Abstract
Chronic stress and the associated elevation in corticosteroid levels increase muscle protein catabolism. We hypothesized that the glucocorticoid receptor (GR)-regulated restriction of muscle glucose availability may play a role in the increased protein catabolism during chronic stress. To test this, we generated a ubiquitous GR knockout (GRKO) zebrafish to determine the physiological consequence of glucocorticoid stimulation on muscle metabolism and growth. Adult GRKO zebrafish had higher body mass, and this corresponded to an increased protein and lipid, but not carbohydrate, content. GRKO fish were hypercortisolemic, but they elicited a higher cortisol response to an acute stressor. However, the stressor-induced increase in plasma glucose level observed in the wild type was completely abolished in the GRKO fish. Also, the muscle, but not liver, capacity for glucose uptake was enhanced in the GRKO fish, and this corresponded with a higher hexokinase activity in the mutants. Zebrafish lacking GR also showed a higher capacity for protein synthesis, including increased phosphorylation of eukaryotic initiation factor 4B, higher expression of heat shock protein cognate 70, and total protein content. A chronic fasting stressor reduced body mass and muscle protein content in adult zebrafish, but this decrease was attenuated in the GRKO compared with the wild-type fish. Metabolomics analysis revealed that the free pool of amino acid substrates used for oxidation and gluconeogenesis were lower in the fasted GRKO fish muscle compared with the wild type. Altogether, chronic stressor-mediated GR signaling limits muscle glucose uptake, and this may play a role in protein catabolism, leading to the growth suppression in fish.
Keywords: atrogenes, CRISPR/Cas9, glucocorticoid receptor, glucose, intermediary metabolism, stress
INTRODUCTION
Glucocorticoids (GCs) are one of the most primitive regulators of metabolic homeostasis. These steroid hormones, named for their ability to regulate glucose levels, are released during stress and are the hallmark of the conserved stress response in vertebrates. The glucose response is essential to fuel the increased energy demand during stress, and GCs facilitate this by enhancing liver gluconeogenesis (9, 33, 61). GCs also increase the availability of other energy substrates, including free fatty acids and amino acids from endogenous stores, to offset the increased energy demand associated with stress coping (9, 33, 61). The metabolic effect of GCs is primarily mediated by activation of the glucocorticoid receptor (GR), a ligand-bound transcription factor that is highly conserved in vertebrates (9, 57). In teleosts, the primary GC is cortisol and is produced in response to the hypothalamus-pituitary-interrenal (HPI) axis activation (42), which is analogous to the hypothalamic-pituitary-adrenal (HPA) axis activation of higher vertebrates (65). The adrenal cortex is not present as a distinct gland in teleosts, and the corticosteroid-producing interrenal cells are distributed throughout the head kidney region (42, 65). In both cases of HPA/HPI axis activation, corticotropin-releasing hormone is secreted from the hypothalamus, stimulating the pituitary to release proopiomelanocortin, a precursor protein cleaved into adrenocorticotropic hormone (ACTH). ACTH binds to the melanocortin 2 receptor in the interrenal cells, stimulating steroidogenesis, and the associated increase cortisol production (9, 42).
Although an acute stressor-mediated cortisol increase is essential for stress adaption, a chronic increase of GCs has a negative impact on growth and weight maintenance that is highly conserved in vertebrates (26, 34). The molecular mechanisms of GC regulation of skeletal muscle protein metabolism have been well characterized in mammalian models. For instance, GC stimulation causes muscle atrophy by regulating REDD1 (regulated in development and DNA damage response 1), leading to suppression of mammalian target of rapamycin complex 1 signaling and an associated reduction in protein synthetic rates (8, 23). In mammals, it has been postulated that muscle mass is regulated primarily by alterations in protein synthetic rates, and that changes in muscle protein degradation are secondary and adaptive (49). Other genes that are upregulated during muscle wasting in mammals include two muscle-specific E3 ubiquitin ligases: muscle RING finger 1 (MuRF1) and muscle atrophy box, as well as myostatin (33). While there are orthologs of these genes in fish, it is unknown whether these genes are under transcriptional control of GR. Indeed, there is evidence to suggest that the function of these genes, in particular, myostatin (mystnb), is not conserved in lower vertebrates (20). A direct role of GR signaling in modulating weight changes comes from conditional knockouts and pharmacological studies in mammals. Ubiquitous GR knockouts (GRKOs) in mice lead to lethality, due to delayed lung development (60). Mice lacking muscle GR (GRmKO) had increased muscle mass, but smaller adipose tissue (56). Whereas mice lacking liver GR had impaired growth hormone signaling, reduced growth (60), and increased lipid stores (44). These studies highlight the tissue-specific metabolic changes associated with GR signaling. However, the systems-level changes associated with GR signaling, including glucose regulation and its effect on muscle protein metabolism and growth, are unknown.
Muscle makes up over 50% of adult fish mass, and, therefore, any change to this organ system, including enhanced energy substrate mobilization to offset the increased energy demand during stress, will be reflected in lower growth rate (41, 50). As in mammals, GR signaling also increases glucose production via hepatic gluconeogenesis in teleosts (12, 30). However, whether GCs modulate glucose uptake in target tissues during chronic stress, as in mammals, is unknown (6). While many teleosts are considered to be glucose intolerant due to their limited capacity to clear a bolus of glucose, this does not appear to be the case in zebrafish (Danio rerio) (37, 39, 40, 43, 66). Recently mifepristone, a GR antagonist, was approved for use in type II diabetes; however, the physiological consequences of using this drug to modulate metabolism at the systems level is unknown (6). Indeed, the physiological impact of increased glucose uptake on muscle intermediary metabolism, and whether this is regulated by GR signaling, is unclear in any animal model. A lack of muscle glucose sensitivity has been shown to promote protein catabolism in mammals (28, 46), but whether GR signaling is involved in this process is unclear. Against this backdrop, we tested the hypothesis that GR signaling mediates the GC-induced reduction in muscle glucose uptake, causing changes to protein synthesis/breakdown, leading to lower body mass during hypercortisolemia. Using zebrafish, we generated ubiquitous GRKO using CRISPR/Cas9 mutagenesis. Zebrafish is the only teleost identified so far lacking a GR paralog (1, 2), and, unlike mammalian models (44, 56, 60), GRKO are not lethal in this species (14, 24, 67). This makes zebrafish an ideal model to explore the metabolic changes associated with the absence of GR at the organismal level and eliminates compensatory mechanisms evident in the conditional mammalian knockouts (56). Our results indicate that the lack of a functional GR enhances muscle glucose utilization and reduces the anti-anabolic and catabolic capacities of this tissue, suggesting a direct role for GR signaling in the growth suppression with chronic stress. Given the conserved nature of GCs action, our results suggest that GRKO zebrafish is an excellent model for translational research to determine the fundamental roles of stress on energy substrate repartitioning and nutrient homeostasis (54).
MATERIALS AND METHODS
Zebrafish maintenance.
Adult (9-mo-old) zebrafish (Tupfel long fin strain) were maintained according to standard protocols. All procedures were performed according to a protocol approved by the University of Calgary Animal Care Committee (AC17-0079) and followed the guidelines set by the Canadian Council for Animal Care. All fish were maintained in 10-liter tanks on a recirculating system with a 14:10-h light-dark cycle (Tecniplast). Water was maintained at 28.5°C, pH 7.6, and 750-µS conductivity. Animals were fed twice daily with Gemma micro 300 diet [Skretting; 3% body weight; composition: protein (59%), oil (14%), ash (14%), fiber (0.2%), and phosphorus (1.3%)] in the morning and live Artemia (San Francisco Bay Brand) in the afternoon. We used CRISPR/Cas9 mutagenesis to generate the ubiquitous GRKO zebrafish, and this was described recently (17). Briefly, 7 base pairs have been deleted in exon 2 of the nr3c1 gene (corresponding to the NH2-terminal domain of the GR protein), resulting in a nonfunctional, truncated protein (17). In all cases, fish were euthanized before the first feed (10:30 AM).
Body composition measurements.
Moisture content was the difference in weight before and after drying the tissue for 48 h at 70°C in an oven (Thermo Fisher Scientific). Dehydrated tissue was used for total lipid content determination using the Folch method (34). Briefly, tissue was transferred to a 1.5-ml centrifuge tube and homogenized in 1 ml chloroform-methanol (2:1). The homogenate was incubated for 30 min, after which the supernatant was removed to a clean glass tube, washed with 2× volume of 0.9% saline, and vortexed for 30 s. The phases were allowed to separate for 30 min on an orbital shaker, after which the lipid phase was transferred to a preweighed glass tube using a microcapillary pipette. The chloroform from this lipid/chloroform phase was allowed to evaporate overnight, and the weight of lipids was determined gravimetrically. Lipid content was measured from female fish with gonads excised and, therefore, measured lipids in the somatic tissue. Total protein was measured using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Glucose and glycogen contents were analyzed, as described previously (34).
Feeding trials.
Individual fish were placed in 1-liter of water in a 2-liter clear tank and fed 10 pellets at a time. Additional pellets were added when the fish had eaten all previous pellets. Pellets were counted at 5-min intervals over a 20-min period and expressed as the total weight of pellets (average pellet weight 4.8 ± 0.1 mg) consumed per fish. Trials were carried out with a minimum of five independent fish in a day and repeated on 2 different days.
Stress experiment.
Fish were subjected to an acute handling disturbance. Briefly, adult zebrafish were exposed to a 1-min air exposure. Fish were sampled by euthanasia in 0.5 mg/l of MS-222 (buffered 1:2 with sodium bicarbonate) either before (0 h) or 0.5, 1, and 2 h poststressor exposure. Fish were weighed, gonads and liver removed, and all tissues were frozen at −80°C for later analysis.
Cortisol and glucose determination.
Whole body cortisol was extracted and quantified using an ELISA, as previously described for zebrafish (16). The intra-assay and interassay variations were <5 and <15%, respectively. Blood glucose level was determined by FreeStyle glucose strips and meter (Abbott, Mississauga, ON, Canada), as described previously for zebrafish (11). The detection limit of this instrument was 1.1 mmol/l.
In vivo 2-NB-deoxy-d-glucose uptake.
Glucose uptake in vivo followed the protocol of Itoh et al. (27) with modifications. Briefly, fish were injected intraperitoneally with 25 µmol/kg of 2-[N-7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino-2-deoxy-d-glucose (2-NBDG), a fluorescent analog of 2-deoxyglucose. Fish were allowed to recover for 1 h and euthanized with 0.5 mg/l of MS-222 (buffered 1:2 with sodium bicarbonate). White epaxial muscle and livers were removed and immediately homogenized in 50 mM Tris + protease inhibitor using a sonicator (Fisher Scientific, 6 × 3 s bursts, setting 3–4). Samples were centrifuged at 13,000 g for 1 min, and the supernatant was added to a black 96-well plate and read by a Paradigm plate reader (excitation/emission 465/540 nm; Molecular Devices). Saline-injected fish were used as background controls. Preliminary experiments revealed that zebrafish muscle and liver took up 2-NBDG in a dose-dependent manner.
Immunodetection.
SDS-PAGE and Western blotting were performed as previously described (17). For the dot blot, 2 µl of denatured protein samples in Laemmli’s buffer (normalized to 2 mg/ml protein) were added to a nitrocellulose membrane and dried for 2 h at 37°C. Immunodetection was carried out as described previously (17). Primary antibodies are as follows: anti-phospho-eIF4B (eukaryotic initiation factor-4B) [Cell Signaling Technologies no. 3591T, 1:1,000; the antibody for total eIF4B (3592T) did not cross-react with zebrafish]; anti-ubiquitin (Sigma U5379, 1:100); anti-insulin (Agilent Technologies, no. A056401-2, 1:500); and anti-HSP70 (heat shock protein 70)/HSC70 (heat shock cognate 70) (SPA-812, 1:1,000). Equal loading for eIF4B and HSC/HSP70 Western blots was assessed using Ponceau staining (0.1% wt/vol in 5% acetic acid; Bioshop). All densiometric quantification was done with ImageJ software (National Institutes of Health, Bethesda, MD).
Fasting study.
Age-matched wild-type (WT) zebrafish were randomly assigned to two 3-liter tanks (12 fish/tank). Additionally, GRKO fish (age-matched with WT) were also randomly assigned to two 3-liter tanks. All fish were held on a recirculating system. One WT and one GRKO tank were fed 3% of their body weight/day, and the other tank of WT and GRKO fish were fasted for 7 days. On day 7, fish were euthanized with MS-222, and muscle was removed and stored at −80 for further analysis. Total muscle protein concentration was calculated as protein × total muscle weight. Muscle weight was taken as 50% of the total body mass (41).
Transcript abundance.
Transcript levels of specific genes were measured by quantitative real-time PCR. Total RNA was extracted from larvae using Ribozol reagent (VWR), according to the manufacturer’s instructions, and quantified using a SpectraDrop Micro-Volume microplate (VersaMax, Molecular Devices). One microgram of RNA was treated with DNase I (Thermo Scientific) to remove genomic contamination before cDNA synthesis using the high-capacity cDNA reverse transcription kit (Applied Biosystems), according to the manufacturer’s protocols. Quantitative real-time PCR was carried out using a QuantStudio 3 real-time PCR system (Applied Biosystems), with a Sso Advanced SYBR green master mix (Bio-Rad). Ct values for target genes were normalized against β-actin, and expression was calculated by the ∆∆Ct method (36). Gene-specific primers are in Table 1.
Table 1.
Primers used for quantitative real-time PCR
| Target | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Source, ref. no. |
|---|---|---|---|
| murf1a | GGAAGAAAACTGCCAGGCACAG | CTGGGTGATCTGCTCCAGAAGATG | 55 |
| murf1b | CAGGACAATGCTCAACGTGCC | CTTGCTCTTTGCCAATACGCTCTAAGAG | 55 |
| redd1 | ATGCAAGATCAGTTGATTTCCAGCC | TCAGCATTCTTCAATCAGGAGCTCT | 18 |
| myostatinb | AGACCGCTGTGGCTGCTCAT | GCGGAAAGCACTGGTAATGT | 45 |
| pepck/pck1 | AGTGGGACAAAGCCATGACC | TCAGCTCCACCCCTATCTTG | |
| Β-actin | TGTCCCTGTATGCCTCTGGT | AAGTCCAGACGGAGGATG | 45 |
Enzyme activities.
Enzyme activities were measured as described previously (26). Whole body homogenate was stored frozen in 50% glycerol buffer (50% glycerol, 21 mM Na2HPO4, 0.5 mM EDTA, 0.2% BSA, and 5 mM β-mercaptoethanol, pH 7.5). Enzyme kinetics were measured in 50 mM imidazole (pH 7.4) at 340 nm using a microplate reader (VersaMax, Molecular Devices).
Hexokinase (EC 2.7.1.1): 1 mM glucose, 5 mM MgCl2, 10 mM KCl, 0.25 mM NADH, 2.5 mM phosphoenolpyruvate (PEP), 5 U/ml lactate dehydrogenase (LDH), and 2.5 U/ml pyruvate kinase. Reaction started with 1 mM ATP.
Pyruvate kinase (EC 2.7.1.40): 3 mM KCl, 10 mM MgCl2, 0.12 mM NADH, 2.5 mM ADP, 20 U/ml LDH. Reaction started with 2.5 mM PEP.
Alanine aminotransferase (EC 2.6.1.2): 0.12 mM NADH, 200 mM l-alanine, 0.025 mM pyridoxal 5-phosphate, and 12 U/ml LDH. Reaction started with 10.5 mM α-ketoglutarate.
Aspartate aminotransferase (EC 2.6.1.1): 7 mM α-ketoglutarate, 0.025 mM pyridoxal 5-phosphate, 0.12 mM NADH, and 8 U/ml malate dehydrogenase. Reaction started with 40 mM aspartic acid.
Malic enzyme (EC 1.1.1.40): 1 mM MgCl2, 0.4 mM NADP. Reaction started with 1 mM l-malate.
ATP citrate lyase (EC 4.2.3.8): 20 mM citrate, 0.2 mM coenzyme A, 0.1 mM NADH, 10 mM MgSO4, 10 U/ml malate dehydrogenase. Reaction started with 5 mM ATP.
Glucose-6-phosphate dehydrogenase (EC 1.1.1.49): 7 mM MgCl2, 0.4 mM NADP. Reaction started with 1 mM glucose-6-phosphate.
Muscle metabolomics.
Epaxial muscle from the dorsal, posterior part of the fish was homogenized in 50 mM Tris and extracted using methanol. Briefly, muscle homogenate was diluted 5× in 50% ultrapure methanol and incubated on ice for 15 min. Samples were then centrifuged for 10 min at maximum speed at 4°C. The supernatant was then analyzed using liquid chromatography (Vanquish UHPLC, Thermo Fisher Scientific)-mass spectrometry (LC-MS; Q-Exactive HF hybrid Quadruple-orbitrap, Thermo Fisher Scientific) at the University of Calgary’s Metabolomics Research Facility (CMRF). Spectral intensity data were analyzed by the CMRF, using an in-house metabolite library in MAVEN (Metabolomic Analysis and Visualization Engine). Peak intensity data were normalized to muscle wet weight and analyzed using MetaboAnalyst (https://www.metaboanalyst.ca/).
Statistics.
Data are shown as means ± SE, and statistical comparisons were carried out using Sigma Plot 13 (Systat Software). All data were analyzed using a t-test (P < 0.05), unless otherwise stated. Muscle metabolomics was analyzed using two-way ANOVA (false discovery rate corrected P < 0.05; Fisher’s least significant difference post hoc) in MetaboAnalyst. Weight data (Fig. 1B), stress response data (Fig. 2) and fasting data (Fig. 6) were analyzed with a two-way ANOVA (Holm-Sidak post hoc). All data were transformed to meet the assumptions of normality and equal variance. Untransformed data are shown in Figs. 1–7, and a significance level of P < 0.05 was used in all cases.
Fig. 1.
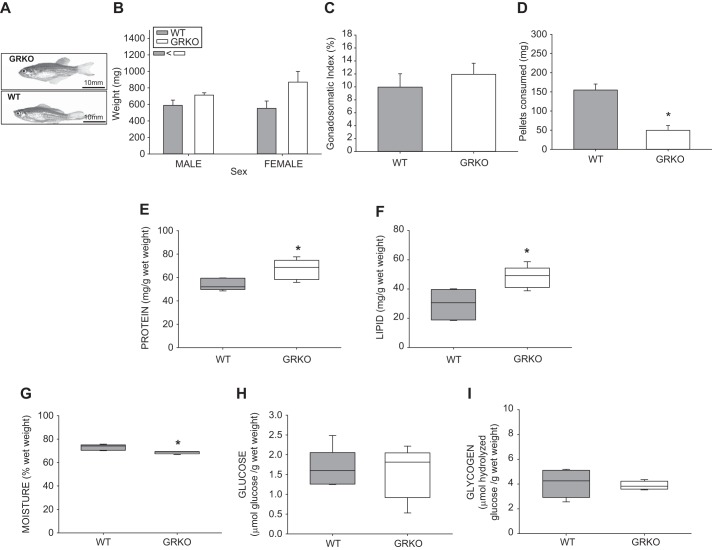
Glucocorticoid receptor knockout (GRKO) zebrafish are larger than wild type (WT), with different body composition. A: representative image of GRKO adult female zebrafish and a WT female zebrafish. B: the body mass of GRKO zebrafish, both male and female, was higher than that of their WT counterparts. C: however, the gonadosomatic index was not significantly different between the two groups. The GRKO fish consumed less food (D), had greater total protein content (E), higher total lipid content (F), and less moisture (G) compared with the WT controls. There was no change in carbohydrate levels, including whole body glucose (H) and glycogen contents (I). Values are means ± SE (n = 4–6). B, inset: main effect (two-way ANOVA; P < 0.05) is shown. *Significantly different from the WT (t-test; P < 0.05).
Fig. 2.
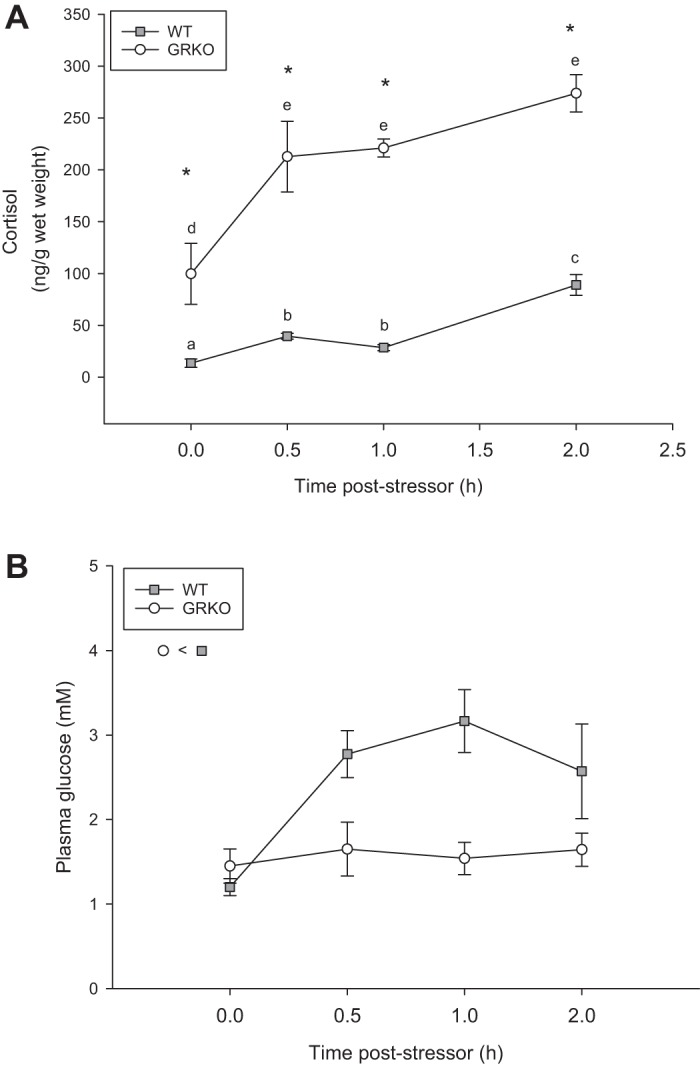
Glucocorticoid receptor knockout (GRKO) have an impaired glucose response to stress. Adult zebrafish (mixed sex) were subjected to a 1-min air exposure and sampled at different time points poststressor. A: GRKO zebrafish (open circles) are hypercortisolemic compared with the wild type (WT) (shaded squares), and both groups increase cortisol levels by 30 min poststressor. B: GRKO fish had lower blood glucose levels compared with the WT, regardless of sampling time poststressor exposure. Values are means ± SE (n = 4–6). a–eTime points with different letters are significantly different for the WT and the GRKO fish. *Significant difference from the WT at each time point. B, inset: significant main effect (two-way ANOVA/Holm-Sidak; P < 0.05) is shown.
Fig. 6.
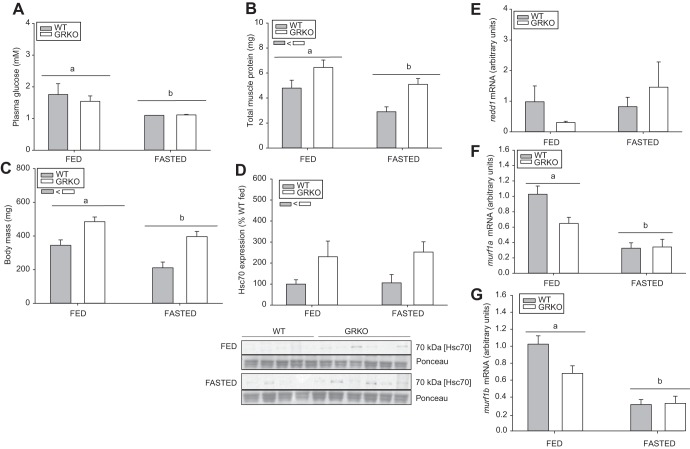
Glucocorticoid receptor knockout (GRKO) fish have higher body mass and protein content postfasting. Adult wild-type (WT) and GRKO zebrafish (mixed sex) were subjected to a 7-day fasting stressor. A: fasting reduced blood glucose levels in both groups. B: GRKO fish had higher muscle protein content compared with the WT in both the fed and fasted state. C: GRKO fish, regardless of nutritional status, had higher body mass compared with the WT. D: heat shock cognate 70 (HSC70)/heat shock protein 70 protein levels were also higher in the GRKO fish, independent of feeding status. The data are shown as percent WT fed. E–G: muscle-specific expression of genes involved in protein catabolism, including redd1 (E), murf1a (F), and murf1b (G). Values are means ± SE. a,bDifferent letters denote a significant effect of fasting, while significant genotype effect is shown as an inset below the legend box (two-way ANOVA/Holm-Sidak, P < 0.05).
Fig. 7.
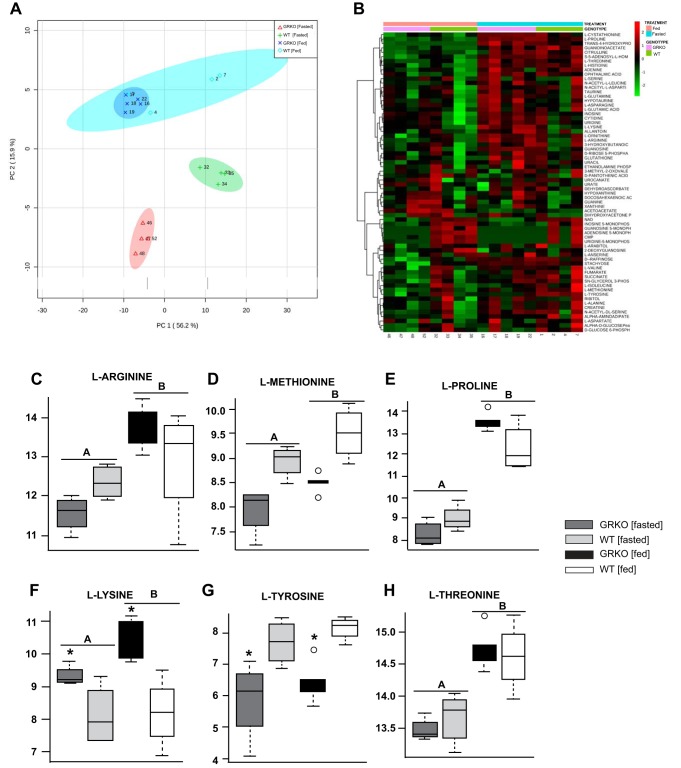
Muscle metabolomics. The muscle metabolite profile was analyzed from fed and fasted glucocorticoid receptor knockout (GRKO) and wild-type (WT) zebrafish muscle was analyzed using liquid chromatography-mass spectrometry. A: principal component (PC) analysis. B: heat map of all measured metabolites. Box plots of metabolites include gluconeogenic amino acids l-arginine (C), l-methionine (D), l-proline (E), l-tyrosine (G), and l-threonine (H), and ketogenic amino acid l-lysine (F). Values are means ± SE (n = 4–6). All reported statistics are main effects of two-way ANOVA/false discovery rate corrected, P < 0.05. A,BBars with different letters denote significant differences between nutritional state. *Significant differences between genotype.
RESULTS
Loss of GR increases body mass and alters body composition.
Adult GRKO fish had higher body weight compared with WT fish, independent of sex (Fig. 1, A and B; P = 0.002). Female GRKO fish had 1.5-fold higher body mass (n = 6; 870.76 ± 129.4 mg) compared with WT fish (n = 12; 551.93 ± 89.1 mg; Fig. 1, A and B), and male body size of the GRKO fish (n = 6; 709.4 ± 27.9 mg) was also larger compared with WT (n = 12; 589.2 ± 63.4 mg). As the gonadosomatic index was not different between the WT and GRKO female fish (Fig. 1C, n = 6, P = 0.479), the differences observed were due to the somatic tissues mass. The higher body mass was also not a reflection of increased appetite, as the GRKO fish ate only a third of the food consumed by WT fish (P = 0.0191; Fig. 1D). The adult GRKO mutants differed in body composition, and the measurements were carried out with somatic tissue of female fish. GRKO had higher protein (Fig. 1E; P = 0.0217) and total lipid content (Fig. 1F; P = 0.007), while moisture content (Fig. 1G; P = 0.00561) was lower compared with the WT. There were no changes in whole body glucose or glycogen content between the two groups (Fig. 1, H and I, respectively).
GRKO zebrafish have a functional HPI axis, but an impaired glucose response to stress.
We next tested whether glucose regulation was central to the GR-mediated metabolic programming. To determine whether target tissue glucose utilization was involved in modulating the nutrient stores during stress, we first measured whole body cortisol and blood glucose levels poststressor exposure (Fig. 2, A and B, respectively). GRKO fish were hypercortisolemic compared with the WT at every time point poststressor exposure (P < 0.001). There was a significant interaction between genotype and time for cortisol levels poststressor (P = 0.043; Fig. 2A). Within WT, cortisol levels increased threefold from 13.6 ± 7.9 to 39.6 ± 5.7 ng/g wet wt at 0.5 h poststress (P < 0.001). This remained elevated for the rest of the 2-h sampling (Fig. 2A). Within the GRKO, basal cortisol levels (99.7 ± 58.8 ng/g wet wt) increased twofold to 212.8 ± 68.0 ng/g wet wt at 0.5 h poststressor (P < 0.001) and remained elevated for the duration of the 2-h sampling time point (Fig. 2A). There was only a treatment effect on blood glucose levels (Fig. 2B). WT had higher glucose levels compared with GRKO zebrafish, which were at the detection limit of the glucose strips (P = 0.015; Fig. 2B), and there was no interaction between genotype and time poststressor exposure for blood glucose levels.
Loss of GR increased glucose uptake in the muscle.
We then tested whether the lack of a glucose response in the GRKO was due to a faster glucose clearance from the circulation. Therefore, we tested glucose uptake in target tissues in vivo by injecting fish interperitoneally with 2-NBDG, a fluorescent analog to 2-deoxyglucose commonly used to trace glucose utilization in neurons (27). Injection of 2-NBDG raised blood glucose levels in the WT (n = 8, 5.1 ± 0.7 mM), but not in the GRKO fish (n = 6, 1.4 ± 0.2 mM) compared with the uninjected and saline-injected controls (Fig. 3A). There was no change in 2-NBDG fluorescence in the liver of the GRKO fish compared with the WT (Fig. 3B; P = 0.0921). Unlike the liver, the GRKO fish muscle had double the amount of 2-NBDG [162,604 ± 10,087 relative fluorescent units (RFU)/mg tissue] compared with the WT muscle (79,105 ± 23,075 RFU/mg tissue), indicating an increased rate of glucose uptake (twice the rate; P = 0.01) over the 1 h postinjection (Fig. 3C).
Fig. 3.
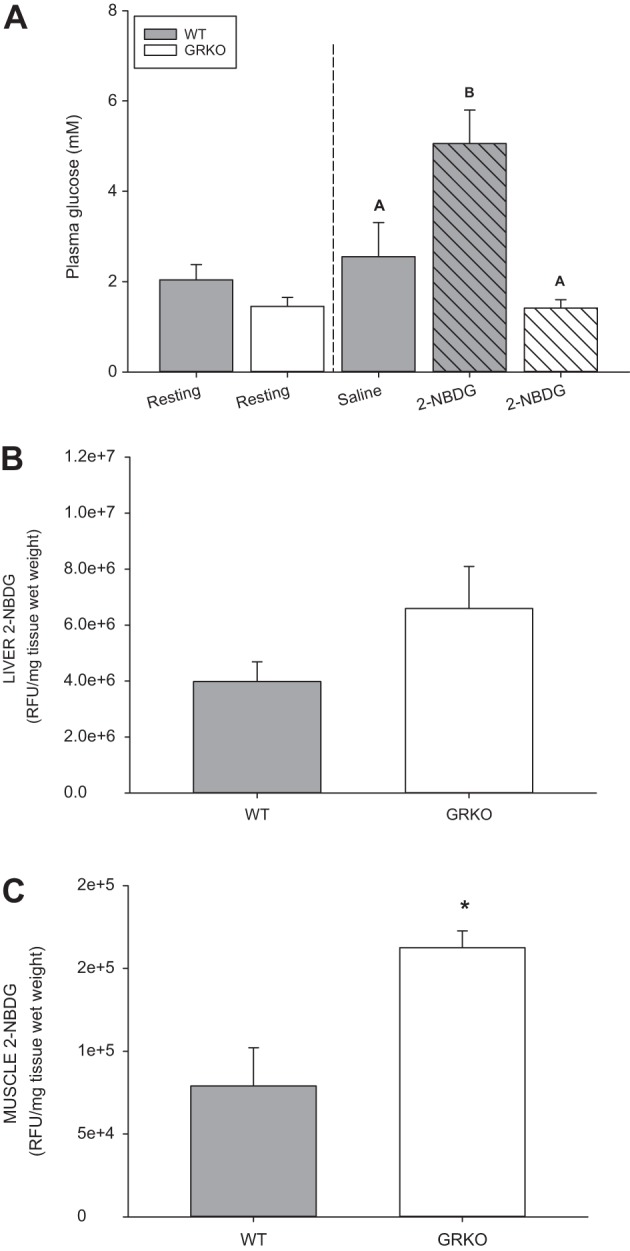
Glucocorticoid receptor knockout (GRKO) increased glucose clearance due to increased muscle uptake. Glucose levels from adult zebrafish (mixed sex) were taken at resting (noninjected) and 1 h postinjection with a fluorescent glucose analog, 2-[N-7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino-2-deoxy-d-glucose (2-NBDG). A: injection of 2-NBDG in wild-type (WT) fish (shaded hatched bar) caused an increase in blood glucose levels at 1 h postinjection (vertical dashed line) compared with the saline-injected controls (shaded bars) and the GRKO fish (open hatched bar). Resting levels (noninjected fish) are depicted before the vertical dotted line. Relative fluorescence units of 2-NBDG in the liver (B) and the white muscle (C) are shown. Values are means ± SE (n = 4–6). A,BBars with different letters are significantly different (one-way ANOVA; P < 0.05). *Significantly different from the WT (t-test; P < 0.05). RFU, relative fluorescent units.
We further confirmed an increased potential for glucose utilization by measuring hexokinase activity (pre-2NBDG injection). Adult GRKO fish had greater than twofold maximal hexokinase activity (1.1 ± 0.2 U/g) compared with the WT (0.414 ± 0.025 U/g; Fig. 4A). To test whether insulin may play a role in the increased tissue glucose capacity in the GRKO fish, we determined insulin expression. Dot-blot analysis of whole body insulin showed that the expression was similar in the GRKO and WT fish at either the resting state or post-2NBDG injection (P = 0.058). However, injection with 2-NBDG increased insulin levels above resting (P = 0.001; Fig. 4B).
Fig. 4.
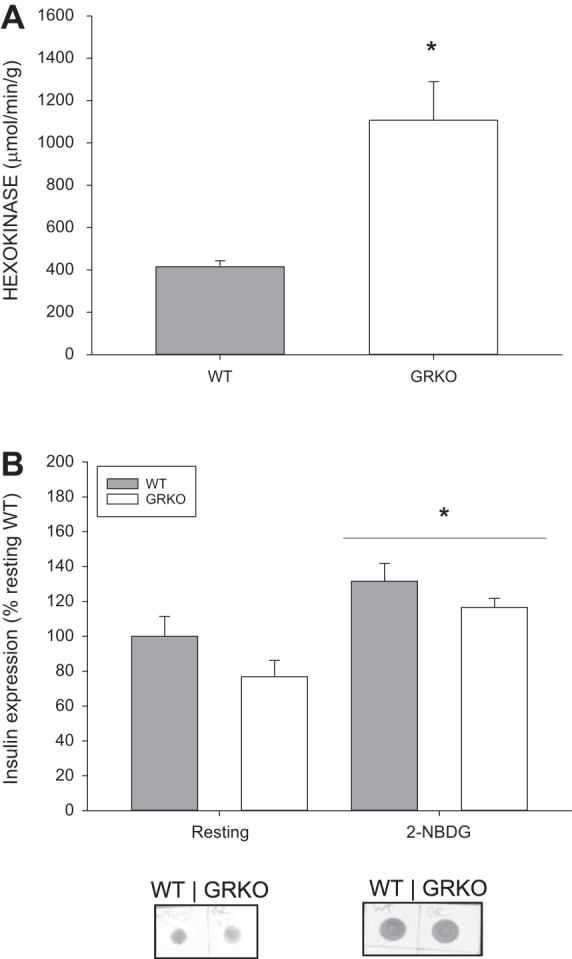
Glucocorticoid receptor knockout (GRKO) fish have higher hexokinase activity but no change in insulin expression. A: hexokinase activity (μmol·min−1·g tissue−1) was higher in the GRKO fish compared with the WT. *Significantly different from the wild type (WT) (t-test; P < 0.05). B: insulin expression was measured by dot-blot using an anti-insulin antibody (1:500) in the whole body homogenates of WT and GRKO fish either before (resting) or after 2-[N-7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino-2-deoxy-d-glucose (2-NBDG) injection. Values are means ± SE (n = 4–6). *Significantly different from resting insulin levels (t-test; P < 0.05).
Loss of GR promotes protein synthesis.
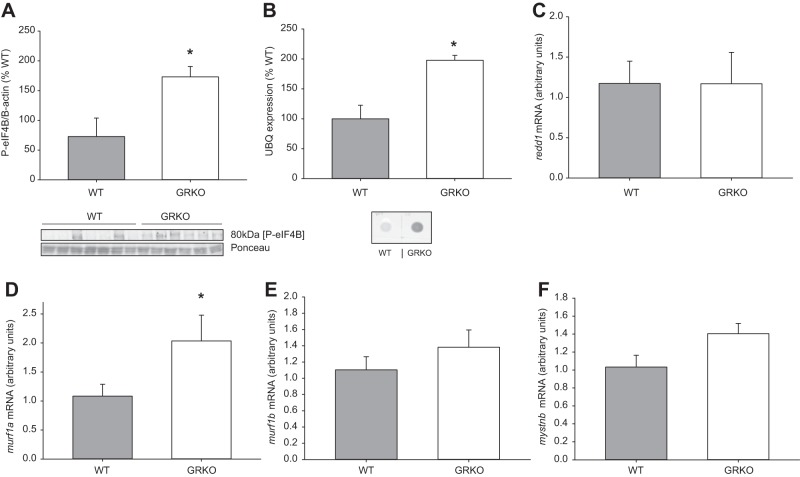
As GRKO zebrafish are larger with increased total protein, we next assessed the protein synthetic capacity of GRKO zebrafish compared with WT by measuring the phosphorylation of eIF4B, a marker for enhanced translation capacity. GRKO fish had twofold higher phosphorylation of eIF4B compared with the WT (P = 0.026; Fig. 5A). The mutants also showed a higher expression of polyubiquitinated proteins compared with the WT (Fig. 5B). Targets of GC-induced muscle catabolism were also measured to assess the capacity of the fish to induce muscle wasting. There were no differences in the whole body transcript abundance of redd1 (Fig. 5C; P = 0.992), mystnb (Fig. 5F; P = 0.0559), and murf1b (Fig. 5D; P = 0.321). The transcript abundance of murf1a was upregulated in the GRKO fish (Fig. 5E; P = 0.05).
Fig. 5.
Glucocorticoid receptor knockout (GRKO) fish have greater protein synthetic capacity. A: translational capacity was measured in whole body wild-type (WT) and GRKO zebrafish (mixed sex) injected with 2-[N-7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino-2-deoxy-d-glucose (2-NBDG) by measuring the phosphorylation of eukaryotic initiation factor-4B (eIF4B), which was normalized to ponceau. A representative Western blot of anti-phospho-eIF4B (80 kDa; 1:1,000) and ponceau is shown below the bar graph. B: ubiquitin (UBQ) expression was assessed by dot blot of whole body homogenate from WT and GRKO zebrafish using an anti-UBQ antibody (1:100). Targets of glucocorticoid-induced muscle catabolism were also measured, including redd1 (C), murf1a (D), murf1b (E), and mystnb (F). Values are means ± SE (n = 5–6). *Significance from the WT (t-test; P < 0.05).
Loss of GR attenuates protein catabolism.
To further test our hypothesis that glucose regulation is central to GR-mediated metabolic changes to adult growth, we next restricted glucose availability by fasting. We hypothesized that, if increased glucose uptake was contributing to increased protein synthesis, the GRKO fish would spare proteins under a fasting stressor. The blood glucose levels were lower in the fasted fish compared with the fed fish, regardless of genotype (Fig. 6A, P = 0.017). Fasted fish had significantly less protein compared with the fed fish (P = 0.006; Fig. 6B); however, GRKO fish showed significantly less protein breakdown compared with the WT, regardless of the nutritional status (P = 0.002; Fig. 6B). The 7-day fasting stressor also reduced the body mass of WT fish by ~32% (P = 0.012) compared with the fed WT fish (Fig. 6B). Although, there was no significant interaction between genotype and nutritional status, the reduction in body mass with fasting was less pronounced in the GRKO fish (~19%) compared with the fed GRKO fish (P = 0.54; Fig. 6C). The molecular chaperone, HSC70 was also evaluated as a measure of global protein synthesis, as these are important for the folding of nascent polypeptides (5). Independent of fed status, the HSC70 protein expression was greater in the GRKO fish (Fig. 6D; P = 0.048). To determine the effect of GR signaling on muscle-specific atrogene expression, we measured redd1, murf1a, and murf1b transcript abundance in the fed and fasted fish (Figs. 6, E–G). There was no effect of genotype (P = 0.0.682) or treatment (P = 0.259) on redd1 (Fig. 6E); however, both murf1 paralogs (Fig. 6, F and G) were lower in the fasted fish compared with the fed fish (murf1a, P < 0.001; muf1b, P < 0.001).
Muscle metabolomics.
To further analyze the physiological significance due to the absence of a functional GR, we examined the muscle metabolome under fed and fasting conditions. A principal component analysis shows a clear separation of GRKO and WT muscle under fasting conditions, with little difference between the two groups in the fed state (Fig. 7A). This was further reflected in the heatmap, which revealed distinct metabolite profiles between the fasted GRKO and WT fish (Fig. 7B). The majority of changes observed were in the amino acid profiles. The gluconeogenic amino acids, including l-arginine, l-methionine, l-proline, and l-threonine, were higher in the fasted fish muscle compared with the fed fish (Figs. 7, C–E, H). Other amino acids, including l-tyrosine and the ketogenic l-lysine, were affected by genotype. l-Tyrosine was significantly lower in the GRKO compared with the WT (Fig. 7G) fish, whereas l-lysine was significantly higher in the GRKO compared with the WT (Fig. 7I).
DISCUSSION
Here, using a loss of function approach, our results reveal a key role for GR signaling in the cortisol-mediated growth suppression in fish. The larger body mass, including higher muscle protein content in the GRKO zebrafish, even with fasting, indicates a role for GR signaling in affecting growth during stress in fish. A conserved response to chronic GC stimulation involves enhanced protein catabolism, leading to muscle atrophy and suppressed growth (30, 38, 42). Our results indicate that GR-mediated reduction in muscle glucose uptake may be a major driver in promoting the protein breakdown, leading to reduced growth with chronic stress in fish.
Zebrafish lacking GR are hypercortisolemic (11, 19, 49, 15) (Fig. 2A) and have a larger body mass. However, it is well known that hypercortisolemia associated with chronic stress will lead to muscle wasting (42). This indicates that a functional GR is essential for the loss of muscle mass during chronic stress and may involve changes in energy substrate repartitioning. A hallmark of chronic GC stimulation is hyperglycemia due to enhanced liver gluconeogenesis. This process is dependent on amino acids, produced by muscle proteolysis, to provide C3 substrates for gluconeogenesis (see reviews in Refs. 3, 12, 34). The necessity of GR signaling in mediating this response is supported in the present study, where hypercortisolemia did not lead to hyperglycemia in the GRKO fish. Although hyperglycemia may be a combination of altered glucose production and clearance, few studies have examined the role of stress and/or cortisol in delaying glucose clearance in fish (7, 11, 43, 62). In mammals, the delay in postabsorptive rates of systemic glucose clearance is a result of insulin resistance in peripheral tissues, including skeletal muscle and adipocytes (51). While peripheral tissues, such as the muscle, are involved in glucose clearance in both mammals and fish (7, 47), the effect of elevated GCs on this response is unknown. Similar to mammals, insulin-mediated glucose regulation occurs in fish, and the muscle upregulates insulin-sensitive glucose transporters (47). Poor glucose utilization of peripheral tissues is considered a factor for glucose intolerance in fishes (43). This is evident in the present study, as WT fish have a greater than twofold increase in plasma glucose levels following a stressor (Fig. 2B), which was maintained over the 2-h sampling period, supporting slower clearance of this metabolite (43). Interestingly, GRKO fish did not have a glucose response to stress. Given that the stressor-induced increase in glucose levels is usually associated with epinephrine-mediated glycogenolysis (13, 42), we hypothesized that the lack of hyperglycemia in the GRKO fish is due to a faster glucose clearance from the circulation as a result of target tissue uptake (6). However, we cannot discount the possibility that gluconeogenesis was also reduced in the GRKO fish, leading to reduced production of glucose (42), but the lack of change in the transcript abundance of phosphoenolpyruvate carboxykinase in the GRKO compared with WT (Table 1) argues for a faster clearance rather than lower production.
To test whether the lack of GR signaling leads to enhanced glucose clearance, we followed the uptake of a glucose tracer (2-NBDG) into the muscle and liver of zebrafish (Fig. 3). Injection of the tracer increased blood glucose in the WT but not the GRKO fish, again suggesting faster clearance in the absence of GR signaling. The increase in blood glucose levels post-2-NBDG injection seen in our WT fish was also observed in mammals (12, 29). This may involve the activation of the sympathetic nervous system and the HPA axis, as 2-deoxyglucose-induced hyperglycemia was abolished in adrenalectomized rats (29). The absence of an increase in blood glucose levels in the saline control group pre- and postinjection argues against injection as a stressor eliciting the glucose response (Fig. 3A). This suggests that 2-NBDG may affect the cortisol response in fish, but this remains to be tested. This further emphasizes that the lack of glucose response in the GRKO fish injected with 2-NBDG is due to a faster clearance of glucose in the absence of GR signaling, as GRKO fish are naturally hypercortisolemic (Fig. 2A). This notion was further supported by an enhanced 2-NBDG uptake by the muscle, but not the liver (Fig. 3, B and C). To our knowledge, this is the first study to show that GR signaling modulates glucose uptake by the muscle in fish. Higher hexokinase activity in the GRKO fish indicates a higher tissue capacity for glucose utilization. The increased muscle glucose uptake capacity did not involve changes in whole body insulin expression in the GRKO fish (Fig. 4B). However, we cannot rule out the possibility that GR deficiency will enhance insulin sensitivity. Mammalian studies have clearly shown that corticosteroids inhibit the insulin-mediated muscle glucose uptake, and this involves target tissue insulin resistance (22). The lack of an antibody for zebrafish glucose transporter-4 (GLUT-4) precluded us from testing whether the higher recruitment of this protein on muscle membrane may be responsible for the increased muscle glucose uptake in the GRKO fish. However, there was no change in whole body glycogen content (Fig. 1I) or in the activities of glycolytic enzymes (Table 2). As these are metabolic targets for insulin-mediated muscle glucose regulation, our results suggest possible metabolic reorganization specific to GR signaling and independent of insulin responsiveness. The reduced food consumption in the GRKO fish, despite higher body mass, may be due to the enhanced brain capacity for glucose uptake, which suppresses appetite (19). Although our results suggest a role for GR signaling in modulating feeding behavior, further studies are clearly warranted to specifically address the molecular mechanisms involved in the GR regulation of nutrient homeostasis in vertebrates. Together, these results underscore a direct role for GR signaling in reducing muscle glucose uptake in fish, while the mechanism of action remains to be elucidated. We propose this lack of glucose uptake by the muscle during stress may be a driver in the physiological reorganization of the tissue, favoring reduced protein synthesis and increased protein catabolism.
Table 2.
Enzyme activities
| Enzyme | Wild-Type Activity | GRKO Activity | Significance (P < 0.05) |
|---|---|---|---|
| Malic enzyme | 0.992 ± 0.14 | 0.110 ± 0.040 | |
| G6PDH | 0.415 ± 0.132 | 0.606 ± 0.099 | |
| Citrate lyase | 4.3 ± 0.5 | 3.1 ± 0.4 | |
| Pyruvate kinase | 30.0 ± 2.1 | 27.6 ± 2.1 | |
| Alanine aminotransferase | 2.1 ± 0.4 | 1.1 ± 0.1 | 0.0598 |
| Aspartate aminotransferase | 13.0 ± 3.0 | 15.2 ± 1.0 | |
| PEPCK (mRNA) | 1.0 ± 0.2 | 0.7 ± 0.2 | 0.441 |
Values are means ± SE (n = 6). Activity is shown as μmol·min−1·g wet wt−1. Activities of enzymes involved in intermediary metabolism, including glycolysis (pyruvate kinase), amino acid catabolism (alanine and aspartate aminotransferases), and lipid biosynthesis [glucose-6-phosphate dehydrogenase (G6PDH), citrate lyase, malic enzyme], are shown. A marker of gluconeogenic activity [phosphoenolpyruvate carboxykinase (PEPCK)] was measured by quantitative PCR, and mRNA transcript abundance of this enzyme is listed under activity.
Glucose is a known regulator of protein synthesis in mammalian skeletal muscle, and, along with insulin, has the ability to increase the phosphorylation of eukaryotic initiation factor 4E (eIF4E) (28, 46). This initiation factor plays an important role in protein translation and is used as a marker of the protein synthetic capacity of the cell (41). Glucose, and its hexose derivatives, interact with eIF4E binding proteins, phosphorylating them and causing them to dissociate from eIF4E (46). The eIF4E will then form a heterocomplex with other proteins, including eIF4B, promoting translation. Phosphorylation of eIF4B is also important to the aforementioned translation heterocomplex, and a loss of this protein reduces translation efficiencies (53). In our study, GRKO fish showed a marked increase in phosphorylation of eIF4B, suggesting an increase in muscle protein synthetic capacity due to the loss of GR function. This is further reflected at the system level by the higher body mass and whole body protein content in the GRKO fish (Figs. 1E and 6C). Also, the higher protein ubiquitination seen in the GRKO fish is usually associated with increased protein synthetic capacity, as nearly 30% of newly synthesized proteins are degraded in a proteasome-dependent manner (63). However, we cannot rule out the possibility that there is also concurrent protein breakdown that may be mediated by the mineralocorticoid receptor (MR) signaling. This seems plausible, given that murf1a, a known atrogene (33), was upregulated in the GRKO, in which MR is the only functioning corticosteroid receptor. Consequently, lack of GR signaling exerts a predominantly net anabolic effect by increasing the protein synthetic capacity, and this may be associated with the enhanced capacity for muscle glucose uptake in the GRKO fish.
As stress increases the energy demand (4, 52), there is a greater demand for tissue-specific energy substrate repartitioning to fuel the metabolic processes (42). Indeed, GC stimulation enhances muscle proteolysis, releasing amino acids for use as substrates for hepatic gluconeogenesis (39, 42). Stressor-mediated increases in glucose levels are necessary to fuel the aerobic tissues, including brain and gills, to cope with the increased energy demand. For instance, in brown trout (Salmo trutta), the uptake rates of glucose were highest in the brain, kidney, spleen, and gills, and lowest in red muscle, heart, and white muscle (7). As muscle constitutes >50% of adult fish mass (41), even small changes in glucose utilization by this tissue may limit the availability for aerobic tissues, which are critical for coping with stress. Indeed, despite a low uptake rate, muscle in brown trout accumulated the most glucose because of the larger mass of this tissue (7). Therefore, limiting muscle glucose uptake to facilitate reallocation of this fuel to other tissues may be an evolutionarily conserved role of GR signaling in stress adaptation. While it is well known that certain atrogenes are under the transcriptional control of GR in mammals, this has yet to be shown in fish. Also, there is some evidence that myostatin does not have the same role in fish as it does in mammals (20), but genes that are known to be essential to protein catabolism, including redd1, remain unchanged in the GRKO, despite the high cortisol levels in the present study. In mammals, several obesity-related factors are known to upregulate redd1 mRNA/protein levels, including hyperinsulinemia, hyperlipidemia, and hypoxia (35). Failure to upregulate this gene in the GRKO fish further emphasizes the importance of GR signaling in regulating whole body metabolic homeostasis. When muscle-specific atrogene transcript abundance was examined (Fig. 7, E–G), there was also no change in redd1, while fasting reduced transcription of murf1a and murf1b. In mammals, redd1 gene and protein expression is increased 24 h postfasting (58). The lack of increase in muscle redd1 gene expression in this study, despite lower body mass in the fasted fish, may suggest changes in mRNA stability and/or turnover, but this remains to be determined. While these results suggest atrogene regulation by GR, it is clear from our study that the cortisol-driven muscle wasting is abolished in the GRKO fish. Interestingly, the murf1a paralog was upregulated in the GRKO fish, which indicates that it may be under the transcriptional control of another transcription factor sensitive to the increased cortisol levels, such as the MR. The lack of consensus between whole body and muscle murf1a and murf1b transcript abundance in the present study suggests that, in addition to tissue-specific glucose uptake (Fig. 3, B and C), the role of GR signaling on atrogene regulation may also be tissue specific.
To test whether GR signaling has a direct role in weight loss, we measured body mass and protein content in 7-day fasted zebrafish. Our prediction was that body mass and protein content loss will be lower in the absence of GR signaling. Indeed, GRKO fish had larger body mass (Fig. 6B) and higher muscle protein content (Fig. 6D) relative to the WT during fasting. Also, the protein expression of HSC70 was higher in the GRKO fish, regardless of the nutritional state of the animal, suggesting that the overall protein synthetic capacity was higher in the absence of GR in fish (31, 59). Importantly, HSC70 also interacts with CHIP (COOH-terminal of HSP70-interacting protein), which is thought to coordinate the cellular balance between folding and degradation of proteins (31). While the physiological role for GR signaling may be primarily to enhance protein catabolism, a reduction in protein synthesis may also be an important part of the GC-driven muscle atrophy. Mice with a conditional GRmKO also showed increased muscle mass and reduced muscle loss postfasting (56), confirming a conserved GR-dependent phenotype. Cortisol-induced muscle wasting is a well-known effect of Cushing’s syndrome (22), and the maintenance of protein levels in the GRKO fish, despite hypercortisolemia, suggests a key role for GR signaling in protein sparing. This was further evident from the overall change in the amino acid profile of the fasted GRKO and WT zebrafish (Fig. 7, A and B), in which fasting resulted in higher arginine, methionine, proline, and threonine (Fig. 7, C–E, H), suggesting increased proteolysis compared with fed fish. GRKO fish had lower levels of tyrosine, but not lysine, independent of fasting, suggesting a reduced capacity for proteolysis (21).
A limitation of this study was the lack of antibodies that were specific to fish proteins. For instance, mammalian antibodies for insulin receptor B and GLUT-4 failed to cross-react with our zebrafish samples. Also, an ortholog of glut4 in zebrafish has not yet been identified, which limited our ability to establish whether glucose uptake was due to differences in transporter availability using transcript abundance (37). We were also limited by the small size of the animal, which limited the number of measurements we could make on a single tissue sample. For example, it was not possible to get enough blood to measure circulating glucose and insulin levels. Despite these limitations, the single copy of GR (compared with two paralogs in all other teleost fish) makes the zebrafish an ideal translational model to examine the conserved nature of GC-driven glucose regulation and nutrient homeostasis. While our study has shed light on the whole body metabolic changes evident in the absence of GR, we cannot differentiate the metabolic actions that are GR isoform specific. For instance, we know that GR-α and GR-β have unique functions in animals (32), and the cross talk between these two isoforms may also modulate target tissue metabolism (10). While our CRISPR-Cas9 method deleted both the α- and β-splice variant, generating knockout of the splice variant of GR in the future may help to elucidate the conserved functions of this receptor in the metabolic adaptation to stress in animals.
In conclusion, we report that cortisol-GR signaling plays a key role in body mass regulation during stress in fish. GRKO enhances muscle glucose uptake and protein content, leading to increased body mass in zebrafish. This study emphasizes the effect associated with a lack of functional GR on intermediary metabolism at the systems level and provides the physiological underpinnings to a global antagonism of GR. Our results underscore the physiological significance of GR in the regulation of muscle glucose availability during stress, and this has a potent effect on muscle protein homeostasis. These findings have important implications in aquaculture, where enhancing growth rate with better feed utilization is paramount, and manipulation of GR abundance may be one possible way to achieve this. Also, GR manipulation may be an approach to increase target tissue glucose sensitivity (6) in humans suffering from type 2 diabetes (6, 64), and the knockout zebrafish model has the potential to shed light on the physiological consequences of this treatment at the systems level.
GRANTS
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grant to M. M.Vijayan. E. Faught was the recipient of a NSERC doctoral scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.F. and M.M.V. conceived and designed research; E.F. performed experiments; E.F. analyzed data; E.F. and M.M.V. interpreted results of experiments; E.F. prepared figures; E.F. drafted manuscript; E.F. and M.M.V. edited and revised manuscript; E.F. and M.M.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ramen Sood and Blake Carrington at National Institutes of Health for assistance with establishing our CRISPR/Cas9 strains, and Dr. Ian Lewis and Ryan Groves at the University of Calgary metabolomics facility for assistance with metabolome analysis.
REFERENCES
- 1.Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Physiol Regul Integr Comp Physiol 294: R711–R719, 2008. doi: 10.1152/ajpregu.00671.2007. [DOI] [PubMed] [Google Scholar]
- 2.Alsop D, Vijayan MM. Molecular programming of the corticosteroid stress axis during zebrafish development. Comp Biochem Physiol A Mol Integr Physiol 153: 49–54, 2009. doi: 10.1016/j.cbpa.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Aluru N, Vijayan MM. Stress transcriptomics in fish: a role for genomic cortisol signaling. Gen Comp Endocrinol 164: 142–150, 2009. doi: 10.1016/j.ygcen.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Barton BA, Schreck CB. The metabolic cost of acute physical stress in Juvenile Steelhead. Trans Am Fish Soc 116: 257–263, 1987. doi:. [DOI] [Google Scholar]
- 5.Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science 248: 850–854, 1990. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- 6.Bernal-Sore I, Navarro-Marquez M, Osorio-Fuentealba C, Díaz-Castro F, Del Campo A, Donoso-Barraza C, Porras O, Lavandero S, Troncoso R. Mifepristone enhances insulin-stimulated Akt phosphorylation and glucose uptake in skeletal muscle cells. Mol Cell Endocrinol 461: 277–283, 2018. doi: 10.1016/j.mce.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Blasco J, Fernàndez-Borràs J, Marimon I, Requena A. Plasma glucose kinetics and tissue uptake in brown trout in vivo: effect of an intravascular glucose load. J Comp Physiol B 165: 534–541, 1996. doi: 10.1007/BF00387514. [DOI] [Google Scholar]
- 8.Britto FA, Begue G, Rossano B, Docquier A, Vernus B, Sar C, Ferry A, Bonnieu A, Ollendorff V, Favier FB. REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab 307: E983–E993, 2014. doi: 10.1152/ajpendo.00234.2014. [DOI] [PubMed] [Google Scholar]
- 9.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 67: 259–284, 2005. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 10.Chatzopoulou A, Roy U, Meijer AH, Alia A, Spaink HP, Schaaf MJM. Transcriptional and metabolic effects of glucocorticoid receptor α and β signaling in zebrafish. Endocrinology 156: 1757–1769, 2015. doi: 10.1210/en.2014-1941. [DOI] [PubMed] [Google Scholar]
- 11.Eames SC, Philipson LH, Prince VE, Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish 7: 205–213, 2010. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edmonds BK, Edwards GL. Dorsomedial hindbrain participation in glucoprivic feeding response to 2DG but not 2DG-induced hyperglycemia or activation of the HPA axis. Brain Res 801: 21–28, 1998. doi: 10.1016/S0006-8993(98)00528-9. [DOI] [PubMed] [Google Scholar]
- 13.Fabbri E, Capuzzo A, Moon TW. The role of circulating catecholamines in the regulation of fish metabolism: an overview. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 120: 177–192, 1998. doi: 10.1016/S0742-8413(98)10017-8. [DOI] [PubMed] [Google Scholar]
- 14.Facchinello N, Skobo T, Meneghetti G, Colletti E, Dinarello A, Tiso N, Costa R, Gioacchini G, Carnevali O, Argenton F, Colombo L, Dalla Valle L. nr3c1 null mutant zebrafish are viable and reveal DNA-binding-independent activities of the glucocorticoid receptor. Sci Rep 7: 4371, 2017. [Erratum in Sci Rep 8: 4445, 2018.] doi: 10.1038/s41598-017-04535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faught E, Aluru N, Vijayan MM. The molecular stress response. : Biology of Stress in Fish: Fish Physiology, edited by Schreck CB, Tort L, Farrell AP, Brauner C. London: Elsevier, 2016, vol. 35, chapt. 4, p. 113–166. doi: 10.1016/B978-0-12-802728-8.00004-7. [DOI] [Google Scholar]
- 16.Faught E, Best C, Vijayan MM. Maternal stress-associated cortisol stimulation may protect embryos from cortisol excess in zebrafish. R Soc Open Sci 3: 160032, 2016. doi: 10.1098/rsos.160032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faught E, Vijayan MM. The mineralocorticoid receptor is essential for stress axis regulation in zebrafish larvae. Sci Rep 8: 18081, 2018. doi: 10.1038/s41598-018-36681-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Q, Zou X, Lu L, Li Y, Liu Y, Zhou J, Duan C. The stress-response gene redd1 regulates dorsoventral patterning by antagonizing Wnt/β-catenin activity in zebrafish. PLoS One 7: e52674, 2012. doi: 10.1371/journal.pone.0052674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher DJ. The physiological control of appetite in fish. Comp Biochem Physiol A Physiol 78: 617–628, 1984. doi: 10.1016/0300-9629(84)90608-X. [DOI] [Google Scholar]
- 20.Galt NJ, Froehlich JM, Remily EA, Romero SR, Biga PR. The effects of exogenous cortisol on myostatin transcription in rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol A Mol Integr Physiol 175: 57–63, 2014. doi: 10.1016/j.cbpa.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garvey SM, Dugle JE, Kennedy AD, McDunn JE, Kline W, Guo L, Guttridge DC, Pereira SL, Edens NK. Metabolomic profiling reveals severe skeletal muscle group-specific perturbations of metabolism in aged FBN rats. Biogerontology 15: 217–232, 2014. doi: 10.1007/s10522-014-9492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geer EB, Islam J, Buettner C. Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinol Metab Clin North Am 43: 75–102, 2014. doi: 10.1016/j.ecl.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon BS, Steiner JL, Williamson DL, Lang CH, Kimball SR. Emerging role for regulated in development and DNA damage 1 (REDD1) in the regulation of skeletal muscle metabolism. Am J Physiol Endocrinol Metab 311: E157–E174, 2016. doi: 10.1152/ajpendo.00059.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffiths BB, Schoonheim PJ, Ziv L, Voelker L, Baier H, Gahtan E. A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Front Behav Neurosci 6: 68, 2012. doi: 10.3389/fnbeh.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ings JS, Servos MR, Vijayan MM. Exposure to municipal wastewater effluent impacts stress performance in rainbow trout. Aquat Toxicol 103: 85–91, 2011. doi: 10.1016/j.aquatox.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Itoh Y, Abe T, Takaoka R, Tanahashi N. Fluorometric determination of glucose utilization in neurons in vitro and in vivo. J Cereb Blood Flow Metab 24: 993–1003, 2004. doi: 10.1097/01.WCB.0000127661.07591.DE. [DOI] [PubMed] [Google Scholar]
- 28.Jeyapalan AS, Orellana RA, Suryawan A, O’Connor PMJ, Nguyen HV, Escobar J, Frank JW, Davis TA. Glucose stimulates protein synthesis in skeletal muscle of neonatal pigs through an AMPK- and mTOR-independent process. Am J Physiol Endocrinol Metab 293: E595–E603, 2007. doi: 10.1152/ajpendo.00121.2007. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson S, Ahrén B. Contribution of adrenergic nerves and the adrenals to 2-deoxy-D-glucose-induced insulin and glucagon secretion in the mouse. Int J Pancreatol 10: 207–215, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, Raissy HH, Van Natta ML, Tonascia J, Strunk RC; CAMP Research Group . Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med 367: 904–912, 2012. doi: 10.1056/NEJMoa1203229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Löwe T, Hoppe T. Protein quality control gets muscle into shape. Trends Cell Biol 18: 264–272, 2008. doi: 10.1016/j.tcb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform β: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci 66: 3435–3448, 2009. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo T, Harris CA, Wang JC. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol 380: 79–88, 2013. doi: 10.1016/j.mce.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazaro-Côté A, Sadoul B, Jackson LJ, Vijayan MM. Acute stress response of fathead minnows caged downstream of municipal wastewater treatment plants in the Bow River, Calgary. PLoS One 13: e0198177, 2018. doi: 10.1371/journal.pone.0198177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipina C, Hundal HS. Is REDD1 a metabolic éminence grise? Trends Endocrinol Metab 27: 868–880, 2016. doi: 10.1016/j.tem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Maddison LA, Joest KE, Kammeyer RM, Chen W. Skeletal muscle insulin resistance in zebrafish induces alterations in β-cell number and glucose tolerance in an age- and diet-dependent manner. Am J Physiol Endocrinol Metab 308: E662–E669, 2015. doi: 10.1152/ajpendo.00441.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick SD, Shrimpton JM, Carey JB, O’Dea MF, Sloan KE, Moriyama S, Björnsson BT. Repeated acute stress reduces growth rate of Atlantic salmon parr and alters plasma levels of growth hormone, insulin-like growth factor I and cortisol. Aquaculture 168: 221–235, 1998. doi: 10.1016/S0044-8486(98)00351-2. [DOI] [Google Scholar]
- 39.Milligan CL. The role of cortisol in amino acid mobilization and metabolism following exhaustive exercise in rainbow trout (Oncorhynchus mykiss Walbaum). Fish Physiol Biochem 16: 119–128, 1997. doi: 10.1007/BF00004669. [DOI] [Google Scholar]
- 40.Milligan CL. A regulatory role for cortisol in muscle glycogen metabolism in rainbow trout Oncorhynchus mykiss Walbaum. J Exp Biol 206: 3167–3173, 2003. doi: 10.1242/jeb.00538. [DOI] [PubMed] [Google Scholar]
- 41.Mommsen TP. Paradigms of growth in fish. Comp Biochem Physiol B Biochem Mol Biol 129: 207–219, 2001. doi: 10.1016/S1096-4959(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 42.Mommsen TP, Vijayan MM, Moon TW. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9: 211–268, 1999. doi: 10.1023/A:1008924418720. [DOI] [Google Scholar]
- 43.Moon TW. Glucose intolerance in teleost fish: fact or fiction? Comp Biochem Physiol B Biochem Mol Biol 129: 243–249, 2001. doi: 10.1016/S1096-4959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 44.Mueller KM, Kornfeld JW, Friedbichler K, Blaas L, Egger G, Esterbauer H, Hasselblatt P, Schlederer M, Haindl S, Wagner KU, Engblom D, Haemmerle G, Kratky D, Sexl V, Kenner L, Kozlov AV, Terracciano L, Zechner R, Schuetz G, Casanova E, Pospisilik JA, Heim MH, Moriggl R. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology 54: 1398–1409, 2011. doi: 10.1002/hep.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nesan D, Kamkar M, Burrows J, Scott IC, Marsden M, Vijayan MM. Glucocorticoid receptor signaling is essential for mesoderm formation and muscle development in zebrafish. Endocrinology 153: 1288–1300, 2012. doi: 10.1210/en.2011-1559. [DOI] [PubMed] [Google Scholar]
- 46.Patel J, Wang X, Proud CG. Glucose exerts a permissive effect on the regulation of the initiation factor 4E binding protein 4E-BP1. Biochem J 358: 497–503, 2001. doi: 10.1042/bj3580497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Polakof S, Panserat S, Soengas JL, Moon TW. Glucose metabolism in fish: a review. J Comp Physiol B 182: 1015–1045, 2012. doi: 10.1007/s00360-012-0658-7. [DOI] [PubMed] [Google Scholar]
- 49.Rennie MJ, Edwards RHT, Emery PW, Halliday D, Lundholm K, Millward DJ. Depressed protein synthesis is the dominant characteristic of muscle wasting and cachexia. Clin Physiol 3: 387–398, 1983. doi: 10.1111/j.1475-097X.1983.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 50.Sadoul B, Vijayan MM. Stress and growth. : Biology of Stress in Fish: Fish Physiology, edited by Schreck CB, Tort L, Farrell AP, Brauner C. London: Elsevier, 2016, vol. 35, chapt. 5, p. 167–205. doi: 10.1016/B978-0-12-802728-8.00005-9. [DOI] [Google Scholar]
- 51.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806, 2001. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 52.Schreck CB, Tort L. The concept of stress in fish. : Biology of Stress in Fish: Fish Physiology, edited by Schreck CB, Tort L, Farrell AP, Brauner C. London: Elsevier, 2016, vol. 35, chapt. 1, p. 1–34. doi: 10.1016/B978-0-12-802728-8.00001-1. [DOI] [Google Scholar]
- 53.Sen ND, Zhou F, Harris MS, Ingolia NT, Hinnebusch AG. eIF4B stimulates translation of long mRNAs with structured 5′ UTRs and low closed-loop potential but weak dependence on eIF4G. Proc Natl Acad Sci USA 113: 10464–10472, 2016. doi: 10.1073/pnas.1612398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seth A, Stemple DL, Barroso I. The emerging use of zebrafish to model metabolic disease. Dis Model Mech 6: 1080–1088, 2013. doi: 10.1242/dmm.011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu H, Langenbacher AD, Huang J, Wang K, Otto G, Geisler R, Wang Y, Chen JN. The Calcineurin-FoxO-MuRF1 signaling pathway regulates myofibril integrity in cardiomyocytes. eLife 6: e27955, 2017. doi: 10.7554/eLife.27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimizu N, Maruyama T, Yoshikawa N, Matsumiya R, Ma Y, Ito N, Tasaka Y, Kuribara-Souta A, Miyata K, Oike Y, Berger S, Schütz G, Takeda S, Tanaka H. A muscle-liver-fat signalling axis is essential for central control of adaptive adipose remodelling. Nat Commun 6: 6693, 2015. doi: 10.1038/ncomms7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stolte EH, van Kemenade BM, Savelkoul HFJ, Flik G. Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J Endocrinol 190: 17–28, 2006. doi: 10.1677/joe.1.06703. [DOI] [PubMed] [Google Scholar]
- 58.de Theije CC, Schols AMWJ, Lamers WH, Neumann D, Köhler SE, Langen RCJ. Hypoxia impairs adaptation of skeletal muscle protein turnover- and AMPK signaling during fasting-induced muscle atrophy. PLoS One 13: e0203630, 2018. doi: 10.1371/journal.pone.0203630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thulasiraman V, Yang CF, Frydman J. In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J 18: 85–95, 1999. doi: 10.1093/emboj/18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tronche F, Kellendonk C, Reichardt HM, Schütz G. Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev 8: 532–538, 1998. doi: 10.1016/S0959-437X(98)80007-5. [DOI] [PubMed] [Google Scholar]
- 61.Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol 275: 43–61, 2007. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 62.Vijayan MM, Moon TW. The stress response and the plasma disappearance of corticosteroid and glucose in a marine teleost, the sea raven. Can J Zool 72: 379–386, 1994. doi: 10.1139/z94-054. [DOI] [Google Scholar]
- 63.Wang F, Canadeo LA, Huibregtse JM. Ubiquitination of newly synthesized proteins at the ribosome. Biochimie 114: 127–133, 2015. doi: 10.1016/j.biochi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147: 4160–4168, 2006. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 65.Wendelaar Bonga SE. The stress response in fish. Physiol Rev 77: 591–625, 1997. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- 66.Zang L, Shimada Y, Nishimura N. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci Rep 7: 1461, 2017. doi: 10.1038/s41598-017-01432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ziv L, Muto A, Schoonheim PJ, Meijsing SH, Strasser D, Ingraham HA, Schaaf MJM, Yamamoto KR, Baier H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol Psychiatry 18: 681–691, 2013. doi: 10.1038/mp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]