Abstract
Purpose
To evaluate differences in diagnostic yield of intra-uterine foetal (iuMR) and post-mortem MRI (PMMR) for complex brain malformations, using autopsy as the reference standard.
Methods
In this retrospective, multicentre study spanning 2 years, we reviewed 13 terminated singleton pregnancies with a prenatal ultrasound finding of complex foetal cerebral abnormalities, referred for both iuMR and PMMR. The iuMR and PMMR studies of the brain were reported independently by two groups of radiologists, blinded to each other’s reports. Descriptive statistics were used to compare differences in intracranial abnormalities with autopsy (and genetic testing, where present) as reference standard.
Results
The median gestational age at termination was 24.6 weeks (IQR 22–29) with median time between delivery and PMMR of 133 h (IQR 101–165). There was full concordance between iuMR and PMMR findings and autopsy in 2/13 (15.3%) cases. Partial concordance between both imaging modalities was present in 6/13 (46.2%) and total discordance in the remainder (5/13, 38.5%). When compared to autopsy, PMMR missed important key findings specifically for neuronal migration and cerebellar anomalies, whereas iuMR appeared to overcall CSF space abnormalities which were less crucial to reaching the final overall diagnosis.
Conclusions
iuMR should be performed to improve foetal phenotyping where there is a prenatal ultrasound for complex foetal brain abnormalities. Reliance on PMMR alone is likely to result in misdiagnosis in a majority of cases.
Electronic supplementary material
The online version of this article (10.1007/s00234-019-02218-9) contains supplementary material, which is available to authorized users.
Keywords: Fetus, Autopsy, Magnetic resonance imaging, Brain, Congenital, Termination of pregnancy
Introduction
Foetal brain malformations occur in 2–3 per 1000 pregnancies [1–3] and are amongst the commonest reasons for terminations of pregnancy [4, 5] given their poor long-term outcomes [6–8]. A genetic basis is frequently responsible [9–13], and there is a risk of disease recurrence in future pregnancies depending upon the nature of the abnormality [14]. An accurate prenatal diagnosis is therefore of utmost importance, for targeting subsequent genetic testing of the fetus and for parental counselling regarding recurrence risk.
Prenatal ultrasound is the primary imaging modality for detecting congenital anomalies, with a high concordance rate for brain abnormalities of approximately 75–80% [15–17] (when compared with post-natal imaging and/or autopsy). Recent technological advances, specifically in ultrafast MRI sequences, mean that intra-uterine foetal MRI (iuMR) is becoming more widely available. It is reported to improve upon the sonographic accuracy for foetal brain anomalies by an additional 16% [18, 19] and help in refining prognostic information for 20% of cases [20–22]. Nevertheless, iuMR is not widely available even in tertiary obstetric centres and some women find undertaking the examination physically challenging [23, 24]. Both clinician and patient may also perceive the additional information as unnecessary, if there is a high confidence in the sonographic diagnosis and a post-mortem examination following termination of pregnancy has been agreed to confirm the diagnosis.
Post-mortem diagnoses of foetal brain malformations have traditionally been provided by brain autopsy, although declining rates of parental consent [25–32] for this invasive procedure have led to perinatal post-mortem MRI (PMMR) becoming an increasingly utilised adjunct, and sometimes replacement, tool. Large cohort studies have shown a high concordance rate of 74–92% with brain autopsy [31, 33], and PMMR can also provide additional information over autopsy, especially where there is marked intracranial maceration [31]. In some centres, the brain is no longer examined when PMMR is normal.
It could be therefore argued that given the high concordance rates of both iuMR and PMMR with formal autopsy, only one of these imaging studies is necessary. A recent work by Izzo et al. [34] has challenged this perception. Using the combined results from iuMR and PMMR in the same 53 foetuses as a reference standard, they found that iuMR detected 79% of brain abnormalities but only 45% were detected by PMMR [34], suggesting some loss of information if only performing PMMR. The true diagnostic accuracy rate in their study remains unknown, as they lacked autopsy data in their cohort.
The aim of our study was to evaluate differences in diagnostic yield of foetal brain malformations for iuMR and PMMR, using autopsy as the reference standard. We intended to determine whether one or both are required for accurate diagnoses of foetal brain malformations when malformation was suspected based on prenatal US.
Materials and methods
Ethical approval and consent
A site-specific waiver of formal ethics approval was obtained from three different participating institutions to allow for retrospective data collection and sharing of anonymised clinical and imaging information. All women taking part provided verbal consent for iuMR studies and written informed consent was obtained from all parents for PMMR and autopsy, where performed.
Study cohort
A retrospective review of the radiology information systems (RIS) of three different institutions was conducted for a 2-year period spanning 1 January 2015–1 January 2017. Each of the three institutions had capabilities of performing both iuMR and PMMR examinations on site.
Cases were reviewed based on the following inclusion criteria:
Tertiary prenatal ultrasound study suggesting a foetal brain malformation (at ≥ 19 weeks gestation)
Subsequent iuMR showing a brain abnormality (not necessarily the same abnormality)
Termination of pregnancy for foetal brain abnormality by parental request
Perinatal PMMR
No formal exclusion criteria were set. Demographic details obtained for each patient included date of birth/death, gestational age at termination of pregnancy, prenatal history, method used to terminate pregnancy and prenatal sonographic diagnosis (thus the referral indications for iuMR and PMMR examinations). The time between termination of pregnancy and PMMR was calculated in hours, taking the time of foetal demise as either time of foetal intracardiac injection, time of maternal administration of oral mifepristone or time of foetal demise during delivery. The results of a conventional post-mortem examination by a foetal pathologist at the institution of origin were collated, where this was available.
Magnetic resonance imaging
iuMR and PMMR examinations were performed according to local institutional protocols on either a 3T Ingenia (Philips Healthcare, Amsterdam, The Netherlands), 3T GE Excite (GE Medical Systems, Milwaukee, USA) or 1.5T Avanto (Siemens, Erlangen, Germany) MRI scanner.
Intra-uterine fetal MRI
For all cases, the entire fetus was imaged in three orthogonal planes with T2-weighted single shot echoplanar imaging with the addition of T1 axial-, diffusion- and susceptibility-weighted imaging in the axial plane for the cranial portion of the study. The intracranial diagnoses were predominantly made by assessment of the T2-weighted imaging, and the imaging parameters for this sequence are given in Table 1.
Table 1.
Intra-uterine MR parameters for axial T2-weighted acquisition of intracranial structures
| Site 1 3T Philips Ingenia (N = 9) |
Site 2 3T GE Excite (N = 2) |
Site 3 3T Siemens Magnetom Verio (N = 2) |
|
|---|---|---|---|
| Coil | 32-channel body array | 8-channel cardiac coil | 6-channel body + spine array; 12 channels |
| T2 W seq | Ultrafast SE | SSFSE | HASTE |
| TR/TE (ms) | 10,000/3000 | 4000/327 | 2000/103 |
| FOV (mm) | 180 (dS zoom) | 260 (dep on patient) | 340 |
| Slice | 3 mm | 3 mm/skip 0.3 | 3 mm |
| Matrix | 184 × 150 | 256 × 224 | 256 × 256 |
| Voxel | 1.0 × 1.1 × 3 | 1.0 × 1.1 × 3.3 | 1.3 × 1.3 × 3 |
| Excitations | 2 | 1 | 1 |
| Bandwidth (Hz/pixel) | 452.9 | 31.25 | 781 |
seq sequence, TR repetition time, TE echo time, TI inversion time, ax axial, cor coronal, sag sagittal, FOV field of view, Hz Hertz, SE spin echo, SSFSE single shot fast spin echo, HASTE half-Fourier acquisition single shot turbo spin echo
Perinatal post-mortem MRI
PMMR was performed within 4–5 days of delivery and in all cases. The entire fetus was imaged, with T2-weighted imaging in three orthogonal planes and T1, susceptibility-weighted (SWI) and diffusion-weighted imaging (DWI) in the axial plane only. Scan parameters for T2-weighted imaging performed at PMMR are provided in Table 2.
Table 2.
Postmortem MR parameters for axial T2-weighted acquisition of the foetal head
| Site 1 1.5T Siemens Symphony (N = 8) |
Site 1 3T Philips Ingenia (N = 1) |
Site 2 3T GE Excite (N = 2) |
Site 3 3T Siemens Magnetom Verio (N = 2) |
|
|---|---|---|---|---|
| Coil | 16-channel head | 8-channel paediatric head | 12-channel knee or 16-channel head | 12-channel head with 4-channel neck |
| T2 W seq (ax/cor/sag) | SPACE | TSE–ETL 30 | FSE (ax/cor/sag) | TSE |
| TR/TE |
3200/388 TI 1100 |
3500/160 | 3320/110 | 2500 / 73 |
| FOV | 250 | 100 |
160 (ax) 140 (sag/cor) |
150 |
| Slice (mm) | 1 | 3 no skip | 3/skip 0.3 | 3 |
| Matrix | 512 × 516 | 168 × 168 |
192 × 320 (ax) 192 × 352 (cor/sag) |
163 × 256 |
| Voxel (mm) | 0.5 × 0.5 × 1.0 | 0.6 × 0.6 × 3 | 0.7 × 0.4 × 3.3 | 0.7 × 0.6 × 3 |
| Excitations | 1 | 1 | 2 | 1 |
| Bandwidth (Hz/pixel) | 435 | 31.25 | 222 |
seq sequence, TR repetition time, TE echo time, TI inversion time, ax axial, cor coronal, sag sagittal, FOV field of view, Hz Hertz, TSE turbo spin echo, ETL echo train length, FSE fast spin echo, SPACE sampling perfection with application optimised contrasts using different flip angle evolution
Termination of pregnancy
Feticide by intracardiac injection was performed by qualified obstetricians only where foetuses were ≥ 23 weeks of gestation. The agent used to procure asystole was either lignocaine or potassium chloride (KCl). Oral mifepristone was administered, followed by misoprostol 24–48 h later to induce labour and delivery in all cases. Foetuses were then kept in cool storage within the mortuary at 4 °C until the time of PMMR.
Image analysis
All radiologists were provided with the same clinical information: estimated date of delivery (or death), gestational age of fetus, prenatal ultrasound findings and clinical indication for iuMR or PMMR examination. None of the radiologists had access to the prenatal ultrasound images or autopsy results. A structured reporting template for recording the intracranial anatomical abnormalities and overall diagnosis was completed for all MRI examinations (see online electronic supplementary material).
The iuMR examinations were independently reported by a single radiologist with 15 years of experience in foetal imaging and without knowledge of the PMMR results. The PMMR studies were reported by two paediatric radiologists in consensus with 12 and 3 years post-mortem imaging experience, blinded to the iuMR results. The radiologists interpreting the PMMR were not involved in providing the primary clinical report for the PMMR. The radiologist interpreting the iuMRs was not involved in providing the original clinical interpretation for iuMR studies performed at sites 2 and 3, only at site 1.
Autopsy and genetic testing
An autopsy was defined as any examination by a foetal pathologist, regardless of whether this was limited to external review, examination of the unfixed brain or organ retention with histopathological sectioning, dictated by parental consent. The pathologists were blinded to the PMMR interpretations undertaken for this study but had access to the iuMR reports. Genetic testing was performed at the discretion of foetal medicine specialists and pathologists in selected cases.
Statistical analysis
Our primary outcome was level of concordance for final diagnosis of foetal brain malformation on iuMR and PMMR, with foetal autopsy where available as our reference standard. Descriptive statistics were performed and the case results collated in Microsoft Excel (Microsoft, Seattle, USA).
Results
Study cohort
Thirteen women and their singleton foetuses met our inclusion criteria with nine, two and two cases from institution sites 1, 2 and 3, respectively. The prenatal ultrasound findings and thus referral indications for further MRI imaging with gestational age of fetus are provided in Table 3.
Table 3.
Individual case diagnoses and referral indications for foetuses included in this study
| GA | Prenatal ultrasound findings | Intracardiac injection (ToP) | Intra-uterine foetal MRI diagnosis | PMMR diagnosis | Autopsy diagnosis | Comments | Genetic testing |
|---|---|---|---|---|---|---|---|
| Concordant cases | |||||||
| 29 | ACC | KCl |
ACC Left frontal cortical sulcation anomaly |
ACC Left frontal cortical sulcation anomaly |
ACC Left frontal cortical sulcation anomaly |
All three investigations concordant (Fig. 1) | Normal microarray |
| 22 | Enlarged posterior fossa | None | DWM | DWM | DWM | All three investigations concordant | Normal microarray |
| Partial concordance | |||||||
| 22 |
Severe VM Small cerebellum TGA Vertebral anomalies |
None |
VM, aqueductal stenosis RES |
VM Vertebral anomalies |
VACTERL Vertebral and cardiac defects RES |
Both imaging concordant for VM iuMR and PMMR discordant for RES and vertebral anomalies The autopsy confirms RES and vertebral anomalies with additional cardiac defects (Fig. 2) |
None |
| 23 |
ACC VM Microcephaly Small cerebellum No vermis |
Lignocaine |
ACC Enlarged ganglionic eminences Delayed sulcation PVNH Possible tubulinopathy or dystroglycanopathy |
ACC, VM |
Lissencephaly spectrum Technically limited due to maceration |
iuMR and PMMR concordant for ACC and VM, although iuMR demonstrated additional PVNH Brain autopsy limited by maceration, lissencephaly spectrum diagnosed, ACC, and VM not able to detect |
WES–TUBB1 pathogenic mutation (tubulin gene) |
| 22 |
VM Small cerebellum Microphthalmia |
None |
Bilateral VM Small cerebellum Microphthalmia Possible aqueduct stenosis |
Bilateral VM Small cerebellum. Microphthalmia ACC |
Small cerebellum Microphthalmia |
Imaging concordant for VM, small cerebellum, and microphthalmia Imaging discordant for aqueduct stenosis and ACC Autopsy did not identify the ACC or aqueductal stenosis |
Array CGH = gain of chromosome 2q33.1, 0.24 Mb in size Variant of unknown clinical significance |
| 22 |
Partial ACC Interhemispheric cyst |
None |
Hypogenesis CC Persistent BPC |
Hypogenesis CC | Hypogenesis CC |
iuMR and PMMR concordant for hypogenesis of CC but discordant for BPC Autopsy agrees with PMMR findings |
Normal microarray |
| 31 | Severe VM | Lignocaine |
ACC Abnormal bilateral cortical sulcation |
ACC | ACC |
iuMR and PMMR concordant for ACC but not cortical malformation Autopsy confirms PMMR findings |
None |
| 19 | Posterior fossa cyst | None |
Hypogenesis CC DWM |
ACC DWM |
No consent for brain autopsy |
Imaging concordant for DWM, discordant for ACC No consent for brain autopsy (Fig. 4) |
None |
| Discordant cases | |||||||
| 30 |
Bilateral VM Bilateral fixed flexion thumb deformity |
None Intrapartum cephalocentesis performed |
Severe VM Small dysmorphic cerebellum Aqueductal stenosis Suspected L1 CAM mutation |
Large extra-axial haematomas Ventricles disrupted and collapsed |
Extensive ventricular and extra-axial haematoma Collapsed ventricles Aqueductal stenosis Cerebellar heterotopias Absent medullary pyramids on histology |
iuMR and PMMR discordant Autopsy identified many anomalies suspected on iuMR, and genetic testing confirmed the suspicions Intrapartum cephalocentesis hampered PMMR interpretation (Fig. 5) |
Single-gene L1CAM mutation |
| 23 |
Posterior fossa abnormality Small cerebellum |
Lignocaine | Molar tooth malformation, absent cerebellar vermis | Normal | Absent cerebellar vermis |
iuMR and PMMR discordant for absent cerebellar vermis Autopsy confirms iuMR findings (Fig. 6) |
None |
| 29 | Bilateral VM | Lignocaine + KCl | MPPH syndrome | Normal |
Postaxial polydactyly Non-diagnostic brain autopsy |
iuMR and PMMR discordant Autopsy non-diagnostic Genetic testing confirms iuMR suspicions (Fig. 7) |
WES—pathogenic PI3K mutation consistent with diagnosis of MPPH |
| 28 | VM | KCl |
Unilateral intraventricular haemorrhage Periventricular venous haemorrhagic infarction |
Normal | Non-diagnostic brain autopsy |
iuMR and PMMR discordant Autopsy non-diagnostic |
None |
| 24 | Severe VM | Lignocaine |
Bilateral VM Subependymal nodular heterotopia Filamin A mutation suggested |
Unilateral VM ACC | No consent for brain autopsy |
iuMR and PMMR discordant for nodular heterotopia and ACC No consent for brain autopsy |
FLNA mutation and maternal cranial imaging negative |
ACC absent corpus callosum, BPC Blake’s pouch cyst, CC corpus callosum, CSP cavum septum pellucidum, FLNA filamin A, iuMR intra-uterine foetal MR, KCl potassium chloride, L1CAM L1 cell adhesion molecule, MPPH megalencephaly, perisylvian polymicrogyria, polydactyly and hydrocephalus, PMG polymicrogyria, PMMR perinatal postmortem MRI, RES rhombencephalosynapsis, TGA transposition of the great arteries, ToP termination of pregnancy, VACTERL vertebral, anal, cardiac, tracheoesophageal, renal and limb anomalies, VM ventriculomegaly, WES whole exome sequencing
The median gestational age at termination of pregnancy was 24.6 weeks with an interquartile range (IQR) of 22 to 29 weeks. The iuMR preceded the termination of pregnancy by less than 6 days in all cases. Median time between termination of pregnancy and post-mortem MRI was 133 h (i.e. 5.5 days; IQR 101–165 h, 4.2–6.9 days).
Termination of pregnancy was achieved via intracardiac lignocaine (n = 4), potassium chloride (n = 2), both lignocaine and potassium chloride together (n = 1) or no intracardiac injections (n = 6). In this latter subgroup, one foetal death occurred during Caesarean section where intrapartum cephalocentesis was performed due to severe cerebral ventricular dilatation. In the remaining five foetuses, only oral mifepristone and misoprostol were administered to the mother to induce delivery.
Autopsy and genetic testing
Pathological assessment of the brain at autopsy was possible in 11 of the 13 cases. Of these, two cases were non-diagnostic and one was technically limited due to maceration-related changes.
Genetic testing was performed in eight (8/11, 72.7%) cases. A normal microarray was found in three cases. One case demonstrated an abnormality of unknown significance (gain of chromosome 2q33.1, 0.24 Mb in size). One test was negative for suspected filamin A mutation and three cases were positive for a specific abnormality (one PI3K mutation, one L1 cell adhesion molecule (L1CAM) mutation and one TUBB1 mutation).
Foetal brain malformation diagnoses
Details of the iuMR, PMMR and autopsy findings for each case are outlined in Table 3. There were two cases where iuMR and PMMR were concordant; six cases where they were partially concordant and five cases for complete discordance.
Concordant cases
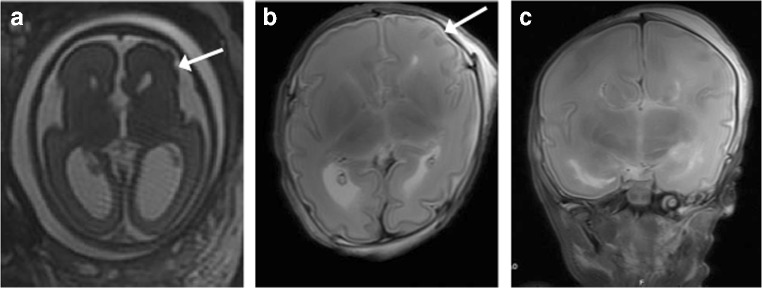
Both iuMR and PMMR findings were concordant in two cases (2/13; 15.3%), one of Dandy Walker malformation and the other with complete callosal agenesis and unilateral frontal oversulcation (Fig. 1). The autopsy results were also concordant with both cases.
Fig. 1.
Agenesis of the corpus callosum (ACC) with unilateral left frontal oversulcation in a 29-week gestation fetus. a Axial T2-weighted imaging of the foetal brain on iuMR demonstrates the lack of callosal tissue and oversulcation in the left frontal lobe (white arrow). b Axial T2-weighted PMMR in the same fetus shows similar results, although the oversulcation (white arrow) is less marked. c Coronal T2-weighted PMMR of the fetus shows the typical steer-horn appearance in absent corpus callosum
Partially concordant cases
There was partial concordance in six cases (6/13, 46.2%), of which five had autopsy correlation.
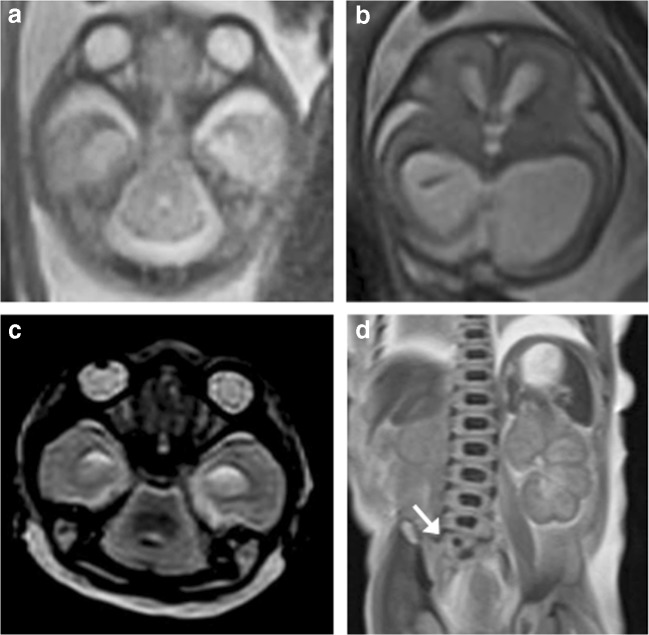
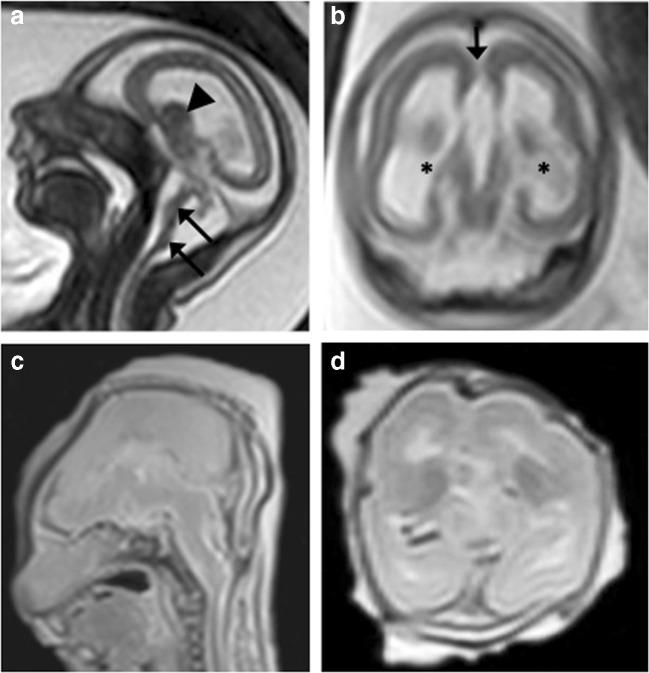
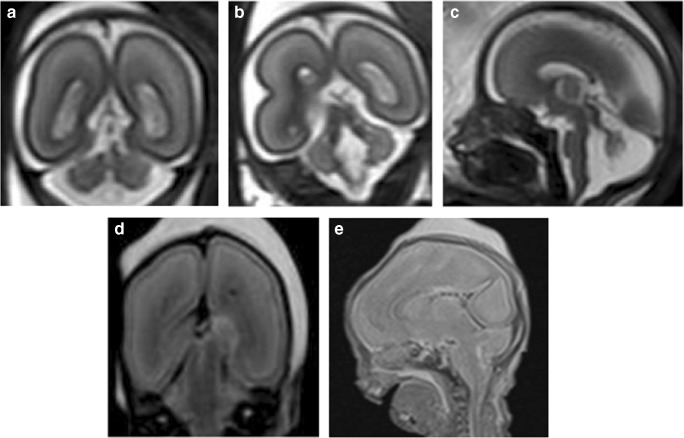
Of these five cases, PMMR ‘overcalled’ ventriculomegaly and ACC in one case, missed a rhomboencephalosynapsis (RES) in a case with vertebral, anal, cardiac, tracheoesophageal, renal and limb anomalies (VACTERL) (Fig. 2) and neuronal migration anomaly in a third case (Fig. 3). In the other two cases, the PMMR agreed with the autopsy findings. The iuMR ‘overcalled’ a Blake’s pouch cyst (BPC) for one case, abnormal bilateral cortical sulcation in another case and did not identify the vertebral anomalies in the case with VACTERL (Fig. 2).
Fig. 2.
VACTERL spectrum anomalies in a 22-week gestation fetus. a, b Axial T2-weighted iuMR images at two different levels demonstrate a typical ‘ball’-shaped appearance of rhombencephalosynapsis (RES) and also lateral and third ventricular dilatation suggestive of aqueductal stenosis. c The T2-weighted PMMR image also shows a deficient cerebellar vermis with hemispheric fusion, although this was not appreciated. d Coronal T2-weighted PMMR of the body did however demonstrate a vertebral segmental anomaly (white arrow) that was not seen on iuMR
Fig. 3.
Tubulinopathy in a 23-week gestation fetus. a Axial T2-weighted iuMR demonstrates microcephaly, severely thinned hemispheric parenchyma, thinly kinked brainstem (black arrows), small malformed cerebellum with enlarged ganglionic eminence (arrowhead). b Coronal single shot T2-weighted iuMR image demonstrates bilateral lateral ventriculomegaly (asterisks) and an absent corpus callosum (black arrow). c, d Coronal and axial T2-weighted PMMR images show decompressed lateral ventricles. There is also difficulty in appreciating the intra-uterine cerebellar abnormalities
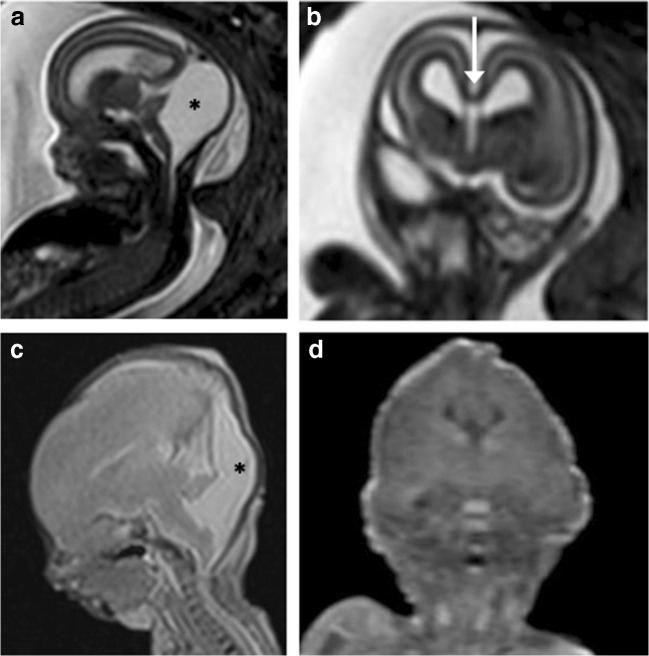
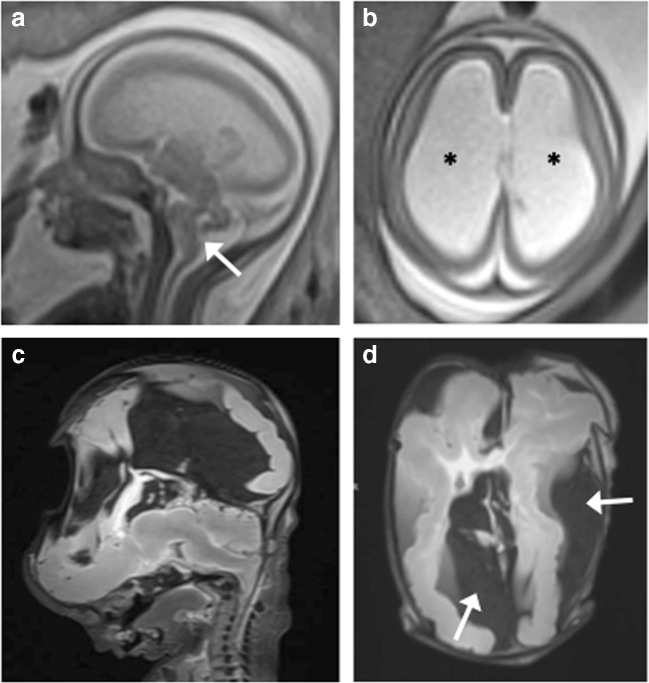
In the one case where there was no autopsy correlation, both imaging modalities described a DWM but could not agree where there was absence or hypoplasia of the corpus callosum (Fig. 4).
Fig. 4.
Dandy−Walker malformation (DWM) with callosal agenesis in a 19-week gestation fetus. a Sagittal iuMR showing upwardly rotated cerebellar vermis with large retrocerebellar CSF space (asterisk) enlarging the posterior fossa, consistent with an enlarged Blake’s pouch cyst. b Coronal T2-weighted iuMR demonstrates a thinned corpus callosum (white arrow) at the base of the anterior hemispheric fissure. c Sagittal T2-weighted and d coronal T1-weighted PMMR images were interpreted as a Dandy–Walker malformation with agenesis of the corpus callosum given the ‘steer-horn’ appearances of the lateral ventricles on the coronal view and posterior fossa cyst (asterisk)
Discordant cases
In the remainder of the cases (5/13, 38.5%), the findings between PMMR and iuMR were discordant. Of these, autopsy correlation was available for two cases. There was no parental consent for autopsy in one case, and in two cases, the autopsy was non-diagnostic.
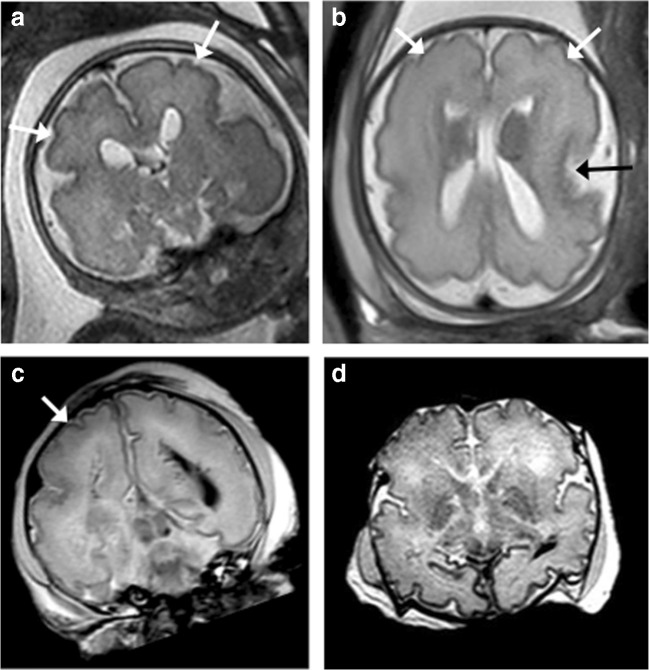
In the two cases with autopsy reference, the PMMR did not identify any features of the final intracranial pathology. This included one case of suspected L1CAM mutation where the iuMR was able to demonstrate multiple findings (i.e. ventriculomegaly, dysmorphic cerebellum, kinked brainstem, aqueductal stenosis). However, given that this fetus had required cephalocentesis to allow delivery of the head via caesarean section, PMMR was only able to show extensive intracranial haemorrhage (Fig. 5). The other case was of a 23-week gestation fetus with an absent cerebellar vermis on iuMR, where the autopsy confirmed underlying Joubert’s syndrome (Fig. 6).
Fig. 5.
L1CAM (cell adhesion molecule) abnormality in a 30-week gestation fetus, with aqueductal stenosis. The fetus was also reported to have adducted thumbs on prenatal ultrasound examination. a Coronal and b axial T2-weighted iuMR images demonstrate kinking of the pontomesencephalic junction (white arrow), with severe ventriculomegaly (asterisks). The ganglionic eminences were not enlarged. c Sagittal and d axial T2-weighted PMMR following intrapartum cephalocentesis revealed collapsed ventricles and large extra-axial haematomas (white arrows). The haematomas were sustained during delivery but misinterpreted at PMMR as having been sustained prenatally
Fig. 6.
Abnormal cerebellar features in a 23-week gestation fetus. a, b Coronal T2-weighted iuMR images reveal features suggestive of an absent cerebellar vermis. The ‘buttocks sign’ is show in image a, and in image b, the ‘molar tooth appearance’ of elongated superior cerebellar peduncles is featured. c Sagittal T2-weighted iuMR image depicts the characteristic ‘figure of 7’ appearance of the elongated superior cerebellar peduncles and small cerebellum. d, e Coronal and sagittal T2-weighted PMMR images do not demonstrate the cerebellar abnormalities
In the two non-diagnostic autopsy cases, the PMMR was reported as normal; however, in one of these cases, the iuMR reported features of MPPH syndrome, which was later confirmed from genetic whole genome sequencing results (Fig. 7) and in the other iuMR demonstrated intraventricular haemorrhage and periventricular venous infarction. There was no genetic testing in this latter case.
Fig. 7.
Megalencephaly polymicrogyria polydactyly and hydrocephalus (MPPH) syndrome due to PIK3R2 mutation in a 29-week gestation fetus. a Coronal and b axial T2-weighted iuMR images demonstrate bilaterally enlarged cerebral hemispheres and perisylvian polymicrogyria (white arrows). There was also a misshapen Sylvian fissure (black arrow). c Coronal and d axial T2-weighted PMMR images also demonstrate polymicrogyria (white arrows), particularly of the right cerebral hemisphere although this was harder to appreciate than on iuMR
In the final case where brain autopsy was declined, the PMMR depicted an absent corpus callosum and unilateral ventriculomegaly. The iuMR demonstrated bilateral ventriculomegaly with subependymal nodular heterotopia. A potential diagnosis for filamin A mutation was suggested but subsequently the genetic testing was found to be negative.
Discussion
This study showed poor complete concordance between iuMR and PMMR for foetal brain malformations, particularly where conditions relied on a constellation of individual findings. Given that accurate phenotyping often facilitates specific genetic diagnoses, we interpret these results to mean that information on recurrence risk may be missed if PMMR examination is performed in the absence of iuMR, particularly after feticide has been performed. This has implications for parental genetic testing, pre-implantation genetic testing of embryos and tertiary referral for neurosonography and foetal MR for subsequent pregnancies.
The only other study in the literature comparable to our work was published by Izzo et al. [34], supporting similar conclusions. The authors reported that iuMR was able to demonstrate 79% of foetal brain abnormalities, compared with 45% by PMMR, using the combined results from iuMR and PMMR as a reference standard. For both modalities, the discrepancies were mostly due to false negative (i.e. ‘missed’) findings. At PMMR, these were more commonly due to CSF spaces, posterior fossa and brainstem-related findings as well as microcephaly and macrocephaly. For iuMR, the discrepancies were mainly related to lamination abnormalities or haemorrhagic lesions. Both modalities were reported to be similarly effective at identifying midline malformations and abnormalities of the ganglionic eminences.
In our study, the discrepant findings were due to both under- and overdiagnosis of callosal agenesis and underdiagnosis of other abnormalities that were key to diagnostic specificity, phenotyping and counselling about recurrence risk. These included brainstem and cerebellar malformations with specific pathological correlates (absent cerebellar vermis, kinking of the brainstem in L1CAM and tubulinopathy), perisylvian polymicrogyria, subependymal heterotopias and enlarged ganglionic eminences. It is interesting to note that only 20% (1/5) of the discordant cases did not have an intracardiac injection for termination of pregnancy, compared with 65% (4/6) partially concordant and 50% (1/2) concordant cases. In the case of the one discordant case that did not have intracardiac injection, there was intrapartum cephalocentesis performed during the delivery. These discrepant findings, although not statistically significant given our small numbers, seem to suggest that foetal intervention plays a part in the level of disagreement between the iuMR and PMMR findings. Feticide may increase foetal cerebral oedema and tissue lysis and mask some of the more subtle intracranial post-mortem findings. It may be possible that PMMR findings would show better concordance with iuMR and eventual autopsy in cases where feticide was not carried out, such as in stillbirths, intra-uterine deaths or cases of slightly lower gestational ages that do not require intracardiac injection for termination of pregnancy.
Whilst we demonstrated a greater disparity in iuMR and PMMR results, it is important to note several differences between our two studies. Firstly, the iuMR studies performed by Izzo et al. [34] were conducted on a 1.5-T magnet, rather than 3-T magnet in our case, and secondly over half of our foetuses (compared to none in the Izzo et al. study [34]) underwent termination of pregnancy with intracardiac potassium chloride injection. Finally, our case cohort were of a slightly more advanced gestational age which may have allowed certain anomalies to be better appreciated on iuMR, such as delayed sulcation and abnormalities of the Sylvian fissure. We also used independent radiologist reporting for iuMR and PMMR and deliberately used genetic testing and conventional PM (sometimes referred to as ‘genetic post-mortem’), where available, as the reference standard.
Our results contrast to the work by Whitby et al. [35], where they assessed the diagnostic accuracy of prenatal ultrasound and iuMR for foetal neurological anomalies, using the combined autopsy and PMMR examination as the reference standard. They found that in their 12 study subjects, iuMR provided a correct diagnosis for all cases, demonstrating a high level of concordance with PMMR and autopsy findings. This is unsurprising, as the accuracy or yield of PMMR was not assessed independently of the autopsy findings.
To our knowledge, there are no other studies that have assessed diagnostic accuracy rates for iuMR performed at 3 T and PMMR in the same cohort of patients, although several larger trials have assessed the accuracy for PMMR and iuMR individually, with autopsy or postnatal imaging as the reference standards.
For the detection of brain malformations on PMMR, an overall concordance rate of 73.8% (sensitivity 79.0%, specificity 71.7%) was reported for the detection of brain malformations on PMMR in an unselected population of 218 foetuses within the Magnetic Resonance Imaging Autopsy Study (MARIAS trial) [31] and Ashwin et al. [33] also found a high concordance rates of 92.2% (sensitivity 94.0%, specificity 90.9%) in a different cohort of 166 unselected foetuses. In these studies, the majority of the discrepancies were false positive findings, relating to intracranial haemorrhage, neuronal migrational and callosal anomalies. The true positive findings were usually due to a single intracranial abnormality rather than a combination of findings, and no specific details on genetic testing or unifying or syndromal diagnoses were provided.
These studies likely reflect the accuracy of PMMR for major rather than minor intracranial abnormalities, particularly as foetal neuropathology is very challenging even for the foetal pathologist, and autopsy may have been difficult to perform on some foetuses at early gestation. We do not challenge the use of PMMR as an excellent screening tool in unselected cases, but where significant neuropathology is identified on antenatal imaging our experience from this study showed that there were abnormalities which could not be seen on PMMR that were indisputably present in utero, confirmed at genetic post-mortem, and that would have been ‘missed’ if PMMR was the only imaging performed. The reasons for these discrepancies may have included anatomical distortion from intrapartum trauma [36], post-mortem cerebral oedema, collapse of cystic structures [37], descent and compression of posterior fossa structures or lack of recognition of severe macro- or microcephaly as diagnostic clues, due to the absence of normative data for post-mortem foetal biometry. There is also the possibility that foetal brain appearances may be less apparent on MR imaging following the iatrogenic effects from agents used to produce feticide [38]. Finally, whilst our post-mortem interval (time between delivery/death to imaging) was broadly consistent with other studies showing high PMMR concordance rates [31, 33] (i.e. less than 7 days), it has been suggested that a lower diagnostic yield may be seen when foetuses are imaged after a prolonged intra-uterine retention rate and later than 24 h from delivery [34]. However, this is challenging for foetuses greater than 24 weeks of gestation when intracardiac injection is routinely performed prior to onset of labour, and thus foetal demise precedes delivery by at least 36–48 h. For these reasons, the use of iuMR is required to ensure key diagnostic information is not ‘lost’ as a result of inevitable post-mortem changes [39].
The largest multicentre trial on iuMR diagnostic accuracy for foetal brain malformations (i.e. Magnetic Resonance Imaging to Enhance the Diagnosis of Fetal Developmental Brain Abnormalities In Utero (MERIDIAN) study) found a high concordance rate of 93% for iuMR [20] with autopsy and postnatal imaging and showed that iuMR was beneficial in aiding clinical management, regardless of whether the prenatal ultrasound findings are related to ventriculomegaly [40], commissural [41] and posterior fossa anomalies [42]. Nonetheless, there was a 7% error rate by iuMR (40/570 in the MERIDIAN cohort [43]), and imaging quality during iuMR can vary due to foetal motion artefacts, imaging technique and lack of isovolumetric images for multiplanar reconstruction of complex anatomy, due to the time-consuming nature of 3D acquisitions [44]. As such, post-mortem examinations will still be important for corroborating prenatal findings, and PMMR still has a place for families where invasive autopsies are refused [45, 46] as was the case in two of our subjects whose families permitted external PM only. In addition, in two other cases, post-mortem maceration adversely affected the ability of the pathologist to accurately perform a comprehensive autopsy.
Limitations of this study include the retrospective nature of the work and small sample size. To address our specific research question, there was a referral or selection bias, given that the MRI examinations were conducted for foetuses with suspicion of brain malformations based on tertiary prenatal US. In addition, because this was a retrospective study, our pathologists were not blinded to either the iuMR findings or the PMMR results. However, complete agreement of the iuMR diagnosis with the results of whole exome or specific genetic testing in three cases could not be influenced by unblinding of the pathologist.
The strengths of this study include the multicentre origin of the case load, iuMR performance at 3 T and the use of ‘expert’ readers in the independent interpretation of iuMR and PMMR, blinded to each other’s radiological opinions. We also include the autopsy data and genetic testing results, where available, as our reference standard against which the index tests were measured.
In summary, we advocate performing iuMR for accurate diagnosis of foetal brain malformations in cases where there is a known abnormality or suspicion based on tertiary prenatal ultrasound, independent of post-mortem imaging. iuMR can provide additional information for improved phenotyping of the brain malformations and thus a better chance of targeting and interpreting genetic testing. This is particularly important in the age of next-generation sequencing technology using whole exome sequencing (WES) [11, 47], as a single gene or panel testing may be more appropriate and cost-effective. Further work in a larger sample size with complete autopsy as a reference standard will enable a more robust evidence basis to inform future clinical practice.
Conclusion
Where tertiary prenatal ultrasound detects or suggests a foetal brain abnormality, iuMR should be performed to improve foetal phenotyping and diagnostic accuracy. Reliance on PMMR alone may result in misdiagnosis and incorrect phenotyping in the majority of cases.
Electronic supplementary material
(DOCX 13 kb)
Abbreviations
- ACC
Absent corpus callosum
- BPC
Blake’s pouch cyst
- CC
Corpus callosum
- CSF
Cerebrospinal fluid
- CSP
Cavum septum pellucidum
- ETL
Echo train length
- FLNA
Filamin A
- FOV
Field of view
- FSE
Fast spin echo
- HASTE
Half-Fourier acquisition single shot turbo spin echo
- iuMR
Intra-uterine magnetic resonance imaging
- KCl
Potassium chloride
- L1CAM
L1 cell adhesion molecule
- MARIAS
Magnetic Resonance Imaging Autopsy Study
- MERIDIAN
Magnetic Resonance Imaging to Enhance the Diagnosis of Fetal Developmental Brain Abnormalities In Utero
- PMG
Polymicrogyria
- MPPH
Megalencephaly, perisylvian, polymicrogyria, polydactyly and hydrocephalus
- MRI
Magnetic resonance imaging
- PMI
Post-mortem interval
- PMMR
Post-mortem magnetic resonance imaging
- RES
Rhombencephalosynapsis
- SE
Spin echo
- SPACE
Sampling perfection with application optimised contrasts using different flip angle evolution
- SSFSE
Single shot fast spin echo
- TE
Echo time
- TGA
Transposition of the great arteries
- TI
Inversion time
- ToP
Termination of pregnancy
- TR
Repetition time
- TSE
Turbo spin echo
- VACTERL
Vertebral, anal, cardiac, tracheoesophageal, renal and limb anomalies
- VM
Ventriculomegaly
- WES
Whole exome sequencing
Funding
This study was funded by a RCUK/UKRI Innovation Fellowship and Medical Research Council (MRC) Clinical Research Training Fellowship, jointly sponsored by the Royal College of Radiologists (Grant Ref: MR/R00218/1). Funding was also received from the National Institute for Health Research (NIHR) Career Development Fellowship (NIHR-CDF-2017-10-037), Great Ormond Street Children’s Charity and the Great Ormond Street Hospital NIHR Biomedical Research Centre.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boyd PA, Tonks AM, Rankin J, Rounding C, Wellesley D, Draper ES, BINOCAR Working Group Monitoring the prenatal detection of structural fetal congenital anomalies in England and Wales: register-based study. J Med Screen. 2011;18(1):2–7. doi: 10.1258/jms.2011.010139. [DOI] [PubMed] [Google Scholar]
- 2.Boyle B, Addor MC, Arriola L, Barisic I, Bianchi F, Csaky-Szunyogh M, de Walle HEK, Dias CM, Draper E, Gatt M, Garne E, Haeusler M, Kallen K, Latos-Bielenska A, McDonnell B, Mullaney C, Nelen V, Neville AJ, O'Mahony M, Queisser-Wahrendorf A, Randrianaivo H, Rankin J, Rissmann A, Ritvanen A, Rounding C, Tucker D, Verellen-Dumoulin C, Wellesley D, Wreyford B, Zymak-Zakutnia N, Dolk H. Estimating Global Burden of Disease due to congenital anomaly: an analysis of European data. Arch Dis ChildFetal Neonatal Ed. 2018;103(1):F22–F28. doi: 10.1136/archdischild-2016-311845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groen H, Bouman K, Pierini A, Rankin J, Rissmann A, Haeusler M, Yevtushok L, Loane M, Erwich J, de Walle HEK. Stillbirth and neonatal mortality in pregnancies complicated by major congenital anomalies: findings from a large European cohort. Prenat Diagn. 2017;37(11):1100–1111. doi: 10.1002/pd.5148. [DOI] [PubMed] [Google Scholar]
- 4.Vaknin Z, Lahat Y, Barel O, Ben-Ami I, Reish O, Herman A, Maymon R. Termination of pregnancy due to fetal abnormalities performed after 23 weeks' gestation: analysis of indications in 144 cases from a single medical center. Fetal Diagn Ther. 2009;25(2):291–296. doi: 10.1159/000229501. [DOI] [PubMed] [Google Scholar]
- 5.Aslan H, Yildirim G, Ongut C, Ceylan Y. Termination of pregnancy for fetal anomaly. Int JGynaecol Obstet. 2007;99(3):221–224. doi: 10.1016/j.ijgo.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 6.Breeze AC, Alexander PM, Murdoch EM, Missfelder-Lobos HH, Hackett GA, Lees CC. Obstetric and neonatal outcomes in severe fetal ventriculomegaly. Prenat Diagn. 2007;27(2):124–129. doi: 10.1002/pd.1624. [DOI] [PubMed] [Google Scholar]
- 7.Volpe P, Muto B, Passamonti U, Rembouskos G, De Robertis V, Campobasso G, Tempesta A, Volpe G, Fanelli T. Abnormal sonographic appearance of posterior brain at 11-14 weeks and fetal outcome. Prenat Diagn. 2015;35(7):717–723. doi: 10.1002/pd.4598. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez RM, Garcia-Diaz L, Marquez J, Fajardo M, Rivas E, Garcia-Lozano JC, Antinolo G. Hemimegalencephaly: prenatal diagnosis and outcome. Fetal Diagn Ther. 2011;30(3):234–238. doi: 10.1159/000329937. [DOI] [PubMed] [Google Scholar]
- 9.Xiang J, Zhang L, Jiang W, Zhang Q, Wang T, Li H. Prenatal diagnosis and genetic analysis of a fetus with Joubert syndrome. Biomed Res Int. 2018;2018:1–6. doi: 10.1155/2018/7202168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Encha-Razavi F. Identification of brain malformations: neuropathological approach. Childs Nerv Syst. 2003;19(7–8):448–454. doi: 10.1007/s00381-003-0764-7. [DOI] [PubMed] [Google Scholar]
- 11.Best S, Wou K, Vora N, Van der Veyver IB, Wapner R, Chitty LS. Promises, pitfalls and practicalities of prenatal whole exome sequencing. Prenat Diagn. 2018;38(1):10–19. doi: 10.1002/pd.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Normand EA, Braxton A, Nassef S, Ward PA, Vetrini F, He W, Patel V, Qu C, Westerfield LE, Stover S, Dharmadhikari AV, Muzny DM, Gibbs RA, Dai H, Meng L, Wang X, Xiao R, Liu P, Bi W, Xia F, Walkiewicz M, Van den Veyver IB, Eng CM, Yang Y. Clinical exome sequencing for fetuses with ultrasound abnormalities and a suspected Mendelian disorder. Genome Med. 2018;10(1):74. doi: 10.1186/s13073-018-0582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark GD. Brain development and the genetics of brain development. Neurol Clin. 2002;20(4):917–939. doi: 10.1016/S0733-8619(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 14.Glinianaia SV, Tennant PW, Rankin J. Risk estimates of recurrent congenital anomalies in the UK: a population-based register study. BMC Med. 2017;15(1):20. doi: 10.1186/s12916-017-0789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi AC, Prefumo F. Correlation between fetal autopsy and prenatal diagnosis by ultrasound: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:201–206. doi: 10.1016/j.ejogrb.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Carroll SGM, Porter H, Abdel-Fattah S, Kyle PM, Soothill PW. Correlation of prenatal ultrasound diagnosis and pathologic findings in fetal brain abnormalities. Ultrasound Obstet Gynecol. 2000;16(2):149–153. doi: 10.1046/j.1469-0705.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 17.Eros FR, Simonyi A, Tidrenczel Z, Szabo I, Rigo J, Jr, Beke A. Efficacy of prenatal ultrasound in craniospinal malformations according to fetopathological and postnatal neonatological, pathological results. Fetal Pediatr Pathol. 2018;37(3):166–176. doi: 10.1080/15513815.2018.1461282. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis D, Mooney C, Cohen J, Papaioannou D, Bradburn M, Sutton A, Griffiths PD. A systematic review and meta-analysis to determine the contribution of MR imaging to the diagnosis of foetal brain abnormalities in utero. Eur Radiol. 2017;27(6):2367–2380. doi: 10.1007/s00330-016-4563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths PD, Bradburn M, Campbell MJ, Connolly DJA, Cooper CL, Jarvis D, Kilby MD, Mason G, Mooney C, Robson SC, Wailoo A, Group MC. Change in diagnostic confidence brought about by using in utero MRI for fetal structural brain pathology: analysis of the MERIDIAN cohort. Clin Radiol. 2017;72(6):451–457. doi: 10.1016/j.crad.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths PD, Bradburn M, Campbell MJ, Cooper CL, Graham R, Jarvis D, Kilby MD, Mason G, Mooney C, Robson SC, Wailoo A. Use of MRI in the diagnosis of fetal brain abnormalities in utero (MERIDIAN): a multicentre, prospective cohort study. Lancet. 2017;389:538–546. doi: 10.1016/S0140-6736(16)31723-8. [DOI] [PubMed] [Google Scholar]
- 21.van Doorn M, Oude Rengerink K, Newsum EA, Reneman L, Majoie CB, Pajkrt E. Added value of fetal MRI in fetuses with suspected brain abnormalities on neurosonography: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2016;29:2949–2961. doi: 10.3109/14767058.2015.1109621. [DOI] [PubMed] [Google Scholar]
- 22.Rossi AC, Prefumo F. Additional value of fetal magnetic resonance imaging in the prenatal diagnosis of central nervous system anomalies: a systematic review of the literature. Ultrasound Obstet Gynecol. 2014;44:388–393. doi: 10.1002/uog.13429. [DOI] [PubMed] [Google Scholar]
- 23.Lie MLS, Graham RH, Robson SC, Griffiths PD. MRI for fetal developmental brain abnormalities: perspectives from the pregnant patient. Qual Health Res. 2018;28(8):1295–1307. doi: 10.1177/1049732318764390. [DOI] [PubMed] [Google Scholar]
- 24.van der Knoop BJ, Vermeulen RJ, Verbeke J, Pistorius LR, de Vries JIP. Fetal MRI, lower acceptance by women in research vs. clinical setting. J Perinat Med. 2018;46:983–990. doi: 10.1515/jpm-2016-0360. [DOI] [PubMed] [Google Scholar]
- 25.Shojania KG, Burton EC. The vanishing nonforensic autopsy. N Engl J Med. 2008;358(9):873–875. doi: 10.1056/NEJMp0707996. [DOI] [PubMed] [Google Scholar]
- 26.Dickinson JE, Prime DK, Charles AK. The role of autopsy following pregnancy termination for fetal abnormality. Aust N Z J Obstet Gynaecol. 2007;47:445–449. doi: 10.1111/j.1479-828X.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 27.Cronin RS, Li M, Wise M, Bradford B, Culling V, Zuccollo J, Thompson JMD, Mitchell EA, McCowan LME. Late stillbirth post mortem examination in New Zealand: maternal decision-making. Aust N Z J Obstet Gynaecol. 2018;58:667–673. doi: 10.1111/ajo.12790. [DOI] [PubMed] [Google Scholar]
- 28.Cartlidge PH, Dawson AT, Stewart JH, Vujanic GM. Value and quality of perinatal and infant postmortem examinations: cohort analysis of 400 consecutive deaths. BMJ. 1995;310:155–158. doi: 10.1136/bmj.310.6973.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brodlie M, Laing IA, Keeling JW, McKenzie KJ. Ten years of neonatal autopsies in tertiary referral centre: retrospective study. BMJ. 2002;324:761–763. doi: 10.1136/bmj.324.7340.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thayyil S, Sebire NJ, Chitty LS, Wade A, Chong W, Olsen O, Gunny RS, Offiah AC, Owens CM, Saunders DE, Scott RJ, Jones R, Norman W, Addison S, Bainbridge A, Cady EB, Vita ED, Robertson NJ, Taylor AM. Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet. 2013;382:223–233. doi: 10.1016/S0140-6736(13)60134-8. [DOI] [PubMed] [Google Scholar]
- 31.Arthurs OJ, Thayyil S, Pauliah SS, Jacques TS, Chong WK, Gunny R, Saunders D, Addison S, Lally P, Cady E, Jones R, Norman W, Scott R, Robertson NJ, Wade A, Chitty L, Taylor AM, Sebire NJ. Diagnostic accuracy and limitations of post-mortem MRI for neurological abnormalities in fetuses and children. Clin Radiol. 2015;70:872–880. doi: 10.1016/j.crad.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter B, Tait G, Barnes M, Adkins G, Naylor C, Begum N. Increasing the information available to coroners: the effect on autopsy decision-making. Med Sci Law. 2009;49(2):101–108. doi: 10.1258/rsmmsl.49.2.101. [DOI] [PubMed] [Google Scholar]
- 33.Ashwin C, Hutchinson JC, Kang X, Langan D, Jones R, Norman W, Cannie M, Jani J, Sebire NJ, Arthurs OJ. Learning effect on perinatal post-mortem magnetic resonance imaging reporting: single reporter diagnostic accuracy of 200 cases. Prenat Diagn. 2017;37:566–574. doi: 10.1002/pd.5043. [DOI] [PubMed] [Google Scholar]
- 34.Izzo G, Talenti G, Falanga G, Moscatelli M, Conte G, Scola E, Doneda C, Parazzini C, Rustico M, Triulzi F, Righini A (2018) Intrauterine fetal MR versus postmortem MR imaging after therapeutic termination of pregnancy: evaluation of the concordance in the detection of brain abnormalities at early gestational stage. Eur Radiol. 10.1007/s00330-018-5878-0 [DOI] [PubMed]
- 35.Whitby EH, Variend S, Rutter S, Paley MN, Wilkinson ID, Davies NP, Sparey C, Griffiths PD. Corroboration of in utero MRI using post-mortem MRI and autopsy in foetuses with CNS abnormalities. Clin Radiol. 2004;59:1114–1120. doi: 10.1016/j.crad.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Arthurs OJ, Barber JL, Taylor AM, Sebire NJ. Normal perinatal and paediatric postmortem magnetic resonance imaging appearances. Pediatr Radiol. 2015;45:527–535. doi: 10.1007/s00247-014-3166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebire NJ, Miller S, Jacques TS, Taylor AM, Rennie JM, Kendall G, Chitty LS. Post-mortem apparent resolution of fetal ventriculomegaly: evidence from magnetic resonance imaging. Prenat Diagn. 2013;33:360–364. doi: 10.1002/pd.4065. [DOI] [PubMed] [Google Scholar]
- 38.Diedrich J, Drey E, Society of Family Planning Induction of fetal demise before abortion. Contraception. 2010;81:462–473. doi: 10.1016/j.contraception.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths PD, Mooney C, Bradburn M, Jarvis D. Should we perform in utero MRI on a fetus at increased risk of a brain abnormality if ultrasonography is normal or shows non-specific findings? Clin Radiol. 2018;73:123–134. doi: 10.1016/j.crad.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Robson SC, Vollmer B, Mason G, Griffiths PD, Brackley K, Bradburn M, Connolly DJA, Gawne-Cain ML, Griffiths DI, Kilby MD, Mandefield L, Mooney C. Anatomical subgroup analysis of the MERIDIAN cohort: ventriculomegaly. Ultrasound Obstet Gynecol. 2017;50:736–744. doi: 10.1002/uog.17475. [DOI] [PubMed] [Google Scholar]
- 41.Griffiths PD, Brackley K, Bradburn M, Connolly DJA, Gawne-Cain ML, Griffiths DI, Kilby MD, Mandefield L, Mooney C. Anatomical subgroup analysis of the MERIDIAN cohort: failed commissuration. Ultrasound Obstet Gynecol. 2017;50:753–760. doi: 10.1002/uog.17502. [DOI] [PubMed] [Google Scholar]
- 42.Griffiths PD, Brackley K, Bradburn M, Connolly DJA, Gawne-Cain ML, Kilby MD, Mandefield L, Mooney C, Robson SC, Vollmer B, Mason G. Anatomical subgroup analysis of the MERIDIAN cohort: posterior fossa abnormalities. Ultrasound Obstet Gynecol. 2017;50:745–752. doi: 10.1002/uog.17485. [DOI] [PubMed] [Google Scholar]
- 43.Batty R, Gawne-Cain ML, Mooney C. Analysis of errors made on in utero MR studies of the foetal brain in the MERIDIAN study. Eur Radiol. 2019;29:195–201. doi: 10.1007/s00330-018-5508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frick N, Fazelnia C, Kanzian K, Hitzl W, Fischer T, Forstner R, Bogner G. The reliability of fetal MRI in the assessment of brain malformations. Fetal Diagn Ther. 2015;37:93–101. doi: 10.1159/000363652. [DOI] [PubMed] [Google Scholar]
- 45.Lewis C, Hill M, Arthurs OJ, Hutchinson C, Chitty LS, Sebire NJ. Factors affecting uptake of postmortem examination in the prenatal, perinatal and paediatric setting. BJOG. 2018;125(2):172–181. doi: 10.1111/1471-0528.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis C, Latif Z, Hill M, Riddington M, Lakhanpaul M, Arthurs OJ. "We might get a lot more families who will agree": Muslim and Jewish perspectives on less invasive perinatal and paediatric autopsy. PLoS One. 2018;13(8):e0202023. doi: 10.1371/journal.pone.0202023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandler N, Best S, Hayward J, Faravelli F, Mansour S, Kivuva E, Tapon D, Male A, DeVile C, Chitty LS. Rapid prenatal diagnosis using targeted exome sequencing: a cohort study to assess feasibility and potential impact on prenatal counseling and pregnancy management. Genet Med. 2018;20:1430–1437. doi: 10.1038/gim.2018.30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)