Abstract
Background and Purpose
Cerebrospinal fluid (CSF) biomarkers of Alzheimer's disease (AD) could be misleading in idiopathic normal-pressure hydrocephalus (iNPH). We therefore investigated the CSF biomarkers in 18F-florbetaben amyloid-negative positron-emission tomography (PET) [amyloid PET(−)] iNPH, amyloid-positive PET [amyloid PET(+)] AD, and cognitively normal (CN) subjects.
Methods
Ten amyloid PET(+) AD patients (56.7±5.6 years old, mean±standard deviation), 10 amyloid PET(−) iNPH patients (72.8±4.5 years old), and 8 CN subjects (61.2±6.5 years old) were included. We measured the levels of β-amyloid (Aβ)40, Aβ42, total tau (t-tau) protein, and phosphorylated tau (p-tau) protein in the CSF using enzyme-linked immunosorbent assays.
Results
The level of Aβ42 and the Aβ42/Aβ40 ratio in the CSF were significantly lower in AD than in iNPH or CN subjects. The Aβ40 level did not differ significantly between AD and iNPH (p=1.000), but it did between AD and CN subjects (p=0.032). The levels of both t-tau and p-tau were higher in AD than in iNPH or CN subjects. The levels of Aβ42, Aβ40, t-tau, and p-tau were lower in iNPH than in CN subjects, but there was no significant difference after controlling for age.
Conclusions
Our results suggest that the mechanism underlying low CSF Aβ levels differs between amyloid PET(−) iNPH and amyloid PET(+) AD subjects. The lower levels of all CSF biomarkers in iNPH patients might be due to reduced clearances from extracellular fluid and decreased brain metabolism of the periventricular zone in iNPH resulting from glymphatic dysfunction.
Keywords: Alzheimer's disease biomarkers, idiopathic normal pressure hydrocephalus, amyloid positron-emission tomography, cerebrospinal fluid
INTRODUCTION
Idiopathic normal-pressure hydrocephalus (iNPH) is characterized by the clinical triad of symptoms of cognitive impairment, gait difficulty, and urinary incontinence, and is accompanied by ventricular enlargement in brain imaging.1 Shunt surgery is thought to alleviate these symptoms in some iNPH patients, but other coexisting neurodegenerative diseases compromise the effectiveness of this approach and thus should be investigated thoroughly before surgery. Coexisting Alzheimer's disease (AD) has been reported in 30–60% of iNPH patients and is associated with poor shunt response and progressive cognitive decline after shunt surgery.2,3 AD is usually evaluated by analyzing the levels of β-amyloid (Aβ)42, total tau (t-tau) protein, and 181-threonine-phosphorylated tau (p-tau) protein in the cerebrospinal fluid (CSF) using enzyme-linked immunosorbent assays (ELISAs).4 Low Aβ42 levels are thought to reflect brain amyloid deposition, while high t-tau and p-tau levels indicate neurodegeneration. Among these CSF biomarkers, the change in Aβ42 levels occurs earlier than those in the other two tau-associated markers. A specific decrease in Aβ42 combined with no change in t-tau and p-tau levels has been considered a preclinical marker for coexisting AD in iNPH patients, without involving any substantial neuronal injury.5,6,7,8
Some researchers have recently reported that CSF AD biomarkers may be misleading in iNPH patients.9 A recent meta-analysis suggested that iNPH is associated with significantly low CSF levels of Aβ42, t-tau, and p-tau.10 Various hypotheses have been proposed for explaining the reduced levels of AD biomarkers in iNPH. One hypothesis is that tight sulci over the cortex of iNPH patients could compromise the convective flow of interstitial fluid and the clearance of amyloid precursor protein (APP) fragments.9 Other authors have proposed that reduced periventricular metabolism accompanied by decreased levels of APP-derived proteins but no major cortical degeneration could explain these results.6 However, these studies did not include 18F-florbetaben amyloid positron-emission tomography (PET) analyses, which probably affected the interpretation of the levels of CSF AD biomarkers in iNPH patients.
The aim of the present study was to measure CSF AD biomarkers in amyloid-positive PET [amyloid PED(+)] AD, amyloid-negative PET [amyloid PED(−)] iNPH, and cognitively normal (CN) subjects, and identify the mechanisms underlying the observed changes.
METHODS
Subjects
Ten probable-iNPH and 10 probable-AD patients were prospectively recruited at Ajou University Hospital (Suwon, Korea) from March 2015 to February 2017. Eight CN subjects were recruited at Chung-Ang University Hospital (Seoul, Korea). All three groups were recruited using convenience sampling. The selection criteria for probable-iNPH patients included the following: 1) insidious onset at an age ≥40 years, disease duration ≥6 months, progressive disease course, and no other neurological, psychiatric, or medical conditions other than the expected disease symptoms; 2) ventricular enlargement that was not entirely attributable to cerebral cortical atrophy, as detected by magnetic resonance imaging (MRI); 3) gait disturbance with or without cognitive deficits or urinary disturbances; and 4) no evidence of high intracranial pressure (70–245 mm H2O).11 The Evans' index was assessed using 3-Tesla MRI (Philips 3.0T Achieva; Best, the Netherlands), and defined as the maximal frontal horn ventricular width divided by the transverse inner diameter of the skull. This index is indicative of ventriculomegaly if it is ≥0.3.11
The clinical syndrome in patients with iNPH was characterized and graded using a NPH scale modified from Larsson et al.12 and Krauss et al.13 that assessed gait [1=normal, 2=walk without any assistive device but insecure, 3=walk with cane, 4=walk with bimanual support (walker), 5=walk with an assistant, and 6=wheelchair-bound], urinary disturbance (0=normal, 1=sporadic incontinence or urge phenomena, 2=frequent incontinence or urge phenomena, and 3=no or minimal control of bladder function), and cognitive deficit (0=normal, 1=minimal attention or memory deficits, 2=considerable attention or memory deficits but oriented to situational context, and 3=not or only marginally oriented to situational context). Patients were evaluated before performing a lumbar puncture (LP).
All probable-AD patients were selected based on the criteria proposed by the National Institute of Neurological and Communicative Diseases and Stroke and the Alzheimer's Disease and Related Disorders Association.14 In patients with iNPH and AD, white-matter hyperintensities (WMH) were assessed using the Clinical Research Center for Dementia of South Korea (CREDOS) rating scale.15 We excluded patients with a history of significant hearing or visual impairments that could interfere with interviews, a history of other neurological disorders (e.g., idiopathic Parkinson's disease, dementia with Lewy bodies, or active epilepsy), psychiatric illnesses (e.g., schizophrenia, mental retardation, major depression, or mania), or significant alcohol and/or other substance abuse, as well as patients taking psychotropic medications. CN subjects were chosen if they scored higher than the cutoff value in each cognitive domain test. The cutoff value for each test score was one standard deviation below the published norm for the age and education group.16
All patients provided written informed consent unless they had impaired decisional capacity, in which case caregivers provided consent and patients provided assent. This study was approved by the Ajou Institutional Review Board (AJIRB-BMR-OBS-15-262).
Sample collection
All iNPH subjects underwent LP in the L3-4 or L4-5 spinal vertebrae interspace between 10 a.m. and 12 p.m. to drain 30–50 mL of CSF. During the procedure, 10-mL aliquots of CSF were collected in polypropylene tubes after discarding the first 3–4 mL. Bloody or cloudy samples were not used. AD and CN subjects underwent LP using the same protocol except for CSF drainage. No serious adverse events were reported. The CSF samples were immediately centrifuged for 15 min at 2,000 g to remove cells, and the obtained supernatants were stored in polypropylene tubes and immediately frozen at −80℃ until being analyzed.
ELISA
The Aβ42 and Aβ40 levels in the CSF were measured using a sandwich ELISA method (INNOTEST Aβ42 and Aβ40, Fujirebio, Ghent, Belgium) specifically designed for measuring each Aβ type, in accordance with the manufacturer's instructions. The CSF t-tau level was determined using a sandwich ELISA method (INNOTEST hTAU-Ag, Fujirebio) specifically designed to measure all tau isoforms irrespective of their phosphorylation status. The CSF p-tau level was measured at position 181 using a sandwich ELISA method [INNOTEST Phospho-Tau(181P), Fujirebio] specifically designed to measure threonine p-tau. All biomarker levels were measured in duplicate and in accordance with the manufacturer's instructions.
Amyloid PET
All iNPH and AD subjects underwent 18F-florbetaben PET. Brain PET/computed tomography (CT) images were obtained with a Discovery ST scanner (GE Healthcare, Milwaukee, WI, USA). An average of 300 MBq of 18F-florbetaben was injected intravenously, and scanning was initiated 90 min later. A noncontrast brain CT scan was performed in the automatic mode (120 kV, 30–100 mA, and section width=3.75 mm) for attenuation correction, and was immediately followed by PET imaging in the three-dimensional mode for 20 min. Motion artifacts were minimized by immobilizing the subject's head in a head holder. PET images were obtained by iterative reconstruction using an ordered subset expectation maximization algorithm with 4 iterations and 21 subsets.
Interpretation and analysis of amyloid PET scans
A board-certified nuclear medicine physician and neuro-PET expert reviewed the amyloid PET images on an Advantage workstation (Advantage Sim, version 4.4, GE Healthcare). The examiners were blinded to all clinical data related to the patients. Grayscale transaxial brain images were visually assessed, and the regional uptakes of the tracer in the lateral temporal, frontal, and posterior cingulate cortex/precuneus, as well as in the parietal cortex were scored using previously described criteria17 (1=no Aβ load, 2=minor Aβ load, and 3=significant Aβ load). A score of 1 was interpreted as an amyloid PET(−) scan, and a score of 2 or 3 was interpreted as an amyloid PET(+) scan.
18F-florbetaben uptake in the brain was quantified using recent T1-weighted MRI scans (MRI 18F-florbetaben scan interval=1.3±1.8 years, mean±standard deviation). All MRI and PET images were processed using the PMOD Neuro tool (PNEURO, version 3.7, PMOD Technologies, Zürich, Switzerland). Automated segmentation was applied to the MRI images to remove the white matter and CSF. The segmented MRI scans and the respective PET images were coregistered and then spatially normalized according to the standard Montreal Neurological Institute T1 template. An automated anatomic labeling atlas18 was subsequently applied. The mean cortical standardized uptake value ratio (SUVR) was calculated in selected regions of interest including the frontal, lateral temporal, parietal, and posterior cingulate cortices, as assessed by visual scoring criteria. The PET images were analyzed quantitatively as described previously.19
Statistical analyses
The Kolmogorov-Smirnov test was used to determine whether continuous variables conformed to a normal distribution. Welch's analyses of variance with Games-Howell post-hoc tests were performed, since the assumption of variance homogeneity was not met in Levene's test. Fisher's exact test was used to compare dichotomous variables. The levels of CSF biomarkers were compared across the three groups using a univariate general linear model while adjusting for age. All statistical analyses were performed using SPSS (version 18.0, SPSS Inc., Chicago, IL, USA), and statistical significance was considered to be present at p<0.05.
RESULTS
The demographic and clinical characteristics of all of the study subjects are presented in Table 1. The iNPH patients were significantly older (72.8±4.5 years) than the AD patients (56.7±5.6 years, p<0.001) and CN subjects (61.2±6.5 years, p=0.003). The symptom scores in patients with iNPH were 2.0±0.0 for gait, 1.6±0.5 for urinary disturbance, and 1.6±0.5 for cognitive deficit. The degree of WMH was moderate in two iNPH patients and one AD patient, and mild in all of the other patients. Visual assessments of the amyloid PET scans revealed that the 10 AD subjects were amyloid PET(+) and the 10 iNPH subjects were amyloid PET(−). The cortical SUVR was significantly higher in AD patients (1.97±0.25) than in iNPH patients (1.37±0.16, p<0.001).
Table 1. Demographic and clinical characteristics of the subjects.
| iNPH (n=10) | AD (n=10) | CN (n=8) | p1 | p2 | p3 | |
|---|---|---|---|---|---|---|
| Age (years) | 72.8±4.5 | 56.7±5.6 | 61.2±6.5 | <0.001 | 0.003 | 0.294 |
| Sex (male/female) | 7/3 | 4/6 | 2/6 | 0.370 | 0.153 | 0.638 |
| Education (years) | 10.6±3.4 | 10.4±4.9 | 13.0±2.4 | 0.990 | 0.185 | 0.286 |
| K-MMSE score | 20.2±2.9 | 12.4±4.2 | 28±2 | <0.001 | <0.001 | <0.001 |
| CDR | 0.85±0.47 | 1.15±0.62 | 0.37±0.23 | 0.465 | 0.038 | 0.009 |
| CDR-SB | 4.15±3.38 | 6.80±3.35 | 0.94±0.32 | 0.211 | 0.036 | 0.001 |
| Evans' index | 0.37±0.04 | 0.26±0.03 | - | <0.001 | - | - |
| Cortical SUVR | 1.37±0.16 | 1.97±0.25 | - | <0.001 | - | - |
Data are mean±standard-deviation values.
AD: Alzheimer's disease, CDR: Clinical Dementia Rating, CDR-SB: CDR Sum of Boxes, CN: cognitively normal, iNPH: idiopathic normal-pressure hydrocephalus, K-MMSE: Korean version of the Mini Mental State Examination, p1: iNPH vs. AD, p2: iNPH vs. CN, p3: AD vs. CN, SUVR: standardized uptake value ratio.
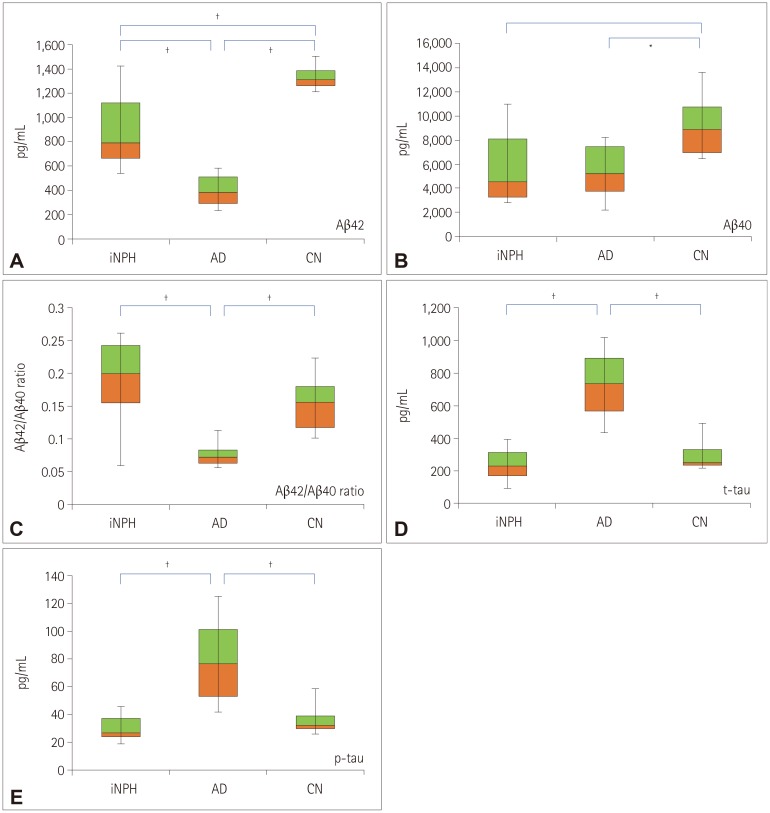
The levels of the assessed AD biomarkers in the CSF are described in Table 2 and plotted in Fig. 1. The CSF Aβ42 level was significantly lower in AD patients than in iNPH patients and CN subjects (p<0.001 and p<0.004, respectively; Fig. 1A). The CSF Aβ40 level was also significantly lower in AD patients than in CN subjects (p<0.032; Fig. 1B), but it did not differ significantly between the iNPH and AD groups. Although the statistical significance was not high enough, Aβ42 and Aβ40 levels were numerically lower in iNPH patients than in CN subjects. The ratio of Aβ42 to Aβ40 levels was significantly lower in AD than in iNPH patients and CN subjects (p=0.001 and p=0.003, respectively; Fig. 1C), while it did not differ between the iNPH and CN groups.
Table 2. AD biomarker levels in the cerebrospinal fluid.
| iNPH | AD | CN | p1 | p2 | p3 | |
|---|---|---|---|---|---|---|
| Aβ42 (pg/mL) | 892.8±311.4 | 391.8±120.2 | 1330.0±99.7 | <0.001 | 0.089 | <0.001 |
| Aβ40 (pg/mL) | 5504.0±2867.1 | 5462.5±2192.2 | 9179.6±2596.9 | 1.000 | 0.082 | 0.032 |
| Aβ42/Aβ40 ratio | 0.187±0.068 | 0.077±0.020 | 0.155±0.044 | 0.001 | 0.178 | 0.003 |
| t-tau (pg/mL) | 238.9±103.7 | 720.7±220.1 | 294.5±95.1 | <0.001 | 1.000 | <0.001 |
| p-tau (pg/mL) | 30.4±9.5 | 78.1±30.6 | 36.2±11.1 | 0.001 | 0.560 | <0.001 |
Data are mean±standard-deviation values.
Aβ: β-amyloid, AD: Alzheimer's disease, CN: cognitively normal, iNPH: idiopathic normal-pressure hydrocephalus, t-tau: total tau, p1: iNPH vs. AD, p2: iNPH vs. CN, p3: AD vs. CN, p-tau: phosphorylated tau.
Fig. 1. AD biomarker levels in the cerebrospinal fluid of iNPH, AD, and CN subjects. Box plots of Aβ42 (A), Aβ40 (B), Aβ42/Aβ40 ratio (C), t-tau (D), and p-tau (E). Each box plot shows the median, first and third quartiles, and range. *p<0.05, †p<0.01 (student t-test). AD: Alzheimer's disease, iNPH: idiopathic normal-pressure hydrocephalus. Aβ: β-amyloid, AD: Alzheimer's disease, CN: cognitively normal, iNPH: idiopathic normal-pressure hydrocephalus, p-tau: phosphorylated tau, t-tau: total tau.
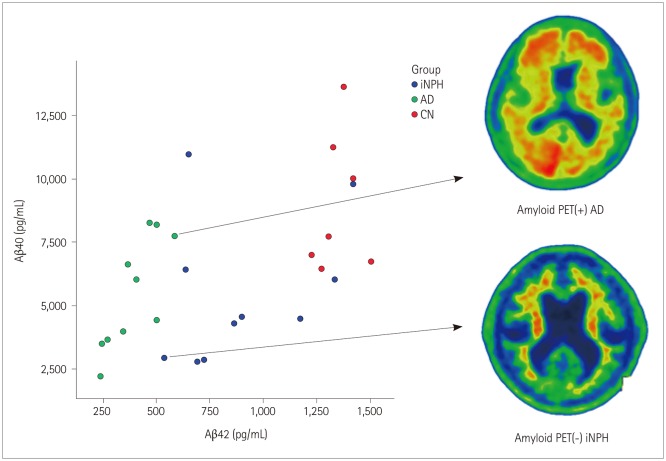
We also assessed the CSF levels of t-tau and p-tau. We found that both t-tau and p-tau levels were significantly higher in AD than in iNPH and CN subjects (t-tau, p<0.001 and p<0.001, respectively; p-tau, p<0.001 and p<0.001, respectively; Fig. 1D and E), while they did not differ between the iNPH and CN subjects. The CSF levels of Aβ42 and Aβ40 in each subject group as well as representative amyloid PET images of amyloid PET(+) AD and amyloid PET(−) iNPH patients are presented in Fig. 2.
Fig. 2. Correlation between Aβ42 and Aβ40 levels in iNPH, AD, and CN subjects. The scatter plot shows the correlation between Aβ42 and Aβ40 levels. Representative images from an amyloid PET(+) AD scan and an amyloid PET(−) iNPH scan are shown on the right. Aβ: β-amyloid, AD: Alzheimer's disease, amyloid PET(+): amyloid positron-emission tomography-positive, amyloid PET(−): amyloid positron-emission tomography-negative, CN: cognitively normal, iNPH: idiopathic normal-pressure hydrocephalus.
DISCUSSION
In this study we found that the levels of both Aβ40 and Aβ42 in the CSF numerically decreased proportionately in amyloid PET(−) iNPH patients compared to CN subjects, although the statistical significance was not high enough. In contrast, amyloid PET(+) AD patients showed a disproportionate decrease in these levels. We also found that the Aβ42/Aβ40 ratio can be used to successfully discriminate between iNPH and AD. Finally, we have revealed differences in t-tau and p-tau levels between amyloid PET(+) AD patients and amyloid PET(−) iNPH patients. To our knowledge, this is the first study to investigate AD biomarkers in the CSF of amyloid PET(−) iNPH patients. We suggest that different mechanisms underlie the low Aβ42 observed in iNPH and AD patients.
There have been inconsistencies in the reported levels of Aβ42 in iNPH and AD patients, with some reports of overlapping levels7,20 but others of lower Aβ42 levels in AD than iNPH patients.5,8,10 These discrepancies might be due to the inclusion of different numbers of patients in the iNPH groups, possibly with coexisting AD and iNPH, since no amyloid PET analyses were performed in these previous studies. In our study we used amyloid PET to distinguish between amyloid PET(−) iNPH and amyloid PET(+) AD patients, and confirmed that the Aβ42 levels are decreased in both patients, but more so in those with AD.
Our ELISA results demonstrated that the Aβ42/Aβ40 ratio is significantly higher in iNPH than AD patients, with no differences found between the iNPH and CN subjects. In line with a previous study,8 these results suggest that Aβ42 and Aβ40 levels are differentially decreased in AD and iNPH patients. The disproportionate decrease in Aβ42 level has been shown to be a signature pattern in amyloid PET(+) AD patients, due to the higher tendency of Aβ42 to be deposited in amyloid plaques.21 In contrast, the similarities in the Aβ42/Aβ40 ratio between the amyloid PET(−) iNPH and CN subjects suggest that there is no selective deposition of Aβ42 in iNPH.
The distinct profile of CSF AD biomarkers in amyloid PET(−) iNPH patients in the present study differ from that of preclinical AD patients. Previous studies employing imaging and CSF AD biomarkers found that a decrease in Aβ42 levels in amyloid PET(−) subjects was the earliest change before the amyloid deposition in the brain parenchyma of preclinical AD patients.22 Accordingly, the decreased Aβ42 levels in iNPH have been attributed to an underlying early or preclinical AD pathology. However, a recent study investigating the pattern of CSF AD biomarkers in preclinical AD patients showed that the disproportionate decrease in Aβ42 than Aβ40 levels is still observed even in the early stages of preclinical AD.23 Longitudinal observations of changes in the Aβ42/Aβ40 ratio and the CSF t-tau and p-tau levels in amyloid PET(−) iNPH patients could elucidate the distinct CSF AD biomarker patterns during the initiation and progression of AD neuropathology in iNPH.
The mechanism underlying the low levels of Aβ isoforms in iNPH has been highly controversial. Jeppson et al.6 analyzed a panel of Aβ isoforms (Aβ38, Aβ40, and Aβ42) and soluble APP (sAPPα and sAPPβ) isoforms in iNPH and healthy individuals, and showed that all of these biomarkers were reduced in iNPH presurgically but restored after shunt surgery. Those authors proposed that these findings could be explained by reduced periventricular amyloid metabolism and/or reduced clearance into the CSF. On the other hand, Graff-Radford suggested that glymphatic dysfunction could be causing the low CSF Aβ isoforms. Indeed, there is a recent report of glymphatic dysfunction in iNPH patients, which was detected by using glymphatic MRI after injection with an intrathecal contrast agent.9,24 Meanwhile, transient glymphatic dysfunction caused by sleep deprivation has been reported to increase CSF Aβ levels; however, little is known about the chronic glymphatic dysfunction in iNPH patients.25,26 A longitudinal serial evaluation of glymphatic function in association with CSF AD biomarkers is therefore required to elucidate how it affects the levels of these biomarkers.
A meta-analysis found that tau levels were lower in NPH than in AD,10 which is consistent with our results. Tau levels in our iNPH patients were significantly lower than those in the AD patients and numerically lower than those in CN subjects. Previous studies have found that the level of tau increases with age.27,28 In our study, the patients with NPH were older than the AD and CN subjects, but lower levels of tau suggest that the pathophysiology differs between AD and NPH. Our study supports that reduced clearance from extracellular fluid and decreased brain metabolism of the periventricular zone in iNPH may contribute to this phenomenon of reduced CSF t-tau and p-tau levels in iNPH.9
The present study was subject to some limitations. Firstly, the sample was relatively small. Secondly, iNPH and AD patients were prospectively recruited from a single hospital, while the CN subjects were recruited from another hospital, which could have caused selection bias. Furthermore, the CN subjects were enrolled from the community, which meant their brain MRI and amyloid PET data were not available. However, we could measure consistent values for the CSF AD biomarkers, indicating the absence of AD pathology in these subjects. Finally, our AD patients had early-onset AD and the pathomechanism of amyloidopathy of early-onset AD differs from that of late-onset AD. Further studies with larger samples and a longitudinal design are therefore required.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0479) to S.Y.M; by a grant of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT (NRF-2016R1C1B2010206) to J.C.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure. A treatable syndrome. N Engl J Med. 1965;273:117–126. doi: 10.1056/NEJM196507152730301. [DOI] [PubMed] [Google Scholar]
- 2.Leinonen V, Koivisto AM, Savolainen S, Rummukainen J, Tamminen JN, Tillgren T, et al. Amyloid and tau proteins in cortical brain biopsy and Alzheimer's disease. Ann Neurol. 2010;68:446–453. doi: 10.1002/ana.22100. [DOI] [PubMed] [Google Scholar]
- 3.Golomb J, Wisoff J, Miller DC, Boksay I, Kluger A, Weiner H, et al. Alzheimer's disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry. 2000;68:778–781. doi: 10.1136/jnnp.68.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 5.Lim TS, Choi JY, Park SA, Youn YC, Lee HY, Kim BG, et al. Evaluation of coexistence of Alzheimer's disease in idiopathic normal pressure hydrocephalus using ELISA analyses for CSF biomarkers. BMC Neurol. 2014;14:66. doi: 10.1186/1471-2377-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeppsson A, Zetterberg H, Blennow K, Wikkelsø C. Idiopathic normal-pressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80:1385–1392. doi: 10.1212/WNL.0b013e31828c2fda. [DOI] [PubMed] [Google Scholar]
- 7.Kapaki EN, Paraskevas GP, Tzerakis NG, Sfagos C, Seretis A, Kararizou E, et al. Cerebrospinal fluid tau, phospho-tau181 and β-amyloid1-42 in idiopathic normal pressure hydrocephalus: a discrimination from Alzheimer's disease. Eur J Neurol. 2007;14:168–173. doi: 10.1111/j.1468-1331.2006.01593.x. [DOI] [PubMed] [Google Scholar]
- 8.Jingami N, Asada-Utsugi M, Uemura K, Noto R, Takahashi M, Ozaki A, et al. Idiopathic normal pressure hydrocephalus has a different cerebrospinal fluid biomarker profile from Alzheimer's disease. J Alzheimers Dis. 2015;45:109–115. doi: 10.3233/JAD-142622. [DOI] [PubMed] [Google Scholar]
- 9.Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology. 2014;83:1573–1575. doi: 10.1212/WNL.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Liu C, Zhang J, Relkin N, Xing Y, Li Y. Cerebrospinal fluid Aβ42, t-tau, and p-tau levels in the differential diagnosis of idiopathic normal-pressure hydrocephalus: a systematic review and meta-analysis. Fluids Barriers CNS. 2017;14:13. doi: 10.1186/s12987-017-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S4–S16. doi: 10.1227/01.neu.0000168185.29659.c5. [DOI] [PubMed] [Google Scholar]
- 12.Larsson A, Wikkelsö C, Bilting M, Stephensen H. Clinical parameters in 74 consecutive patients shunt operated for normal pressure hydrocephalus. Acta Neurol Scand. 1991;84:475–482. doi: 10.1111/j.1600-0404.1991.tb04998.x. [DOI] [PubMed] [Google Scholar]
- 13.Krauss JK, Regel JP, Vach W, Jüngling FD, Droste DW, Wakhloo AK. Flow void of cerebrospinal fluid in idiopathic normal pressure hydrocephalus of the elderly: can it predict outcome after shunting. Neurosurgery. 1997;40:67–73. doi: 10.1097/00006123-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Moon SY, Na DL, Seo SW, Lee JY, Ku BD, Kim SY, et al. Impact of white matter changes on activities of daily living in mild to moderate dementia. Eur Neurol. 2011;65:223–230. doi: 10.1159/000318161. [DOI] [PubMed] [Google Scholar]
- 16.Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25:1071–1076. doi: 10.3346/jkms.2010.25.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabri O, Seibyl J, Rowe C, Barthel H. Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 2015;3:13–26. doi: 10.1007/s40336-015-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 19.Choi WH, Um YH, Jung WS, Kim SH. Automated quantification of amyloid positron emission tomography: a comparison of PMOD and MIMneuro. Ann Nucl Med. 2016;30:682–689. doi: 10.1007/s12149-016-1115-6. [DOI] [PubMed] [Google Scholar]
- 20.Santangelo R, Cecchetti G, Bernasconi MP, Cardamone R, Barbieri A, Pinto P, et al. Cerebrospinal fluid amyloid-β 42, total tau and phosphorylated tau are low in patients with normal pressure hydrocephalus: analogies and differences with Alzheimer's disease. J Alzheimers Dis. 2017;60:183–200. doi: 10.3233/JAD-170186. [DOI] [PubMed] [Google Scholar]
- 21.Lewczuk P, Matzen A, Blennow K, Parnetti L, Molinuevo JL, Eusebi P, et al. Cerebrospinal fluid Aβ42/40 corresponds better than Aβ42 to amyloid PET in Alzheimer's disease. J Alzheimers Dis. 2017;55:813–822. doi: 10.3233/JAD-160722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmqvist S, Mattsson N, Hansson O Alzheimer's Disease Neuroimaging Initiative. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain. 2016;139:1226–1236. doi: 10.1093/brain/aww015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toledo JB, Bjerke M, Da X, Landau SM, Foster NL, Jagust W, et al. Nonlinear association between cerebrospinal fluid and florbetapir F-18 β-amyloid measures across the spectrum of Alzheimer disease. JAMA Neurol. 2015;72:571–581. doi: 10.1001/jamaneurol.2014.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutphen CL, Jasielec MS, Shah AR, Macy EM, Xiong C, Vlassenko AG, et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 2015;72:1029–1042. doi: 10.1001/jamaneurol.2015.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140:2104–2111. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paternicò D, Galluzzi S, Drago V, Bocchio-Chiavetto L, Zanardini R, Pedrini L, et al. Cerebrospinal fluid markers for Alzheimer's disease in a cognitively healthy cohort of young and old adults. Alzheimers Dement. 2012;8:520–527. doi: 10.1016/j.jalz.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Sjögren M, Vanderstichele H, Agren H, Zachrisson O, Edsbagge M, Wikkelsø C, et al. Tau and Aβ42 in cerebrospinal fluid from healthy adults 21-93 years of age: establishment of reference values. Clin Chem. 2001;47:1776–1781. [PubMed] [Google Scholar]