Highlights
-
•
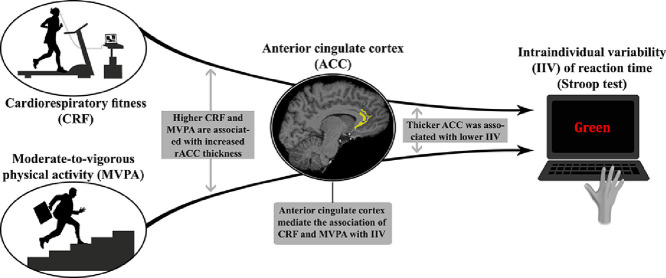
In younger adults, higher CRF and MVPA were associated with greater ACC thickness.
-
•
Greater thickness of the ACC was associated with lower IIV of reaction time in an executive functioning task.
-
•
The association between IIV and both CRF and MVPA was mediated by ACC thickness, suggesting that the structure of this region is a mechanism linking fitness and PA to cognitive performance.
-
•
Our results demonstrate the importance of including neurobiological markers when examining associations between MVPA and cognitive outcomes in a young adult sample.
Keywords: Accelerometry, Aerobic fitness, Cognition, Executive control, Within-person fluctuations
Abstract
Background
Higher levels of cardiorespiratory fitness (CRF) and greater amounts of physical activity have been associated with lower intraindividual variability (IIV) in executive function in children and older adults. In the present study, we examined whether CRF, measured as maximal oxygen uptake (VO2max), and daily volume of moderate-to-vigorous intensity physical activity (MVPA) were associated with IIV of reaction time during performance of the incongruent condition of the Stroop task in younger adults. Further, we examined whether the thickness of the cingulate cortex was associated with regulating variability in reaction time performance in the context of CRF or physical activity.
Methods
CRF (measured as VO2max), accelerometry-measured MVPA, Stroop performance, and thickness of the rostral anterior cingulate cortex (rACC) derived from magnetic resonance imaging data were collected in 48 younger adults (age = 24.58 ± 4.95 years, mean ± SD). Multiple regression was used to test associations between IIV during the Stroop task and CRF, MVPA, and rACC thickness. Mediation was tested using maximum likelihood estimation with bootstrapping.
Results
Consistent with our predictions, higher VO2max was associated with greater rACC thickness for the right hemisphere and greater daily amounts of MVPA were associated with greater rACC thickness for both the left and right hemispheres. Greater thickness of the right rACC was associated with lower IIV for the incongruent condition of the Stroop task. CRF and MVPA were not directly associated with IIV. However, we did find that IIV and both CRF and MVPA were indirectly associated via the thickness of the right rACC.
Conclusion
These results indicate that higher CRF and greater daily volume of MVPA may be associated with lower IIV during the Stroop task via structural integrity of the rACC. Randomized controlled trials of MVPA would provide crucial information about the causal relations between these variables.
Graphical Abstract
1. Introduction
Higher cardiorespiratory fitness (CRF) levels and a greater amount of moderate-to-vigorous intensity physical activity (MVPA) are frequently associated with better mental health and cognitive performance, especially in children and older adults.1, 2, 3, 4 For example, executive function, a cognitive domain associated with the maintenance of goals, working memory, attentional control, and inhibition, is often positively associated with both MVPA and CRF in both children and older adults.5, 6, 7, 8, 9 However, the relationship of MVPA and CRF to executive function is more poorly understood in early adulthood.1, 10, 11 The dearth of research in young adults is likely related to the perception that young adults generally perform quite well on cognitive tasks, leaving limited room for MVPA-related improvements in cognition.2 This assumes, however, that there is restricted variability in cognitive function in young adults and that exposure to MVPA would not explain individual variability in cognitive performance. It also assumes that increases in MVPA would have limited cognitive benefits. These are assumptions that should be tested empirically rather than taken for granted. Prior literature indicates rather equivocal results; some studies have demonstrated positive associations between physical activity (PA) and cognitive performance in young adults, including performance on tasks such as the Stroop test,12,13 although other studies have shown negligible or nonsignificant associations.14, 15, 16, 17
There have been several reasons postulated for the more equivocal associations of MVPA and CRF to cognitive performance in younger adults. For example, there may be ceiling effects on cognitive tests that preclude an assessment of individual differences in performance. In fact, many prior studies use younger adults as a comparison sample for children or older adults. When this is the case, the cognitive tests and instruments used are often optimized for assessing individual variability in cognitive performance in these other age groups, and not in younger adults. There has also been limited use of instruments that objectively measure PA or CRF in prior studies with younger adults.11 Thus, the use of computer-based tests that have increased sensitivity for detecting individual variability in cognitive performance in a young adult age range, the use of objective and gold-standard measures of CRF (e.g., the maximal oxygen uptake (VO2max) test), and accelerometry-derived MVPA data may impact associations by minimizing floor and ceiling effects and improving precision and reliability.18, 19, 20, 21
A promising method for studying the relationships of MVPA and CRF to cognitive function is to use a measure of intraindividual variability (IIV) of response time (RT), which has been validated as an important metric of executive function.22 Specifically, IIV in performance can be measured by examining trial-by-trial fluctuations in RT and reflects dynamic variability in attentional or executive control.23 A growing body of evidence in older adults suggests that it is an important index of information processing efficiency,24 a sensitive indicator of neurobiological integrity,22 and a potential predictor of memory failures,23 dementia,25 and mortality.26
In preadolescents, middle-aged adults, and older adults, higher CRF and greater amounts of PA are associated with reduced IIV on inhibitory control, executive function,9,27, 28, 29, 30, 31 and psychomotor tests.31 Also, in a cross-sectional study of adults 50–90 years old, greater aerobic fitness attenuated the effect of age on IIV. Furthermore, variation in executive performance partly mediated the association.31 These findings indicate that CRF and PA may influence brain systems involved in regulating and reducing trial-by-trial fluctuations in performance.
Unfortunately, the neuroanatomic and neurophysiological correlates of IIV, especially in relation to CRF and MVPA, remain poorly understood. However, several studies have shown associations between neuroimaging metrics and IIV. For example, although IIV was not correlated with frontal brain volume,32 it was associated with inhibitory success related to increased activation of frontal lobe areas, including the anterior cingulate cortex (ACC);24 and higher ACC activation was associated with less IIV during a color-word error awareness task.33 Indeed, greater activity in the default mode network and lower activation of the ACC precedes longer RT during performance of the color-word Stroop test,34, 35 suggesting that lower activation of the ACC could be a biomarker of IIV and inconsistency in RT. Furthermore, there is evidence that older adults who are more physically active have lower IIV and reduced age-related attenuation of prefrontal cortex activity during a task-switching paradigm.30 Thus, the prior literature indicates that the ACC in particular may support attentional control operations that allow for more consistent performance during tasks of executive function. In addition, prior work with older adults has indicated that better CRF is associated with greater volume7,36 and better function of the ACC,8 and that exercise interventions also increase ACC volume37 and functional connectivity of the ACC.38 Thus, the ACC reflects a top neurobiological candidate pathway for regulating IIV.
To our knowledge, there have not been published studies examining the associations of CRF and MVPA with IIV and brain morphology in a young adult sample. Our aim in this study was to test associations of CRF and MVPA with measures of IIV and ACC thickness in a sample of younger adults. Specifically, we aimed to examine the associations of CRF (VO2max) and daily accumulated volume of MVPA with IIV during the Stroop task in younger adults and further relate these associations to the thickness of the ACC. By using a computerized cognitive task with a measurement of IIV in RT, measures of ACC thickness, and objective measures of MVPA and CRF, we are able to test important hypotheses about associations between these measures in young adults. Indeed, cognitive performance and brain function may be modifiable, and MVPA might be a low-cost approach for improving cognitive and brain function, even in young adults. We predicted that higher CRF and greater amounts of MVPA would be associated with lower IIV during the Stroop task and that this association would be statistically mediated by thickness of the ACC. Such associations would highlight the importance of measuring brain morphology in the context of MVPA and CRF in younger adults to better understand individual variability in cognitive performance.
2. Methods
2.1. Study overview
Participants completed all of the assessments in a 1-month period. The first session included cognitive assessments and screening for color blindness, and a second session included a maximal CRF test and fitting for an accelerometer armband device to measure MVPA. Participants also completed a magnetic resonance imaging (MRI) scan. Procedures and protocols have been previously published39, 40 and are briefly described herein.
2.2. Participants
A total of 60 young adults (age: 24.58 ± 4.95 years, mean ± SD; 57% females) were recruited from the University of Pittsburgh and surrounding community. To be eligible, participants had to be between 18 and 40 years of age, native English speakers, and without a history of cardiovascular or cardiometabolic (e.g., type 2 diabetes) or physical conditions that would prevent exercising. They also had to be free of neurological conditions that could affect central nervous system functioning, such as history of head trauma, brain tumors, stroke, psychiatric conditions, or epilepsy. Participants were also excluded for color blindness by use of the Pseudoisochromatic Plates for Testing Color Vision, 16 Plate edition. The present study was approved by the University of Pittsburgh Institutional Review Board, and informed written consent was obtained before data collection. All procedures were in accordance with the Declaration of Helsinki.
2.3. CRF assessment
Participants performed a modified Bruce protocol to evaluate maximal CRF (VO2max). The protocol began with a 1-min warm-up, with participants walking at a speed of 3.0 mph on a motor-driven treadmill at 0 incline. The test itself began at 3.5 mph and 2% grade. Every 2 min, the treadmill speed increased by 0.5 mph and the incline increased by 2%. An exercise physiologist continuously monitored oxygen uptake, heart rate, and blood pressure in accordance with the American College of Sports Medicine guidelines. Participants wore nose clips, and all expired air was collected at 15-s intervals (on average) in a gas-analysis machine via a mouthpiece connected to a two-way valve (ParvoMedics TrueOne 2400 metabolic measurement system; Parvo Medics, Sandy, UT, USA). The mouthpiece was supported by headgear, and the equipment was worn until maximal VO2 was achieved. VO2max was defined as the maximum oxygen consumption value registered (expressed in mL/kg/min). One of 2 criteria defined the maximum effort level: (1) a plateau in VO2peak between 2 or more workloads (0.15 L/min or 2.0 mL/kg/min) or (2) when 2 of the following 3 criteria were met: a respiratory exchange ratio of greater than 1.1, a heart rate within 10 beats of a participant's age-predicted maximum, or a rating of perceived exertion of 17 or greater.
2.4. PA assessment
To estimate the daily accumulated total volume of MVPA, participants were given an accelerometer armband (Body Media SenseWear®, Model MF-SW, BodyMedia Inc., Pittsburgh, PA, USA). The energy expenditure estimation was based on integrated information from a 3-axis accelerometer and other physiological sensors (heat flux sensor, skin temperature sensor, near-body ambient temperature sensor, and galvanic skin response sensor). The armband accelerometer has been demonstrated to be a valid method, with good accuracy and reliability, for monitoring energy expenditure, PA patterns, and sleep quantity and quality both in laboratory conditions and free-living conditions in different populations.41, 42, 43 The analysis in the present study was generally based off data collected over periods of approximately 7 days.41, 42, 43, 44
Participants were instructed to wear the monitoring device for 7 days on the upper-left brachial region, located equally between the acromion and olecranon processes, at all times, except when in water (e.g., when showering, bathing, or swimming). The devices were worn, on average, for 6.14 ± 0.93 days. The main outcome was the average minutes of MVPA per day, which was calculated as any 1-min epoch (collected every 1 s and averaged over the 1-min period) that achieved a level of energy expenditure of 3.0 metabolic equivalent of tasks or greater.
2.5. Cognitive test: Stroop task
A computerized version of the classic color–word Stroop task was used to assess executive functioning. Participants were presented with words that were printed in an ink color that was either congruent (e.g., the word RED printed in red ink), neutral (e.g., the word CAR printed in red ink), or incongruent (e.g., the word RED printed in blue ink) to the word's meaning. Stimuli were randomly presented for 1000 ms, and participants were instructed to respond as quickly as possible to indicate the color of the word, while ignoring the word's semantic identity. Participants responded by pressing a key on a standard keyboard with the index, middle, and ring fingers of their right hand. The test included 48 incongruent trials, 54 congruent trials, and 63 neutral trials. Several practice trials were completed before starting the task to acquaint the participant with the instructions. The Stroop effect was calculated by subtracting mean RT on congruent trials from the mean RT on incongruent trials and dividing the result by the RT on congruent trials. Stimuli were presented using E-Prime 2.0 software (Psychology Software Tools, Inc., Pittsburgh, PA, USA).
2.6. Image acquisition and preprocessing
A total of 48 of the 60 participants (80%) completed the MRI scan. The 12 participants who did not complete the imaging scan were excluded for MRI safety eligibility reasons (e.g., claustrophobia) and did not differ in any demographic characteristics from the total sample. The scan was conducted within 2 weeks of VO2max testing. All images were collected on a 3T head-only Siemens Allegra MRI scanner (Allegra; Siemens, Munich, Germany). High-resolution T1-weighted brain images were acquired using a 3-dimensional magnetization prepared rapid gradient echo imaging protocol with 176 contiguous axial slices, 1 mm in thickness, collected in ascending fashion parallel to the anterior and posterior commissures (echo time of 2.48 ms, repetition time of 1400 ms, field of view of 256 mm, acquisition matrix of 256 × 256 mm, flip angle of 8 degrees).
FreeSurfer software package (Version 5.3.0; Laboratory for Computational Neuroimaging, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, USA) was used to semiautomatically calculate the thickness of the ACC. For each participant, the image underwent skull stripping, transformation to standard space, creation of representations of the gray/white matter boundaries, calculation of cortical thickness as the distance between the gray/white matter boundary and the pial surface, and visual inspection for quality control of these steps for each subject.45, 46 Our a priori region-of-interest was the rostral ACC (rACC) because of its established associations with Stroop task performance, CRF, and prior studies of IIV.
2.7. Statistical analysis
The main outcome of interest was IIV from the Stroop task. Both the standard deviation of reaction time (SDRT) and coefficient of variation of reaction time (CVRT) were calculated. Both SDRT and CVRT are metrics used to assess IIV.22 Outliers were removed for each condition, for each subject. Intraindividual outliers were defined as any response 150 ms or less and values ± 3 SD from the mean.31 Only correct responses were included in the analysis. Normality was assessed for each variable using a Shapiro-Wilk test before proceeding. Spearman correlations were conducted between IIV, age, mean RT, and accuracy in congruent, incongruent, and neutral conditions, as well as with the Stroop effect (Table 1). The Friedman test and Bonferroni correction post hoc test were applied to investigate possible differences in performance between conditions. Results were considered statistically significant with a corrected p <0.05 (two tailed).
Table 1.
Correlations analysis between fitness, PA, and performance measures on the Stroop task.
| Age | CRF | MVPA | RT Cong | RT Incong | RT neutral | Accuracy Cong | Accuracy Incong | Accuracy neutral | Stroop effect | SDRT Cong | SDRT Incong | SDRT neutral | CVRT Cong | CVRT Incong | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRF | –0.153 | –0.244 | |||||||||||||
| MVPA | –0.365⁎⁎ | 0.650⁎⁎ | –0.277* | ||||||||||||
| RT Cong | 0.211 | –0.071 | –0.056 | 0.325* | |||||||||||
| RT Incong | 0.176 | –0.133 | –0.121 | 0.817⁎⁎ | 0.608⁎⁎ | ||||||||||
| RT neutral | 0.144 | –0.082 | –0.127 | 0.869⁎⁎ | 0.859⁎⁎ | 0.471⁎⁎ | |||||||||
| Accuracy Cong | –0.010 | 0.045 | 0.049 | 0.262* | 0.229 | 0.161 | –0.178 | ||||||||
| Accuracy Incong | –0.040 | 0.110 | 0.074 | 0.118 | 0.069 | –0.011 | 0.711⁎⁎ | –0.227 | |||||||
| Accuracy neutral | 0.113 | –0.038 | –0.131 | 0.217 | 0.193 | 0.099 | 0.715⁎⁎ | 0.723⁎⁎ | –0.126 | ||||||
| Stroop effect | –0.156 | 0.108 | 0.196 | –0.247 | –0.714⁎⁎ | –0.427⁎⁎ | –0.148 | –0.022 | –0.138 | –0.632⁎⁎ | |||||
| SDRT Cong | 0.195 | –0.076 | –0.067 | 0.660⁎⁎ | 0.643⁎⁎ | 0.689⁎⁎ | –0.129 | –0.203 | –0.096 | –0.320* | 0.507⁎⁎ | ||||
| SDRT Incong | 0.285* | –0.228 | –0.274* | 0.511⁎⁎ | 0.778⁎⁎ | 0.632⁎⁎ | –0.062 | –0.147 | –0.017 | –0.709⁎⁎ | 0.620⁎⁎ | 0.961⁎⁎ | |||
| SDRT neutral | 0.125 | 0.043 | –0.156 | 0.454⁎⁎ | 0.559⁎⁎ | 0.626⁎⁎ | –0.155 | –0.245 | –0.135 | –0.427⁎⁎ | 0.545⁎⁎ | 0.644⁎⁎ | 0.594⁎⁎ | ||
| CVRT Cong | 0.085 | –0.065 | –0.044 | 0.279* | 0.345⁎⁎ | 0.358⁎⁎ | –0.326* | –0.319* | –0.299* | –0.240 | 0.867⁎⁎ | 0.495⁎⁎ | 0.427⁎⁎ | 0.471⁎⁎ | |
| CVRT Incong | 0.267* | –0.244 | –0.277* | 0.325* | 0.608⁎⁎ | 0.471⁎⁎ | –0.178 | –0.227 | –0.126 | –0.632⁎⁎ | 0.507⁎⁎ | 0.961⁎⁎ | 0.594⁎⁎ | 0.471⁎⁎ | 1.000 |
| CVRT neutral | 0.143 | 0.032 | –0.144 | 0.261* | 0.366⁎⁎ | 0.408⁎⁎ | –0.259* | –0.312* | –0.209 | –0.345⁎⁎ | 0.416⁎⁎ | 0.540⁎⁎ | 0.957⁎⁎ | 0.385⁎⁎ | 0.539⁎⁎ |
Abbreviations: Cong = congruent; CRF = cardiorespiratory fitness; CVRT = coefficient of variation of reaction time; Incong = incongruent; MVPA = moderate-to-vigorous intensity physical activity; PA = physical activity; RT = reaction time; SDRT = standard deviation of reaction time.
p < 0.05,
p < 0.01.
To test the relevant contribution of each of the variables, we conducted hierarchical multiple linear regression analyses to test the relationships of CRF and MVPA to the rACC, the relationships between the rACC and IIV, the association of CRF and MVPA to IIV, and to test if MVPA was associated with CRF. Regression models were conducted controlling for age, education, and sex.
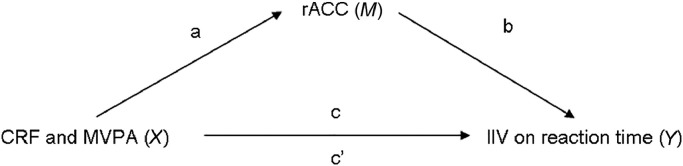
We examined whether the thickness of the rACC (defined as M in the mediation model) statistically mediated the association between either CRF or MVPA (defined as X in the mediation model) and IIV (defined as Y in the mediation model). The approach we used to test for mediation is based on modern views of mediation, which do not require the presence of a simple association between X and Y to test for indirect effects (Fig. 1). Thus, statistical mediation can be tested in observational studies by using the PROCESS macro for SPSS (Version 24.0; IBM Corp., Armonk, NY, USA), which uses maximum likelihood linear regression to estimate the coefficients of the mediation model. The macro then uses a bootstrapping procedure in which the data are resampled 5000 times and asymmetric confidence intervals (CIs) are generated to estimate the significance of the indirect effect. The indirect effect of X on Y through a mediator (M) is significant if the CIs do not overlap with zero.47 This approach is considered a more robust inferential procedure compared with more traditional mediation approaches (e.g., Sobel) because it does not depend on the same assumptions about the product of the sampling distributions. Thus, a requirement to test for statistical mediation is that a- and b-, but not c-, paths need to be significant (Fig. 1). In line with this approach, we tested whether an association between CRF or MVPA with IIV could be statistically mediated by rACC thickness when accounting for age, education, and sex. Indirect mediation was considered statistically significant based on the bias-corrected 95%CI not overlapping with 0. Analysis were completed using SPSS Version 24.0 (IBM Corp.).
Fig. 1.
Mediation model in which predictors CRF or MVPA (X) are associated with (a-path) rACC thickness (mediator (M)). Associations between the mediator (b-path) with dependent variable IIV (Y), direct (c’), and total effects (c) are also illustrated. CRF = cardiorespiratory fitness; IIV = intraindividual variability; MVPA = moderate-to-vigorous intensity physical activity; rACC = rostral anterior cingulate cortex.
3. Results
Means ± SD are reported for all demographic, MVPA, CRF, and Stroop task parameters in Table 2. There were no sex differences in Stroop performance.
Table 2.
Demographics, fitness, PA, and Stroop task performance.
| Variable | mean ± SD or % |
|---|---|
| Age (year) | 24.58 ± 4.98 |
| Sex (female) | 57 |
| Education (year) | 15.98 ± 2.11 |
| CRF (mL/kg/min) | 41.78 ± 7.89 |
| MVPA (min/day) | 159.41 ± 77.01 |
| Stroop accuracy (%) | |
| Congruent | 0.913 ± 0.07⁎ |
| Incongruent | 0.844 ± 0.09 |
| Neutral | 0.896 ± 0.07⁎ |
| Stroop RT (ms) | |
| Congruent | 476.664 ± 41.91⁎ |
| Incongruent | 513.612 ± 72.27 |
| Neutral | 493.088 ± 49.18⁎,## |
| Stroop effect | –0.079 ± 0.10 |
| Stroop SDRT (ms) | |
| Congruent | 86.013 ± 20.98⁎ |
| Incongruent | 119.123 ± 48.87 |
| Neutral | 101.062 ± 31.70⁎,# |
| Stroop CVRT | |
| Congruent | 0.179 ± 0.03* |
| Incongruent | 0.226 ± 0.06 |
| Neutral | 0.203 ± 0.05⁎,# |
Friedman Test: *p < 0.0005 in comparison with the incongruent condition; #p < 0.005, ##p < 0.0005 in comparison to congruent condition.
Abbreviations: CRF = cardiorespiratory fitness; CVRT = coefficient of variation of reaction time; MVPA = moderate-to-vigorous intensity physical activity; RT = reaction time; SDRT = standard deviation of reaction time.
There were statistically significant differences between congruent, incongruent, and neutral conditions on accuracy (χ2(2) = 49.520, p < 0.0005), RT (χ2(2) = 39.186, p < 0.0005), SDRT (χ2(2) = 50.881, p < 0.0005), and CVRT (χ2(2) = 39.288, p < 0.0005). The incongruent condition was less accurate and had a longer RT than both other conditions (p < 0.0005). The incongruent condition also showed greater SDRT and higher CVRT than congruent (p < 0.0005) and neutral (p = 0.013) conditions. Consistent with a facilitation effect, we found a shorter RT (p = 0.005) for the congruent compared with the neutral condition, and there was lower SDRT (p < 0.0005) and smaller CVRT (p = 0.002) for the congruent compared with the neutral condition.
3.1. Regression analysis
Inconsistent with our predictions, there were no significant associations between MVPA and IIV (SDRT: β = –0.30, p = 0.540; CVRT: β = –0.280, p = 0.070) or CRF and IIV (SDRT: β = –0.210, p = 0.220; CVRT: β = –0.210, p = 0.210).
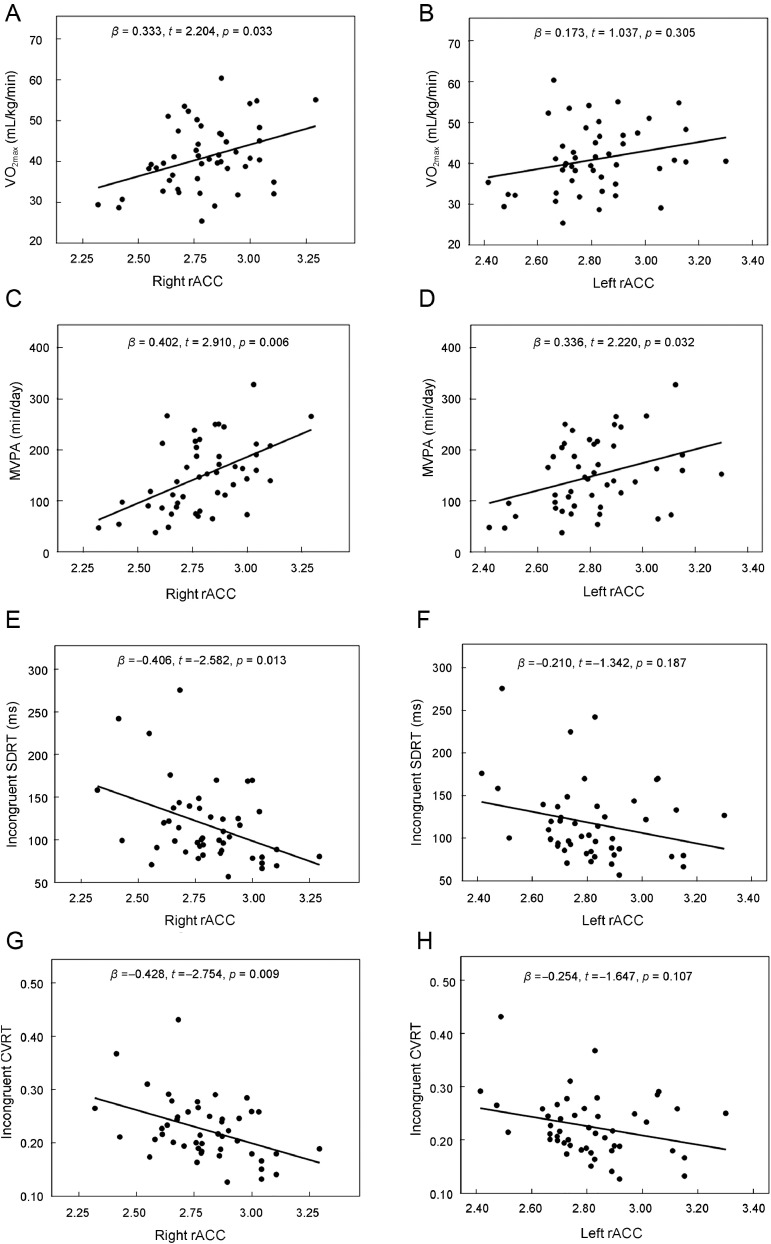
In contrast, hierarchical regression analysis confirmed our hypothesis that higher CRF was associated with greater rACC thickness. This effect was specific for the right hemisphere (β = 0.333, p = 0.033) but not the left hemisphere (β = 0.173, p = 0.305). We also found that greater amounts of MVPA were associated with greater left (β = 0.336, p = 0.032) and right (β = 0.402, p = 0.006) rACC thickness (Fig. 2 A–D).
Fig. 2.
rACC associations with aerobic fitness (A & B), physical activity (C & D), and IIV across reaction time (E–H). Plots represent regression associations of cardiorespiratory fitness and MVPA with rACC thickness (cm3), as well as associations of SDRT and CVRT from the incongruent condition of the Stroop test to rACC thickness. Upper and bottom graphics represent associations with right and left rACC, respectively. The β, t, and p values for each regression are indicated in each graphic. CVRT = coefficient of variation of reaction time; MVPA = moderate-to-vigorous intensity physical activity; rACC = rostral anterior cingulate cortex; SDRT = standard deviation of reaction time; VO2max = maximal oxygen uptake.
Also consistent with our hypothesis, rACC thickness was associated with IIV. Greater thickness of the right rACC was associated with lower IIV for the incongruent condition (SDRT: β = –0.406, p = 0.013; CVRT: β = –0.428, p = 0.009) but not the left rACC (SDRT: β = –0.210, p = 0.187; CVRT: β = –0.254, p = 0.107) (Fig. 2 E–H). No significant associations were found for the other Stroop conditions (data not shown).
We found that MPVA was associated with CRF (MVPA: β = 0.570, t = 5.09, p < 0.001). Therefore, we examined whether associations with the rACC would remain significant when both MVPA and CRF were included in the regression model. We found that MVPA was still associated with right rACC thickness after the inclusion of CRF as a covariate in a hierarchical regression model (MVPA, β = 0.320, t = 2.00, p = 0.051). However, CRF was no longer associated with right rACC thickness after adjustment for MVPA (β = 0.130, t = 0.75, p = 0.460).
3.2. Mediation analysis
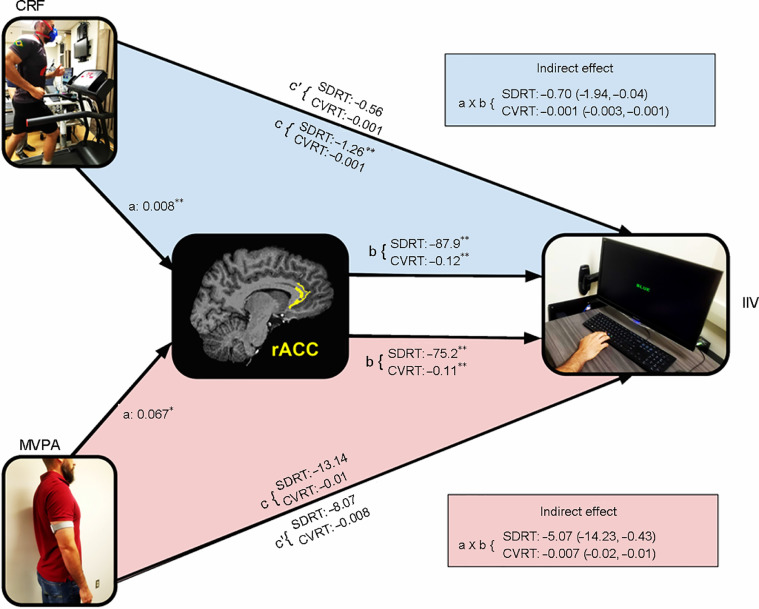
We conducted a mediation analysis testing the hypothesis that the thickness of the rACC would constitute an indirect (mediating) pathway between CRF and/or MVPA on IIV (Fig. 1). Considering that the a- and b- paths were both significant and the c- path was not, we were able to test for mediation.48 The 95%CI corrected for bias did not include 0 for the association between higher CRF (VO2max) and IIV (SDRT: –0.70 (95%CI: –1.94 to –0.04), CVRT: –0.001 (95%CI: –0.003 to –0.001)). This finding suggests that the relationship between CRF and IIV for the incongruent condition was statistically mediated by a greater thickness of the right rACC (Fig. 3). Similar results were found for daily accumulated volume of MVPA. That is, the association of a greater number of minutes of MVPA to SDRT and CVRT for the incongruent condition was statistically mediated by greater thickness of the right rACC (SDRT: –5.07 (95%CI: –14.23 to –0.43), CVRT: –0.007 (95%CI: –0.02 to –0.01)) (Fig. 3).
Fig. 3.
Schematic representation of mediation results. The a-path is significant for both CRF and MVPA, and the b-paths are significant for SDRT and CVRT. Note that c and c’ are not significant (except for CRF and SDRT). Indirect mediation was significant for both predictive variables (i.e., CRF and MVPA). Data in parentheses are 95% bias corrected lower-level confidence interval and upper-level confidence interval, respectively (5000 bootstrap samples). * p ≤ 0.05, ** p ≤ 0.01. CRF = cardiorespiratory fitness; CVRT = coefficient of variation of reaction time; MVPA = moderate-to-vigorous intensity physical activity; SDRT = standard deviation of reaction time.
4. Discussion
We initially predicted that CRF and MVPA would be associated with lower IIV in younger adults. Inconsistent with this prediction, we did not find a significant direct association between these variables. However, we discovered that, when accounting for rACC thickness, such an association was indeed significant. That is, we found that both CRF and MVPA were related to rACC thickness and that, in turn, a greater rACC thickness was associated with lower IIV. Thus, an association of MVPA and CRF with IIV emerges when accounting for variation of rACC thickness. This finding is important because it demonstrates both the significance of including neurobiological information into these regression models and highlights the role of the rACC in IIV. In addition to this finding, our results expand the current literature by demonstrating that CRF and MVPA in young adults are associated with neurobiological markers of brain health (i.e., rACC thickness) and aspects of executive function. Finally, we demonstrate the importance of the rACC in the context of IIV during the Stroop task.
Animal studies have demonstrated that rats housed in an enriched environment with running wheels had better performance on spatial and reversal learning tasks that were associated with greater expression of early genes (c-Fos) in the ACC.49 Another study in rodents demonstrated that an acute bout of exercise increased expression of C-fos messenger RNA in the ACC.50 Consistent with this work, a single study with younger adults showed that increased activation of the ACC was related to increased glutamate levels after an acute bout of intense exercise.51 These evidence suggests that PA affects the molecular and cellular milieu of the ACC, which could influence behavior on a trial-by-trial basis.
Consistent with rodent work showing associations between PA and the ACC, Colcombe and colleagues37 demonstrated that a 12-month aerobic exercise intervention improved CRF and increased ACC volume in older adults. This finding is in agreement with another intervention study with older adults, which found that an increased amount of MVPA was associated with memory improvement and greater change in ACC volume.52 In addition, Cahill et al.53 found an increased ACC thickness and hippocampal volume in adult mice after 4 weeks of voluntary exercise. Taken together, studies of both humans and rodents argue that the morphology of the ACC is highly responsive to PA, and its plasticity is consonant with the modifiability of well-established PA-sensitive areas like the hippocampus and dentate gyrus.54 The present data are also in agreement with data from a recent large cross-sectional study of 834 adults ranging in age from 25 to 83 years.55 In this study, there were significant associations reported between MVPA and bilateral ACC volume. In the present study, we demonstrate for the first time that greater MVPA and higher CRF are associated with greater rACC thickness in younger adults.
Functional neuroimaging studies have confirmed that the ACC is a key structure in trial-by-trial IIV. Reduced activation of the ACC before the presentation of a stimulus produces a longer RT and higher IIV,34,35 and higher activation of the ACC is associated with reduced IIV in RT.24,33 Despite these associations, at least 1 study found that IIV was not associated with morphological aspects of the ACC in younger adults.32 However, there is evidence of age-related morphological and biochemical changes in the ACC associated with increased IIV, including reductions in grey matter myelinization,56 white matter integrity,57 and dopamine receptors.58 Our results provide the first evidence that right rACC thickness is associated with reduced IIV of RT in younger adults.
Finally, we found that associations between MVPA and right rACC thickness remained significant even after controlling for VO2max, indicating the possibility that the association between CRF and rACC thickness is being driven by daily accumulated total volume of MVPA, independent of one's fitness level. In addition, prior studies examining associations between CRF and IIV have been conducted in children, adolescents, middle-aged adults, and older adults. The lack of a direct association between CRF and IIV in the present study might be explained by the young adult age range and the elevated level of performance on the Stroop task. Given that very few prior studies have examined both CRF and MVPA as part of the same research, the relative importance of these parameters with respect to IIV is difficult to discern from the extant literature.
The majority of studies examining the Stroop task, IIV, and ACC have analyzed effects across both cerebral hemispheres and have not commented on laterality. Thus, we did not develop any a priori predictions about hemispheric differences and can only speculate about the lateralized effects found in this study. However, the results of several studies have been consistent with our results in showing that the right ACC is more responsive to IIV than the left. For example, in studies with older adults, decreased prestimulus activation in the right ACC, but not the left, was associated with longer RT34 and was positively associated with low-level PA.59 The right hemisphere ACC and right prefrontal cortex are more involved in supporting and resolving response conflict processes, whereas the left hemisphere has a more general role in supporting non-response-related conflict.60 Thus, we speculate that the lateralized effects that we find between IIV and the right rACC might be related to the dominance of the right hemisphere in processing response conflict, which would in turn influence IIV in conditions that elicit response conflict (i.e., incongruent conditions).
Our results should be interpreted in the context of several limitations. First, the cross-sectional design of the study inherently limits our ability to derive causal conclusions. Only in the context of experimental manipulations in which MVPA is systematically manipulated could we determine whether MVPA is causally linked with lower IIV or greater rACC thickness. Second, we used a hypothesis-driven approach to focus on the ACC, but there are likely many other brain areas associated with CRF, MVPA, and IIV. In fact, in this same sample we have previously reported that higher CRF is associated with differences in functional connectivity of the hippocampus.40 Future studies should examine other brain areas that might further explain variation in IIV in the context of MVPA and CRF. Third, other analytical approaches for assessing these associations (e.g., ex-Gaussian) should be conducted in future studies because the distributions of many of these variables often do not follow a Gaussian distribution. An ex-Gaussian analysis was not performed in this study because many of the participants had fewer than 40 correct trials in the incongruent condition, which would likely violate assumptions for such an analysis.22 Fourth, we decided to focus our analysis on MVPA at the expense of light-intensity activity. We decided this approach was acceptable given the emphasis on MVPA in health outcomes, the lack of prior data in young adults, and the desire to limit our numbers of comparisons. Finally, we found that rACC thickness statistically mediated the association of MVPA and CRF with IIV, but most estimates indicate that much larger sample sizes are needed to reliably test statistical mediation. Thus, our mediation results should be interpreted in the context of power and effect sizes for reliably and validly testing mediation.
5. Conclusion
We demonstrate for the first time that higher CRF and greater amounts of MVPA in a younger adult sample are associated with increased rACC thickness. We also demonstrate that a thicker rACC was associated with lower IIV during the incongruent condition of the Stroop task. Finally, we found evidence that an association between MVPA and CRF and IIV was statistically mediated by rACC thickness. These results indicate that the positive associations of CRF and MVPA with metrics of brain health and function are not limited to children and older adults but are also present in younger adult samples. Our results also demonstrate the importance of considering neurobiological markers to help better understand associations of MVPA and CRF with cognitive endpoints.
Acknowledgments
Acknowledgments
The authors thank the BACH lab research assistant, Leighanne Ollinger, for her assistance with data entry. This research was supported by funding to KIE from the National Institute of Diabetes and Digestive and Kidney Diseases (http://www.niddk.nih.gov/Pages/default.aspx), grant number R01 DK095172. CMS was supported by the National Institute of Mental Health (https://www.nimh.nih.gov/index.shtml), grant T32 MH109986. JBTN was supported by CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (http://www.capes.gov.br/), Finance Code 001. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ contributions
KIE, JBT, and NVOBT designed the study and conducted the statistical analysis; MEW, CMS, HH, FU, and JCW were involved in data collection and the brain segmentation procedures; All fitness and physical activity data were collected, analyzed, and written by KIE and GAG. All authors were involved with reading and editing the final version of the manuscript, and agree with the order of authorship.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Kamijo K., Takeda Y. Regular physical activity improves executive function during task switching in young adults. Int J Psychophysiol. 2010;75:304–311. doi: 10.1016/j.ijpsycho.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Hillman C.H., Erickson K.I., Kramer A.F. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 3.Masley S.C., Roetzheim R., Clayton G., Presby A., Sundberg K., Masley L.V. Lifestyle markers predict cognitive function. J Am Coll Nutr. 2017;36:617–623. doi: 10.1080/07315724.2017.1336128. [DOI] [PubMed] [Google Scholar]
- 4.Guidelines Advisory Committee Physical-Activity . U.S. Department of Health and Human Services; Washington, DC: 2018. 2018 Physical Activity Guidelines Advisory Committee scientific report. [Google Scholar]
- 5.Engeroff T., Ingmann T., Banzer W. Physical activity throughout the adult life span and domain-specific cognitive function in old age: a systematic review of cross-sectional and longitudinal data. Sports Med. 2018;48:1405–1436. doi: 10.1007/s40279-018-0920-6. [DOI] [PubMed] [Google Scholar]
- 6.de Greeff J.W., Bosker R.J., Oosterlaan J., Visscher C., Hartman E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: a meta-analysis. J Sci Med Sport. 2018;21:501–507. doi: 10.1016/j.jsams.2017.09.595. [DOI] [PubMed] [Google Scholar]
- 7.Colcombe S.J., Kramer A.F., Erickson K.I., Scalf P., McAuley E., Cohen N.J. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong C.N., Chaddock-Heyman L., Voss M.W., Burzynska A.Z., Basak C., Erickson K.I. Brain activation during dual-task processing is associated with cardiorespiratory fitness and performance in older adults. Front Aging Neurosci. 2015;7:154. doi: 10.3389/fnagi.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raine L.B., Kao S.C., Pindus D., Westfall D.R., Shigeta T.T., Logan N. A large-scale reanalysis of childhood fitness and inhibitory control. J Cogn Enhanc. 2018;2:170–192. [Google Scholar]
- 10.Verburgh L., Königs M., Scherder E.J., Oosterlaan J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis. Br J Sports Med. 2014;48:973–979. doi: 10.1136/bjsports-2012-091441. [DOI] [PubMed] [Google Scholar]
- 11.Cox E.P., O'Dwyer N., Cook R., Vetter M., Cheng H.L., Rooney K. Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: a systematic review. J Sci Med Sport. 2016;19:616–628. doi: 10.1016/j.jsams.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Giles G.E., Cantelon J.A., Eddy M.D., Brunyé T.T., Urry H.L., Mahoney C.R. Habitual exercise is associated with cognitive control and cognitive reappraisal success. Exp Brain Res. 2017;235:3785–3797. doi: 10.1007/s00221-017-5098-x. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda K., Ikeda S., Mitsutake T., Nakahara M., Nagai Y., Ikeda T. Factors influencing executive function by physical activity level among young adults: a near-infrared spectroscopy study. J Phys Ther Sci. 2017;29:470–475. doi: 10.1589/jpts.29.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman C.H., Weiss E.P., Hagberg J.M., Hatfield B.D. The relationship of age and cardiovascular fitness to cognitive and motor processes. Psychophysiology. 2002;39:303–312. doi: 10.1017/s0048577201393058. [DOI] [PubMed] [Google Scholar]
- 15.Newson R.S., Kemps E.B. Relationship between fitness and cognitive performance in younger and older adults. Psychol Health. 2008;23:369–386. doi: 10.1080/08870440701421545. [DOI] [PubMed] [Google Scholar]
- 16.Scisco J.L., Leynes P.A., Kang J. Cardiovascular fitness and executive control during task-switching: an ERP study. Int J Psychophysiol. 2008;69:52–60. doi: 10.1016/j.ijpsycho.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Hayes S.M., Forman D.E., Verfaellie M. Cardiorespiratory fitness is associated with cognitive performance in older but not younger adults. J Gerontol B Psychol Sci Soc Sci. 2016;71:474–482. doi: 10.1093/geronb/gbu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soares F.C., de Oliveira T.C., de Macedo L.D., Tomás A.M., Picanço-Diniz D.L., Bento-Torres J. CANTAB object recognition and language tests to detect aging cognitive decline: an exploratory comparative study. Clin Interv Aging. 2015;10:37–48. doi: 10.2147/CIA.S68186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods A.L., Garvican-Lewis L.A., Rice A.J., Thompson K.G. The ventilation-corrected parvoMedics trueOne 2400 provides a valid and reliable assessment of resting metabolic rate (RMR) in athletes compared with the Douglas Bag method. Int J Sport Nutr Exerc Metab. 2016;26:454–463. doi: 10.1123/ijsnem.2015-0315. [DOI] [PubMed] [Google Scholar]
- 20.Löllgen H., Leyk D. Exercise testing in sports medicine. Dtsch Arztebl Int. 2018;115:409–416. doi: 10.3238/arztebl.2018.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westerterp K.R. Physical activity assessment with accelerometers. Int J Obes Relat Metab Disord. 1999;23(Suppl. 3):S45–S49. doi: 10.1038/sj.ijo.0800883. [DOI] [PubMed] [Google Scholar]
- 22.Haynes B.I., Bauermeister S., Bunce D. A systematic review of longitudinal associations between reaction time intraindividual variability and age-related cognitive decline or impairment, dementia, and mortality. J Int Neuropsychol Soc. 2017;23:431–445. doi: 10.1017/S1355617717000236. [DOI] [PubMed] [Google Scholar]
- 23.Haynes B.I., Kliegel M., Zimprich D., Bunce D. Intraindividual reaction time variability predicts prospective memory failures in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2018;25:132–145. doi: 10.1080/13825585.2016.1268674. [DOI] [PubMed] [Google Scholar]
- 24.Bellgrove M.A., Hester R., Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Kochan N.A., Bunce D., Pont S., Crawford J.D., Brodaty H., Sachdev P.S. Reaction time measures predict incident dementia in community-living older adults: the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2016;24:221–231. doi: 10.1016/j.jagp.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kochan N.A., Bunce D., Pont S., Crawford J.D., Brodaty H., Sachdev P.S. Is intraindividual reaction time variability an independent cognitive predictor of mortality in old age? Findings from the Sydney Memory and Ageing Study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C.T., Pontifex M.B., Raine L.B., Chaddock L., Voss M.W., Kramer A.F. Aerobic fitness and response variability in preadolescent children performing a cognitive control task. Neuropsychology. 2011;25:333–341. doi: 10.1037/a0022167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore R.D., Wu C.T., Pontifex M.B., O'Leary K.C., Scudder M.R., Raine L.B. Aerobic fitness and intra-individual variability of neurocognition in preadolescent children. Brain Cogn. 2013;82:43–57. doi: 10.1016/j.bandc.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagot D., Chicherio C., Albinet C.T., André N., Audiffren M. The impact of physical activity and sex differences on intraindividual variability in inhibitory performance in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2017;26:1–23. doi: 10.1080/13825585.2017.1372357. [DOI] [PubMed] [Google Scholar]
- 30.Kimura K., Yasunaga A., Wang L.Q. Correlation between moderate daily physical activity and neurocognitive variability in healthy elderly people. Arch Gerontol Geriatr. 2013;56:109–117. doi: 10.1016/j.archger.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Bauermeister S., Bunce D. Aerobic fitness and intraindividual reaction time variability in middle and old age. J Gerontol B Psychol Sci Soc Sci. 2016;71:431–438. doi: 10.1093/geronb/gbu152. [DOI] [PubMed] [Google Scholar]
- 32.Lövdén M., Schmiedek F., Kennedy K.M., Rodrigue K.M., Lindenberger U., Raz N. Does variability in cognitive performance correlate with frontal brain volume? Neuroimage. 2013;64:209–215. doi: 10.1016/j.neuroimage.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson B.P., Pinar A., Fornito A., Nandam L.S., Hester R., Bellgrove M.A. Left anterior cingulate activity predicts intra-individual reaction time variability in healthy adults. Neuropsychologia. 2015;72:22–26. doi: 10.1016/j.neuropsychologia.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Tam A., Luedke A.C., Walsh J.J., Fernandez-Ruiz J., Garcia A. Effects of reaction time variability and age on brain activity during Stroop task performance. Brain Imaging Behav. 2015;9:609–618. doi: 10.1007/s11682-014-9323-y. [DOI] [PubMed] [Google Scholar]
- 35.Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 36.Colcombe S.J., Erickson K.I., Raz N., Webb A.G., Cohen N.J., McAuley E. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 37.Colcombe S.J., Erickson K.I., Scalf P.E., Kim J.S., Prakash R., McAuley E. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 38.Burdette J.H., Laurienti P.J., Espeland M.A., Morgan A., Telesford Q., Vechlekar C.D. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stillman C.M., Watt J.C., Grove G.A., Wollam M.E., Uyar F., Mataro M. Physical activity is associated with reduced implicit learning but enhanced relational memory and executive functioning in young adults. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stillman C.M., Uyar F., Huang H., Grove G.A., Watt J.C., Wollam M.E. Cardiorespiratory fitness is associated with enhanced hippocampal functional connectivity in healthy young adults. Hippocampus. 2018;28:239–247. doi: 10.1002/hipo.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johannsen D.L., Calabro M.A., Stewart J., Franke W., Rood J.C., Welk G.J. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc. 2010;42:2134–2140. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- 42.Koehler K., Drenowatz C. Monitoring energy expenditure using a multi-sensor device-applications and limitations of the sensewear armband in athletic populations. Front Physiol. 2017;8:983. doi: 10.3389/fphys.2017.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malavolti M., Pietrobelli A., Dugoni M., Poli M., Romagnoli E., De Cristofaro P. A new device for measuring resting energy expenditure (REE) in healthy subjects. Nutr Metab Cardiovasc Dis. 2007;17:338–343. doi: 10.1016/j.numecd.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Tian Q., Glynn N.W., Erickson K.I., Aizenstein H.J., Simonsick E.M., Yaffe K. Objective measures of physical activity, white matter integrity and cognitive status in adults over age 80. Behav Brain Res. 2015;284:51–57. doi: 10.1016/j.bbr.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 46.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preacher K.J., Hayes A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 48.Zhao X., Lynch J.G., Chen Q. Reconsidering Baron and Kenny: myths and truths about mediation analysis. J Consum Res. 2010;37:197–206. [Google Scholar]
- 49.Sampedro-Piquero P., Zancada-Menendez C., Begega A. Housing condition-related changes involved in reversal learning and its c-Fos associated activity in the prefrontal cortex. Neuroscience. 2015;307:14–25. doi: 10.1016/j.neuroscience.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 50.Timofeeva E., Huang Q., Richard D. Effects of treadmill running on brain activation and the corticotropin-releasing hormone system. Neuroendocrinology. 2003;77:388–405. doi: 10.1159/000071311. [DOI] [PubMed] [Google Scholar]
- 51.Maddock R.J., Casazza G.A., Fernandez D.H., Maddock M.I. Acute modulation of cortical glutamate and GABA content by physical activity. J Neurosci. 2016;36:2449–2457. doi: 10.1523/JNEUROSCI.3455-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruscheweyh R., Willemer C., Krüger K., Duning T., Warnecke T., Sommer J. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2011;32:1304–1319. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Cahill L.S., Steadman P.E., Jones C.E., Laliberté C.L., Dazai J., Lerch J.P. MRI-detectable changes in mouse brain structure induced by voluntary exercise. Neuroimage. 2015;113:175–183. doi: 10.1016/j.neuroimage.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 54.Voss M.W., Vivar C., Kramer A.F., van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jochem C., Baumeister S.E., Wittfeld K., Leitzmann M.F., Bahls M., Schminke U. Domains of physical activity and brain volumes: a population-based study. Neuroimage. 2017;156:101–108. doi: 10.1016/j.neuroimage.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Grydeland H., Walhovd K.B., Tamnes C.K., Westlye L.T., Fjell A.M. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci. 2013;33:18618–18630. doi: 10.1523/JNEUROSCI.2811-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mella N., de Ribaupierre S., Eagleson R., de Ribaupierre A. Cognitive intraindividual variability and white matter integrity in aging. Sci World J. 2013;2013 doi: 10.1155/2013/350623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacDonald S.W., Karlsson S., Rieckmann A., Nyberg L., Bäckman L. Aging-related increases in behavioral variability: relations to losses of dopamine D1 receptors. J Neurosci. 2012;32:8186–8191. doi: 10.1523/JNEUROSCI.5474-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flöel A., Ruscheweyh R., Krüger K., Willemer C., Winter B., Völker K. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 60.Milham M.P., Banich M.T., Webb A., Barad V., Cohen N.J., Wszalek T. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]