therefore, CPAP therapy and stringent management of other coronary risk factors might be effective for stabilization of unstable plaque and secondary prevention of adverse cardiovascular events in patients with OSA.
Limitations
The sample size of this retrospective, cross–sectional study conducted at a single medical center was small, and the results require confirmation in a prospective study including a larger number of patients. Second, we could not analyze microchannels of < 50 μm due to the limit of the device resolution. Third, there is an inherent discrepancy between characteristics assessed by OFDI and actual histopathological findings [58]. Further analyses using higher resolution OFDI might enable more detailed assessment of intraplaque microstructures in patients undergoing PCI. Fourth, we did not have information on inflammatory biomarkers such as high–sensitivity CRP, ICAM-1 and E-selectin. Fifth, the analysis of the risk factors was done on a per lesion basis although the patient characteristics were analyzed on a per patient basis.
Conclusion
This OFDI analysis suggests that OSA is associated with plaque instability in patients with CAD. More intensive medical management is required for patients with OSA for stabilization of coronary atherosclerotic plaques.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Mr. Ryosuke Kato and Mr. Kensuke Ito for OFDI analysis. We also thank Mr. Satoru Miura for quantitative coronary analysis.
Compliance with ethical standards
Conflict of interest
The authors have no potential conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haba-Rubio J, Marques-Vidal P, Andries D, Tobback N, Preisig M, Vollenweider P, Waeber G, Luca G, Tafti M, Heinzer R. Objective sleep structure and cardiovascular risk factors in the general population: the HypnoLaus Study. Sleep. 2015;38:391–400. doi: 10.5665/sleep.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–933. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. doi: 10.1093/sleep/31.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy P, Kohler M, McNicholas WT, Barbe F, McEvoy RD, Somers VK, Lavie L, Pepin JL. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- 10.Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, Aurigemma C, Prati F, Crea F. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J. 2015;36:1377–1384. doi: 10.1093/eurheartj/ehv029. [DOI] [PubMed] [Google Scholar]
- 11.Satogami K, Ino Y, Kubo T, Tanimoto T, Orii M, Matsuo Y, Ota S, Yamaguchi T, Shiono Y, Shimamura K, Katayama Y, Aoki H, Nishiguchi T, Ozaki Y, Yamano T, Kameyama T, Kuroi A, Kitabata H, Tanaka A, Hozumi T, Akasaka T. Impact of plaque rupture detected by optical coherence tomography on transmural extent of infarction after successful stenting in ST-segment elevation acute myocardial infarction. JACC Cardiovasc Interv. 2017;10:1025–1033. doi: 10.1016/j.jcin.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Xing L, Higuma T, Wang Z, Aguirre AD, Mizuno K, Takano M, Dauerman HL, Park SJ, Jang Y, Kim CJ, Kim SJ, Choi SY, Itoh T, Uemura S, Lowe H, Walters DL, Barlis P, Lee S, Lerman A, Toma C, Tan JWC, Yamamoto E, Bryniarski K, Dai J, Zanchin T, Zhang S, Yu B, Lee H, Fujimoto J, Fuster V, Jang IK. Clinical significance of lipid-rich plaque detected by optical coherence tomography: a 4-year follow-up study. J Am Coll Cardiol. 2017;69:2502–2513. doi: 10.1016/j.jacc.2017.03.556. [DOI] [PubMed] [Google Scholar]
- 13.Uemura S, Ishigami K, Soeda T, Okayama S, Sung JH, Nakagawa H, Somekawa S, Takeda Y, Kawata H, Horii M, Saito Y. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J. 2012;33:78–85. doi: 10.1093/eurheartj/ehr284. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto Y, Okura H, Kume T, Kawamoto T, Neishi Y, Hayashida A, Yamada R, Imai K, Saito K, Yoshida K. Plaque characteristics of thin-cap fibroatheroma evaluated by OCT and IVUS. JACC Cardiovasc Imaging. 2011;4:638–646. doi: 10.1016/j.jcmg.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Dai J, Tian J, Hou J, Xing L, Liu S, Ma L, Yu H, Ren X, Dong N, Yu B. Association between cholesterol crystals and culprit lesion vulnerability in patients with acute coronary syndrome: An optical coherence tomography study. Atherosclerosis. 2016;247:111–117. doi: 10.1016/j.atherosclerosis.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Iannaccone M, Quadri G, Taha S, D'Ascenzo F, Montefusco A, Omede P, Jang IK, Niccoli G, Souteyrand G, Yundai C, Toutouzas K, Benedetto S, Barbero U, Annone U, Lonni E, Imori Y, Biondi-Zoccai G, Templin C, Moretti C, Luscher TF, Gaita F. Prevalence and predictors of culprit plaque rupture at OCT in patients with coronary artery disease: a meta-analysis. Eur Heart J Cardiovasc Imaging. 2016;17:1128–1137. doi: 10.1093/ehjci/jev283. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi Y, Itoh T, Oda H, Uchimura Y, Kaneko K, Sakamoto T, Goto I, Sakuma M, Ishida M, Terashita D, Otake H, Morino Y, Shinke T. Coronary risk factors associated with OCT macrophage images and their response after CoCr everolimus-eluting stent implantation in patients with stable coronary artery disease. Atherosclerosis. 2017;265:117–123. doi: 10.1016/j.atherosclerosis.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.CIR.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 19.Kitabata H, Tanaka A, Kubo T, Takarada S, Kashiwagi M, Tsujioka H, Ikejima H, Kuroi A, Kataiwa H, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Hirata K, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol. 2010;105:1673–1678. doi: 10.1016/j.amjcard.2010.01.346. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Gebregziabher M, Parker AT, Abro JA, Armstrong AM, Schoepf UJ. Independent association between obstructive sleep apnea and noncalcified coronary plaque demonstrated by noninvasive coronary computed tomography angiography. Clin Cardiol. 2012;35:641–645. doi: 10.1002/clc.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent BD, Garvey JF, Ryan S, Nolan G, Dodd JD, McNicholas WT. Severity of obstructive sleep apnoea predicts coronary artery plaque burden: a coronary computed tomographic angiography study. Eur Respir J. 2013;42:1263–1270. doi: 10.1183/09031936.00094812. [DOI] [PubMed] [Google Scholar]
- 22.Turmel J, Series F, Boulet LP, Poirier P, Tardif JC, Rodes-Cabeau J, Larose E, Bertrand OF. Relationship between atherosclerosis and the sleep apnea syndrome: an intravascular ultrasound study. Int J Cardiol. 2009;132:203–209. doi: 10.1016/j.ijcard.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 23.Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW, Expert's OCTRD. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 24.Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G, International Working Group for Intravascular Optical Coherence T (2012) Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 59:1058–1072 [DOI] [PubMed]
- 25.Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, Yu B, Mizuno K, Jang IK. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: a 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. doi: 10.1161/CIRCIMAGING.112.973701. [DOI] [PubMed] [Google Scholar]
- 27.Tahara S, Morooka T, Wang Z, Bezerra HG, Rollins AM, Simon DI, Costa MA. Intravascular optical coherence tomography detection of atherosclerosis and inflammation in murine aorta. Arterioscler Thromb Vasc Biol. 2012;32:1150–1157. doi: 10.1161/ATVBAHA.111.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizukoshi M, Kubo T, Takarada S, Kitabata H, Ino Y, Tanimoto T, Komukai K, Tanaka A, Imanishi T, Akasaka T. Coronary superficial and spotty calcium deposits in culprit coronary lesions of acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol. 2013;112:34–40. doi: 10.1016/j.amjcard.2013.02.048. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, Tuzcu EM, Nissen SE, Nicholls SJ. Spotty calcification and plaque vulnerability in vivo: frequency-domain optical coherence tomography analysis. Cardiovasc Diagn Ther. 2014;4:460–469. doi: 10.3978/j.issn.2223-3652.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milzi A, Burgmaier M, Burgmaier K, Hellmich M, Marx N, Reith S. Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: an intracoronary optical coherence tomography study. Cardiovasc Diabetol. 2017;16:152. doi: 10.1186/s12933-017-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abtahian F, Yonetsu T, Kato K, Jia H, Vergallo R, Tian J, Hu S, McNulty I, Lee H, Yu B, Jang IK. Comparison by optical coherence tomography of the frequency of lipid coronary plaques in current smokers, former smokers, and nonsmokers. Am J Cardiol. 2014;114:674–680. doi: 10.1016/j.amjcard.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 32.Yonetsu T, Kato K, Uemura S, Kim BK, Jang Y, Kang SJ, Park SJ, Lee S, Kim SJ, Jia H, Vergallo R, Abtahian F, Tian J, Hu S, Yeh RW, Sakhuja R, McNulty I, Lee H, Zhang S, Yu B, Kakuta T, Jang IK. Features of coronary plaque in patients with metabolic syndrome and diabetes mellitus assessed by 3-vessel optical coherence tomography. Circ Cardiovasc Imaging. 2013;6:665–673. doi: 10.1161/CIRCIMAGING.113.000345. [DOI] [PubMed] [Google Scholar]
- 33.Koide M, Matsuo A, Shimoo S, Takamatsu K, Kyodo A, Tsuji Y, Mera K, Tsubakimoto Y, Isodono K, Sakatani T, Inoue K, Fujita H. Cholesterol crystal depth in coronary atherosclerotic plaques: a novel index of plaque vulnerability using optical frequency domain imaging. PLoS One. 2017;12:e0180303. doi: 10.1371/journal.pone.0180303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato A, Hoshi T, Kakefuda Y, Hiraya D, Watabe H, Kawabe M, Akiyama D, Koike A, Aonuma K. In vivo evaluation of fibrous cap thickness by optical coherence tomography for positive remodeling and low-attenuation plaques assessed by computed tomography angiography. Int J Cardiol. 2015;182:419–425. doi: 10.1016/j.ijcard.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41:15S–22S. doi: 10.1016/S0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 36.Chen CY, Chen CL, Yu CC, Chen TT, Tseng ST, Ho CH. Association of inflammation and oxidative stress with obstructive sleep apnea in ischemic stroke patients. Sleep Med. 2015;16:113–118. doi: 10.1016/j.sleep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, Kimura H. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–1679. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 38.Forstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 39.Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation. 2000;101:1500–1502. doi: 10.1161/01.CIR.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 40.Davignon J, Ganz P (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109:III27-32 [DOI] [PubMed]
- 41.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.ATV.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 42.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 43.Konishi T, Funayama N, Yamamoto T, Morita T, Hotta D, Nomura R, Nakagaki Y, Murahashi T, Kamiyama K, Yoshimoto T, Aoki T, Nishihara H, Tanaka S. Pathological quantification of carotid artery plaque instability in patients undergoing carotid endarterectomy. Circ J. 2017;82:258–266. doi: 10.1253/circj.CJ-17-0204. [DOI] [PubMed] [Google Scholar]
- 44.Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–1569. [PubMed] [Google Scholar]
- 45.Cortese R, Gileles-Hillel A, Khalyfa A, Almendros I, Akbarpour M, Khalyfa AA, Qiao Z, Garcia T, Andrade J, Gozal D. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: Potential role of CD36. Sci Rep. 2017;7:43648. doi: 10.1038/srep43648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gileles-Hillel A, Almendros I, Khalyfa A, Zhang SX, Wang Y, Gozal D. Early intermittent hypoxia induces proatherogenic changes in aortic wall macrophages in a murine model of obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190:958–961. doi: 10.1164/rccm.201406-1149LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, Duimel H, Frederik PM, van Hinsbergh VW, Virmani R, Daemen MJ. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53:1517–1527. doi: 10.1016/j.jacc.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep. 2002;25:59–65. doi: 10.1093/sleep/25.1.59. [DOI] [PubMed] [Google Scholar]
- 49.Tual-Chalot S, Gagnadoux F, Trzepizur W, Priou P, Andriantsitohaina R, Martinez MC. Circulating microparticles from obstructive sleep apnea syndrome patients induce endothelin-mediated angiogenesis. Biochim Biophys Acta. 2014;1842:202–207. doi: 10.1016/j.bbadis.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Jia L, Fan J, Cui W, Liu S, Li N, Lau WB, Ma X, Du J, Nie S, Wei Y. Endothelial cell-derived microparticles from patients with obstructive sleep apnea hypoxia syndrome and coronary artery disease increase aortic endothelial cell dysfunction. Cell Physiol Biochem. 2017;43:2562–2570. doi: 10.1159/000484508. [DOI] [PubMed] [Google Scholar]
- 51.Stiefel P, Sanchez-Armengol MA, Villar J, Vallejo-Vaz A, Moreno-Luna R, Capote F. Obstructive sleep apnea syndrome, vascular pathology, endothelial function and endothelial cells and circulating microparticles. Arch Med Res. 2013;44:409–414. doi: 10.1016/j.arcmed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Ayers L, Ferry B, Craig S, Nicoll D, Stradling JR, Kohler M. Circulating cell-derived microparticles in patients with minimally symptomatic obstructive sleep apnoea. Eur Respir J. 2009;33:574–580. doi: 10.1183/09031936.00107408. [DOI] [PubMed] [Google Scholar]
- 53.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS, Investigators Coordinators S. CPAP for Prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 56.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 57.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 58.Phipps JE, Hoyt T, Vela D, Wang T, Michalek JE, Buja LM, Jang IK, Milner TE, Feldman MD. Diagnosis of thin-capped fibroatheromas in intravascular optical coherence tomography images: effects of light scattering. Circ Cardiovasc Interv. 2016;9:e003163. doi: 10.1161/CIRCINTERVENTIONS.115.003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.