Abstract
Purpose
Uveitis is a serious inflammatory disease of the uvea, frequently leading to visual impairment and irreversible blindness. Here, we investigated the anti-inflammatory effect of minocycline on rat endotoxin-induced uveitis (EIU) and retinal inflammation.
Methods
For in vivo studies, the rat EIU model was induced with intravitreal injection of lipopolysaccharide (LPS). Minocycline was administered intraperitoneally 2 h before and after the LPS injection. The severity of the ocular inflammation was evaluated with slit-lamp photography, aqueous humor cell counting, protein quantitative determination, and histological analysis. Retinal microglia were labeled with a fluorescent dye 4Di-10ASP. Microglial activity and inflammatory cytokine production were analyzed with immunofluorescence and real-time PCR. For the in vitro studies, BV-2 microglia cells were stimulated with LPS or cotreated with minocycline for 6 h. Toll-like receptor (TLR) 2/4 levels were determined with real-time PCR and western blotting.
Results
The LPS-challenged eyes displayed severe inflammation in all ocular structures, including a large number of anterior chamber cells, fibrin exudation, hypopyon, and infiltrated inflammatory cells in the vitreous and retina. Immunostaining of the retinal whole-mounts also revealed numerous retinal microglia were activated promptly, and then more and more peripheral leukocytes were recruited and infiltrated in the LPS-injected retinas. Additionally, the production of tumor necrosis factor-α (TNF-α), chemokine (C-C motif) ligand 2 (CCL-2), interleukin-1 beta (IL-1β), and IL-6 was dramatically increased. However, minocycline treatment strongly inhibited microglia activation, decreased inflammatory cytokine production, prevented peripheral inflammatory cell recruitment, and significantly attenuated ocular inflammation. Finally, we demonstrated the mechanism of the microglia inactivation effect of minocycline is via suppression of TLR4 signaling.
Conclusions
This study indicates minocycline is far beyond an antibiotic. It not only attenuates rat EIU but also inhibits retinal inflammation through inactivating microglia, inhibiting inflammatory cell recruitment and inflammatory cytokine production.
Introduction
Uveitis is a serious inflammatory disease of the uvea, frequently leading to blindness and visual disability directly or through ocular complications. Uveitis can be caused by autoimmune response, infection, and injury [1]. In the current treatment, corticosteroids are the mainstay therapy for uveitis, but long-term administration of steroids may result in many systemic or local side effects, such as glaucoma, cataract, and decreased resistance to infection [2]. Therefore, there is an urgent need to develop a more effective and safer anti-inflammatory drug for uveitis.
Minocycline, a second-generation derivative of the tetracycline family, is a clinically available antibiotic, and it has a broad spectrum that is effective against Gram-negative and Gram-positive bacteria. Thus, minocycline has been widely used to treat respiratory infections, reproductive tract infections, skin infections, and suppurative infections [3]. Recently, minocycline has been reported to have various other effects that are independent of its antibacterial activity, including neuroprotective effects via anti-inflammation, immunomodulation, suppression of microglia activation, and inhibition of oxidative stress and neuron apoptosis [3,4]. Additionally, minocycline can penetrate the blood–brain barrier easily owning to the antibiotic’s high lipophilicity [5]. Therefore, minocycline is an ideal candidate for the treatment of central nervous system diseases, including traumatic brain injury and neurodegenerative diseases [6]. However, the anti-inflammatory effects of minocycline on uveitis and retinal inflammation are entirely unknown.
Microglia cells are the resident macrophages of the retina that play a critical role in retinal innate immune defense [7,8]. In the normal retina, microglia are located around vessels in the inner part of the retina; however, they can be activated and migrate in the sub-retinal space in uveitis [9], diabetic retinopathy (DR) [10,11], age-related macular degeneration (AMD) [12], and aging [7]. Previous studies reported that resident microglia migrate toward the photoreceptor cell layer before the circulating macrophages and neutrophils infiltrate the ocular tissues in experimental uveitis or in light-induced retinal damage models [9]. The activated microglia generate a broad range of proinflammatory cytokines (such as tumor necrosis factor-alpha, TNF-α), and toxic mediators (such as nitric oxide, NO), which are involved in the recruitment of peripheral neutrophils and macrophages infiltrating the lesions [13,14], and are even suspected to be neurotoxic to photoreceptor cells [15,16]. This suggests that the activation of microglia may contribute to permanent retinal damage. Thus, microglia are a key regulator in retinal inflammation and a potential modulator of the inflammatory response. Targeting microglia may be a potential therapeutic strategy for treating inflammatory and degenerative diseases of the retina.
Endotoxin-induced uveitis (EIU) is a well-established animal model for studying acute inflammatory uveitis. EIU is induced by footpad or intravitreal injection of lipopolysaccharide (LPS). LPS injection into the mouse foot induces mild anterior uveitis, while intravitreal injection of LPS can induce a greater inflammatory response, leading to dose-dependent infiltration of monocytes and neutrophils in the uveal tract, retina, and vitreous [17]. Intraocular inflammation begins 4–6 h after LPS injection, peaks at 24 h, and then starts to resolve after 2 or 3 days [17,18].
In the present study, we first examined whether minocycline can inhibit ocular inflammation in rat EIU. We then focused on microglia cells to explore their functions and cellular mechanisms in retinal inflammation in EIU. Finally, we revealed the mechanism underlying the ability of minocycline to attenuate retinal inflammation is to modulate microglia activation.
Methods
In vivo labeling of retinal microglia
All experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animal experiments were approved by the Animal Use and Care Committee of Zhongshan Ophthalmic Center at the Sun Yat-Sen University, Guangzhou, China. The labeling of retinal microglia in rat eyes was performed as described previously [9,19]. Briefly, female Sprague-Dawley rats weighing 180–200 g were anesthetized, and a lateral canthotomy was performed on them, followed by a superior conjunctival peritomy. Dissection was performed posteriorly until the retrobulbar optic nerve was identified. A slit was created in the optic nerve sheath, parallel to the direction of the nerve fibers. Scissors were used to transect the optic nerve within the sheath, and the fluorescent dye 4 N-4-(4-didecylaminostyryl)-N methylpyridinium iodide (4Di-10ASP, Molecular Probes, Eugene, OR) was packed at the cut surface of the optic nerve. The lateral canthotomy was then sutured. The axotomy was performed on the right eyes only.
To examine the labeling of the retinal microglia, the rats were killed on postaxotomy days 14 and 28. The enucleated eyes were fixed in 4% paraformaldehyde (PFA) at 4 °C overnight, and were split to discard the anterior segment and vitreous. The retinas were separated from the choroids and radially divided into four segments, and then were mounted with antifade mounting medium on a slide and examined using a confocal microscope (Carl Zeiss, Overkochen, Germany).
Induction of rat EIU
The model of EIU in rat eyes was performed using 4Di-10ASP labeling rats at 28 days or ordinary rats weighing 180–200 g. The right eyes of the rats were given intravitreal injection with 0.5 µg/µl of Escherichia coli LPS (Sigma-Aldrich, MO) 2 μl or sterile PBS (1X; 135 mM NaCl, 4.7 mM KCl, 10 mM Na2HPO4, 2 mM NaH2PO4, pH 7.4) alone as a control. Before the eye injection, the pupils of the rats were dilated with a mixture of 0.5% phenylephrine hydrochloride and 0.5% tropicamide (Santen, Suzhou, China), and then the anesthesia was inhaled generally with isoflurane and topically with dicaine eye drop. The injections were made in the midvitreous using a 33-gauge needle (Hamilton, Reno, NV). The left eyes of the rats were left as the control group. At the desired time points after the intravitreal injection, the rats were euthanized by inhaling 5% of isoflurane, and the retinas were harvested for real time-PCR or whole-mount staining.
Minocycline administration and clinical evaluation of anterior inflammation
To study the effects of minocycline treatment on EIU, the rats received intraperitoneal administration of minocycline (Sigma-Aldrich, Louis, MO) at a dose of 45 mg/kg or an equal volume of PBS as solvent control 2 h before the LPS injection and after the injection. After the LPS injection for 6 and 24 h, the rats were anesthetized with isoflurane inhalation, and then the severity of the anterior inflammation was evaluated and photographed with slit-lamp biomicroscopy. After that, the rats were euthanized by inhaling 5% of isoflurane, and the eyes were harvested for histological analysis and whole-mount staining.
Cell counting and protein concentration of aqueous humor
For cell counting and the measurement of the protein concentration in the anterior chamber, aqueous humor was collected with an anterior chamber puncture 24 h after the LPS injection. Cell counting was performed according to previously reports [20]. Briefly, the aqueous humor was diluted with an equal volume of trypan blue (Sigma-Aldrich, Louis, MO), and then the number of viable and non-viable cells was counted manually with a hemocytometer.
For the protein measurement, the aqueous humors were centrifuged for 5 min at 300 ×g, and the supernatant was obtained. The protein concentration was determined with the bicinchoninic acid assay (BCA)-100 Protein Quantitative Analysis Kit (Biocolor Biotechnology Co., Ltd., Shanghai, China).
Histological analysis
For the histological analysis, the eyes of the rats were collected 24 h after the LPS challenge and fixed in 4% PFA overnight. After dehydration and clarification, they were embedded in paraffin and sectioned serially at 4 μm thickness via the pupil–optic nerve plane and then were stained with hematoxylin and eosin. The sections were evaluated with a light microscope (Carl Zeiss).
Immunofluorescent staining of the retina whole-mount
For immunofluorescent staining, the retinas were permeated and blocked with 1% Triton X-100 in 1% bovine serum albumin (BSA) for 1 h at room temperature (RT) after separation. After that, the retinas were stained with primary antibody CD11b (Serotec, Oxford, UK), Iba-1 (Wako Chemicals, Osaka, Japan), interleukin-1 beta (IL-1β; Abcam, Cambridge, MA), and chemokine (C-C motif) ligand 2 (CCL-2; Novus Biologicals, Littleton, CO) for 18 h at 4 °C. After washing with PBS, the retinas were incubated with the appropriate secondary antibodies conjugated with fluorescent dye (Alexa Fluor 555 or 488, Cell Signaling, Danvers, MA) at RT for 1 h. Finally, the retinas were flat-mounted and examined with a confocal microscope.
Cell culture and treatment
The mouse microglial cell line BV-2 was kindly provided by Professor Xialin Liu at the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center (Guangzhou, China). The cell line was previously identified with microglia markers Iba-1 and CD11b [21]. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Life Technologies, Grand Island, NY) containing 10% fetal bovine serum (FBS; Gibco, Life Technologies) at 37 °C in a humidified atmosphere of 5% CO2. For LPS stimulation, the cells were seeded in six-well plates and incubated with 100 ng/ml of LPS for 6 h. Minocycline (50 μg/ml) was added 1 h before treatment with LPS. After stimulation, cells were harvested for further analysis.
STR analysis
The mouse microglial cell line BV-2 was authenticated using short tandem repeat (STR) analysis and interspecies contamination testing (Creative Bioarray, Beijing, China). DNA was extracted by a commercial kit from CORNING (AP-MN-BL-GDNA-250G, Corning, NY). The twenty STRs including Amelogenin locus were amplified by six multiplex PCR and separated on ABI 3730XL Genetic Analyzer. Data were analyzed by GeneMapper® ID-X v1.2 software (Applied Biosystems, Suzhou, China). The cells were verified to be of mouse origin, and no multiple alleles and cross contamination of human cells were detected (Appendix 1).
Immunofluorescent staining for cultured cells
Immunofluorescent staining for cultured cells was performed according to previously described protocols [22]. The primary antibodies IL-1β (1:200, Abcam) and CCL-2 (1:200, Novus) were used. Images were obtained with confocal microscopy.
Real-time PCR
The RNA from BV-2 cells or rat retinas was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), and then was synthesized into cDNAs using the PrimeScriptTM RT Master Mix kit (Takara, Siga, Japan). Quantitative PCR was preformed using the TB Green Premix Ex Taq RT–PCR kit (Takara) according to the manufacturer’s instructions. The standard PCR conditions were 30 s at 95 °C, followed by 5 s at 95 °C, 34 s at 60 °C. The PCR reactions were run on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). The results were normalized to the expression of an internal control glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Western blotting
The total protein of the BV-2 microglia was extracted using ice-cold lysis buffer (RIPA; Biocolors, Shanghai, China) containing a protease inhibitor cocktail. About 30 μg of protein for each sample was mixed with 5X sodium dodecyl sulfate (SDS) loading buffer and subjected to 12% SDS–polyacrylamide gel electrophoresis (PAGE). After the electroblotting, the membranes were blocked with 5% non-fat milk in PBS with Tween 20 (PBST) for 1 h at RT, and then were incubated with primary antibodies against Toll-like receptor (TLR) 2 and TLR4 (1:1,000, Cell Signaling Technology) at 4 °C overnight. β-actin (Abcam) was used as an internal protein loading control.
Statistical analysis
All results were repeated three or more times. The data are presented as mean ± standard error of the mean (SEM) and analyzed by using a Student t test. A p value of less than 0.05 was considered statistically significant.
Results
Minocycline attenuates ocular inflammation in rat EIU
Minocycline not only has an excellent antibacterial effect but also exerts anti-inflammatory and neuroprotective effects in brain infection and neurodegenerative disorders [23,24]. We first investigated whether minocycline treatment can attenuate ocular inflammation in rat EIU. As shown in Figure 1A, all the challenged eyes that received 1 µg of LPS displayed obvious manifestations of acute anterior inflammation, including chemosis, dilated iris stroma vessels, 3+ to 4+ anterior chamber cells, fibrin exudation, and hypopyon at 6 and 24 h post-infection. The number of inflammatory cells and protein exudation in the aqueous humors were increased by 300-fold and 7.4-fold, respectively (Figure 1B,C). However, in the minocycline treatment group, the manifestations of anterior inflammation were dramatically attenuated. No obvious fibrin exudation and hypopyon were observed in the minocycline treatment eyes, and inflammatory cell infiltration and protein exudation were distinctly reduced compared to the eyes treated with PBS (Figure 1). Furthermore, the histologic analysis of the eyeball sections also revealed that the normal rat eyes displayed normal and well-distinguished layers, whereas the eyes from the LPS-injected group exhibited severe inflammation in all ocular structures. The anterior chamber, iris, and ciliary body were completely filled with fibrin and infiltrated inflammatory cells, and several inflammatory cells were observed migrating from the vitreous humor into the retina (Figure 2). However, the inflammatory response in the minocycline treatment eyes was obviously alleviated. Less fibrin and fewer infiltrated inflammatory cells were seen in the anterior chamber and around the iris and the ciliary body, and fewer inflammatory cells infiltrated into the retinas in the minocycline treatment eyes (Figure 2). Therefore, these results demonstrate that minocycline can effectively attenuate ocular inflammation in rat EIU.
Figure 1.
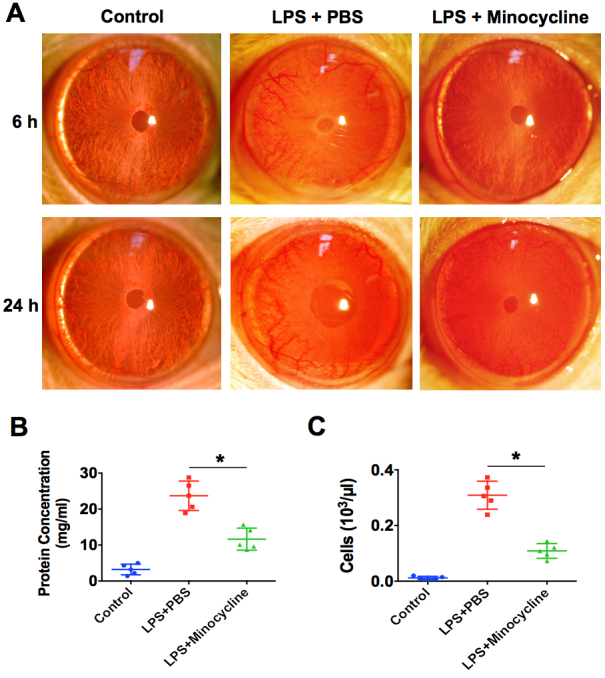
Minocycline attenuates the anterior inflammation in rat EIU. A: Treatment with 45 mg/kg of minocycline or PBS intraperitoneally 2 h before and after lipopolysaccharide (LPS) injection. Representative images of anterior segment of eyes were obtained with slit-lamp biomicroscopy after LPS injection for 6 h and 24 h (n=8 per group). B: The number of inflammatory cells in the aqueous humor were counted after 24 h (n=5 per group). *p<0.05 compared with the LPS plus PBS group. C: The protein concentrations of aqueous humor were quantified after 24 h (n=5 per group). *p<0.05 compared with the LPS plus PBS group.
Figure 2.
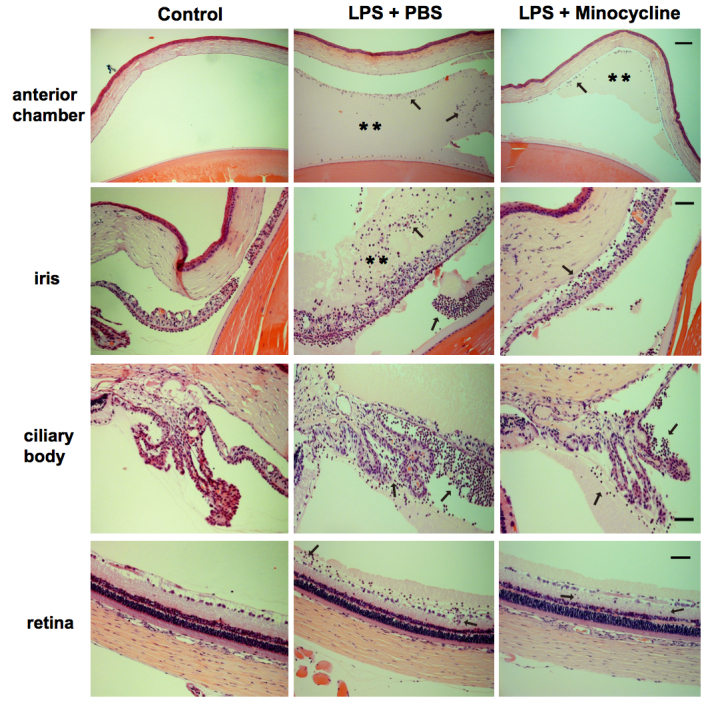
Minocycline attenuates LPS-induced inflammation in all ocular tissues. Histopathologic sections from the normal control eyes, and lipopolysaccharide (LPS) stimulation with or without minocycline treatment eyes were stained with hematoxylin-eosin. Representative images from light microscopy display that the anterior chamber, iris, and ciliary body are filled with fibrin (**) and infiltrated inflammatory cells (black arrows), and several inflammatory cells (black arrows) infiltrate into the retina in the PBS-treated retinas. However, minocycline treatment attenuates the inflammation response in all ocular tissues (n=6 per group). Scale bars: 100 μm for anterior chamber sections; 50 μm for iris, ciliary bodies, and retina sections.
In vivo labeling of retinal microglia
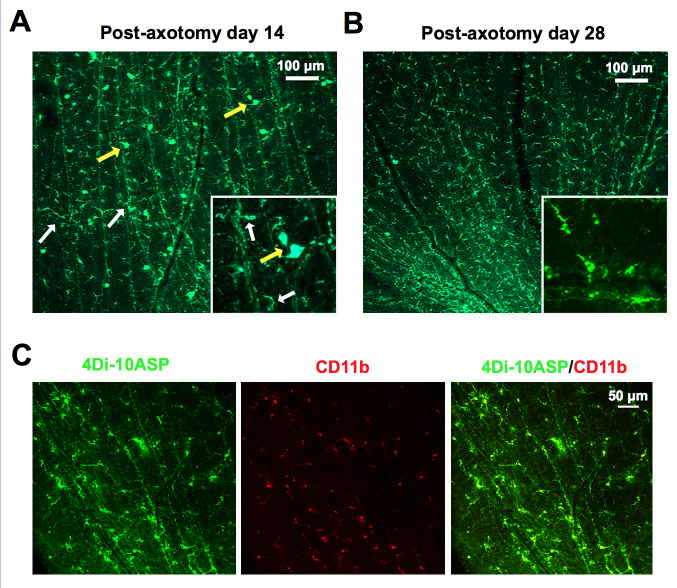
Labeling of retinal microglia using 4Di-10ASP in vivo is a well-established method for studying the role of retinal microglia, which can distinguish the activated retinal microglia from infiltrating macrophages [9,25]. Consistent with a previous study, at 14 days after 4Di-10ASP labeling, the whole-mount of the retinas revealed microglia and ganglion cells could be labeled with 4Di-10ASP. The ganglion cells were larger, measuring approximately 30 µm, while the microglia were smaller, measuring approximately 10 to 14 µm (Figure 3A). Nevertheless, at postaxotomy day 28, the ganglion cells disappeared, and the labeled cells presented highly branched morphology and were also positive for CD11b (a microglial marker; Figure 3B,C). These results suggest that only retinal microglia can be labeled with 4Di-10ASP on day 28. Therefore, we established an EIU model that used the axotomized rats at 28 days.
Figure 3.
In vivo labeling of retinal microglia with Fluorescent 4Di-10ASP. A: The whole-mount of the retinas shows the staining of the microglia (small ramified cells, white arrows) and ganglion cells (large cell bodies, yellow arrows) after optic nerve transection and 4Di-10ASP was packed for 14 days. Scale bars: 100 μm. Inset: higher magnification. B: Representative images from the whole-mount of the retinas show microglia staining after labeled with 4Di-10ASP for 28 days. Scale bars: 100 μm. Inset: higher magnification. C: The retinas, which were labeled with 4Di-10ASP for 28 days, were stained with the microglial marker CD11b (red), and the representative images were obtained with confocal microscopy. Scale bars: 50 μm.
Microglia can be rapidly activated and peripheral leukocytes were recruited to the retina in EIU
To test whether retinal microglia cells are activated in response to LPS stimulation, as occurs in the brain, EIU was induced in the axotomized rats at 28 days, and the eyes were collected at 3, 6, 12, and 24 h post-injection. Microglia morphological change was determined by immunostaining of retinal whole-mounts. At 3 h post-infection, CD11b+/4Di-10ASP+ cells (microglia) showed resting microglia characterized by small cells with rounded soma and various branching processes in the normal control retinas. However, in the LPS-injected retinas, the CD11b+/4Di-10ASP+ cells lost their highly branched morphology, and became large cell bodies and short processes, like the morphology of the amoeboid type (Figure 4A). At 6 h and 12 h post-infection, more and more CD11b+/4Di-10ASP- peripheral leukocytes were recruited and infiltrated in the LPS-injected retinas, increasing by approximately 15- and 60-fold, respectively (Figure 4A). Moreover, the results from real-time PCR also demonstrated that the expression of chemotactic factor CCL-2 and proinflammatory cytokines TNF-α, IL-1β, and IL-6 was upregulated dramatically in the LPS-injected retinas for 24 h (Figure 4B). Taken together, these results indicate that retinal microglia can be activated promptly, then peripheral leukocytes are recruited and infiltrate into the retinas, and various chemotactic factors and proinflammatory cytokines are markedly increased in the LPS-induced EIU model.
Figure 4.
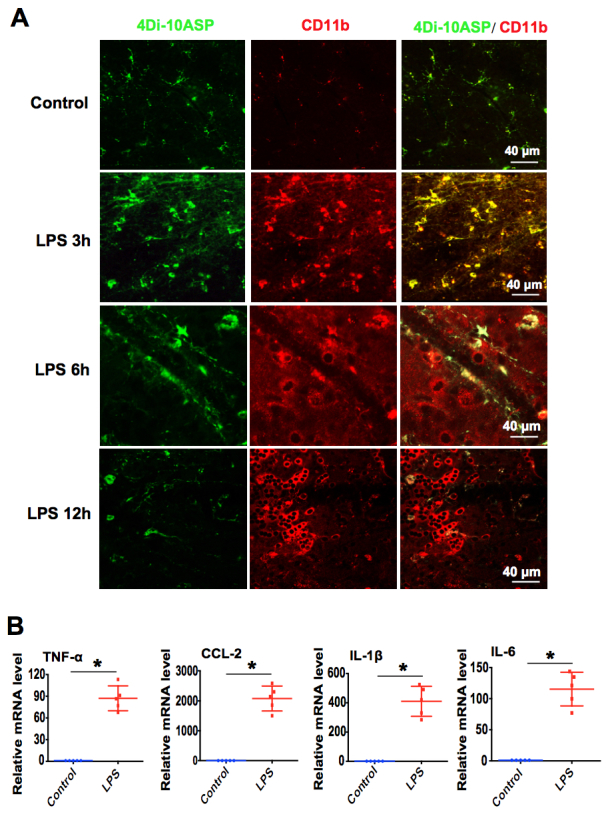
Microglia can be rapidly activated and peripheral leukocytes were recruited to the retina in EIU. A: One microgram of lipopolysaccharide (LPS) was injected into the vitreous of the 4Di-10ASP labeling rats to induce endotoxin-induced uveitis (EIU). The retinas were harvested and stained with a microglial marker CD11b (red) after infection for 3, 6, and 12 h. Representative immunofluorescence images of the retinal whole-mounts were obtained with confocal microscopy (n=6 per group). Scale bars: 40 μm. B: Twenty-four hours after the LPS injection, the retinas were harvested, and real-time PCR was used to analyze the mRNA expression of the chemotactic factor CCL-2, and proinflammatory cytokines TNF-α, IL-1β, and IL-6 (n=5 per group). *p<0.05 compared with the normal control retinas.
Minocycline inhibits LPS-induced the proinflammatory response of microglia in vitro
We next investigated whether minocycline treatment affects the activation of BV-2 microglial cells and the production of proinflammatory cytokines in vitro. The expression of chemotactic factor CCL-2 and proinflammatory cytokines TNF-α, IL-1β, and IL-6 was analyzed in the LPS-activated BV-2 cells for 6 h that were treated with or without minocycline. The results for real-time PCR displayed that LPS stimulation statistically significantly induced CCL-2 and the expression of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 in BV-2 microglial cells. Strikingly, minocycline treatment could attenuate the LPS-induced expression of IL-1β and CCL-2, but had no effects on TNF-α and IL-6 (Figure 5A). Furthermore, the immunofluorescent staining results also demonstrated that minocycline could decrease the LPS-induced expression of IL-1β and CCL-2 (Figure 5B). These results imply that minocycline can inhibit LPS-induced microglia activation and proinflammatory cytokine production.
Figure 5.
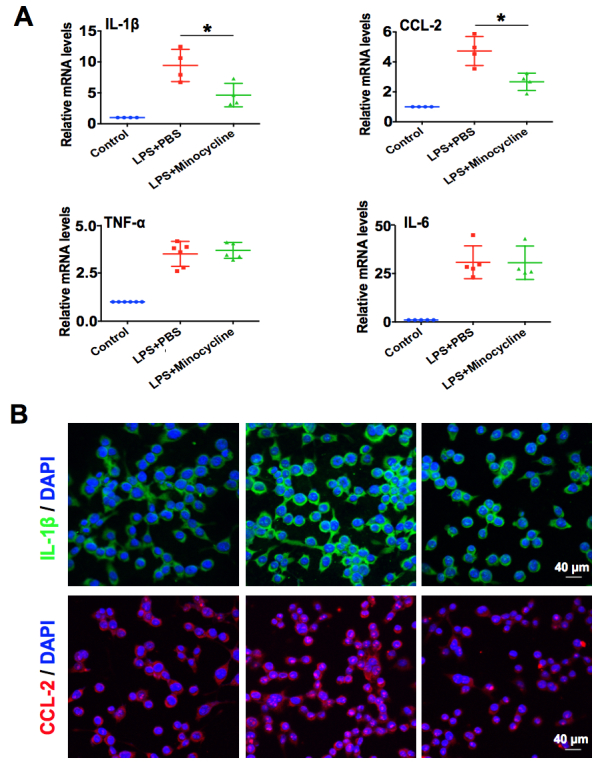
Minocycline inhibits LPS-induced the proinflammatory response of microglia in vitro. A: Real-time PCR analysis of IL-1β, CCL-2, TNF-α, and IL-6 mRNA expression in BV-2 cells exposed to 100 ng/ml of lipopolysaccharide (LPS) with or without minocycline (50 μg/ml) for 6 h. *p<0.05 compared with the LPS plus PBS group. B: Immunofluorescent staining analysis of IL-1β (green) and CCL-2 (red) expression in BV-2 microglial cells exposed to 100 ng/ml of LPS with or without minocycline (50 μg/ml) for 6 h (n=3 per group). Scale bars: 40 μm.
Minocycline attenuates LPS-induced retinal inflammation via suppression of microglia activation
Previously, retinal microglia activation and inflammatory cytokine production were reported to be the critical causes of retina damage during retinal inflammation [8,26]. To further determine how minocycline attenuates LPS-induced retinal inflammation in EIU, we evaluated microglia activation and inflammatory cytokine production using retina whole-mount staining and real-time PCR. As illustrated in Figure 6A, the results of whole-mount staining showed that increased numbers of Iba-1 (which primarily expresses in the activated microglia) positive cells were detected in the LPS-challenged retinas at 3 h post-infection. In contrast, almost no Iba-1 positive cells were seen in the minocycline-treated retinas. Additionally, the LPS challenge sharply induced the production of IL-1β and CCL-2 in the retinas at 24 h post-infection; however, minocycline treatment drastically reduced the expression of both (Figure 6A). Moreover, the results from real-time PCR also demonstrated that minocycline treatment could downregulate TNF-α, CCL-2, IL-1β, and IL-6 mRNA expression in response to LPS challenge in vivo (Figure 6B). Therefore, the results indicated that minocycline attenuates LPS-induced retinal inflammation in EIU via modulation of the microglial inflammatory response.
Figure 6.
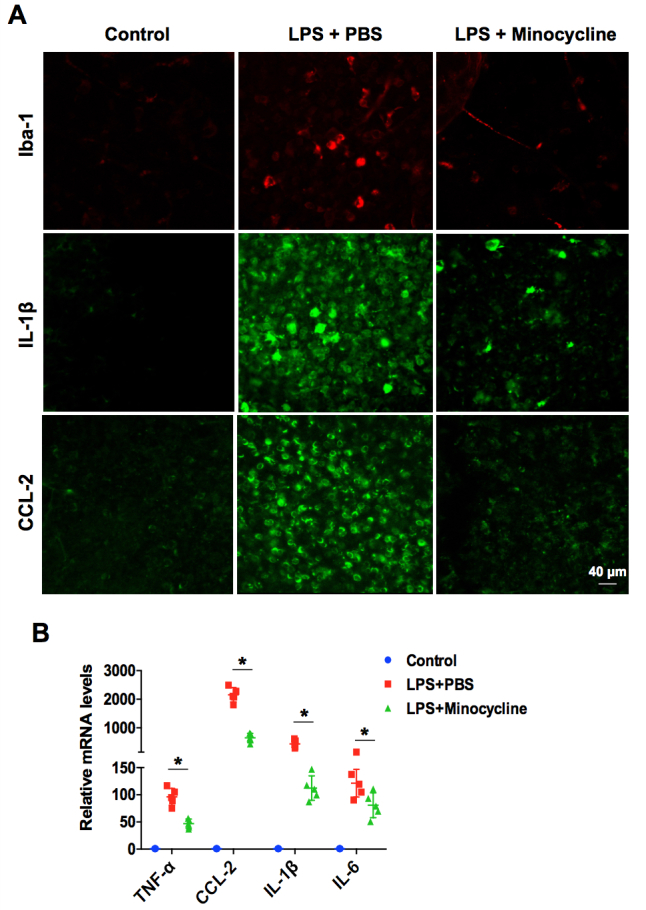
Minocycline attenuates LPS-induced retinal inflammation via suppression of microglia activation. A: Immunofluorescent staining of retinal whole-mounts show iba-1 (red) at 3 h post-infection, and interleukin-1 beta (IL-1β; green) and chemokine (C-C motif) ligand 2 (CCL-2; green) at 24 h in the lipopolysaccharide (LPS) stimulation with or without minocycline treatment eyes (n=4 per group). Scale bar: 40 μm. B: Real-time PCR analysis of the mRNA expression of tumor necrosis factor-α (TNF-α), CCL-2, IL-1β, and IL-6 in LPS stimulation with or without minocycline treatment eyes at 24 h post-infection. *p<0.05 compared with the LPS treated with PBS group.
Minocycline suppresses LPS-induced retinal microglia activation through downregulation of TLR4 signaling
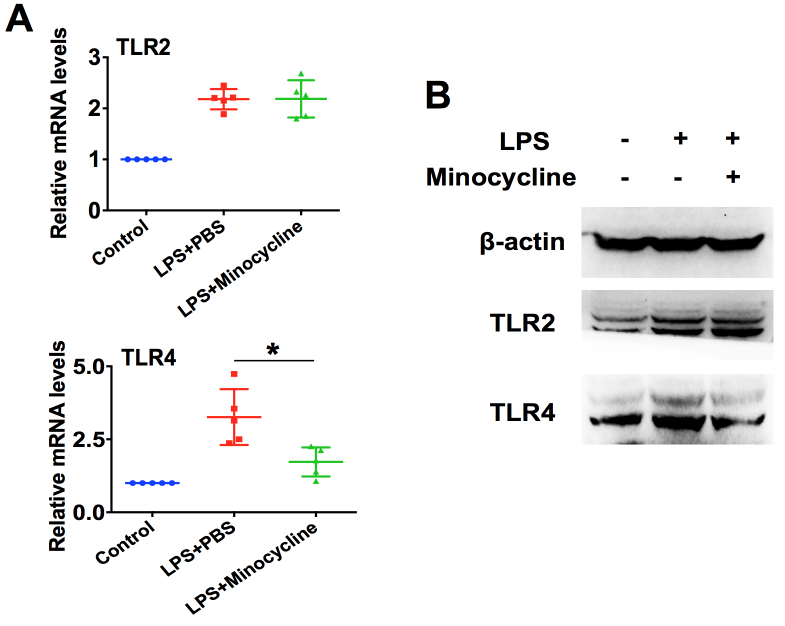
TLRs play a vital role in the innate immune surveillance by microglia [27]. TLR2 recognizes lipoproteins, yeast peptidoglycans, and Gram-positive bacteria cell walls [28], and TLR4 is essential for Gram-positive bacterial recognition, including LPS [29]. To understand how minocycline suppresses LPS-induced retinal microglia activation, we assessed the role of TLR2 and TLR4 in this process. As illustrated in Figure 7, LPS infection could upregulate the expression of TLR2 and TLR4 at the mRNA and protein levels in BV-2 microglial cells, and pretreatment with minocycline could inhibit the TLR4 expression induced by LPS. Nevertheless, minocycline did not affect TLR2 expression (Figure 7A,B). These findings revealed that minocycline suppresses LPS-induced retinal microglia activation through downregulation of TLR4 signaling.
Figure 7.
Minocycline suppresses LPS-induced retinal microglia activation through downregulation of TLR4 signaling. A: Real-time PCR analysis of Toll-like receptor (TLR) 2 and TLR4 mRNA expression in BV-2 microglial cells exposed to 100 ng/ml of lipopolysaccharide (LPS) with or without minocycline (50 μg/ml) for 6 h (n=5 per group). *p<0.05 compared with the LPS treated with PBS group. B: Western blotting of TLR2 and TLR4 protein expression (n=4 per group).
Discussion
Uveitis often has a devastating effect on ocular tissues and visual function. Corticosteroids are the only available anti-inflammatory treatment used. However, the use of steroids may induce many local and systemic side effects [30]. Minocycline is a broad-spectrum antibiotic, and it also has anti-inflammatory and neuronal protective effects in brain inflammation and neurodegenerative disease [6,24]. However, the effects of minocycline on uveitis remain unclear. In this study, we demonstrated, for the first time, that minocycline has a strong anti-inflammatory effect on rat EIU. Minocycline can effectively attenuate anterior and retinal inflammation induced by LPS. The results from cultured microglia cells in vitro and the rat EIU model in vivo revealed that retinal microglia can be promptly activated and involved in retinal inflammation in the EIU model, but minocycline can inhibit retinal inflammation through suppressing microglia activation, inhibiting inflammatory cell recruitment and inflammatory cytokine production.
In vivo labeling of retinal microglia with fluorescent dye 4Di-10ASP is a well-established method reported by Thanos and Richter [25]. The dye initially appears in the retinal ganglion cells through retrograde axoplasmic transport. The dye is then taken up by the retinal microglia as a result of their phagocytosis of the dye-labeled ganglion cells. The retinal microglia containing the dye remain labeled for up to 12 months [19]. Thus, the long-term labeling of microglia offers an opportunity for evaluating the role of retinal microglia in ocular diseases. In this study, we found microglia and ganglion cells can be labeled with 4Di-10ASP at 14 days after axotomy. Then the ganglion cells disappear, and only retinal microglia are labeled at postaxotomy day 28. These results are consistent with those of a previous study [19]. Therefore, we established an EIU model using the axotomized rats at 28 days in the present study.
Microglia cells form an important part of the retinal innate immune defense. Normally, microglia reside in the ganglion layer and the inner and outer plexiform layers of the retina. However, under pathogenic conditions, microglia can be activated and respond within an extremely short time, and undergo structural changes, from a resting surveillance state to an active ameboid form [31]. The activation of microglia may secrete various inflammatory mediators to further promote immune cells to recruit to the retina [32]. Therefore, microglial reactivity is a hallmark of various retinal degenerative and inflammatory diseases, including uveitis, retinitis pigmentosa, DR, glaucoma, and AMD [26]. In this study, we also demonstrated that retinal microglia are activated with changes in morphology response to LPS rapidly after challenge for 3 h. Then numerous peripheral leukocytes are recruited and infiltrate in the infected retinas. Furthermore, a wide range of proinflammatory cytokines, such as IL-1β, TNF-α, IL-6, and CCL-2, were increased in BV-2 microglia cells and the EIU model in vitro and in vivo, which can worsen retinal damage. Thus, these results suggest that microglia play an important role in LPS-induced retinal inflammation in EIU.
Minocycline, which is a broad-spectrum antibiotic, has anti-inflammatory effects independent of its anti-microbial activity. Minocycline has been proved to be a potent inhibitor for microglia activation, and a neuronal protector in brain ischemia and neurodegenerative disease models [33-37]. The beneficial effects of minocycline in ocular diseases have also been reported in a light-induced retinal degeneration model, glaucoma, diabetic retinopathy and branch retinal vein occlusion ischemic model [15,38-41]. However, no studies are available on the effects of minocycline on uveitis. In this study, we reported, for the first time, that minocycline can strongly alleviate LPS-induced inflammation response in the rat EIU model. Less fibrin exudation and fewer infiltrated inflammatory cells were seen in the anterior chamber and around the iris and the ciliary body, and fewer inflammatory cells infiltrated into the retinas in the minocycline treatment group. In addition, minocycline can significantly suppress retinal microglia cell activation and the production of proinflammatory cytokines TNF-α, CCL-2, IL-1β, and IL-6 in vivo. However, minocycline had no effects on TNF-α and IL-6 in vitro. We speculate that the inconsistent results for TNF-α and IL-6 in vitro and in vivo were due to the difference in the nature of the models. In vivo, various types of cells are involved in the immune response and produce inflammatory factors, rather than a single kind of cell as in an in vitro study.
TLRs have been shown to recognize pathogen-associated molecular patterns and initiate innate immune responses. At present, ten TLRs have been identified in humans and nine in mice, including TLR1–9 [42]. It has been reported that TLR2 associates with TLR6 to recognize triacyl lipopeptides from bacteria, and TLR4 recognizes LPS in association with a LPS-binding protein, CD14, and MD-2 [42]. TLR signaling activation leads to the induction of nuclear factor kappa beta (NF-κB), ultimately leading to chemokine and proinflammatory cytokine production, and upregulation of the cell surface molecules involved in the initiation of adaptive immune responses [43]. In addition, microglia express all of the recently identified TLRs and form an important part of the retinal innate immune defense [27]. Stimulation of quiescent microglia with various TLR agonists, including LPS (TLR4), peptidoglycan (TLR2), polyinosinic-polycytidylic acid (TLR3), and CpG DNA (TLR9), can activate the microglia to produce proinflammatory cytokines and chemokines [27]. Based on the dominant roles of TLR signaling in the innate immune response of microglia, it can be postulated that the effects of minocycline are likely to occur via suppressing TLR signaling. Thus, we examined the expression of TLR2 and TLR4 that recognize bacteria, and demonstrated minocycline treatment can suppress TLR4 expression directly. Therefore, these data indicate that the mechanism of the microglia inactivation effect of minocycline is via suppressing TLR4 signaling.
In summary, using the EIU model in vitro and in vivo, these results provide evidence that minocycline can strongly alleviate the LPS-induced inflammation response in the rat EIU model. Microglia can be activated promptly and play a critical role in retinal inflammation. Minocycline treatment can inhibit retinal inflammation through suppressing microglia activation, and inhibiting inflammatory cell recruitment and inflammatory cytokine production in vitro and in vivo. Finally, we also demonstrated the mechanism of the microglia inactivation effect of minocycline is via directly suppressing TLR4 signaling. These encouraging results suggest that minocycline has potential as a therapeutic approach for the treatment of uveitis.
Acknowledgments
This research was funded by the Science and Technology Planning Foundation of Guangdong Province (2016A020215092).
Appendix 1. STR analysis
To access the data, click or select the words “Appendix 1.”
References
- 1.Vallejo-Garcia JL, Asencio-Duran M, Pastora-Salvador N, Vinciguerra P, Romano MR. Role of inflammation in endophthalmitis. Mediators Inflamm. 2012;2012:196094. doi: 10.1155/2012/196094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478–83. doi: 10.1097/00055735-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337–52. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortolanza M, Nascimento GC, Socias SB, Ploper D, Chehin RN, Raisman-Vozari R, Del-Bel E. Tetracycline repurposing in neurodegeneration: focus on Parkinson’s disease. J Neural Transm (Vienna) 2018;125:1403–1415. doi: 10.1007/s00702-018-1913-1. [DOI] [PubMed] [Google Scholar]
- 5.Kielian T, Esen N, Liu SL, Phulwani NK, Syed MM, Phillips N, Nishina K, Cheung AL, Schwartzman JD, Rulhe JJ. Minocycline modulates neuroinflammation independently of its antimicrobial activity in Staphylococcus aureus-induced brain abscess. Am J Pathol. 2007;171:1199–214. doi: 10.2353/ajpath.2007.070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–79. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–68. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76:463–71. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 9.Rao NA, Kimoto T, Zamir E, Giri R, Wang R, Ito S, Pararajasegaram G, Read RW, Wu GS. Pathogenic role of retinal microglia in experimental uveoretinitis. Invest Ophthalmol Vis Sci. 2003;44:22–31. doi: 10.1167/iovs.02-0199. [DOI] [PubMed] [Google Scholar]
- 10.Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17:463–71. doi: 10.1017/s0952523800173122. [DOI] [PubMed] [Google Scholar]
- 11.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–32. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 12.Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez-Punaro Mde L, Segura M, Contreras I, Lachance C, Houde M, Lecours MP, Olivier M, Gottschalk M. In vitro characterization of the microglial inflammatory response to Streptococcus suis, an important emerging zoonotic agent of meningitis. Infect Immun. 2010;78:5074–85. doi: 10.1128/IAI.00698-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin HY, Tang CH, Chen JH, Chuang JY, Huang SM, Tan TW, Lai CH, Lu DY. Peptidoglycan Induces Interleukin-6 Expression Through the TLR2 Receptor, JNK, c-Jun, and AP-1 Pathways in Microglia. J Cell Physiol. 2011;226:1573–82. doi: 10.1002/jcp.22489. [DOI] [PubMed] [Google Scholar]
- 15.Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–65. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Xu GZ, Liu W, Ni YQ, Zhou WT.Role of Fractalkine/CX3CR1 Interaction in Light-Induced Photoreceptor Degeneration through Regulating Retinal Microglial Activation and Migration. PLoS One 20127e35446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum JT, Woods A, Kezic J, Planck SR, Rosenzweig HL. Contrasting ocular effects of local versus systemic endotoxin. Invest Ophthalmol Vis Sci. 2011;52:6472–7. doi: 10.1167/iovs.11-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–6. [PubMed] [Google Scholar]
- 19.Bodeutsch N, Thanos S. Migration of phagocytotic cells and development of the murine intraretinal microglial network: An in vivo study using fluorescent dyes. Glia. 2000;32:91–101. doi: 10.1002/1098-1136(200010)32:1<91::aid-glia90>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Merida S, Sancho-Tello M, Almansa I, Desco C, Peris C, Moreno ML, Villar VM, Navea A, Bosch-Morell F. Bevacizumab Diminishes Inflammation in an Acute Endotoxin-Induced Uveitis Model. Front Pharmacol. 2018;9:649. doi: 10.3389/fphar.2018.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Z, Zhou T, Sun X, Zheng Y, Cheng B, Li M, Liu X, He C. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 2018;25:180–9. doi: 10.1038/cdd.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Xiao W, Chen W, Liu X, Wu M, Bo Q, Luo Y, Ye S, Cao Y, Liu Y. MicroRNA-26a and −26b inhibit lens fibrosis and cataract by negatively regulating Jagged-1/Notch signaling pathway. Cell Death Differ. 2017;24:1990. doi: 10.1038/cdd.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 24.Adembri C, Selmi V, Vitali L, Tani A, Margheri M, Loriga B, Carlucci M, Nosi D, Formigli L, De Gaudio AR. Minocycline But Not Tigecycline Is Neuroprotective and Reduces the Neuroinflammatory Response Induced by the Superimposition of Sepsis Upon Traumatic Brain Injury. Crit Care Med. 2014;42:E570–82. doi: 10.1097/CCM.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 25.Thanos S, Kacza J, Seeger J, Mey J. Old dyes for new scopes: the phagocytosis-dependent long-term fluorescence labelling of microglial cells in vivo. Trends Neurosci. 1994;17:177–82. doi: 10.1016/0166-2236(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 26.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 28.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 29.Savva A, Roger T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol. 2013;4:387. doi: 10.3389/fimmu.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn JP. Uveitis. Primary Care. 2015;42:305. doi: 10.1016/j.pop.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: insights from novel mouse models. Immunobiology. 2010;215:685–91. doi: 10.1016/j.imbio.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Kochan T, Singla A, Tosi J, Kumar A. Toll-like receptor 2 ligand pretreatment attenuates retinal microglial inflammatory response but enhances phagocytic activity toward Staphylococcus aureus. Infect Immun. 2012;80:2076–88. doi: 10.1128/IAI.00149-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95:15769–74. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–71. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM. Inhibition of Microglial Activation Protects Hippocampal Neurogenesis and Improves Cognitive Deficits in a Transgenic Mouse Model for Alzheimer’s Disease. Neurodegener Dis. 2012;9:187–98. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aras M, Urfali B, Serarslan Y, Ozgur T, Ulutas KT, Urfali S, Altas M, Yilmaz N. Protective effects of minocycline against short-term ischemia-reperfusion injury in rat brain. Pediatr Neurosurg. 2013;49:172–8. doi: 10.1159/000362202. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Xu L, Yin J, Zhong Z, Fan H, Li X, Chang Q. Effect of minocycline on cerebral ischemia-reperfusion injury. Neural Regen Res. 2013;8:900–8. doi: 10.3969/j.issn.1673-5374.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scholz R, Sobotka M, Caramoy A, Stempfl T, Moehle C, Langmann T. Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration. J Neuroinflammation. 2015;12:209. doi: 10.1186/s12974-015-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1437–46. doi: 10.1167/iovs.07-1337. [DOI] [PubMed] [Google Scholar]
- 40.Levkovitch-Verbin H, Kalev-Landoy M, Habot-Wilner Z, Melamed S. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch Ophthalmol. 2006;124:520–6. doi: 10.1001/archopht.124.4.520. [DOI] [PubMed] [Google Scholar]
- 41.Sun C, Li XX, He XJ, Zhang Q, Tao Y. Neuroprotective effect of minocycline in a rat model of branch retinal vein occlusion. Exp Eye Res. 2013;113:105–16. doi: 10.1016/j.exer.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 43.Janssens S, Beyaert R. Role of Toll-like receptors in pathogen recognition. Clin Microbiol Rev. 2003;16:637–46. doi: 10.1128/CMR.16.4.637-646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]