Abstract
Individuals with autism spectrum disorder (ASD) report high levels of co-occurring mood disorders. Previous work suggests that people with ASD also experience aberrant responses to social reward compared to typically developing (TD) peers. In the TD population, aberrant reward processing has been linked to anhedonia (i.e., loss of pleasure), which is a hallmark feature of depression. This study examined the interplay between self-reported pleasure from social and non-social rewards, autism symptom severity, loneliness, and depressive symptoms across adults with autism spectrum disorder (ASD; N=49), TD currently depressed adults (TD-dep; N=30), and TD never depressed controls (TD-con; N=28). The ASD cohort reported levels of social and non-social anhedonia that were greater than TD-con but not significantly different from TD-dep. Across cohorts, both social and non-social hedonic capacity moderated the relationship between autism symptoms and loneliness: individuals with low capacity for pleasure experienced elevated loneliness regardless of autism symptom severity, while those with intact capacity for pleasure (i.e., less anhedonia) experienced greater loneliness as a function of increased autism symptoms. Loneliness was the strongest predictor of depressive symptoms across clinical cohorts. Our findings suggest a putative pathway from trait-like anhedonia in ASD to depression via elevated loneliness and indicate that variability in hedonic capacity within the autism spectrum may differentially confer risk for depression in adults with ASD. Results underscore potential mental health benefits of social skills interventions and community inclusion programs for adults with ASD.
Lay Summary
The relationship between autism symptoms and loneliness depended on one’s ability to experience both social and non-social pleasure. Adults who experienced less pleasure reported high levels of loneliness that did not depend autism severity, while adults with high capacity for pleasure were especially lonely if they also had many autism symptoms. Loneliness was the strongest predictor of depressive symptoms, compared to capacity for social and non-social pleasure and autism symptoms.
Introduction
The social motivation hypothesis of autism (Dawson et al., 2004; Dawson, Webb, & McPartland, 2005) posits that individuals with autism spectrum disorder (ASD) show impaired ability to assign appropriate reward value to social stimuli, which leads to diminished pleasure in social interaction and hinders the development and maintenance of social relationships. In adulthood, these persistent social challenges contribute to elevated rates of self-reported loneliness (Hedley, Uljarević, Foley, Richdale, & Trollor, 2018; Mazurek, 2014; Whitehouse, Durkin, Jaquet, & Ziatas, 2009) and may explain rates of depression that are three- to four-fold higher in ASD compared to typically developing (TD) peers (Hudson, Hall, & Harkness, 2018). In the TD literature, general loss of pleasure (i.e., anhedonia) (Pizzagalli, 2014) and deficits in appetitive motivation (Cooper, Arulpragasam, & Treadway, 2018) are considered hallmark features of depression, and loneliness has been shown to prospectively predict depression onset (Cacioppo, Hughes, Waite, Hawkley, & Thisted, 2006) and self-injurious behaviors (Joiner et al., 2009). Thus, aberrant reward processing of social stimuli that is etiologically characteristic of autism may serve as a trait-like vulnerability factor for loneliness and explain alarmingly high rates of depression in adults with ASD. To date, few studies have integrated the depression and ASD literatures to assess the extent to which mechanisms underlying depression in TD individuals operate similarly in individuals with ASD. To address this gap in the literature, the current study aimed to investigate relations among autism symptoms, capacity for social and non-social pleasure, loneliness, and depressive symptoms in adults with ASD, TD depressed individuals, and never-depressed controls.
Social and Non-Social Reward in ASD and Depression
Informed by and consistent with the social motivation hypothesis, previous work has focused on the processing of social reward (i.e., social anhedonia) in ASD, with some studies suggesting a specific deficit in social, but not non-social, reward that is associated with increased autism symptom severity (Chevallier, Grèzes, Molesworth, Berthoz, & Happé, 2012; Cox et al., 2015; Delmonte et al., 2012). However, a recent meta-analysis of fMRI studies concluded that ASD is linked to a more domain-general deficit in reward processing, such that individuals exhibit aberrant neural processing (e.g., both hyper- and hypoactivation in striatal regions) in response to both social and non-social rewards, with preliminary evidence supporting consistent hyperactivation in response to restricted interests (Clements et al., 2018). In the TD literature, reward processing deficits have been associated with anhedonia and major depressive disorder. Importantly, reward processing can be dissected into the component parts of anticipatory “wanting” (i.e., the motivation or drive to obtain a reinforcer) and consummatory “liking” (i.e., the subjective experience of pleasure that may occur in response to a reinforcer) (Berridge, Robinson, & Aldridge, 2009; Treadway & Zald, 2011), which are neurobiologically dissociable (Rizvi et al., 2016; Pizzagalli, 2014). Previous studies indicate that individuals with high levels of anhedonia or depression show deficits in both motivation to obtain rewards (e.g., reduced effort expenditure during the Effort Expenditure for Rewards Task; Treadway, Bossaller, Shelton, & Zald, 2012) and reduced reward sensitivity upon the receipt of reward on both behavioral and neural indices (Proudfit, 2015). In the context of the social motivation hypothesis, social communication deficits and heightened loneliness in ASD may result from reduced effort to seek social connection or reduced pleasure derived from social interaction.
Less work has characterized patterns of social versus non-social reward processing for TD depressed individuals. However, studies suggest that depressed adults experience heightened levels of social anhedonia that subside after they are no longer in a depressive episode, while more trait-like social anhedonia diagnostically differentiates individuals with schizophrenia and psychosis proneness from those with major depressive disorder (Barch, Gold, & Kring, 2017; Blanchard, Horan, & Brown, 2001). Researchers have not yet investigated patterns of social and non-social anhedonia for adults with ASD compared to typically-developing depressed adults. Further, few studies have acknowledged how individual differences in social motivation, which is known to be heterogenous in the ASD population in particular (Wing & Gould, 1979), may differentially predict risk for depression.
Loneliness
Understanding social hedonic processes in ASD is relevant because impaired motivation to orient to social stimuli leads to reduced social engagement, in turn resulting in social isolation, poor friendship quality, and increased rates of loneliness (Locke, Ishijima, Kasari, & London, 2010; Mazurek, 2014; Whitehouse et al., 2009). In the TD literature, loneliness is a well-documented risk factor for depressive symptoms and suicidal ideation (Cacioppo et al., 2006; Joiner et al., 2009) and has been shown to prospectively predict poor cardiovascular health, sleep dysfunction, functional limitations, and mortality (Cacioppo et al., 2002; Luo, Hawkley, Waite, & Cacioppo, 2012). Similarly, in ASD, an emerging body of work has shown that loneliness is associated with heightened levels of anxiety and depression, social disability, lower levels of social support, suicidal ideation, and self-harm behaviors (Hedley, Uljarević, Wilmot, Richdale, & Dissanayake, 2018; Lasgaard, Nielsen, Eriksen, & Goossens, 2010; Mazurek, 2014; White & Roberson-Nay, 2009). Recent work also suggests a temporal sequence from loneliness to depression, such that loneliness mediates the relationship between decreased social support (which was related to autism severity) and depressive symptoms (Hedley, Uljarević, Foley, et al., 2018).
Current Study
To date, much of the work investigating anhedonia has focused on TD individuals with depression. To our knowledge, this is the first study to examine capacity for social and non-social pleasure, loneliness, and depressive symptoms across samples of adults with ASD, TD currently depressed adults (TD-dep), and TD never-depressed controls (TD-con). We aim to identify patterns of social and non-social reward processing in ASD compared to TD-dep and TD-con, and to examine how individual differences in hedonic capacity and autism symptom severity may be associated with loneliness and depressive symptoms. Informed by previous literature, we hypothesize the following:
Regarding between-group differences on primary measures,
-
Individuals with ASD and TD-dep will report lower social and non-social capacity for pleasure relative to TD-con. ASD will exhibit intermediate levels of loneliness and depressive symptoms compared to TD-con and TD-dep (TD-con < ASD < TD-dep).
We tested the subsequent hypotheses in the combined transdiagnostic sample (TD-con + TD-dep + ASD), in the combined TD group (TD-con + TD-dep), and in ASD alone:
Autism symptoms will be negatively associated with capacity for social and non-social pleasure and positively associated with loneliness and depressive symptoms.
The relation between autism symptoms and loneliness will be moderated by capacity for social pleasure; those with greater capacity for social pleasure will report higher levels of loneliness dependent on autism symptom severity. We will also examine capacity for non-social pleasure as a moderator.
Finally, we will assess autism symptoms, capacity for social and non-social pleasure, and loneliness as predictors of depressive symptoms. Given its relevance to the social experience of adults with ASD and that it prospectively predicts depression onset in TD adults, loneliness will be the strongest predictor of depressive symptoms compared to the other measures.
Methods and Materials
Participants and Procedures
A total of 107 participants aged 18–35 years were recruited from three diagnostic cohorts: Individuals with autism spectrum disorder (ASD, n=49), typically developing adults with a current depressive disorder (TD-depressed, n=30), or typically developing comparisons with no history of an ASD or clinically significant depression or anxiety (TD-controls, n=28). Participants were recruited from national and local (mid-Southern United States) resources. Eligibility criteria included verbal IQ>=80; verbal fluency per Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2) (Lord et al., 2012) module selection criteria; reading level >= 5th grade; and no history or concerns of psychotic or bipolar disorders, or current substance use disorders. Participants in the clinical cohorts had existing diagnoses of ASD or depressive disorder, respectively. Table 1 provides demographic information and self-report means and standard deviations by cohort.
Table 1.
Group Differences on Demographic and Primary Variables
| Mean (SD) |
TD-con (N=28) |
ASD (N=49) |
TD-dep (N=30) |
Significance |
|---|---|---|---|---|
| Age | 25.32 (5.28) |
23.98 (26.23) |
26.23 (4.67) |
n.s. |
|
Gender (% Female/Other) |
50%/0% | 37%/2% | 63%/3% | n.s. |
| Verbal IQ | 114.93 (14.00) |
103.63 (12.75) |
109.67 (9.48) |
F(2, 60.96)=6.66, p=0.002 ASD < TD-con, TD-dep |
| Nonverbal IQ | 109.11 (15.30) |
103.04 (19.11) |
105.40 (10.42) |
n.s. |
| BDI-II | 2.39 (2.42) |
11.83 (9.89) |
26.37 (6.77) |
F(2, 57.29)=173.02, p<0.001 TD-con < ASD < TD-dep |
| SRS-2 Total | 43.25 (4.12) |
64.69 (10.46) |
54.66 (8.57) |
F(2, 60.81)=86.55, p<0.001 TD-con < TD-dep < ASD |
| SRS-2 SCI | 42.96 (4.64) |
63.46 (11.71) |
53.17 (8.82) |
F(2, 63.67)=85.90, p<0.001 TD-con < TD-dep < ASD |
| SRS-2 RRB | 43.86 (3.83) |
65.28 (12.18) |
52.66 (8.82) |
F(2, 58.43)=64.70, p<0.001 TD-con < TD-dep < ASD |
| ACIPS | 92.73 (7.51) |
73.47 (18.96) |
78.46 (12.77) |
F(2, 61.37)=25.28, p<0.001 ASD, TD-dep < TD-con |
| TEPS | 88.96 (8.20) |
75.20 (13.66) |
80.71 (13.37) |
F(2, 59.60)=14.61, p<0.001 ASD, TD-dep < TD-con |
| LiCQ | 13.70 (4.27) |
22.94 (7.40) |
28.90 (6.96) |
F(2, 63.59)=56.80, p<0.001 TD-con < ASD < TD-dep |
| ADOS-2 Module 4 | ||||
| Social Affect CS | -- | 6.04 (2.63) |
-- | -- |
| Restricted and Repetitive Behavior CS | -- | 5.51 (2.52) |
-- | -- |
| Total CS | -- | 5.70 (2.82) |
-- | -- |
Note. Means, standard deviations, and between-group comparisons on demographic and primary measures. BDI-II = Beck Depression Inventory, 2nd Edition; SRS-2 = Social Responsiveness Scale, 2nd Edition, SCI = Social Communication and Interaction, RRB = Restricted Interests and Repetitive Behavior (though we used raw SRS-2 scores in our analyses, T scores are reported above for interpretability); ACIPS = Anticipatory and Consummatory Interpersonal Pleasure Scale; TEPS = Temporal Experience of Pleasure Scale; LiCQ = Loneliness in Context Questionnaire; ADOS-2 = Autism Diagnostic Observation Schedule, 2nd Edition; CS=ADOS-2 Comparison Score on 1–10 metric.
Procedures were approved by the Institutional Review Board of Vanderbilt University Medical Center. All participants were assessed at this academic medical center and completed questionnaires in person. The ADOS-2 Module 4 was administered to all participants in the ASD cohort to confirm diagnosis, as well as to any participants without a prior ASD diagnosis who exceeded clinical cut-offs on the Social Responsiveness Scale (SRS-2) (Constantino & Gruber, 2012) or Autism Spectrum Quotient (AQ) (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001). The Structured Clinical Interview for DSM Disorders (SCID-5) (First, Williams, Karg, & Spitzer, 2014) depression module and the Mini International Neuropsychiatric Interview (MINI 5.0) (Sheehan et al., 1998) were administered to all participants to confirm diagnosis and/or assess emotional health history. In the ASD cohort, 73% (36/49) met criteria for lifetime depressive disorders (n = 16, 33%, with current mood concerns per clinical judgment, of which n = 6, 12%, met criteria for current depressive disorder per the SCID-5). In the TD-depressed group, all had current Major Depressive Disorder or Persistent Depressive Disorder.
Measures
Social Responsiveness Scale, Second Edition (SRS-2).
The SRS-2 (Constantino & Gruber, 2012) is a 65-item self-report measure designed to assess social ability in domains related to ASD impairments. Across clinical samples, we conceptualized higher SRS-2 scores to denote greater autism symptom severity. The SRS-2 also yields two DSM-5 compatible subscales, including Social Communication and Interaction (SCI) and Restricted Interests and Repetitive Behavior (RRB), which are each comprised of a subset of relevant items on the SRS-2. The SRS-2 demonstrates high internal consistency, test-retest reliability, and inter-rater reliability. In the current study, the SRS-2 demonstrated high internal consistency for all cohorts (Cronbach’s α = .88, .95, and .94 for TD-Con, ASD, and TD-Dep, respectively).
Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS).
The ACIPS (Gooding & Pflum, 2014) is a 17-item self-report measure designed to assess an individual’s capacity to experience interpersonal and social pleasure. The ACIPS includes seven anticipatory (e.g., “I look forward to seeing people when I’m on my way to a party or get-together”) and 10 consummatory reward items (e.g., “I enjoy watching films about friendships or relationships with friends”). Each item is scored on a 6-point Likert scale ranging from 1 (very false for me) to 6 (very true for me), with lower scores indicating reduced capacity to experience interpersonal pleasure. Given limited ability of this scale to differentiate anticipatory versus consummatory reward (Gooding & Pflum, 2014), we were primarily interested in using the ACIPS total score. In the present sample, the ACIPS total score demonstrated high internal consistency (Cronbach’s α = .83, .95, and .88 for TD-con, ASD, and TD-dep, respectively).
Temporal Experience of Pleasure Scale (TEPS).
The TEPS (Gard, Gard, Kring, & John, 2006) is an 18-item self-report measure used to assess individual differences in anticipatory and consummatory pleasure. In contrast to the ACIPS, the TEPS includes items assessing non-social pleasure and is considered a measure of more general pleasure. Items are rated on Likert scale ranging from 1 (very false for me) to 6 (very true for me), with 10 items assessing anticipatory pleasure (e.g., “When something exciting is coming up in my life, I really look forward to it”) and 8 items assessing consummatory pleasure (e.g., “I love the sound of rain on the windows when I’m lying in my warm bed”). In the present sample, the TEPS demonstrated good internal consistency (Cronbach’s α = .76, .83, and .81 for TD-con, ASD, and TD-dep, respectively). In the present sample, the TEPS total score demonstrated good internal consistency (Cronbach’s α = .76, .83, and .81 for TD-con, ASD, and TD-dep, respectively).
Beck Depression Inventory-II (BDI-II).
The BDI-II (Beck, Steer, & Brown, 1996) is a 21-item self-report measure that asks individuals to respond to statements related to depressive symptoms based on their experience over the past two weeks. The BDI-II is widely used as an indicator of depression severity. Total scores (range = 0–63) were used to assess the level of depressive symptoms for each participant on a continuous scale. In the current study, the BDI-II demonstrated high internal consistency (Cronbach’s α = .91, .91, .72 for TD-con, ASD, TD-dep, respectively).
Loneliness in Context Questionnaire (LiCQ).
LiCQ (Asher & Weeks, 2014) is a 10-item self-report measure that assesses loneliness for adults in different daily contexts. The 10 items of the LiCQ are designed to reflect pure loneliness items that are not confounded by hypothesized causes of loneliness (e.g., “Mornings are a lonely time for me” and “I am lonely with other people”). Items are rated on a Likert scale ranging from 1 (never) to 5 (always). In the current study, internal consistency for the LiCQ was also excellent (Cronbach’s α = .90, .88, .87 for TD-con, ASD, and TD-dep, respectively).
Statistical Analyses
Between-group differences on primary measures.
To identify significant between-group differences on self-report measures, we first computed Levene’s test for homogeneity of variance (Levene, 1960) across cohorts. Significant heterogeneity of variance was detected on all self-report measures (all p’s < 0.05), with the general pattern that TD-con exhibited the lowest variability, followed by TD-dep and ASD with the highest variability. Thus, we proceeded with the Welch adjusted degree of freedom (Welch, 1951) robust alternative to the one-way ANOVA to assess between-group differences in primary measures. Pairwise multiple comparisons were assessed using Fisher’s Least Significant Difference test, which is the ideal post-hoc procedure when comparing three groups (Seaman, Levin, & Serlin, 1991).
Autism symptoms (SRS-2) as predictors of social and non-social pleasure and depressive symptoms.
We used Ordinary Least Squares (OLS) regression to assess the degree to which self-reported autism symptoms predicts social and non-social pleasure (ACIPS and TEPS), loneliness (LiCQ), and depressive symptoms (BDI-II) in the combined transdiagnostic sample, in TD only, and in ASD only. For these analyses, we used the SRS-2 total score (i.e., overall social impairment related to autism symptoms), as well as the Social Communication and Interaction (SCI) and Restricted Interests and Repetitive Behavior (RRB) subscales to examine the potentially unique contributions of the core symptom domains of ASD. As described above, the TD groups were defined based on depression status while the ASD group was allowed to vary in depressive symptoms. Considering our interest in understanding relations between SRS-2 and other measures across a broad range of ASD- and depression-related symptoms, we were particularly interested in comparing the strength and direction of the bivariate relations between the combined TD (TD-con +TD-dep) and ASD groups. To facilitate this goal, we conducted a series of hierarchical regressions for each outcome variable (LiCQ, BDI-II, ACIPS, and TEPS). In Step 1, we included only SRS-2 as the predictor, which allowed us to assess the total relationship between SRS-2 and the outcome variable in the combined, transdiagnostic sample. In Step 2, we included SRS-2 and a dummy variable for diagnostic cohort (ASD vs. TD) as predictors. In this step, the effect of SRS-2 represents the pooled, within-group regression coefficient and assumes that the relationship between SRS-2 and the outcome variable is the same (i.e., parallel) in the two cohorts. Finally, Step 3 included SRS-2, cohort, and their interaction as predictors, which allowed us to assess whether the direction and strength of the relationship between SRS-2 and the outcome variable differed in TD vs. ASD. As such, the hierarchical regression allowed us to assess the relationship between SRS-2 and outcome variables across and within diagnostic cohorts.
To ensure the validity of our regression results, we assessed linearity and bivariate normality using visual inspection of scatterplots, quantile-quantile (Q-Q) plots. We also tested the homoscedasticity assumption using the Breusch-Pagan test for heteroskedasticity (Breusch & Pagan, 1979), which was not significant for any of the linear regression models. Because outliers were detected in the between-groups analysis of primary measures, we also conducted robust regression analyses using M-estimation and Huber weights (Maronna, Martin, & Yohai, 2006). We used the sandwich estimator to calculate robust standard errors and test the significance of relevant coefficients. Of note, OLS and robust regression results did not differ. For brevity, we present the results from standard regression methods; robust regression results are available upon request.
Social and non-social pleasure as a moderator of the relationship between SRS-2 and loneliness (LiCQ).
Consistent with our goal of examining social and non-social pleasure as a shared vulnerability factor conferring risk for loneliness across a broad spectrum of ASD- and depression-related impairment, we examined social and non-social pleasure as potential moderators of the relationship between autism symptoms (SRS-2) and loneliness (LiCQ) using OLS linear regression in the transdiagnostic sample. To explore the contribution of different facets of core autism symptoms, we also conducted the moderation analyses using the SCI and RRB subscales.
Identifying the strongest predictor of depressive symptoms.
Finally, we used multiple linear regression analysis to examine the role of loneliness (LiCQ), social and non-pleasure (ACIPS and TEPS), and autism symptoms (SRS-2) on depressive symptoms (BDI-II) to identify the strongest predictor of depressive symptoms.
Results
Cohort Differences on Self-Report Measures
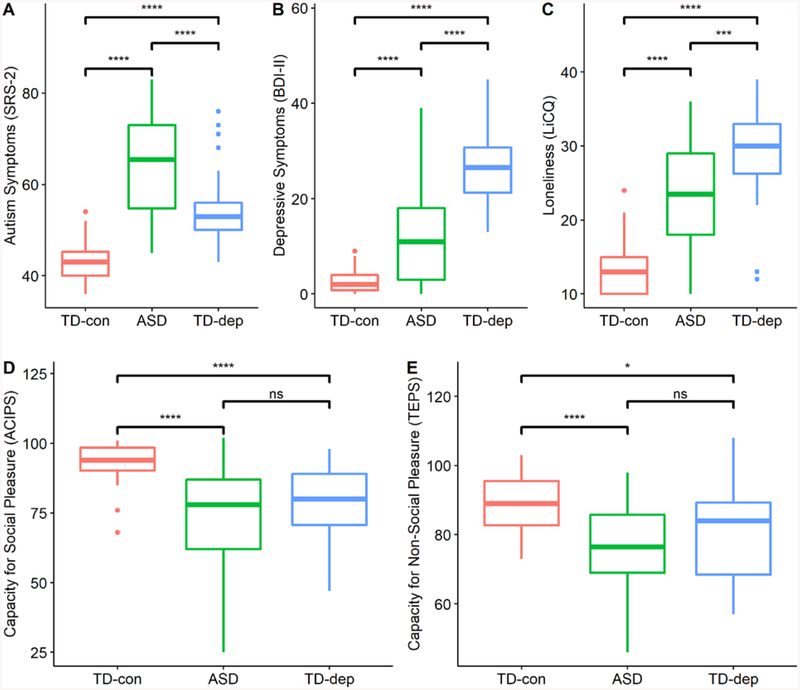
Omnibus ANOVAs and follow-up Fisher LSD tests indicated significant differences among cohorts on measures of autism symptoms, depressive symptoms, and loneliness (see Table 1). As expected, the ASD group demonstrated the highest level of autism symptoms on the SRS-2 (SCI, RRB, and total scores), followed by TD-dep and TD-con, and the TD-dep group demonstrated the highest level of depressive symptoms (BDI-II) and loneliness (LiCQ), followed by ASD and TD-con. A distinct and consistent pattern emerged for between-group differences in measures of capacity for social (ACIPS) and non-social (TEPS) pleasure. The TD-con group reported significantly higher levels of social and non-social pleasure compared to TD-dep and ASD, which were not statistically different from each other (ACIPS: d = −.32; TEPS: d = −.41). Results indicated moderately small effect sizes for the differences between ASD and TD-dep on ACIPS and TEPS, lending support to the similarity between these clinical cohorts on measures of social and non-social pleasure. In contrast, the differences between ASD and TD-dep on loneliness, depressive symptoms, and autism symptoms yielded Cohen’s d values ranging from .8 to 1.85, reflecting large and significant effect sizes. We also examined the pattern of between-group differences with the subset of individuals in the ASD group who were assessed to have current mood problems based on structured diagnostic interviews (n = 16, 33%). Participants with ASD and mood problems showed the same pattern of results as the whole ASD group when compared to TD-con and TD-dep groups. Thus, between-group differences for TD-con, ASD, and TD-dep (the primary comparison groups in this study) are represented by boxplots in Figure 1.
Figure 1.
Box Plots and Between-Group Differences on Primary Measures
Note. Boxplots illustrating between-group differences on (A) autism symptoms (SRS-2), (B) depressive symptoms (BDI-II), (C) loneliness (LiCQ), (D) capacity for social pleasure (ACIPS), and (E) capacity for non-social pleasure (TEPS). **** = p < 0.0001; *** = p < 0.001; * = p < 0.05; ns = non-significant pairwise difference.
Autism Symptoms as a Predictor of Depressive Symptoms, Reward, and Loneliness
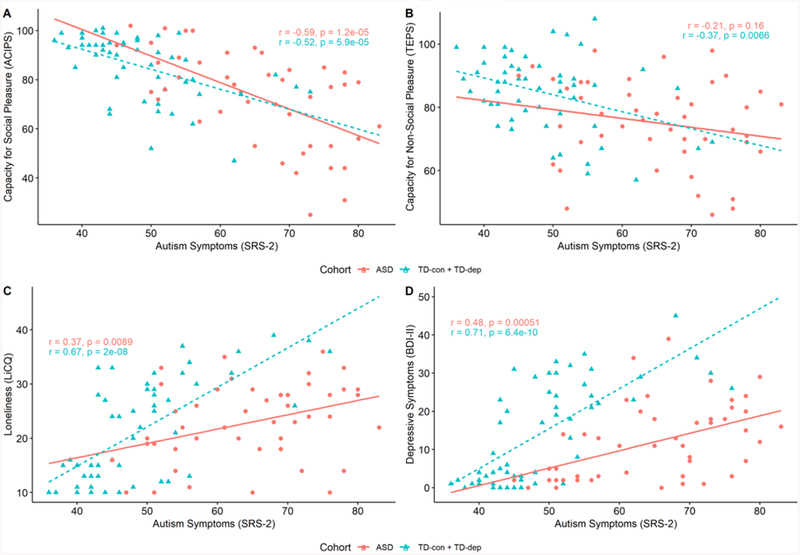
Results from the hierarchical linear regressions are presented in Figure 2. Because results did not vary when using the SRS-2 total score, SCI subscale, or RRB subscale as the independent variable, we report results using the SRS-2 total score. Significant main effects and interactions were found for autism symptoms (SRS-2) and cohort (TD vs. ASD) on loneliness (LiCQ) and depressive symptoms (BDI-II). SRS-2 predicted increased loneliness (LiCQ) and depressive symptoms (BDI-II) in the whole sample, combined TD group, and ASD alone. For the regression models with LiCQ and BDI-II as the outcome variables, respectively, Step 1 (only SRS-2 as the predictor) accounted for 20% and 13% of the variance; Step 2 (SRS-2 and diagnostic cohort as predictors) accounted for 26% and 35% of the variance; and Step 3 (SRS-2, cohort, and their interaction as predictors) accounted for 33% and 41% of the variance. For both outcome variables, model comparison indicated that Step 3, which included SRS-2, cohort (TD vs. ASD), and their interaction as predictors yielded the best fit. The significant SRS-2 × Cohort (TD vs. ASD) interaction terms when predicting LiCQ (F(1, 101) = 10.10, p = 0.002, ΔR2 = .07) and BDI-II (F(1, 102) =10.06, p = 0.002, ΔR2 = .06) indicated that the positive relationships between autism symptoms and loneliness and depressive symptoms were stronger in the combined TD (r(58)=.67, p < 0.001) compared to ASD (r(49)=.37, p < 0.01) cohort. For the regression models with capacity for social and non-social pleasure (ACIPS and TEPS) as dependent variables, Step 1 accounted for 39% and 18% of the variances, respectively, and Steps 2 and 3 did not significantly increment upon the base models. In other words, only the main effect of SRS-2 was significant (ACIPS: t(98) = −6.74, p < 0.001; TEPS: t(98) = −4.72, p < 0.001). These results suggest that greater autism symptomatology significantly predicts decreased capacity for social and non-social pleasure in the whole sample and that the relationship between SRS-2 and ACIPS and TEPS does not differ between the combined TD (ACIPS: r(58) = −0.52, p < 0.001; TEPS: r(58) = −.37, p < 0.01) and ASD (ACIPS: r(49) = −.59 p < 0.001; TEPS: r(49) = −.21, p=0.16) groups. The between-cohort similarity in the relationship between SRS-2 and measures of social and non-social pleasure is further supported by the fact that the increments in R2 for the second and third steps of the hierarchical regressions are not significant and of very small magnitude (range of ΔR2 = [.005, .01]).
Figure 2.
Bivariate Relations Between Autism Traits and Primary Measures
Note. Scatter plots and fitted regression lines for the combined TD cohort (TD-con + TD-dep) and ASD cohort are provided in teal hatched line and red solid line, respectively, to depict the relationship between autism symptoms (SRS-2) and (A) capacity for social pleasure (ACIPS), (B) capacity for non-social pleasure (TEPS), (C) loneliness (LiCQ), and (D) depressive symptoms (BDI-II). Pearson correlations and significance values are also provided in each panel for each group.
Capacity for Social and Non-Social Reward as a Moderators for the Effect of SRS-2 on Loneliness
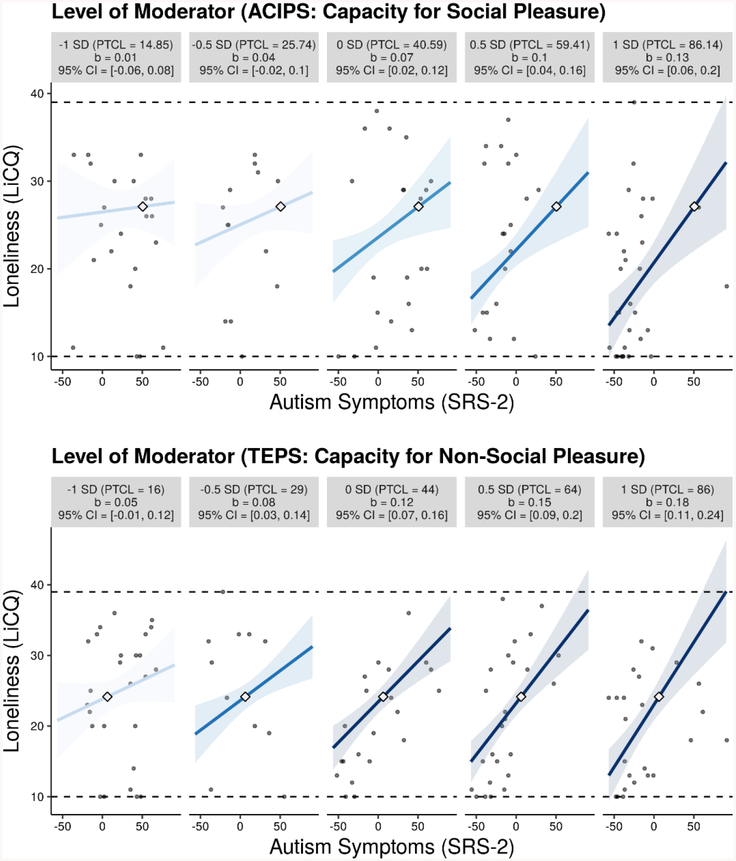
Linear regression was used to examine the moderating role of capacity for social and non-social pleasure in the effect of autism symptoms (SRS-2) on loneliness (LiCQ). As illustrated by Figure 3, both ACIPS (t(96) = 2.52, p = 0.01) and TEPS (t(95) = 2.60, p = 0.01) significantly moderated the relationship between SRS-2 and loneliness. These findings were replicated when we used the SRS-2 DSM-5 compatible subscales (SCI and RRB) as the independent variable, suggesting that the moderation effect was not specific to social communication or restricted interests and repetitive behaviors. For brevity, we provide further interpretation of the interaction effects using the SRS-2 total score.
Figure 3.
Moderating Role of Capacity for Non-Social and Social Pleasure in the Relationship Between Autism Symptoms and Loneliness
Note. Interaction plots were created using interActive, an interaction visualization application in R (R Developmental Core Team, 2016) created by McCabe, Kim, and King (2018). Several simple slopes are presented for levels of the moderator at 1 SD and .5 SD below the mean, at the mean, and .5 SD and 1 SD above the mean to depict the change in association between autism symptoms (SRS-2) and loneliness across multiple levels of the moderator. For interpretability, SRS-2, ACIPS, and TEPS were centered. Each graphic shows the observed data (grey dots), maximum and minimum values of outcome (dashed horizontal lines), coefficient of the simple slope and 95% confidence region (shaded area), and the crossover point (diamond). The x-axes represent the full range of centered SRS-2 scores.
Individuals with lower capacity for social pleasure exhibited high levels of loneliness regardless of their degree of autism symptoms, while those with greater capacity for social pleasure demonstrated a positive relation between social impairment and loneliness that increased in strength with increases in the capacity for social pleasure (see Figure 3). A similar interpretation was found for the SRS-2 × TEPS interaction effect: individuals with higher capacity for non-social pleasure showed a positive, direct relationship between SRS-2 and loneliness, while those with lower capacity for non-social pleasure were lonelier overall, with a mild positive relationship between social impairment and loneliness.
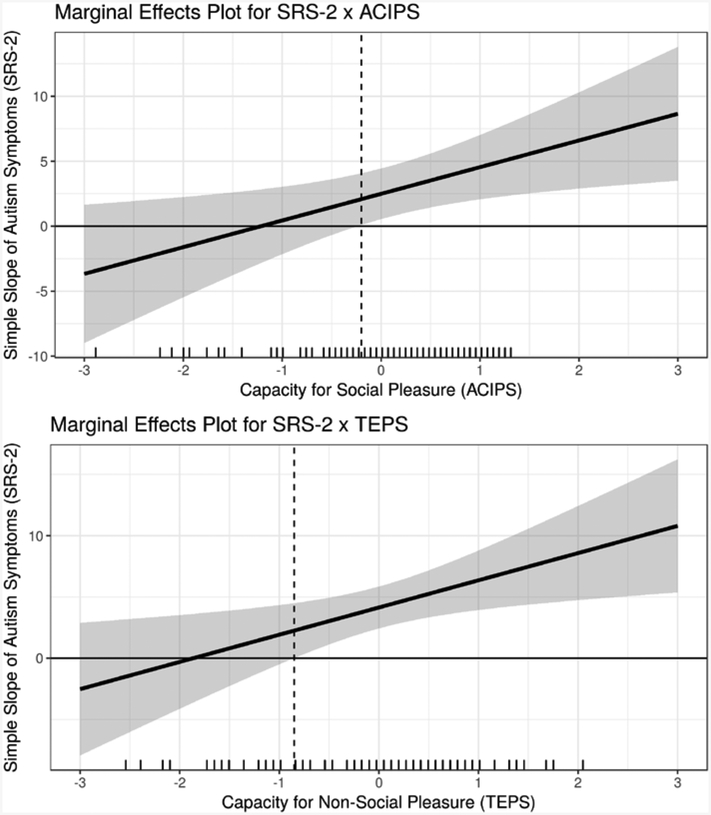
We further probed the interactions using marginal effects plots (also called “region-of-significance” plots) (McCabe, Kim, & King, 2018; Preacher, Curran, & Bauer, 2006) to assess the conditional effect of autism symptoms on loneliness across levels of the moderators. The marginal effects plots are depicted in Figure 4 and indicate the significance, magnitude, and direction of the simple slope (i.e., marginal effect) of SRS-2 across the full hypothetical range of the moderators, ACIPS and TEPS (mean +/− 3 standard deviations). For the SRS-2 × ACIPS interaction, the simple slope of SRS-2 on LiCQ is significant and positive when the ACIPS score is −0.20 standard deviations away from the mean or greater. This range includes 62% of ACIPS observations in the combined sample. For the SRS-2 × TEPS interaction, the simple slope of SRS-2 on LiCQ is significant and positive when the TEPS score is −0.85 standard deviations away from the mean or greater and includes 77% of the observed TEPS scores in the transdiagnostic sample. In ASD alone, 53% and 71% of observed ACIPS and TEPS scores, respectively, are located in the estimated regions of significance. These findings support the validity of the interaction effects both within and across diagnostic cohorts.
Figure 4.
Marginal Effects Plots Depicting Regions of Significance of the SRS-2 X TEPS and SRS-2 X ACIPS Interaction Effects
Note. The marginal-effects plots were created using interActive (McCabe, Kim, and King, 2018) and show the marginal effect of autism symptoms (SRS-2) on loneliness (i.e., the simple slopes of SRS-2 for each interaction effect) across a range of the moderator variables (i.e., ACIPS and TEPS). The shaded areas represent the 95% confidence regions for the marginal effects. Further, the plots also include a marginal rug of the moderators on the horizontal axis that indicates the frequency of observed values of the moderators across the range of the horizontal axis. For the SRS-2 x; ACIPS interaction, the simple slope of SRS-2 on LiCQ is significant and positive when the ACIPS score is −0.20 standard deviations away from the mean or greater; 62% of ACIPS observations are within this region. For the SRS-2 x TEPS interaction, the simple slope of SRS-2 on LiCQ is significant and positive when the TEPS score is −0.85 standard deviations away from the mean or greater; 77% of observed TEPS scores are within this region.
Loneliness as the Strongest Predictor of Depressive Symptoms
To identify the strongest predictor of depressive symptoms, we simultaneously included loneliness, social and non-pleasure, and autism symptoms as predictors of depressive symptoms in a multiple regression framework. Results showed that loneliness (LiCQ) was the strongest predictor of depressive symptoms in the transdiagnostic sample (t(94) = 8.02, p < 0.001, adjusted R2 = .49), combined TD sample (t(48) = 5.50, p<0.001, adjusted R2 = .67), and ASD cohort alone (t(41) = 3.41, p=0.001, adjusted R2 = .33).
Discussion
We aimed to better understand the common co-occurrence of depression in adults with ASD by exploring the relations among social and nonsocial anhedonia, autism symptoms, and loneliness; this study lays groundwork for investigations of anhedonia as a vulnerability factor for depression in adults with ASD. A noteworthy strength of this study was the inclusion of three comparison groups: ASD, TD currently depressed (TD-dep), and TD never-depressed controls (TD-con), which allowed us to conduct a series of between-group and transdiagnostic analyses. A highlight among our findings was that both social and non-social hedonic capacity were significant moderators of the effect of autism symptoms on loneliness. Individuals were particularly likely to experience loneliness if they had both a high level of autism-related impairment and high capacity for social and non-social pleasure. Importantly, those with a low capacity for pleasure experienced relatively high levels of loneliness irrespective of autism symptoms. Marginal effects plots of these interactions identified regions of significance indicating that the effects were relevant to the majority of the TD and ASD cohorts, lending validity to the transdiagnostic nature of these moderation effects.
As we elaborate upon below, the primary findings of this study point to two important conclusions about the co-occurrence of depression in ASD: 1) Trait-like social and non-social anhedonia may both confer vulnerability for depression in adults with ASD, and 2) Individual differences in hedonic capacity moderate the relationship between social impairment and loneliness, a well-documented risk factor for depression (Cacioppo, Hawkley, & Thisted, 2010; Cacioppo et al., 2006). These findings suggest a putative pathway from anhedonia, through loneliness, to depression within ASD that warrants future longitudinal inquiry. These results further our understanding of anhedonia as a potential mechanism that affects social and emotional health in ASD, thus informing targeted treatment development for this population.
Anhedonia as a Trait-like Vulnerability Factor for Depression in ASD
Our first two hypotheses build upon previous reports of significant associations between elevated autism traits and social and non-social anhedonia, with a stronger effect for the social domain (Novacek, Gooding, & Pflum, 2016). Interestingly, and never directly compared in previous work, between-group analyses (Hypothesis 1) showed that our ASD cohort reported levels of social and non-social pleasure that were statistically commensurate with TD depressed adults, yet intermediate levels of loneliness and depressive symptoms compared to TD never-depressed controls and TD depressed adults (TD-con < ASD < TD-dep). In other words, even though ASD was comparable to TD-dep on anhedonia (i.e., our focal candidate mechanism), this did not translate to the same degree of emotional health problems across groups. One possible interpretation is that TD individuals experience anhedonia that results from a depressive state, while those with ASD experience anhedonia that is associated with autism symptomology that then contributes to the development of depression prospectively. This represents an area rich for future exploration with longitudinal data across diagnostic cohorts.
In Hypothesis 2, we assessed the bivariate relations between autism symptoms (SRS-2) and primary measures in the whole sample (TD-con + TD-dep +ASD), in addition to assessing differences in these relations in the TD sample (TD-con + TD-dep) compared to ASD alone. Based on linear association, ASD did not differ from TD comparisons when predicting social and non-social anhedonia, but ASD did differ from TD when predicting psychosocial health states. Again, these findings lend preliminary support for anhedonia (both social and non-social) as a more stable, trait-like experience in ASD that likely contributes to but does not directly map onto depressive symptoms, while TD individuals experience state-dependent anhedonia when they are depressed.
Potential Etiological Pathway from Anhedonia, through Loneliness, to Depression
Though our data is cross-sectional, results from theory-informed specifications of Hypotheses 3 and 4 suggest one potential pathway from anhedonia to depression due to heightened loneliness in ASD. Our moderation analyses (Hypothesis 3) revealed that social and non-social pleasure moderated the relationship between autism symptoms (SRS-2) and loneliness. Individuals with diminished capacity for pleasure were lonelier overall, regardless of autism-related impairment, while loneliness was dependent on degree of autism-related impairment for those who had intact hedonic capacity. Subsequent probing of these interactions showed that levels of anhedonia conferring significant risk for loneliness (dependent on autism symptoms) applied to 53% to 71% of the ASD sample, suggesting that the ASD group was well-represented in the significant interaction effects. Given that social withdrawal is part of the diagnostic criteria for depression and that loneliness and depression are correlated constructs, we also conducted the same moderation analysis with depressive symptoms (BDI-II) as the primary outcome. Results showed that the interaction was significant only for social, but not non-social, pleasure as a moderator. Thus, in the context of greater social impairment, hedonic deficits appear to have a broader impact on loneliness compared to depressive symptoms.
Aberrant reward processing (for both social and non-social stimuli) may have down-stream effects for the orienting, seeking, engaging, and maintaining of rewarding experiences, creating the “perfect storm” for decreased social competency, increased social withdrawal, and loneliness in ASD. In all cohorts, loneliness was the strongest predictor of depressive symptoms. Though we cannot make causal conclusions due to the cross-sectional nature of our study, our findings suggest a putative pathway to depression in ASD, in which trait-like anhedonia interacts with individual variability in social impairment (i.e., autism symptom severity) to confer risk for loneliness and subsequently depression. This pathway warrants attention, as recently published work suggests a potential temporal sequence from loneliness, through depression, to thoughts of self-harm in the ASD population (Hedley, Uljarević, Foley, et al., 2018; Hedley, Uljarević, Wilmot, et al., 2018).
Further, because our measures of capacity for social and non-social pleasure were self-report measures, higher scores may also be influenced by increased insight and awareness into the participant’s social and emotional experience. Thus, another complementary way to interpret the significant interaction effects is that greater insight into one’s experience also strengthens the positive relation between autism symptoms and loneliness. This interpretation is consistent with previous work indicating that individual with “high functioning” ASD are at greater risk for psychosocial symptomatology, including depression and anxiety (Gotham, Bishop, Brunwasser, & Lord, 2014; White, Bray, & Ollendick, 2012; Sterling, Dawson, Estes, & Greenson, 2008)
Importance of Social and Non-Social Reward
Notably, our findings suggest a possible mechanistic role for both social and non-social reward processing for the development of depression in ASD. The original instantiation of the social motivation hypothesis suggested unique motivational deficits for social stimuli in ASD (Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Dawson et al., 2004), leading to a greater focus on social reward processing in previous work. Our findings are consistent with a recent meta-analysis of imaging studies of reward processing in ASD that reported aberrant reward circuitry in striatal regions for both social and non-social rewards (Clements et al., 2018). Taken together, results provide multi-method support (i.e., subjective experience and neural accounts of reward processing) for taking a broader understanding of reward deficits in ASD that includes both social and non-social domains. Though social and communication deficits comprise a core feature of ASD, continued work characterizing non-social or domain-general processing of reward may be just as relevant for understanding social and emotional health in ASD.
Limitations, Strengths, and Future Directions
A notable body of work has established that the processing of reward can be dissociated into the components of anticipatory “wanting” (i.e., the motivation to obtain a reinforcer) and consummatory “liking” (i.e., the subjective experience of pleasure that may occur in response to a reinforcer) (Berridge et al., 2009). Effort-based cognitive tasks are recommended to adequately isolate the consummatory and anticipatory aspects of reward (Treadway & Zald, 2011). Considering the current state of the literature, self-report measurement was considered an appropriate first step for examining patterns of social and non-social anhedonia in ASD compared to two TD comparison groups. Further, self-report allowed us to bypass issues with motor differences that may affect the utility of cognitive tasks, and reliance on standardized stimuli (e.g., static social stimuli) that may not be ecologically valid or salient in ASD. Future studies intend to integrate experimental paradigms that are uniquely designed to parse the anticipatory and consummatory aspects of pleasure using ecologically valid social motivation paradigms (e.g., mobile technology) to facilitate momentary, real-time assessment of social behavior (Fulford, Campellone, & Gard, 2018). Finally, due to the cross-sectional design of the study, we had minimal ability to infer causality or comment on the temporal order of relationships between anhedonia, loneliness, and depression. However, the order in which we conducted statistical analyses and interpreted our results was guided by the social motivation hypothesis in ASD and existing literature. We also hope to pursue this work further using longitudinal study designs that would allow us to probe causal effects.
Conclusions
The current study suggests that variability in hedonic capacity across the autism spectrum may differentially confer risk for depression in adults with ASD. Overall, individuals with ASD exhibit a similar profile of social and non-social anhedonia compared to TD depressed adults. Both within ASD and in our combined transdiagnostic sample, individuals with heightened anhedonia and autism-related impairment reported greater loneliness, which was the strongest predictor of depressive symptoms. Our findings highlight a potential pathway from anhedonia through loneliness to depression. Real-world skills-based and behavioral interventions that target loneliness or increase positive social feedback and opportunity will likely improve emotional health in adults with ASD. Future work should investigate these relations in a longitudinal framework and continue to use well-characterized clinical comparison groups to clarify the mechanisms contributing to depression in this vulnerable population. Detailed probing of reward-processing mechanisms and differential emotional health outcomes in ASD may also inform personalized treatment selection for mood disorders in ASD.
Acknowledgments
The corresponding author (KG) reports a potential conflict of interest, in that she receives royalties from Western Psychological Services as an author of the Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2). The ADOS-2 was used for diagnostic confirmation within this sample but was not an outcome measure in this study. The remaining authors (GH, AT) have no financial interests or potential conflicts of interest related to this work. This work was supported in part by an NIMH training grant (T32 MH018921).
Contributor Information
Gloria T. Han, Department of Psychology, Vanderbilt University
Andrew J. Tomarken, Department of Psychology, Vanderbilt University
Katherine O. Gotham, Department of Psychiatry and Behavioral Sciences, Vanderbilt University Medical Center
References
- Asher S, & Weeks MS (2014). Loneliness and belongingness in the college years In The Handbook Of Solitude: Psychological Perspecives on Social Isolation, Social Withdrawal, and Being Alone (pp. 283–301). [Google Scholar]
- Barch DM, Gold JM, & Kring AM (2017). Paradigms for assessing hedonic processing and motivation in humans: Relevance to understanding negative symptoms in psychopathology. Schizophrenia Bulletin, 43(4), 701–705. 10.1093/schbul/sbx063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, & Clubley E (2001). The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory-II, 78(2), 490–498. [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65–73. 10.1016/j.coph.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JL, Horan WP, & Brown SA (2001). Diagnostic differences in social anhedonia: A longitudinal study of schizophrenia and major depressive disorder. Journal of Abnormal Psychology, 110(3), 363–371. 10.1037/0021-843X.110.3.363 [DOI] [PubMed] [Google Scholar]
- Breusch TS, & Pagan AR (1979). A simple test for heteroscedasticity and random coefficient variation. Econometrica, 47(5), 1287 10.2307/1911963 [DOI] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, … Berntson GG (2002). Loneliness and Health: Potential Mechanisms: Psychosomatic Medicine, 64(3), 407–417. 10.1097/00006842-200205000-00005 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, & Thisted RA (2010). Perceived social isolation makes me sad: Five year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychology and Aging, 25(2), 453–463. 10.1037/a0017216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, & Thisted RA (2006). Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychology and Aging, 21(1), 140–151. 10.1037/0882-7974.21.1.140 [DOI] [PubMed] [Google Scholar]
- Chevallier C, Grèzes J, Molesworth C, Berthoz S, & Happé F (2012). Brief Report: Selective social anhedonia in high functioning autism. Journal of Autism and Developmental Disorders, 42(7), 1504–1509. 10.1007/s10803-011-1364-0 [DOI] [PubMed] [Google Scholar]
- Clements C, Zoltowski A, Yankowitz L, Yerys BE, Schultz RT, & Herrington J (2018). Evaluation of the social motivation hypothesis of autism: A systematic review and meta-analysis. JAMA Psychiatry, 75(8), 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J, & Gruber C (2012). Social Responsiveness Scale, Second Edition (SRS-2) Western Psychological Services. [Google Scholar]
- Cooper JA, Arulpragasam AR, & Treadway MT (2018). Anhedonia in depression: biological mechanisms and computational models. Current Opinion in Behavioral Sciences, 22, 128–135. 10.1016/j.cobeha.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Kohls G, Naples A, Mukerji C, Coffman M, Rutherford H, … McPartland J (2015). Diminished social reward anticipation in the broad autism phenotype as revealed by event-related brain potentials. Social Cognitive and Affective Neuroscience, 10(10), 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, & Brown E (1998). Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders, 28(6), 479–485. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, & Liaw J (2004). Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology, 40(2), 271–283. 10.1037/0012-1649.40.2.271 [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, & McPartland J (2005). Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology, 27(3), 403–424. 10.1207/s15326942dn2703_6 [DOI] [PubMed] [Google Scholar]
- Delmonte S, Balsters JH, McGrath J, Fitzgerald J, Brennan S, Fagan AJ, & Gallagher L (2012). Social and monetary reward processing in autism spectrum disorders. Molecular Autism, 3(1), 7 10.1186/2040-2392-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2014). Structured Clinical Interview for DSM-5 Disorders–Research Version (SCID-5-RV). Arlington: American Psychiatric Assocation. [Google Scholar]
- Fulford D, Campellone T, & Gard DE (2018). Social motivation in schizophrenia: How research on basic reward processes informs and limits our understanding. Clinical Psychology Review, 63, 12–24. 10.1016/j.cpr.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, & John OP (2006). Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality, 40(6), 1086–1102. 10.1016/j.jrp.2005.11.001 [DOI] [Google Scholar]
- Gooding DC, & Pflum MJ (2014). The assessment of interpersonal pleasure: Introduction of the Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) and preliminary findings. Psychiatry Research, 215(1), 237–243. 10.1016/j.psychres.2013.10.012 [DOI] [PubMed] [Google Scholar]
- Gotham K, Bishop SL, Brunwasser S, & Lord C (2014). Rumination and perceived impairment associated with depressive symptoms in a verbal adolescent-adult ASD sample. Autism Research: Official Journal of the International Society for Autism Research, 7(3), 381–391. 10.1002/aur.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley D, Uljarević M, Foley K-R, Richdale A, & Trollor J (2018). Risk and protective factors underlying depression and suicidal ideation in Autism Spectrum Disorder. Depression and Anxiety, 35(7). [DOI] [PubMed] [Google Scholar]
- Hedley D, Uljarević M, Wilmot M, Richdale A, & Dissanayake C (2018). Understanding depression and thoughts of self-harm in autism: A potential mechanism involving loneliness. Research in Autism Spectrum Disorders, 46, 1–7. 10.1016/j.rasd.2017.11.003 [DOI] [Google Scholar]
- Hudson CC, Hall L, & Harkness KL (2018). Prevalence of depressive disorders in individuals with Autism Spectrum Disorder: a Meta-Analysis. Journal of Abnormal Child Psychology 10.1007/s10802-018-0402-1 [DOI] [PubMed] [Google Scholar]
- Joiner TE, Van Orden KA, Witte TK, Selby EA, Ribeiro JD, Lewis R, & Rudd MD (2009). Main predictions of the Interpersonal-Psychological Theory of suicidal behavior: Empirical tests in two samples of young adults. Journal of Abnormal Psychology, 118(3), 634–646. 10.1037/a0016500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasgaard M, Nielsen A, Eriksen ME, & Goossens L (2010). Loneliness and social support in adolescent boys with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 40(2), 218–226. 10.1007/s10803-009-0851-z [DOI] [PubMed] [Google Scholar]
- Levene H (1960). Robust tests for equality of variances. Stanford University Press. [Google Scholar]
- Locke J, Ishijima EH, Kasari C, & London N (2010). Loneliness, friendship quality and the social networks of adolescents with high-functioning autism in an inclusive school setting. Journal of Research in Special Educational Needs, 10(2), 74–81. 10.1111/j.1471-3802.2010.01148.x [DOI] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–Second edition (ADOS-2) Western Psychological Services. [Google Scholar]
- Luo Y, Hawkley LC, Waite LJ, & Cacioppo JT (2012). Loneliness, health, and mortality in old age: A national longitudinal study. Social Science & Medicine (1982), 74(6), 907–914. 10.1016/j.socscimed.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronna RA, Martin RD, & Yohai VJ (2006). Robust Statistics: Theory and Methods. New York: Wiley. [Google Scholar]
- Mazurek MO (2014). Loneliness, friendship, and well-being in adults with autism spectrum disorders: Autism, 18(3), 223–232. 10.1177/1362361312474121 [DOI] [PubMed] [Google Scholar]
- McCabe CJ, Kim DS, & King KM (2018). Improving present practices in the visual display of interactions. Advances in Methods and Practices in Psychological Science, 1(2), 147–165. 10.1177/2515245917746792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novacek DM, Gooding DC, & Pflum MJ (2016). Hedonic capacity in the broader autism phenotype: Should social anhedonia be considered a characteristic feature? Frontiers in Psychology, 7 10.3389/fpsyg.2016.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10(1), 393–423. 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31(4), 437–448. 10.3102/10769986031004437 [DOI] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–459. 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Seaman MA, Levin JR, & Serlin RC (1991). New developments in pairwise multiple comparisons: Some powerful and practicable procedures. Psychological Bulletin, 110(3), 577–586. 10.1037/0033-2909.110.3.577 [DOI] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett K, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl 20), 22–33. [PubMed] [Google Scholar]
- Sterling L, Dawson G, Estes A, & Greenson J (2008). Characteristics associated with presence of depressive symptoms in adults with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 38(6), 1011–1018. 10.1007/s10803-007-0477-y [DOI] [PubMed] [Google Scholar]
- Treadway MT, Bossaller N, Shelton RC, & Zald DH (2012). Effort-based decision-making in Major Depressive Disorder: A translational model of motivational anhedonia. Journal of Abnormal Psychology, 121(3), 553–558. 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, & Zald DH (2011). Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews, 35(3), 537–555. 10.1016/j.neubiorev.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BL (1951). On the comparison of several mean values: An alternative approach. Biometrika, 38(3/4), 330–336. 10.2307/2332579 [DOI] [Google Scholar]
- White SW, & Roberson-Nay R (2009). Anxiety, social deficits, and loneliness in youth with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 39(7), 1006–1013. 10.1007/s10803-009-0713-8 [DOI] [PubMed] [Google Scholar]
- Whitehouse AJO, Durkin K, Jaquet E, & Ziatas K (2009). Friendship, loneliness and depression in adolescents with Asperger’s Syndrome. Journal of Adolescence, 32(2), 309–322. 10.1016/j.adolescence.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Wing L, & Gould J (1979). Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. Journal of Autism and Developmental Disorders, 9(1), 11–29. 10.1007/BF01531288 [DOI] [PubMed] [Google Scholar]