Abstract
Circulating myostatin-attenuating agents are being developed to treat muscle-wasting disease despite their potential to produce serious off-target effects, as myostatin/activin receptors are widely distributed among many nonmuscle tissues. Our studies suggest that the myokine not only inhibits striated muscle growth but also regulates pituitary development and growth hormone (GH) action in the liver. Using a novel myostatin-null label-retaining model (Jekyll mice), we determined that the heterogeneous pool of pituitary stem, transit-amplifying, and progenitor cells in Jekyll mice depletes more rapidly after birth than the pool in wild-type mice. This correlated with increased levels of GH, prolactin, and the cells that secrete these hormones, somatotropes and lactotropes, respectively, in Jekyll pituitaries. Recombinant myostatin also stimulated GH release and gene expression in pituitary cell cultures although inhibiting prolactin release. In primary hepatocytes, recombinant myostatin blocked GH-stimulated expression of two key mediators of growth, insulin-like growth factor (IGF)1 and the acid labile subunit and increased expression of an inhibitor, IGF-binding protein-1. The significance of these findings was demonstrated by smaller muscle fiber size in a model lacking myostatin and liver IGF1 expression (LID-o-Mighty mice) compared with that in myostatin-null (Mighty) mice. These data together suggest that myostatin may regulate pituitary development and function and that its inhibitory actions in muscle may be partly mediated by attenuating GH action in the liver. They also suggest that circulating pharmacological inhibitors of myostatin could produce unintended consequences in these and possibly other tissues.
Keywords: growth hormone, insulin-like growth factor 1, muscle, myostatin, prolactin
INTRODUCTION
Skeletal muscle wasting, in its many forms, contributes to the pathogenesis of several disease states, including cancer (17). Indeed, almost 80% of patients with advanced stages of cancer develop cachexia, the progressive loss of striated muscle and fat, which cannot be remedied with nutritional support (21, 66). This ultimately impairs mobility, directly contributes to mortality, and compromises patient outcomes and treatment efficacy (62). Muscle wasting also occurs with heart failure, end-stage renal disease, chronic obstructive pulmonary disease, peripheral nerve damage, and, of course, with the muscular dystrophies. Catabolic cytokines (e.g., IL-6, transforming growth factor β, TNFα, activin, myostatin, etc.) are primarily responsible for muscle wasting and often converge to influence similar signaling events or intracellular processes (e.g., protein degradation) regardless of disease indication (6, 13, 17). Such conservation suggests that attenuating either the cytokines directly or their receptor signaling pathways could be broadly effective in the clinic.
Because myostatin directly inhibits muscle protein synthesis, stimulates muscle protein degradation, and antagonizes the actions of insulin-like growth factor (IGF)1, which itself is a potent supporter of muscle growth (25, 59, 60), several myostatin-attenuating drugs have recently been developed (17). Most of these drugs are monoclonal antibodies or ligand traps that sequester myostatin in the circulation, prevent its receptor binding and in turn block its promuscle atrophy actions. Clinical testing of these drugs, however, has produced mixed results and off-target effects that include compromised blood vessel integrity and pituitary function. This is due in part to the fact that myostatin receptors (ActRIIb or ACVR2B) are expressed in a variety of nonmuscle tissues (e.g., endothelial cells, pituitary, gonads, liver, etc.) and also because multiple ligands in addition to myostatin bind these receptors. These include the activins (A, B, and AB), growth/differentiating factor 11 (GDF11), and bone morphogenic protein 9 (BMP9) as well as other transforming growth factor-β superfamily members (5, 58). Thus, drugs targeting any or all ActRIIb ligands in circulation are inherently capable of producing significant off-target effects.
Several clinical trials of circulating myostatin-attenuating drugs have nevertheless attempted to enhance muscle mass and function (24, 52, 73). Tested products include ACE-031, a soluble ActRIIb receptor that binds myostatin, GDF11, the activins, and BMP9, all of which are known regulators of muscle development, skeletal patterning, reproduction, and blood vessel integrity. This particular trial was terminated prematurely because of serious nosebleeds and telangiectasia (10) whereas preclinical tests of another ActRIIb-Fc, using cynomolgus macaques, also caused nosebleeds as well as pericardial and/or pleural effusions (10, 50). The failure rate of other circulating myostatin-attenuating therapeutics is actually very well known because of high-profile trials by large pharmaceutical companies (e.g., Wyeth, Amgen, Regeneron, Atara, Eli Lilly, and Pfizer) that either did not meet critical end points or produced significant adverse events (24). Novartis’s ActRIIb monoclonal antibody Bimagrumab (a.k.a. BYM338), for example, recently failed to meet phase III clinical end points (51) and was subsequently demonstrated to interfere with pituitary function (27). Such failures question the efficacy and safety of broadly targeting myostatin or ActRIIb especially as both directly regulate expression of endothelial cell adhesion molecules (30, 77), which could explain how the targeting of ActRIIb ligands in circulation can compromise blood vessel integrity.
Myostatin’s direct and potent actions on striated muscle are very well characterized (55). It is evident nonetheless that myostatin also acts on nonmuscle tissues in addition to the pituitary gland and blood vessels as our previous studies suggest that endocrine myostatin may regulate various aspects of the growth hormone (GH)/IGF1 axis in the liver (72). The current study provides further evidence that myostatin indeed influences the stem, transit-amplifying, and progenitor (STP) cells that contribute to normal and pathological changes in the pituitary and to the relative composition of the gland (69). Data from myostatin-null (Mstn−/−) mice suggest that myostatin regulates somatotrope and lactotrope development whereas in vitro studies suggest that recombinant myostatin can influence GH and prolactin (PRL) secretion. Moreover, myostatin antagonized GH-induced hepatocyte expression of IGF1 and acid labile subunit (ALS), an IGF-binding partner that limits IGF1 clearance and maintains the IGF1 circulating half-life. Myostatin also stimulated hepatocyte IGF-binding protein (IGFBP)1 expression, which has the potential to limit IGF1 bioavailability. These results clearly illustrate developmental and physiological risks from the pharmacological targeting of circulating myostatin, at least as they relate to pituitary and liver function. In addition, they suggest that myostatin influences different aspects of the somatomedin model of growth regulation and could itself be an integral member.
MATERIALS AND METHODS
Hormones and antisera.
Recombinant human GH was obtained from R&D Systems (Minneapolis, MN) and purified human GH, guinea pig antiserum to rat GH, and rabbit antiserum to recombinant rat PRL was obtained from the National Hormone and Peptide Program, Los Angeles Biomedical Research Institute (Torrance, CA). Recombinant human myostatin was expressed and purified from Chinese hamster ovary cells as we previously described (11), and Nicotinamide and Trichostatin A were purchased from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 647-conjugated donkey anti-guinea pig IgG (DAGP-Alexa 647) was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Hoechst 33342 and Alexa Fluor 555-conjugated donkey anti-rabbit IgG (DAR-Alexa 555) were purchased from Life Technologies (Grand Island, NY). Molecular Probe and PermaFluor Aqueous Mounting Medium was purchased from Life Technologies.
Animals.
Mice were housed and bred in a temperature- and humidity-controlled room under a 12-h light/dark cycle. They were fed ad libitum and used in strict accordance with protocols preapproved by the Institutional Animal Care and Use Committee of Washington State University.
As previously reported (35, 43), we quantified quiescent STP cells [i.e., label-retaining cells (LRCs)] by generating doxycycline-inducible label-retaining mice in wild-type and Mstn−/− backgrounds. The wild-type strain was generated by crossing Tg(tetO-HIST1H2BJ/GFP)47Efu/J (i.e., H2B-GFP+/+) and B6.Cg-Gt(ROSA)26Sortm1(rtTA*M2)Jae/J (i.e., M2+/+) mice. The resulting offspring express a histone 2B/green fluorescent protein (GFP) chimera via a reverse tetracycline-controlled M2 transactivator protein (34). Treating pregnant mice with doxycycline labels all embryonic cells with H2B-GFP whereas a chase period without doxycycline dilutes the label, either as cells divide or as H2B-GFP is replaced by endogenous H2B in differentiated cells. The H2B-GFP label is ultimately retained in only quiescent STP cells (67) that are, by definition, heterogenous and may express a variety of cell and tissue-specific markers. An advantage of this and other label-retaining models is that quantifying the STP pool is a measure of a particular tissues total regenerative potential.
The label-retaining Mstn−/− strain was generated by first establishing the H2B-GFP+/+ and M2+/+ strains in Mstn−/− backgrounds. These strains were then crossed to create Mstn−/− label-retaining mice (a.k.a. Jekyll mice) (35, 43). Doxycycline (Research Products International Corporation, Mt. Prospect, IL) was administered to pregnant mice in their drinking water (400 µg/ml in 5% sucrose) from embryonic day 0.5 (E0.5) to E12.5 as this coincides with pituitary development. Offspring were euthanized at birth (0 days old) or when 14, 30, or 60 days old. Whole pituitaries were collected from these mice and from noninduced control (C57Bl/6) and Mstn−/− mice generated in our breeding colony.
The contribution of liver-derived IGF1 to the muscle hypertrophy that develops with myostatin attenuation was estimated by comparing muscle mass and fiber cross-sectional areas in a Mstn−/− mouse model (Mighty) to those in liver Igf1-deficient (LID) mice (74) that are also Mstn−/− [LID-o-Mighty (LOM)]. The LID mouse possesses LoxP sites flanking the fourth exon of the Igf1 gene and utilizes a liver-specific albumin enhancer to drive Cre recombinase expression. Because the liver is the primary source of circulating IGF1, Cre expression in LID mice significantly reduces circulating levels (74). Mighty and LOM mice were generated from B6.Cg-Speer6-ps1Tg(Alb-cre)21Mgn/J [JR no. 003574, Tg(Alb-cre)], B6.129(FVB)-Igf1tm1Dlr/J (JR no. 016831, fl-Igf1) and C57BL/6J-Mstnlean/J (JR no. 009345, Mstn−/−) parental strains (Jackson Laboratory) in five breeding steps. In step 1, Tg(Alb-cre)/Tg(Alb-cre) mice were crossed with fl-Igf1/fl-Igf1 mice to produce Tg(Alb-cre); fl-Igf1/Igf1 mice. In step 2, the latter were backcrossed to Tg(Alb-cre)/Tg(Alb-cre) mice producing Tg(Alb-cre)/Tg(Alb-cre); fl-Igf1/Igf1 mice, which were subsequently crossed in step 3 with Mstn−/− mice to produce Tg(Alb-cre); fl-Igf1/Igf1; Mstn+/− mice. In step 4, the latter were backcrossed to Mstn−/− mice to produce Tg(Alb-cre); fl-Igf1/Igf1; Mstn−/− and fl-Igf1/Igf1; Mstn−/− mice. These mice were then crossed in step 5 to produce Tg(Alb-cre); fl-Igf1/fl-Igf1; Mstn−/− (LOM) mice that lack myostatin and liver-derived IGF1 and fl-Igf1/fl-Igf1; Mstn−/− (Mighty) mice that lack myostatin and possess a Flox’d Igf1 allele.
Tail-snip biopsies were used to genotype LOM and Mighty mice by PCR amplification of Alb-cre using genomic DNA template. Biopsies were incubated in 90 µl of 50 mM NaOH at 95°C for 45 min before neutralizing with 10 µl of 1 M Tris-HCl. Samples were centrifuged, and 5 μl of supernatant were PCR-amplified using EconoTaq DNA Polymerase (VWR) and two different forward primers for wild-type (5′-TGC AAA CAT CAC ATG CAC AC-3′) or mutant (5′-GAA GCA GAA GCT TAG GAA GAT GG-3′) with a common reverse primer (5′-TTG GCC CCT TAC CAT AAC TG-3′). The final working concentration for each primer was 400 nM in a reaction volume of 25 µl/tube. Amplification conditions were as follows: (step 1) 94°C for 2 min followed by 10 cycles of 94°C for 20 s, 65°C for 15 s, and 68°C for 10 s; (step 2) 28 cycles of 94°C for 15 s, 60°C for 15 s, and 72°C for 10 s; (step 3) 72°C for 2 min. Amplicons were visualized on a 2% agarose gel, and Mighty mice were identified by the presence of a 351-kb band (wild-type) whereas LOM mice were identified by 150-kb (artifact), 351-kb, and 390-kb (mutant) bands.
The presence or absence of fl-Igf1 was similarly assessed by PCR using two forward primers (wild-type, 5′-GGC AAA TGG AAA TCC TAT GTC T-3′; mutant, 5′- AAA CCA CAC TGC TCG ACA TTG-3′) with a common reverse primer (5′-CAC TAA GGA GTC TGT ATT TGG ACC-3′). After denaturing at 94°C for 3 min, DNA was amplified for 35 cycles of 30 s at 94°C, 1 min at 60°C, and 1 min at 72°C followed by a final extension for 2 min at 72°C. Wild-type and mutant (Igf1 and Fl-Igf1) alleles were identified by 398- and 275-kb bands, respectively. The Mstn−/− allele was identified by pyrosequencing with 5′-ATG TTA TTT TCA GTT ATC AC-3′ after PCR using 5′-Biotin-GCC ATG ATC TTG CTG TAA CCT-3′ forward and 5′-CGG TTG CTA GAA TGT TAT TTT CAG-3′ reverse primers. After denaturing at 94°C for 5 min, DNA was amplified for 50 cycles of 20 s at 94°C, 10 s at 60°C, and 30 s at 65°C followed by a final extension for 5 min at 65°C.
Muscle histology.
Tibialis anterior (TA), gastrocnemius, and bicep brachii skeletal muscles were removed, weighed and flash frozen in isopentane cooled to −140°C. Frozen TA muscles were then sectioned (eight/muscle) at a thickness of 10 μm on a cryostat and stored at −80°C. Sections were fixed for 45 min in 4% paraformaldehyde/phosphate-buffered saline (PBS), rinsed in ddH2O, and then stained with hematoxylin and eosin. Cross-sectional area of individual fibers was then quantified on at least four images/muscle using ImageJ. These values were used to calculate means representing each individual mouse, which in turn were used to calculate group means. Hearts were removed, cleared of noncardiac tissue, cut open, and rinsed in PBS to remove blood clots. They were then blotted dry before weighing. Tibia were also removed, and their lengths were used to normalize heart mass.
Pituitary preparation for imaging.
Pituitaries from H2B-GFP+/+/M2+/+/Mstn +/+ (wild-type) and H2B-GFP+/+/M2+/+/Mstn−/− (Jekyll) mice were fixed with 4% paraformaldehyde in PBS for 2 h and stored in 70% ethanol at 4°C. Tissues were rinsed with PBS and incubated in a blocking solution (1× PBS with 1% BSA and 0.1% Triton X-100) at 4°C for 2.5 h (0- and 14-day-old pituitaries) or overnight (30- and 60 -day-old pituitaries) and then incubated at 4°C overnight with both primary antibodies, guinea pig antiserum to rat GH, and rabbit antiserum to recombinant rat PRL, at a titer of 1:500 in the blocking solution. They were then incubated with secondary antibodies, DAGP-Alexa 647 (1:500) and DAR-Alexa 555 (1:2,000), and nuclei were subsequently stained with Hoechst 33342 (10 μg/ml PBS), both at 4°C overnight. Propidium iodide (1 μg/ml) was initially used to stain DNA when optimizing LRC labeling (Fig. 1) but was replaced with Hoechst as the former interfered with the Alexa-555 emission signal. Just before imaging, each pituitary was rinsed with PBS and mounted with an antifade, antireflective mounting medium (PermaFluor Aqueous Mounting Medium) on a glass slide.
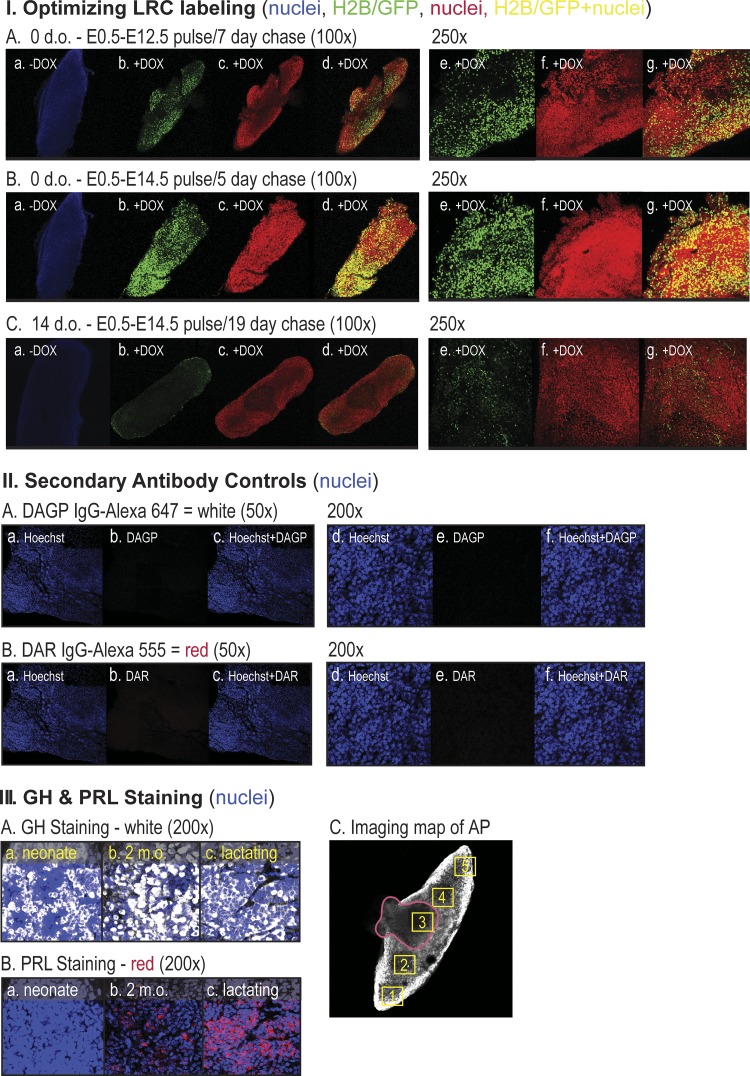
Fig. 1.
Validating LRC induction and immunostaining protocols. I: whole-mount fluorescent confocal microscopic images of anterior pituitaries collected from H2B-GFP+/+/M2+/+/Mstn+/+ (WT) neonates (A and B) or 14 d.o. mice (C). LRC induction was accomplished by adding 0 (a) or 400 µg/ml doxycycline (DOX, b–g) to the drinking water (pulse) of their pregnant mothers starting on embryonic (E) day 0.5 and ending on day 12.5 (A) or 14.5 (B and C) as this period corresponds to pituitary morphogenesis. The gestation period of all mice was 19 days; thus, the chase period without DOX induction varied from 5 to 19 days as indicated. Nuclei were labeled with Hoechst 33342 (blue, a) or propidium iodine (PI, red, c, d, f, g) and specificity of fluorescent signals was confirmed at ×100 and ×250 magnification individually (a–c, e, f) and in merged GFP/PI images (d,g). II: whole-mount immunostaining of pituitaries isolated from noninduced 2-month-old H2B-GFP+/+/M2+/+/Mstn+/+ (WT) female mice using Alexa 647-conjugated donkey anti-guinea pig (DAGP; A) or Alexa 555-conjugated donkey anti-rabbit (DAR; B) antisera. A pituitary was counterstained with Hoechst 33342 (blue) and imaged separately for Hoechst (a, d), Alexa 647 or Alexa 555 (b, e) and merged (c, f). III: anterior pituitary (AP) immunostained with anti-GH and DAGP-Alexa 647 (white) (A) or anti-PRL and DAR-Alexa 555 (red) (B) and counterstained with Hoechst. Pituitaries were removed from neonate (a), 2-month-old (b) and 2-month-old lactating mice (c). Posterior view of an AP with the posterior pituitary remnant outlined (red) (C). Numbered yellow boxes correspond to positions where LRC, somatotrope, and lactotrope numbers and GH and PRL immunofluorescence were quantified in the pars distalis. d.o., days old; GH, growth hormone; LRC, label-retaining cell; m.o., months old; Mstn, myostatin; PRL, prolactin; WT, wild-type.
Image analysis.
Pituitaries were imaged at ×50 and ×200 using a Leica DM 6000CS TCS SP5II Broadband Confocal Microscope. Stack sections (10, 11, 12, 13, 14, 15) were sequentially imaged at several different locations (Fig. 1; scan 1 = GFP, scan 2 = Alexa 555, scan 3 = Alexa 647, scan 4 = DAPI/Hoechst) using a multiphoton laser at 780 nm. The setting of image parameters and three beam paths for GFP, Alexa 555, and Alexa 647 remained consistent throughout imaging, and fluorescent intensity levels equivalent to the pituitary content of both GH and PRL were acquired using the Leica Application Suite Advanced Fluorescent Lite software 2.6.0 (build 7266). The proportion of hormone-producing cells to total cells (cell counts) and relative hormone content (optical density) were quantified using ImageJ 1.46 software. Both maximum and minimum threshold levels (for GFP, Alexa 555, and Alexa 647) as well as particle sizes (for all four signals) were set consistently throughout the analysis.
GH3 cell culture.
Rat pituitary GH3 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA), maintained at 37°C under a humidified atmosphere of 5% CO2, and grown as a serial monolayer in F-12K Medium (ATCC) with 2.5% FBS (Atlas Biologicals, Fort Collins, CO), 15% horse serum (Sigma-Aldrich), and 1% antibiotic/antimycotic solution (Sigma-Aldrich). From the same parent flask, 20,000 cells were seeded in 24-well tissue culture plates (Corning, Tewksbury, MA) with 0.5 ml growth media and allowed to settle for 48 h. Medium was then exchanged for growth media alone (control) or growth media with 2 or 20 nM myostatin. Samples were collected every 24 h for 5 days, and these media were replaced at each sampling. Day 1 samples were collected after 1 h of myostatin treatment. Conditioned media from each well was centrifuged at 800 × g for 5 min to remove floating cells and stored at −80°C. Total RNA from corresponding cells were extracted using TRIzol Reagent (Life Technologies) according to the manufacturer’s protocol and stored at −80°C. Growth assays were also performed using the CellTiter 96 Aqueous One Cell Proliferation Assay (Promega Corporation, Madison, WI) on cells originally seeded at 2 × 104 cells/well in 96 well flat-bottom plates containing growth media and 0, 2, and 20 nM myostatin. Cells were terminated after 6, 24, 30, and 48 h.
Quantifying GH and PRL levels.
PRL concentrations in GH3 conditioned media were initially quantified with rat ELISAs (Molecular Innovations, Novi, MI), but these assays produced highly variable and unreliable results. Although we detected no significant cross-reactivity with GH when using the mouse PRL ELISA from Abcam (data not shown), the sensitivity was far too low to quantify potential differences reliably again. We therefore used a double-antibody RIA Kit from the National Hormone and Peptide Program and custom radiolabeled PRL using chloramine-T. GH levels were quantified using a rodent GH ELISA (Life Technologies) according to the manufacturer’s protocol.
HepG2 cell culture.
HepG2 cells were obtained from ATCC and cultured in DMEM without phenol red, supplemented with 4 mM l-glutamine and 10% FBS (Atlas Biologicals). Cells were seeded at 2x105 cells/well in 12-well plates and preincubated for 24 h. Complete growth media were changed to serum- and phenol red-free DMEM, and cells were again preincubated for 24 h before GH treatment. Cells were then washed twice with 1× PBS before adding fresh medium and treating independently with two different sources of human GH at different doses and for varying periods of time with or without Nicotinamide and Trichostatin A.
Primary human hepatocyte culture.
Normal human hepatocytes were obtained through the Liver Tissue Cell Distribution System (Pittsburg, PA), funded by NIH contract no. N01-DK-7–0004/HHSN276201200017C (29). Cells were verified to be at least 96% pure and 90% viable, respectfully, before shipping and were plated on 6-well plates at a density of 1.5 × 106 cells/well in Eagle MEM (Lonza, Houston, TX) supplemented with the following additions from the SingleQuot kit (Lonza): 100 nM insulin, 100 nM dexamethasone, and 0.5 ml of Gentamicin Sulfate/Amphotericin B. Plates were shipped overnight with heated pads and upon receipt, the medium was replaced with Williams’ E media (Sigma-Aldrich) lacking serum, phenol red and dexamethasone but with insulin-transferrin-sodium selenite supplement (Sigma-Aldrich), 4 mM l-glutamine, and HEPES (Gibco, Life Technologies). Cells were allowed to recover from shipping for 20 h at 37°C under a humidified atmosphere of 5% CO2 and before treating with 2.27 nM of GH (R&D Systems) and varying doses (0.2, 2, and 20 nM) of myostatin for 48 h.
Quantitative RT-PCR.
Total RNA was isolated using Trizol (Life Technologies) and treated with DNAase using the Turbo DNA-free kit (Life Technologies). RNA integrity and purity were assessed routinely by agarose gel electrophoresis and spectrophotometry before reverse transcribing 1 µg using the iScript cDNA synthesis kit (Bio-Rad). All assays conformed to the previously published MiQE quantitative PCR guidelines (8) and used a Bio-Rad CFX96 Real-Time C1000 Touch thermal cycler and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). Samples were amplified for 40 cycles with the following cycling protocol: initialization at 95°C for 2 min, denaturation at 95°C for 5 s, and annealing/extension at 55°C for 30 s. Predesigned and experimentally validated gene-specific primer sets were obtained from Integrated DNA Technologies (Coralville, IA) and Bio-Rad (Table 1). Amplification efficiency was assessed by analyzing the slope of a standard curve generated with 10-fold cDNA dilutions. Amplification products were also subjected to melt-curve analysis to verify amplicon specificity. Several potential reference genes were tested (ACTB, GAPDH, EEF1a1, TBP, HMBS, HPRT1), and HPRT1 proved most suitable for experiments with human hepatocytes and GAPDH for those with GH3 cells. The fold change in the RNA expression level was then determined using the comparative cycle threshold method (∆ΔCT).
Table 1.
Primers used in qRT-PCR assays
| Primer Gene Symbol | Source | Assay ID | Splice Variants Targeted | Size, bp |
|---|---|---|---|---|
| ACTB | Bio-Rad | qHsaCED0036269 | All | 62 |
| Gapdh | IDT | Rn.PT.39a.11180736.g | All | 85 |
| Gh1 | IDT | Rn.PT.58.44236580.gs | All | 121 |
| HPRT1 | IDT | Hs.PT.58.20881146 | All | 111 |
| IGF-1 | Bio-Rad | qHsaCED0038638 | All | 118 |
| IGFALS | IDT | Hs.PT.58.267 | Exon 1–3b | 103 |
| IGFBP1 | IDT | Hs.PT.58.3620731 | All | 103 |
| Prl | IDT | Rn.PT.58.9831337 | All | 132 |
ACTB, actin beta; bp, base pair; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Gh1, growth hormone; HPRT1, hypoxanthine phosphoribosyltransferase 1; IDT, Integrative DNA Technologies; IGF-1, insulin-like growth factor-1; IGFALS, IGF acid labile subunit; IGFBP1, IGF-binding protein-1; Prl, prolactin. qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction.
Statistics.
Differences between means were determined by an ANOVA (one- or two-way) and Tukey’s post hoc test, as appropriate, using GraphPad Prism 6 Software. In each comparison, P ≤ 0.05 was used to determine significance unless otherwise noted.
RESULTS
Myostatin regulates pituitary plasticity.
Surveys of the Allen Brain Atlas (55) and pituitary Gene Expression Omnibus (GEO) databases (GDS2917 and GDS2913) indicate that myostatin is expressed in the hypothalamus but not in the pituitary, which we confirmed by RT-PCR (data not shown). The pituitary is well known, however, to express ActRIIb receptors, suggesting that circulating myostatin could potentially regulate pituitary activity and/or development. We therefore measured the collective number of pituitary STP cells using label-retaining mice with wild-type or Mstn−/− (a.k.a. Jekyll mice) backgrounds (35). Quiescent cells were labeled with a doxycycline-inducible histone 2B/GFP fusion protein followed by a chase period without doxycycline (67). Label is diluted in mitotic cells and replaced by endogenous histone 2B in differentiated cells but is retained in quiescent cells. Quantifying green LRCs is therefore an accurate estimate of the entire heterogeneous STP pool regardless of antigen/marker expression or stage of cellular determination.
Preliminary studies validated the appropriate time frame for the pulse-chase labeling of pituitaries in utero, and this corresponded perfectly with pituitary embryological development, which lasts until E12.5 in mice (Fig. 1IA) (78). Indeed, a longer induction time to E14.5 followed by a 5-day chase resulted in the labeling of most if not all cells (Fig. 1IB), although ~10% of cells remained labeled after 2 wk (Fig. 1IC). This is consistent with the rapid period of STP cell differentiation that occurs after birth (69). Antibody staining specificity was demonstrated by incubating pituitaries with secondary antibodies alone (Fig. 1II), which produced no staining, and by the inclusion of antibodies for GH and PRL, which readily stained somatotropes or lactotropes, respectively, in neonates and in 2-mo-old adult mice (Fig. 1, IIIA and IIIB).
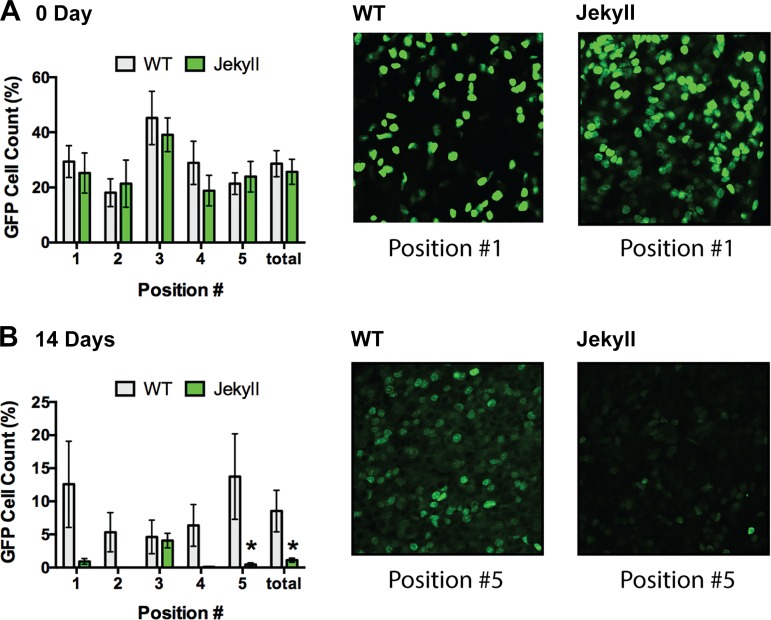
Five separate locations within the pars distalis of each anterior pituitary gland were imaged, including four peripheral regions and the marginal zone (Fig. 1IIIC). This accounted for regional differences as the latter is particularly rich in STP cells (12). LRCs were then quantified in pituitaries from labeled wild-type and Jekyll mice. On day 0, cell counts (i.e., proportion of LRCs) tended to be higher in the marginal zone (region 3), consistent with the known STP distribution (12, 69), and were equal in wild-type and Jekyll neonates (Fig. 2). They also represented ~25% of total nuclei, which is consistent with estimates of the entire heterogeneous STP pool (Sox2+, nestin+, Prop1+, Pit1+, Prx1+/Prx2+, etc.) (69). However, LRCs were almost undetectable by day 14 in most Jekyll regions but represented 5%–15% of total wild-type cells depending on region. The more rapid decline in Jekyll LRCs after birth suggests that myostatin regulates pituitary development, specifically that STP cells more readily differentiate in Mstn−/− pituitaries. We then determined whether this resulted in differential immunostaining of somatotropes and lactotropes, GH- and PRL-secreting cells, respectively, which are coderived from Pit1+ progenitors (69).
Fig. 2.
LRC quantification. Whole-mount pituitaries from 0-, 14-, 30-, and 60-day-old WT and Jekyll mice, of both sexes, were imaged at ×200. The number of LRCs was quantified, and data are expressed as mean % of total cell counts. There were no differences between sexes so data were combined, and no LRCs were detected after 14 days. Significant differences between WT (H2B-GFP+/+/M2+/+/Mstn+/+) and Jekyll (H2B-GFP+/+/M2+/+/Mstn−/−) mice (n = 8, P ≤ 0.05 determined by two-way ANOVA) in each location imaged (numbers on x-axes correspond to pituitary regions imaged, see Fig. 1) and for the entire pituitary (“total”) are indicated by asterisks in the histograms. Representative images of the indicated positions are included on the right. LRC, label-retaining cell; WT, wild-type.
Myostatin regulates somatotrope and lactotrope development.
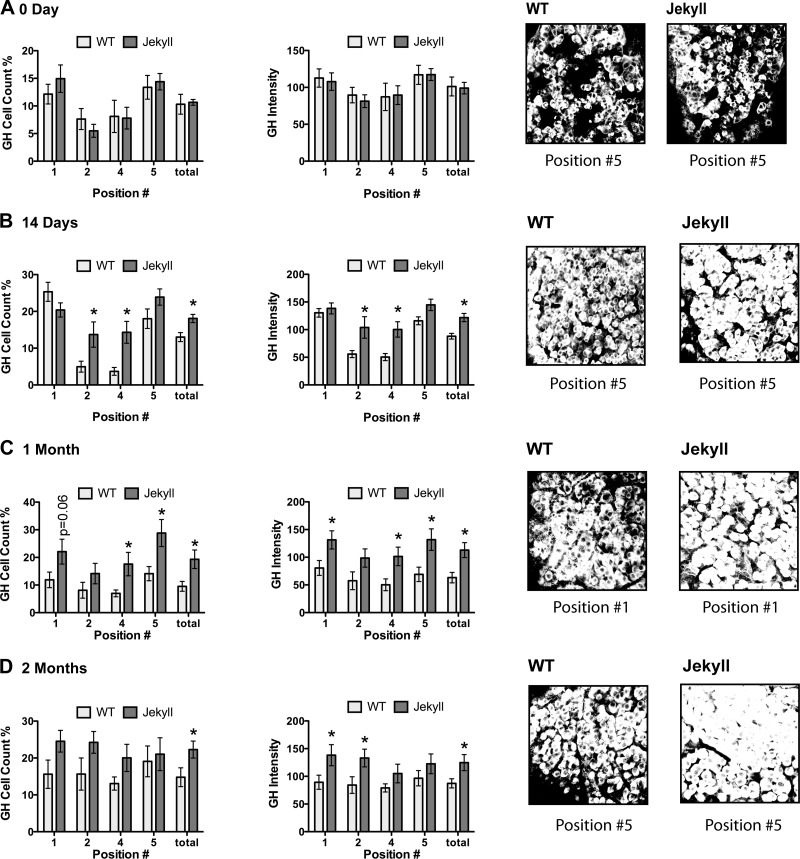
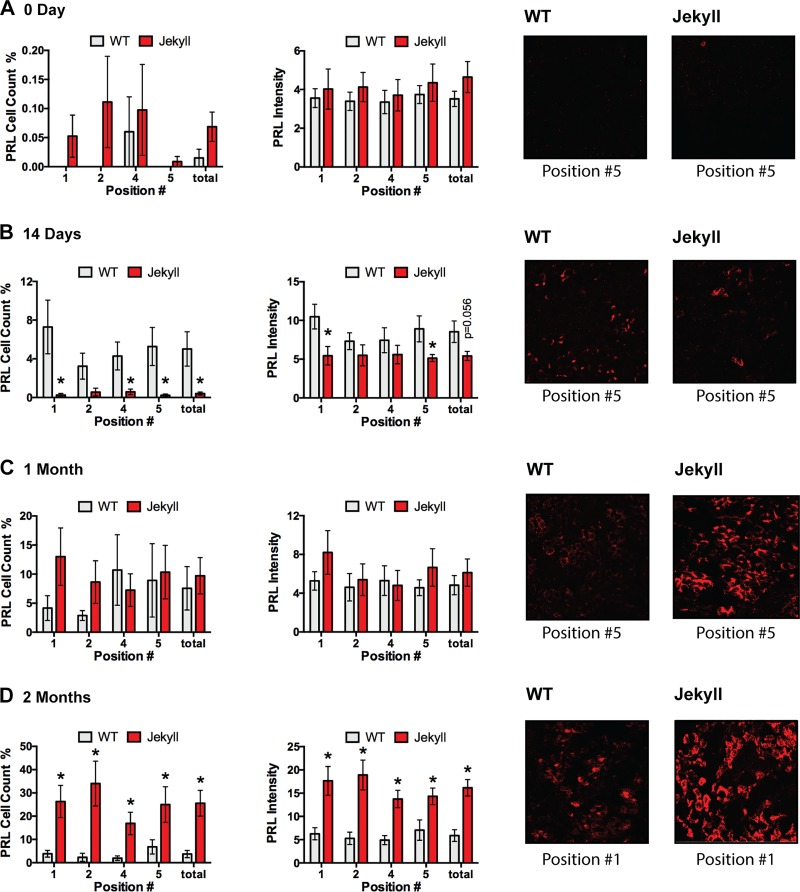
Somatotrope density was greatest in the periphery of neonate pituitaries, again consistent with the known distribution (41), and by day 14, total cell number and GH content were greater in Jekyll mice (Fig. 3). This was most evident in central regions where somatotrope density is normally low. The pattern remained in 1- and 2-mo-old mice despite greater variability and was apparent even in peripheral regions. Although lactotropes share a similar distribution pattern as somatotropes, they were at the limit of detection in all neonates. By day 14, however, they were more readily detected in wild-type mice, albeit with still low levels of staining (Fig. 4). Nevertheless, this pattern eventually reversed as total cell number and PRL content were similar in both groups by 1 mo and three- to fivefold higher in Jekyll mice by 2 mo. These data clearly indicate, for the first time to our knowledge, that the number of GH- and PRL-secreting cells, as well as the total pituitary content of these hormones, is elevated in Mstn−/− mice. This supports the novel concept that myostatin regulates pituitary development.
Fig. 3.
Quantifying somatotrope number and GH content. Somatotrope number and fluorescent intensity (i.e., GH content as optical density units) were quantified in pituitaries from 0-, 14-, 30-, and 60-day-old WT and Jekyll mice (histograms). Significant differences between means of each region are indicated by asterisks (n = 8, P ≤ 0.05 determined by two-way ANOVA) and P values for other noted comparisons are shown. Numbers on x-axes correspond to pituitary regions imaged (Fig. 1) and “total” represents the entire pituitary. Also included are representative images (×200) of the indicated positions. GH, growth hormone; WT, wild-type.
Fig. 4.
Quantifying lactotrope number and PRL content. Lactotrope number and fluorescent intensity (i.e., PRL content as optical density units) were quantified in pituitaries from 0, 14, 30, and 60-day-old WT and Jekyll mice (histograms). Significant differences between means of each region are indicated by asterisks (n = 8, P ≤ 0.05 determined by two-way ANOVA) and P values for other noted comparisons are shown. Numbers on x-axes correspond to pituitary regions imaged (Fig. 1) and “total” represents the entire pituitary. Also included are representative images (×200) of the indicated positions. PRL, prolactin; WT, wild-type.
These general patterns remained the same when data from male and female mice were analyzed separately (Supplemental Figure S1, available online at https://doi.org/10.6084/m9.figshare.7926671.v1), although relative differences in somatotropes and lactotropes were more pronounced in male mice. In fact, all cell counts were 5%–15% at any given time except in male Jekyll mice where somatotrope and lactotrope counts were ~25% and 35%, respectively. These results suggest that sex steroids may influence tissue sensitivity to myostatin or to its absence and are consistent with the previously reported sexual dimorphism in the mass and function of Mstn−/− hearts (36, 56). We next sought to determine whether myostatin directly influences GH or PRL release using GH3 cells cultured with 2 and 20 nM myostatin (Fig. 5).
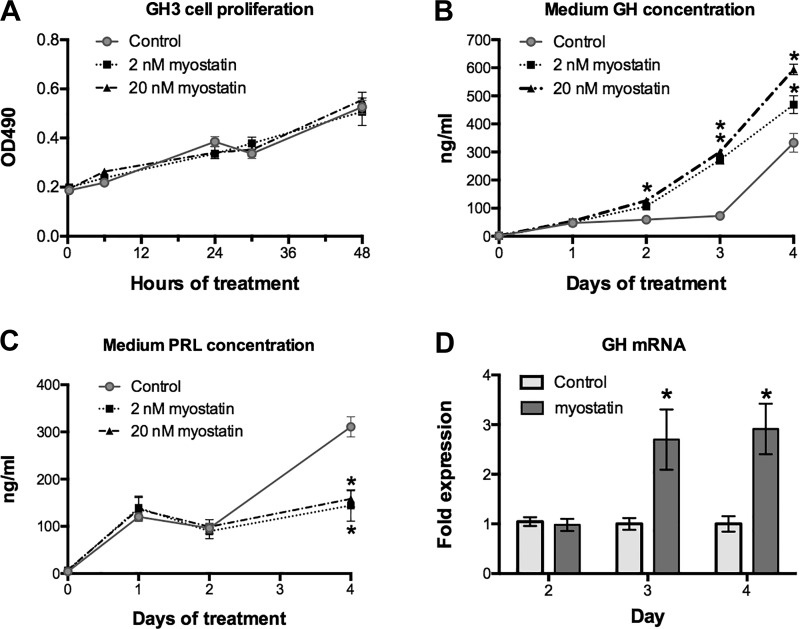
Fig. 5.
Myostatin regulates GH and PRL release from GH3 cells. GH3 cells were cultured with 2 and 20 nM myostatin for 48 h, and cell number was assessed at the indicated times (A). Concentrations of GH and PRL were also measured in conditioned medium of GH3 cells cultured for 4 days with myostatin, changing medium with fresh myostatin on day 2 (n = 12/day/dose, P < 0.05, two-way ANOVA; OD490, optical density units at 490 nm) (B and C). GH mRNA was quantified at the indicated days of treatment by qRT-PCR (D). *Significant differences from controls (P ≤ 0.05; n = 12/group). GH, growth hormone; PRL, prolactin; WT, wild-type.
Myostatin regulates GH and PRL release in vitro.
Neither of these doses influenced cell number over the 4-day culture period yet both stimulated GH secretion and in an apparent dose-dependent manner (Fig. 5, A and B). This resulted in fourfold greater secretion rate among treated cells during the first 3 days that was only because of increased release of stored hormone but also to enhanced GH gene expression (Fig. 5D). Myostatin had the opposite effect on PRL release as both doses prevented the rise in secretion that occurred by day 4 in control cells (Fig. 5C), although it had no effect on PRL mRNA. Activin A shares homology with myostatin and binds the same ActRIIb receptors. It also inhibits PRL secretion from primary rat lactotropes whereas in GH3 cells, it stimulates GH and inhibits PRL secretion at concentrations similar to those used in the current study (49, 64).
We confirmed that myostatin is not expressed in wild-type mouse pituitaries by RT-PCR and by searching GEO databases, which indicated either no expression or extremely low constitutive expression. By contrast, myostatin is expressed in the hypothalamus as well as in striated muscle (55) and must therefore influence pituitary development via the portal vasculature of the median eminence. Either way, this is the first evidence of endocrine or neuroendocrine action for this presumed myokine as it additionally appears to influence the development and activity of somatotropes and lactotropes.
Myostatin inhibits GH action in hepatocytes.
It is well established that myostatin itself is not expressed in the liver (48, 57), which we confirmed with surveys of liver and hepatocyte GEO databases (GDS4506, GDS4427, GDS4387, GDS4390, GDS3068). Thus, we began to determine if myostatin could directly attenuate GH action (3, 42) in HepG2 cells but surprisingly could not demonstrate GH-induced IGF1 expression even when using two independent hormone sources (recombinant and purified), various doses and exposure times, or even by cotreating with the histone deacetylase inhibitors Trichostatin A or Nicotinamide (Supplemental Figure S2, available online at https://doi.org/10.6084/m9.figshare.7926677.v1). Subsequent surveys of GEO databases revealed that HepG2 cells no longer express high levels of GH receptors, which has also been confirmed by others (63, 76). We therefore used primary human hepatocytes obtained from the Liver Tissue Cell Distribution System.
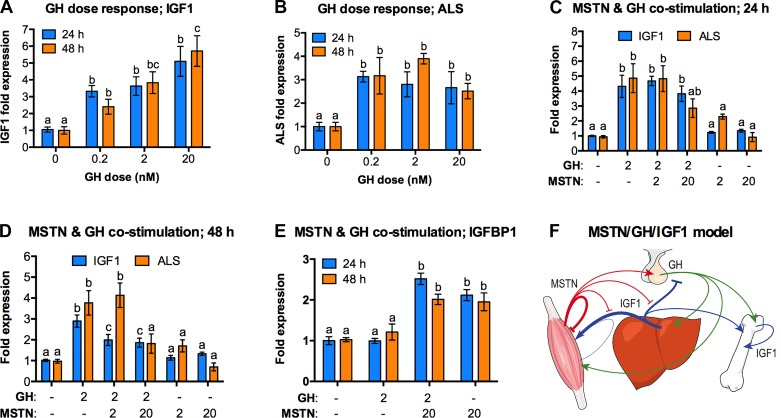
We first verified GH sensitivity by quantifying the GH-induced expression patterns of two critically important mediators of GH action: IGF1 and the ALS. The latter forms a tertiary complex with IGF1 and IGFBP3, which in turn helps to maintain the circulating half-life of IGF1 (2). Cells were treated for 24 and 48 h (Fig. 6, A and B), and GH responsiveness was established for both IGF1 and ALS. We then quantified expression of IGF1, ALS, and IGFBP1, a negative regulator of IGF1 action, in cells treated with GH and/or myostatin (Fig. 6, C–E). Myostatin alone failed to alter basal expression of IGF1 or ALS regardless of dose or treatment time. It similarly had little effect on GH-induced IGF-1 or ALS expression after just 24 h, although 20 nM myostatin slightly suppressed GH-induced ALS expression to levels equivalent to those of controls (Fig. 6C). Nevertheless, 2 and 20 nM myostatin attenuated GH-induced IGF1 expression whereas 20 nM completely suppressed that of ALS at 48 h (Fig. 6D). Both myostatin doses also stimulated IGFBP1 expression at both time points (Fig. 6E).
Fig. 6.
Myostatin attenuates GH action in primary human hepatocytes. Temporal GH responses were established by treating cells for 24 or 48 h with increasing GH doses and quantifying IGF1 (A) and ALS (B) mRNA using qRT-PCR. (C,D,E) Cells were then costimulated with GH and/or myostatin at the indicated doses (nM) and expression of IGF1, ALS, and IGFBP1 was quantified after 24 and 48 h. In each panel, mean differences for each gene are indicated by different letters, shared letters indicate no difference and comparisons were only made within time periods for a specific gene (AB, n = 6; C-E, n = 12; P ≤ 0.05; one-way ANOVA). (F) A proposed model incorporating GH released from the pituitary (green), MSTN from muscle (red), and IGF1 from the liver, muscle, and bone (blue). Arrows indicate stimulation, blocked lines attenuation. ALS, acid labile subunit; GH, growth hormone; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; MSTN, myostatin.
These data strongly suggest that in addition to myostatin’s direct and potent inhibitory actions on striated muscle growth, it has the potential to also attenuate GH’s endocrine and growth-promoting action in the liver. This would presumably alter the circulating levels of IGF1 and different IGFBPs that regulate IGF-bioavailability and thus, organismal growth. In fact, we previously reported such changes in Mstn−/− (a.k.a. Mighty) mice (72) and additionally suggested that myostatin itself regulates the GH/IGF axis or “somadomedin model” of growth regulation. The current study is the first attempt, to our knowledge, to test this model and indeed suggests that myostatin has the potential to regulate key regulatory points in the model (Fig. 6F), specifically GH release and GH-induced hepatic IGF1 production. Myostatin’s ability to stimulate directly IGFBP1 expression could additionally reflect a complimentary means to suppress growth. This particular IGFBP prevents IGF1 from binding and activating its receptor (20) whereas its expression is generally controlled by catabolic factors and insults (e.g., stress hormones, fasting, hypoxia, etc.).
IGF1 in myostatin-null mice.
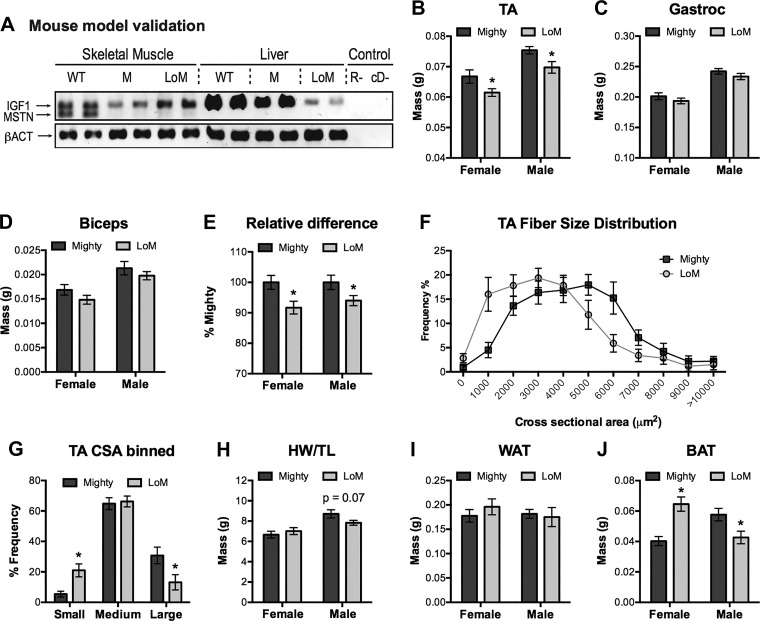
The relative contribution of circulating IGF1 to the enhanced muscularity of Mighty mice was estimated by comparing metrics of muscle mass in Mighty and LOM mice. Both strains lack myostatin whereas LOM mice additionally cannot produce IGF1 in the liver as they were derived from LID mice (74). Circulating IGF1 levels are reduced by ~75% in LID mice, although organismal growth is mostly normal because of a compensatory increase in GH secretion and the loss of IGF1 negative feedback. Normal and substantially reduced IGF1 expression in muscle and liver, respectively, of LOM mice was confirmed by RT-PCR as was the lack of myostatin expression (Fig. 7A). Muscle IGF1 expression was further assessed by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) in age-matched wild-type, Mighty, and LOM mice, and no differences were detected, consistent with a previous report using only wild-type and Mighty mice (72). Such comparisons in the current study are invalid because sibling wild-type mice were not produced from the crosses yielding Mighty and LOM mice and because congenic strains of the latter were not developed. Nevertheless, the mass of several Mighty and LOM skeletal muscles were indeed significantly greater than those in wild-type mice whereas the mass of fat pads was lower, confirming the Mstn−/− phenotype in both strains.
Fig. 7.
IGF1 contributes to muscle hypertrophy in myostatin-null mice. A: IGF1, myostatin (Mstn), and β-actin (βACT) expression in skeletal muscle and liver of wild-type (WT), Mighty (M, fl-Igf1/fl-Igf1; Mstn−/− = Flox’d Igf1 myostatin null) and LID-o-Mighty (LOM, Tg(Alb-cre); fl-Igf1/fl-Igf1; Mstn−/− = liver IGF1-deficient and myostatin null) mice were assessed by RT-PCR (R-, reverse transcriptase negative control; cD-, no cDNA template). B–D: mass of tibialis anterior (TA), gastrocnemius, and bicep brachii skeletal muscles from female and male mice. E: relative difference in the combined mass of all three muscle groups. F and G: TA muscle sections from female mice were stained with H&E, fiber cross-sectional area was quantified using Image-J and mean values were plotted in a frequency distribution (F) and by binned image sizes (G; small, 0–2000 μm; medium, 2000–5999 μm; large; ≥6000 μm). H: heart mass normalized to tibia length. I and J: mass of white and brown adipose tissue, WAT and BAT, respectively. Significant differences within a sex are indicated by asterisks (P < 0.05); notable probability P levels are shown. IGF, insulin-like growth factor.
The mass of TA muscles in LOM mice was significantly lower than that in Mighty mice, and this was true for both sexes (Fig. 7B). Similarly, small but nonsignificant trends were also detected when comparing the mass of gastrocnemius and bicep muscles (Fig. 7, C and D), although when all of these data were combined as a percentage of Mighty mouse values, the overall relative muscle mass was significantly lower in LOM mice (Fig. 7E). These differences were reflected at the cellular level as there were more small myofibers in LOM mice and more large fibers in Mighty mice (Fig. 7, F and G). This was particularly evident when comparing differences of binned fiber sizes and suggests that hepatic-derived IGF1 indeed contributes to the myostatin-null phenotype, albeit the effect is minimal. Such differences were not reflected in cardiac mass or in white adipose tissue (Fig. 7, H and I). By contrast, sexually dimorphic differences were noted for brown adipose tissue as female LOM fat pads were larger than those in Mighty mice whereas the opposite was true in male mice (Fig. 7J).
DISCUSSION
Mammalian myostatin expression is primarily limited to striated muscle and fat (55). The myokine circulates at concentrations well above its affinity for ActRIIb, however, which is expressed in many different tissues, including the pituitary, liver, and of course, striated muscle (5, 58). Thus, myostatin action in nonmuscle tissues should be expected as should the production of off-target effects when attenuating myostatin with circulating ligand traps or antibodies. Such effects could presumably involve the liver and pituitary as our results suggest that myostatin can regulate both. In fact, Bimagrumab, a human monoclonal antibody inhibitor of ActRIIb, was recently demonstrated to alter pituitary function and circulating levels of gonadotropic hormones in human subjects (27).
Quite possibly the most significant finding reported herein is that myostatin may inhibit the development of somatotropes and lactotropes as well as PRL release although conversely stimulating GH release. Differences in cell number between wild-type and Jekyll mice were independently replicated in both sexes and are complemented by myostatin’s direct actions on GH3 cells. They are also consistent with the known actions of activin in GH3 cells and in the pituitary as it suppresses expression of Pit-1, a transcription factor that drives somatotrope and lactotrope development (14, 19, 23, 38, 49, 64). Myostatin circulates at physiologically relevant levels that greatly exceed other ActRIIb ligands (54). It is also expressed in the hypothalamus (55) and thus, its control of the pituitary could be mediated by neuroendocrine as well as endocrine means. Future studies are therefore needed to determine whether muscle- or hypothalamic-derived myostatin is responsible for these actions. They are also needed to determine whether myostatin additionally controls the release of hypothalamic-releasing factors or pituitary sensitivity to such factors.
Liver expression of various IGF axis components are elevated in Mstn−/− neonates and/or adult mice, including IGF1, its receptor, IGFBP3, and IGFBP5 (72). Circulating levels of IGF1 protein are also elevated in adults whereas IGFBP1 and IGFBP2 levels are lower. These results together suggest that total circulating IGF1 as well as the bioavailable fraction are elevated. They also suggest that endocrine myostatin, which originates from striated muscle and not the liver, may directly regulate hepatic production as the liver is the primary source for each circulating factor (37). Direct liver responsiveness to activin and GDF11 has been documented as having indirect responsiveness for myostatin (7, 16, 30, 45). Our results, however, are the first to our knowledge to report a direct effect for myostatin and on GH action. Myostatin attenuation also prevents hepatic steatosis in low-density lipoprotein receptor (Ldlr)-null mice and in a hepatocarcinoma cell line (HepG2 cells) upregulates the expression of lipogenic genes and stimulates lipogenesis (30). Thus, targeting myostatin in the circulation could easily alter other aspects of liver function in addition to IGF1 production.
Our demonstration that myostatin attenuates GH induction of IGF1 and ALS expression, yet stimulates IGFBP1 expression in vitro is consistent with changes in circulating concentrations for these factors in Mstn−/− mice (72). These findings are also consistent with the notion that myostatin regulation of GH-induced IGF1 expression is an important contributor to the myokine’s central actions on the GH/IGF1/IGFBP axis. Myostatin and IGF1 have an antagonistic relationship as IGF1 stimulates myostatin expression in striated muscle, and each factor attenuates one another’s actions locally (25, 26, 47, 56, 61, 68). These findings strongly suggest that myostatin functions as a legitimate hormone rather than as an autocrine factor per se. They also suggest that in addition to the many well-established direct effects on striated muscle growth (55), myostatin is also a potential participant of the somatomedin model (Fig. 6F), as its expression is upregulated by IGF1 and in turn, it attenuates IGF action and stimulates GH secretion.
The somatomedin model is principal to understanding vertebrate growth regulation and is a ubiquitous feature of physiology and endocrinology textbooks. It begins with the release of GH from the anterior pituitary into the general circulation. In the liver, GH then stimulates the production and secretion of IGF1, which in turn mediates many of the anabolic actions of GH. Circulating IGF1 eventually suppresses GH release via a negative feedback loop to the pituitary and to the hypothalamus where it modifies the release of the two primary positive and negative regulators of GH release, GH releasing hormone and somatostatin, respectively. The model has been confirmed in various species, from many different vertebrate classes and includes even basal groups (e.g., cyclostomes) (20). It has also been revised to incorporate the autocrine and paracrine actions of IGF1 produced in peripheral tissues as well as the influences of IGFBPs (37, 40). The primary participants, however, have remained the same as has the general understanding of the GH/IGF axis. Revising this model to include myostatin (Fig. 6F) represents a paradigm shift worthy of future testing.
The teleological significance of myostatin attenuating IGF1 action yet stimulating GH gene expression is remarkably similar to the actions of glucocorticoids. Under conditions of insult, these stress hormones prevent growth factor utilization of metabolites for nonessential processes (i.e., growth, liver IGF1 production, etc.) but stimulate GH gene expression. Glucocorticoids are also primary regulators of IGFBP1 production, which attenuates IGF1 action by preventing binding to the type 1 IGF receptor (20). Myostatin may therefore function similarly as its expression and presumably its secretion are elevated in some catabolic conditions (31, 65), it is necessary for glucocorticoid-induced muscle atrophy (28), it activates signaling pathways that stimulate muscle atrophy (60), and it upregulates IGFBP1 gene expression (Fig. 6E). Circulating IGFBP1 levels in Mstn−/− mice are approximately one-third of those in wild-type mice (72), further supporting the novel suggestion that myostatin functions as a catabolic hormone and possibly as a feedback regulator of somatomediation.
The somatomedin model, however, is not without controversy as several studies have questioned the relative importance of systemic IGF1 to organismal growth. These include those with LID or ALS knock out mice (4, 46, 75). Linear bone growth and even that of tumors is minimally affected in these animals despite a 65%–75% reduction in circulating IGF1. By contrast, body mass is reduced in ALS knock out mice presumably from changes in muscle mass. This suggests that muscle growth may be more dependent upon circulating IGF1 than is bone growth. In fact, circulating IGF1 is highly correlated with striated muscle growth (15, 18, 70) but not with bone growth except under extreme conditions (fasting, hypophysectomy, acromegaly, etc.). Furthermore, GH receptors are expressed at very low levels in striated muscle (22, 32, 33), regardless of species, whereas lean body mass and muscle function are normal in muscle-specific GH receptor knock out mice (71) but suppressed in liver-specific GH receptor knockout mice despite increased muscle IGF1 expression (44). All of this reflects muscle reliance on circulating rather than locally produced IGF1 as well as the potential need to control it with myostatin.
Several studies indicate that bone mass and mineralization are enhanced in Mstn−/− mice or when attenuating myostatin with pharmacological agents (39). Preclinical studies further suggest that the latter are potentially effective in treating different pathological conditions of bone. These effects are largely a compensatory response to increased mechanical loading, although recent studies have demonstrated myostatin to suppress osteocyte-derived exosomal signaling to osteoblasts (53). An emerging model suggests that myostatin and other myokines may function as mechano-humoral factors that directly and indirectly influence bone growth. This is consistent with data presented herein as myostatin’s indirect effects on bone could include attenuating the GH/IGF1 axis.
Muscle growth was minimally affected in LOM mice suggesting that increased circulating and bioavailable IGF1 play a small yet significant role in maintaining the enhanced musculature of Mstn−/− mice. This does not detract from the central hypothesis that circulating IGF1 is more important to muscle growth than is locally produced IGF1 as muscle myostatin mRNA levels were similar in all groups tested. Thus, differences in hepatic rather than muscle IGF1 production explain the reduced muscle fiber size distribution in LOM versus Mighty mice. This also suggests that changes in GH secretion, which increase by two- to threefold in LID mice, are unlikely to have compensated for the loss of hepatic IGF1 production. Future studies are therefore needed to explore the endocrine link between muscle and liver more adequately, possibly by overexpressing myostatin in muscle and interrogating the GH/IGF1/IGFBP axis or by antagonizing IGF1 in Mstn−/− mice. Such studies would definitively determine the relative contribution of circulating IGF1 to the Mstn−/− phenotype as differences in Mighty versus LOM mice could result from the expression of a basal LID phenotype within a Mstn−/− genetic background. These studies would also help inform the development of myostatin-attenuating therapeutics as the myokine has a demonstrable effect on the tissues involved and is well known to antagonize IGF1 action.
GRANTS
These studies were supported by grants from the National Science Foundation (grant no. 1147275 to B. D. Rodgers) and the National Institutes of Health (grant nos. R44CA-221539 to B. D. Rodgers and R01-GM-084186 to T. B. Thompson).
DISCLOSURES
B. D. Rodgers is the founder of AAVogen, Inc., a biotechnology company that develops gene therapeutics for muscle-wasting disease. This includes AVGN7, which attenuates myostatin/activin receptor signaling in striated muscle. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
B.D.R. conceived and designed research; W.C., Y.K.N., N.L., J.A.E., D.M.D., and T.B.T. performed experiments; W.C., Y.K.N., N.L., J.A.E., D.M.D., and B.D.R. analyzed data; W.C., Y.K.N., and B.D.R. interpreted results of experiments; W.C., Y.K.N., N.L., J.A.E., and B.D.R. prepared figures; W.C., Y.K.N., and B.D.R. drafted manuscript; W.C., Y.K.N., N.L., J.A.E., D.M.D., T.B.T., and B.D.R. edited and revised manuscript; B.D.R. approved final version of manuscript.
REFERENCES
- 2.Allard JB, Duan C. IGF-binding proteins: why do they exist and why are there so many? Front Endocrinol (Lausanne) 9: 117, 2018. doi: 10.3389/fendo.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amit T, Hacham H, Daily O, Hertz P, Barkey RJ, Hochberg Z. The Hep G2 cell line in the study of growth hormone receptor/binding protein. Mol Cell Endocrinol 101: 29–36, 1994. doi: 10.1016/0303-7207(94)90216-X. [DOI] [PubMed] [Google Scholar]
- 4.Anzo M, Cobb LJ, Hwang DL, Mehta H, Said JW, Yakar S, LeRoith D, Cohen P. Targeted deletion of hepatic Igf1 in TRAMP mice leads to dramatic alterations in the circulating insulin-like growth factor axis but does not reduce tumor progression. Cancer Res 68: 3342–3349, 2008. doi: 10.1158/0008-5472.CAN-07-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aykul S, Martinez-Hackert E. Transforming growth factor-β family ligands can function as antagonists by competing for type ii receptor binding. J Biol Chem 291: 10792–10804, 2016. doi: 10.1074/jbc.M115.713487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 7: 43442–43460, 2016. doi: 10.18632/oncotarget.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CW, Li L, Houston-Hawkins DE, Matzuk MM. Activins are critical modulators of growth and survival. Mol Endocrinol 17: 2404–2417, 2003. doi: 10.1210/me.2003-0051. [DOI] [PubMed] [Google Scholar]
- 8.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622, 2009. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 9.Cadena SM, Tomkinson KN, Monnell TE, Spaits MS, Kumar R, Underwood KW, Pearsall RS, Lachey JL. Administration of a soluble activin type IIB receptor promotes skeletal muscle growth independent of fiber type. J Appl Physiol (1985) 109: 635–642, 2010. doi: 10.1152/japplphysiol.00866.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, Wilson DM, Sherman ML, Escolar D, Attie KM. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve 55: 458–464, 2017. doi: 10.1002/mus.25268. [DOI] [PubMed] [Google Scholar]
- 11.Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB. The structure of myostatin:follistatin 288: insights into receptor utilization and heparin binding. EMBO J 28: 2662–2676, 2009. doi: 10.1038/emboj.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castinetti F, Davis SW, Brue T, Camper SA. Pituitary stem cell update and potential implications for treating hypopituitarism. Endocr Rev 32: 453–471, 2011. doi: 10.1210/er.2010-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JA, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, Halder T, Zhang G, Li YP, Garcia JM. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle 6: 132–143, 2015. doi: 10.1002/jcsm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang CC, Tan SK, Tai LK, Hsin JP, Wang FF. Evidence for the involvement of protein kinase C in the inhibition of prolactin gene expression by transforming growth factor-beta2. Mol Pharmacol 53: 1054–1061, 1998. [PubMed] [Google Scholar]
- 15.Climent V, Marín F, Picó A. Pharmacologic therapy in growth hormone disorders and the heart. Curr Med Chem 14: 1399–1407, 2007. doi: 10.2174/092986707780831195. [DOI] [PubMed] [Google Scholar]
- 16.Coerver KA, Woodruff TK, Finegold MJ, Mather J, Bradley A, Matzuk MM. Activin signaling through activin receptor type II causes the cachexia-like symptoms in inhibin-deficient mice. Mol Endocrinol 10: 534–543, 1996. doi: 10.1210/mend.10.5.8732684. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 14: 58–74, 2015. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 18.Colao A. The GH-IGF-I axis and the cardiovascular system: clinical implications. Clin Endocrinol (Oxf) 69: 347–358, 2008. doi: 10.1111/j.1365-2265.2008.03292.x. [DOI] [PubMed] [Google Scholar]
- 19.de Guise C, Lacerte A, Rafiei S, Reynaud R, Roy M, Brue T, Lebrun JJ. Activin inhibits the human Pit-1 gene promoter through the p38 kinase pathway in a Smad-independent manner. Endocrinology 147: 4351–4362, 2006. doi: 10.1210/en.2006-0444. [DOI] [PubMed] [Google Scholar]
- 20.Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol 167: 344–351, 2010. doi: 10.1016/j.ygcen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer 44: 1124–1132, 2008. doi: 10.1016/j.ejca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Frick GP, Tai LR, Baumbach WR, Goodman HM. Tissue distribution, turnover, and glycosylation of the long and short growth hormone receptor isoforms in rat tissues. Endocrinology 139: 2824–2830, 1998. doi: 10.1210/endo.139.6.6047. [DOI] [PubMed] [Google Scholar]
- 23.Gaddy-Kurten D, Vale WW. Activin increases phosphorylation and decreases stability of the transcription factor Pit-1 in MtTW15 somatotrope cells. J Biol Chem 270: 28733–28739, 1995. doi: 10.1074/jbc.270.48.28733. [DOI] [PubMed] [Google Scholar]
- 24.Garber K. No longer going to waste. Nat Biotechnol 34: 458–461, 2016. [Erratum in Nat Biotechnol 34: 888, 2016.] doi: 10.1038/nbt.3557. [DOI] [PubMed] [Google Scholar]
- 25.Garikipati DK, Rodgers BD. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: evidence for endocrine-regulated transcript processing. J Endocrinol 215: 177–187, 2012. doi: 10.1530/JOE-12-0260. [DOI] [PubMed] [Google Scholar]
- 26.Garikipati DK, Rodgers BD. Myostatin stimulates myosatellite cell differentiation in a novel model system: evidence for gene subfunctionalization. Am J Physiol Regul Integr Comp Physiol 302: R1059–R1066, 2012. doi: 10.1152/ajpregu.00523.2011. [DOI] [PubMed] [Google Scholar]
- 27.Garito T, Zakaria M, Papanicolaou DA, Li Y, Pinot P, Petricoul O, Laurent D, Rooks D, Rondon JC, Roubenoff R. Effects of bimagrumab, an activin receptor type II inhibitor, on pituitary neurohormonal axes. Clin Endocrinol (Oxf) 88: 908–919, 2018. doi: 10.1111/cen.13601. [DOI] [PubMed] [Google Scholar]
- 28.Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, Ketelslegers JM, Thissen JP. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 148: 452–460, 2007. doi: 10.1210/en.2006-0539. [DOI] [PubMed] [Google Scholar]
- 29.Gramignoli R, Green ML, Tahan V, Dorko K, Skvorak KJ, Marongiu F, Zao W, Venkataramanan R, Ellis EC, Geller D, Breite AG, Dwulet FE, McCarthy RC, Strom SC. Development and application of purified tissue dissociation enzyme mixtures for human hepatocyte isolation. Cell Transplant 21: 1245–1260, 2012. doi: 10.3727/096368911X600939. [DOI] [PubMed] [Google Scholar]
- 30.Guo W, Wong S, Bhasin S. AAV-mediated administration of myostatin pro-peptide mutant in adult Ldlr null mice reduces diet-induced hepatosteatosis and arteriosclerosis. PLoS One 8: e71017, 2013. doi: 10.1371/journal.pone.0071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 121: 419–425, 2010. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill DJ, Freemark M, Strain AJ, Handwerger S, Milner RD. Placental lactogen and growth hormone receptors in human fetal tissues: relationship to fetal plasma human placental lactogen concentrations and fetal growth. J Clin Endocrinol Metab 66: 1283–1290, 1988. doi: 10.1210/jcem-66-6-1283. [DOI] [PubMed] [Google Scholar]
- 33.Hirano T. Hepatic receptors for homologous growth hormone in the eel. Gen Comp Endocrinol 81: 383–390, 1991. doi: 10.1016/0016-6480(91)90165-3. [DOI] [PubMed] [Google Scholar]
- 34.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121: 465–477, 2005. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Jackson MF, Li N, Rodgers BD. Myostatin regulates tissue potency and cardiac calcium-handling proteins. Endocrinology 155: 1771–1785, 2014. doi: 10.1210/en.2013-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson MF, Luong D, Vang DD, Garikipati DK, Stanton JB, Nelson OL, Rodgers BD. The aging myostatin null phenotype: reduced adiposity, cardiac hypertrophy, enhanced cardiac stress response, and sexual dimorphism. J Endocrinol 213: 263–275, 2012. doi: 10.1530/JOE-11-0455. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan SA, Cohen P. The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab 92: 4529–4535, 2007. doi: 10.1210/jc.2007-0526. [DOI] [PubMed] [Google Scholar]
- 38.Lacerte A, Lee EH, Reynaud R, Canaff L, De Guise C, Devost D, Ali S, Hendy GN, Lebrun JJ. Activin inhibits pituitary prolactin expression and cell growth through Smads, Pit-1 and menin. Mol Endocrinol 18: 1558–1569, 2004. doi: 10.1210/me.2003-0470. [DOI] [PubMed] [Google Scholar]
- 39.Laurent MR, Dubois V, Claessens F, Verschueren SM, Vanderschueren D, Gielen E, Jardí F. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol Cell Endocrinol 432: 14–36, 2016. doi: 10.1016/j.mce.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev 22: 53–74, 2001. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 41.Le Tissier PR, Hodson DJ, Lafont C, Fontanaud P, Schaeffer M, Mollard P. Anterior pituitary cell networks. Front Neuroendocrinol 33: 252–266, 2012. doi: 10.1016/j.yfrne.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Li L, He D, Wilborn TW, Falany JL, Falany CN. Increased SULT1E1 activity in HepG2 hepatocytes decreases growth hormone stimulation of STAT5b phosphorylation. Steroids 74: 20–29, 2009. doi: 10.1016/j.steroids.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li N, Yang Q, Walker RG, Thompson TB, Du M, Rodgers BD. Myostatin attenuation in vivo reduces adiposity, but activates adipogenesis. Endocrinology 157: 282–291, 2016. doi: 10.1210/en.2015-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology 155: 1793–1805, 2014. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu A, Dong W, Peng J, Dirsch O, Dahmen U, Fang H, Zhang C, Sun J. Growth differentiation factor 11 worsens hepatocellular injury and liver regeneration after liver ischemia reperfusion injury. FASEB J 32: 5186–5198, 2018. doi: 10.1096/fj.201800195R. [DOI] [PubMed] [Google Scholar]
- 46.Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology 141: 4436–4441, 2000. doi: 10.1210/endo.141.12.7825. [DOI] [PubMed] [Google Scholar]
- 47.Lokireddy S, Mouly V, Butler-Browne G, Gluckman PD, Sharma M, Kambadur R, McFarlane C. Myostatin promotes the wasting of human myoblast cultures through promoting ubiquitin-proteasome pathway-mediated loss of sarcomeric proteins. Am J Physiol Cell Physiol 301: C1316–C1324, 2011. doi: 10.1152/ajpcell.00114.2011. [DOI] [PubMed] [Google Scholar]
- 48.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94: 12457–12461, 1997. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyamoto S, Irahara M, Ushigoe K, Kuwahara A, Sugino H, Aono T. Effects of activin on hormone secretion by single female rat pituitary cells: analysis by cell immunoblot assay. J Endocrinol 161: 375–382, 1999. doi: 10.1677/joe.0.1610375. [DOI] [PubMed] [Google Scholar]
- 50.Morris C. Balancing efficacy versus safety: learning’s from development of myostatin inhibitors. DACC News 29: 8, 2013. [Google Scholar]
- 51.Plumridge H, Falconi M. Drugs Aim to Help Elderly Rebuild Muscle (Online). https://www.wsj.com/articles/drugs-aims-to-help-elderly-rebuild-muscle-1398644791.
- 52.Polkey MI, Praestgaard J, Berwick A, Franssen FME, Singh D, Steiner MC, Casaburi R, Tillmann HC, Lach-Trifilieff E, Roubenoff R, Rooks DS. Activin type II receptor blockade for treatment of muscle depletion in chronic obstructive pulmonary disease: a randomized trial. Am J Respir Crit Care Med 199: 313–320, 2019. doi: 10.1164/rccm.201802-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H, Cardozo C, Wu Y, Divieti Pajevic P, Bonewald LF, Bauman WA, Qin W. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J Biol Chem 292: 11021–11033, 2017. doi: 10.1074/jbc.M116.770941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodgers BD, Eldridge JA. Reduced circulating GDF11 is unlikely responsible for age-dependent changes in mouse heart, muscle, and brain. Endocrinology 156: 3885–3888, 2015. doi: 10.1210/en.2015-1628. [DOI] [PubMed] [Google Scholar]
- 55.Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev 29: 513–534, 2008. doi: 10.1210/er.2008-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodgers BD, Interlichia JP, Garikipati DK, Mamidi R, Chandra M, Nelson OL, Murry CE, Santana LF. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J Physiol 587: 4873–4886, 2009. doi: 10.1113/jphysiol.2009.172544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodgers BD, Weber GM, Sullivan CV, Levine MA. Isolation and characterization of myostatin complementary deoxyribonucleic acid clones from two commercially important fish: Oreochromis mossambicus and Morone chrysops. Endocrinology 142: 1412–1418, 2001. doi: 10.1210/endo.142.4.8097. [DOI] [PubMed] [Google Scholar]
- 58.Sako D, Grinberg AV, Liu J, Davies MV, Castonguay R, Maniatis S, Andreucci AJ, Pobre EG, Tomkinson KN, Monnell TE, Ucran JA, Martinez-Hackert E, Pearsall RS, Underwood KW, Seehra J, Kumar R. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIB. J Biol Chem 285: 21037–21048, 2010. doi: 10.1074/jbc.M110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sartori R, Gregorevic P, Sandri M. TGFβ and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab 25: 464–471, 2014. doi: 10.1016/j.tem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280: 4294–4314, 2013. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 61.Shyu KG, Ko WH, Yang WS, Wang BW, Kuan P. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res 68: 405–414, 2005. doi: 10.1016/j.cardiores.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 62.Stewart GD, Skipworth RJ, Fearon KC. Cancer cachexia and fatigue. Clin Med (Lond) 6: 140–143, 2006. doi: 10.7861/clinmedicine.6-2-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su TS, Liu WY, Han SH, Jansen M, Yang-Fen TL, P’eng FK, Chou CK. Transcripts of the insulin-like growth factors I and II in human hepatoma. Cancer Res 49: 1773–1777, 1989. [PubMed] [Google Scholar]
- 64.Tamura N, Irahara M, Kuwahara A, Ushigoe K, Sugino H, Aono T. Effect of activin on production and secretion of prolactin and growth hormone in cultured rat GH3 cells. Eur J Endocrinol 142: 506–511, 2000. doi: 10.1530/eje.0.1420506. [DOI] [PubMed] [Google Scholar]
- 65.Thomas SS, Mitch WE. Mechanisms stimulating muscle wasting in chronic kidney disease: the roles of the ubiquitin-proteasome system and myostatin. Clin Exp Nephrol 17: 174–182, 2013. doi: 10.1007/s10157-012-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 89: 381–410, 2009. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 67.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science 303: 359–363, 2004. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valdés JA, Flores S, Fuentes EN, Osorio-Fuentealba C, Jaimovich E, Molina A. IGF-1 induces IP3-dependent calcium signal involved in the regulation of myostatin gene expression mediated by NFAT during myoblast differentiation. J Cell Physiol 228: 1452–1463, 2013. doi: 10.1002/jcp.24298. [DOI] [PubMed] [Google Scholar]
- 69.Vankelecom H, Chen J. Pituitary stem cells: where do we stand? Mol Cell Endocrinol 385: 2–17, 2014. doi: 10.1016/j.mce.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 70.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154: 557–568, 2008. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vijayakumar A, Buffin NJ, Gallagher EJ, Blank J, Wu Y, Yakar S, LeRoith D. Deletion of growth hormone receptors in postnatal skeletal muscle of male mice does not alter muscle mass and response to pathological injury. Endocrinology 154: 3776–3783, 2013. doi: 10.1210/en.2013-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams NG, Interlichia JP, Jackson MF, Hwang D, Cohen P, Rodgers BD. Endocrine actions of myostatin: systemic regulation of the IGF and IGF binding protein axis. Endocrinology 152: 172–180, 2011. doi: 10.1210/en.2010-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodhouse L, Gandhi R, Warden SJ, Poiraudeau S, Myers SL, Benson CT, Hu L, Ahmad QI, Linnemeier P, Gomez EV, Benichou O; Study Investigators . A phase 2 randomized study investigating the efficacy and safety of myostatin antibody LY2495655 versus placebo in patients undergoing elective total hip arthroplasty. J Frailty Aging 5: 62–70, 2016. doi: 10.14283/jfa.2016.81. [DOI] [PubMed] [Google Scholar]
- 74.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96: 7324–7329, 1999. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110: 771–781, 2002. doi: 10.1172/JCI0215463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto K, Uda A, Mukai A, Yamashita K, Kume M, Makimoto H, Bito T, Nishigori C, Hirano T, Hirai M. Everolimus-induced human keratinocytes toxicity is mediated by STAT3 inhibition. J Exp Clin Cancer Res 32: 83, 2013. doi: 10.1186/1756-9966-32-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young K, Conley B, Romero D, Tweedie E, O’Neill C, Pinz I, Brogan L, Lindner V, Liaw L, Vary CP. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood 120: 4263–4273, 2012. [Erratum in Blood 132: 2526, 2018.] doi: 10.1182/blood-2012-07-440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87: 933–963, 2007. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]