Abstract
Our recent electrophysiological analysis of mouse retinal pigment epithelial (RPE) cells revealed that in the presence of 10 mM external thiocyanate (SCN−), voltage steps generated large transient currents whose time-dependent decay most likely results from the accumulation or depletion of SCN− intracellularly. In the present study, we investigated the effects of more physiologically relevant concentrations of this biologically active anion. In whole cell recordings of C57BL/6J mouse RPE cells, we found that, over the range of 50 to 500 µM SCN−, the amplitude of transient currents evoked by voltage steps was proportional to the extracellular SCN− concentration. Transient currents were also produced in RPE cells when the membrane potential was held constant and the external SCN− concentration was rapidly increased by pressure-ejecting 500 µM SCN− from a second pipette. Other results indicate that the time dependence of currents produced by both approaches results from a change in driving force due to intracellular SCN− accumulation or depletion. Finally, by applying fluorescence imaging and voltage-clamping techniques to BALB/c mouse RPE cells loaded with the anion-sensitive dye MQAE, we demonstrated that in the presence of 200 or 500 µM extracellular SCN−, depolarizing voltage steps increased the cytoplasmic SCN− concentration to an elevated steady state within several seconds. Collectively, these results indicate that, in the presence of physiological concentrations of SCN− outside the RPE, the conductance and permeability of the RPE cell membranes for SCN− are sufficiently large that SCN− rapidly approaches electrochemical equilibrium within the cytoplasm when the membrane voltage or external SCN− concentration is perturbed.
Keywords: anion conductance, anion permeability, RPE
INTRODUCTION
The retinal pigment epithelium (RPE) is a transporting epithelium that supports optimal visual function by tightly regulating the volume and ionic composition of the extracellular fluid surrounding the inner and outer segments of rod and cone photoreceptors. We recently discovered that mouse RPE cells have a large conductance and permeability for thiocyanate (SCN−) (7). This conductance, which lies predominantly in the basolateral membrane, exhibits an anion selectivity profile as well as other properties that do not match those of any of the Cl− channels reported to be expressed in the RPE, namely ANO1 (8), ANO2 (15), BEST1 (17), CFTR (4), and ClC-2 (5). This discrepancy suggests that the RPE contains an anion channel and/or electrogenic transporter that has not been identified to date.
In our previous study, we showed that the replacement of 10 mM external Cl− with SCN− produced a dramatic, 10-fold increase in the amplitude of whole cell currents in mouse RPE cells, reflecting a relative conductance for SCN− (GSCN/GCl) of ∼40. Aside from the robust increase in current amplitude, a notable feature of currents evoked by voltage steps in the presence of external SCN− is that they underwent time-dependent decay and were followed by tail currents of opposite polarity. The decay in current was shown not to be a consequence of ion channel gating but rather, is likely due to a change in driving force caused by the accumulation or depletion of SCN− within the cytoplasm. We further showed that this anion conductance has a relative permeability for SCN− [thiocyanate-to-chloride permeability ratio (PSCN/PCl)] of 500 or more, reflective of a high degree of selectivity that is rarely observed in anion channels (13).
Our finding that the RPE anion conductance is selective for SCN− might be construed as an interesting biophysical attribute with little or no physiological significance. However, SCN− is biologically active (1, 9, 12, 18, 28) and is actively transported by epithelia lining the digestive (24) and respiratory (11) tracts as well as the fourth ventricle of the brain (20). Present in most extracellular fluids, the level of SCN− in plasma typically ranges from 25 to 100 µM and in smokers can exceed 150 µM (22, 23). In the present study, we investigated the effects of physiological concentrations of SCN− on whole cell currents in isolated mouse RPE cells using two different approaches: 1) by making step changes in membrane voltage while bathing the cell with a solution containing SCN− at a concentration of 500 µM or less; and 2) by rapidly increasing the external SCN− concentration in the vicinity of the cell by pressure-ejecting 500 µM SCN− from a second pipette while holding the membrane potential constant. In addition, we directly measured voltage-dependent changes in cytoplasmic SCN− concentration in isolated mouse RPE cells loaded with the anion-sensitive dye N-(ethoxycarbonylmethyl)-6-methoxyquinolium bromide (MQAE) using fluorescence imaging microscopy.
Taken together, our results indicate that the RPE contains a SCN−-selective conductive mechanism, likely located in the basolateral membrane, that can mediate robust SCN− fluxes into and out of the cell when the anion is present at levels near those found in human plasma. This finding suggests that this conductance could play a significant role in RPE/retina physiology.
MATERIALS AND METHODS
Animals.
All procedures involving mice received Institutional Animal Care and Use Committee (IACUC) approval and were conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals. C57BL/6J or BALB/c mice of both sexes and ages 4 to 16 wk were euthanized by CO2 inhalation before enucleation. Enucleated eyes were hemisected posteriorly to the ora serrata, and the anterior segment and lens were removed. RPE cells were then isolated enzymatically using papain as described previously (7), stored in L-15 medium containing 0.5 mM taurine and 1 mM reduced glutathione at 4°C, and used within 8 h. All solutions used for the isolation and storage of RPE cells were bubbled with 90% N2/10% O2. This measure improved cell quality significantly.
Solutions.
The pipette solution used in most experiments was an NMDG-Cl-based solution containing (in mM) 135 NMDG-Cl, 10 NMDG-HEPES, 5.5 EGTA-NMDG, 0.5 CaCl2, and 2 MgCl2 (pH 7.2). In MQAE imaging experiments, the Cl− concentration was reduced to 5 mM by replacing NMDG-Cl with 135 NMDG-gluconate or 113 mM Cs+-gluconate plus 50 mM mannitol. The free Ca2+ concentration in all pipette solutions was calculated to be ∼30 nM (https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator). The bath solutions contained (in mM) 140 NaA, 10 Na-HEPES, 10 glucose, 1.8 CaCl2, and 1 MgCl2 (pH 7.4), where A represents Cl−, isethionate, or methanesulfonate. In some experiments, Na+ was replaced with NMDG. SCN− was added to bath solutions as a Na+ salt. In most experiments, nonselective cation channels were blocked by the addition of 100 µM GdCl3 to the bathing solution. All chemicals were obtained from Sigma-Aldrich, except for papain and MQAE, which were obtained from Worthington Biochemical and ThermoFisher Scientific, respectively.
Enzymatically dissociated mouse RPE cells were allowed to attach to the glass coverslip bottom of a recording chamber and were constantly superfused with bath solutions delivered by gravity feed. Rapid increases in [SCN−] in the cell’s local environment were generated by the pressure ejection of bath solution containing 0.5 mM SCN− from a second pipette. The tip of this extracellular pipette had a diameter of ∼1 µm and was positioned ∼10 µm from the cell being recorded. Pressure (15 to 25 psi) was applied to the pipette via a Picospritzer II (General Valve).
Electrophysiology.
The amplifier’s circuit ground was connected to a Ag/AgCl electrode separated from the bath by a 3 M KCl/Agar bridge. All experiments were performed at room temperature (22–25°C). Macroscopic membrane currents were recorded using the whole cell configuration of the patch clamp technique. Patch pipettes were pulled from 1.65-mm outer diameter glass capillary tubing (Warner Instruments), using a multistage programmable microelectrode puller (model P-97; Sutter Instruments), and had an impedance of 2–5 MΩ after fire polishing. Series resistance (Rs) was normally <10 MΩ and was compensated 50–80%. Signals were amplified with an Axopatch 200 or Multiclamp 700B amplifier (Molecular Devices). In most experiments, currents were evoked from a holding potential by a series of 1-s voltage steps in range of −120 to +60 mV, in 10 mV increments, with an intersweep interval of at least 3 s. Reversal potential (Erev) and slope conductance (Gs) were determined by linear regression analysis of current-voltage (I-V) plots. Gs was measured as the slope of the I-V curve between −25 mV and +25 mV of Erev.
Junction potentials arising at the pipette tip and the 3 M KCl/Agar-bridge of the reference electrode were calculated using the Junction Potential Calculator function of pClamp 9 (Molecular Devices). Errors in membrane potential (Vm) arising from junction potentials and the current flow across Rs were corrected by the formula
| (1) |
where Vm, uncorr is the apparent Vm, Rs,correct is the series resistance compensation setting of the voltage clamp amplifier expressed as a decimal, and jp is the correction for junction potentials. For simplicity, command potentials are given for voltage clamp protocols, except where noted.
Reversal potentials from I-V plots were used to calculate the relative permeability ratio for SCN− (PSCN/PCl) according to the Goldman-Hodgkin-Katz equation:
| (2) |
where [SCN]o and [Cl]o are the extracellular concentrations of SCN− and Cl−, respectively, [Cl]i is the concentration of Cl− in the pipette solution, z = −1, and F, R, and T have their normal thermodynamic meanings.
Intracellular SCN− imaging.
Nonpigmented RPE cells isolated from BALB/c mice were transferred to a recording chamber on the stage of an inverted microscope (Nikon TE300) and brought into focus through a ×40 water immersion objective lens (NA 0.8) or a ×100 CFI Super Fluor oil objective lens (NA 1.3). Epifluorescence measurements were made after establishing the whole cell recording configuration and waiting 5 min or more for the NMDG-gluconate or Cs+-gluconate based pipette solution (5 mM Cl−) containing 0.5 or 1 mM MQAE free acid to equilibrate with the cytoplasm. The excitation source was a xenon lamp, and the excitation wavelength of 350 ± 15 nm was transmitted using an Optoscan Monochromator (Cairn Research). Emission fluorescence was passed through a 400-nm dichroic mirror and a 460 ± 25-nm emission filter (Omega Optical) and imaged on a cooled charge-coupled device camera (Sensicam). Average fluorescence within a region of interest (ROI) encompassing the basolateral pole of the cell was sampled every 5 s using Imaging Workbench 5.0 software (INDEC Biosciences). The rate of change in fluorescence before and after depolarizing voltage steps due to bleaching and/or loss of MQAE from the cytoplasm was calculated by applying linear regression, averaged, and subtracted from the raw fluorescence data.
Data analysis.
Data acquisition and analysis were carried out using pClamp 9 (Molecular Devices) and SigmaPlot 10 (Systat Software). For clarity of presentation, capacitative transients in some figures are masked.
Statistics.
Statistical analysis was performed using Excel (Microsoft) or Prism 8 (GraphPad). All mice were assumed to be identical such that cells could be treated as coming from a single population. The statistical significance of differences between groups of three or more was calculated by ANOVA or a mixed-effects model (REML), followed by Tukey’s or Sidak’s multiple-comparisons test. Student’s paired t-test was used for comparisons between two groups. Probability values of P < 0.05 were considered statistically significant. The number of experiments reported refer to the number of cells recorded.
RESULTS
Concentration dependence of SCN− currents.
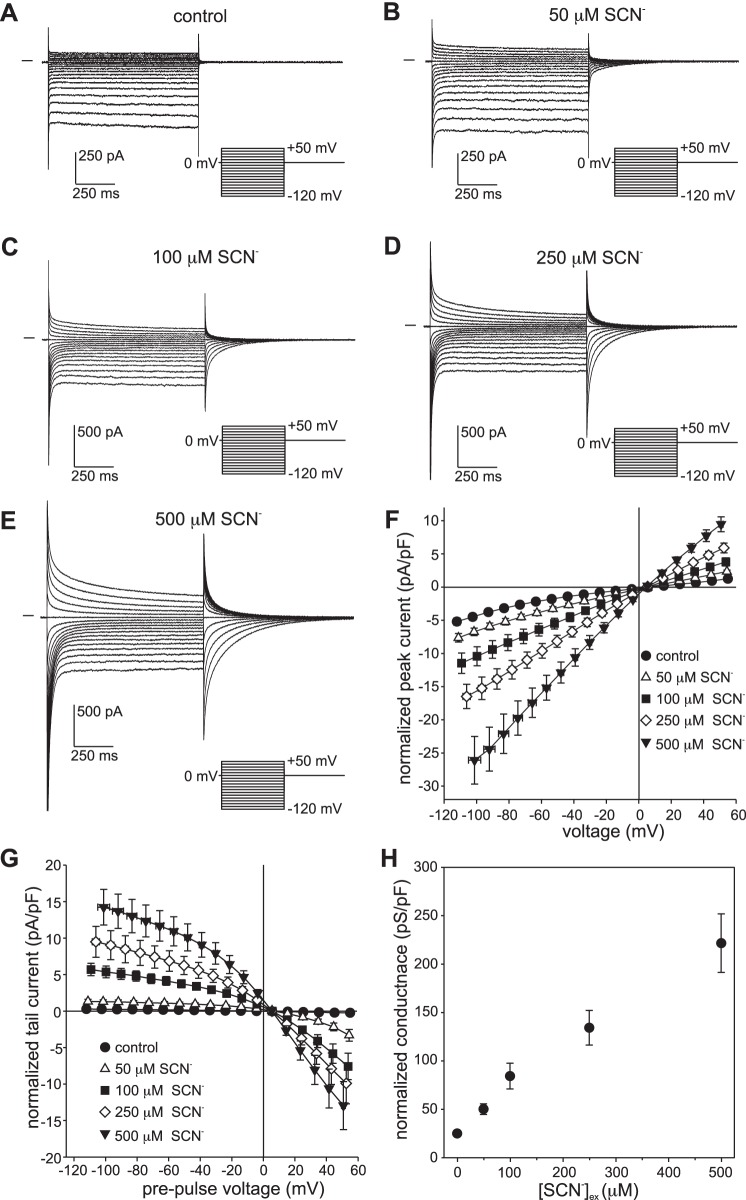
We recently showed that, when exposed to 10 mM external SCN−, voltage-clamped mouse RPE cells exhibited prominent transient currents whose large negative reversal potential indicates that the anion conductance of the RPE cell membranes has a remarkably high relative permeability for SCN− (PSCN/PCl) of ≥500 (7). To determine whether the RPE cell is sensitive to more physiologically relevant concentrations of SCN−, we recorded whole cell currents under voltage clamp conditions in C57BL/6J mouse RPE cells exposed to extracellular SCN− in the range of 50 to 500 µM. Figure 1 shows families of whole cell currents evoked by voltage steps from a holding potential (HP) of 0 mV in an RPE cell exposed to 0 µM SCN− (Fig. 1A), 50 µM (Fig. 1B), 100 µM (Fig. 1C), 250 µM (Fig. 1D), or 500 µM SCN− (Fig. 1E) in the bath. In the absence of external SCN−, currents evoked by depolarizing and hyperpolarizing voltage steps were essentially time independent (Fig. 1A), had a mild, inwardly rectifying current-voltage (I-V) relationship (Fig. 1F), and were largely carried by Cl− (7). In the presence of submillimolar concentrations of external SCN−, outward and inward currents, reflecting SCN− influx and efflux, respectively, increased in amplitude, exhibited time-dependent decay, and were followed by tail currents of opposite polarity (Fig. 1, B–E). The amplitude of the instantaneous current at the onset (peak current) and offset of the voltage step (tail current) was concentration dependent (Fig. 1, F and G), and even in 50 µM SCN−, current amplitude was significantly larger than it was in the absence of SCN− at most voltages (n = 5–7, P < 0.05, two-way ANOVA, followed by Tukey’s multiple-comparisons test). As shown in Fig. 1H, the conductance of RPE cells also increased in proportion to the concentration of external SCN−. It is worth noting that at each of the SCN− concentrations the instantaneous current reversed close to +5.5 mV, which is equal to the membrane potential after correcting for a small voltage error due to the liquid junction potential across the pipette tip. As discussed in more detail in the following sections, this equality likely reflects the intracellular accumulation of SCN− at the holding potential to a concentration near that predicted for electrochemical equilibrium.
Fig. 1.
Dependence of whole cell currents in isolated mouse retinal pigment epithelial (RPE) cells on external thiocyanate (SCN−) concentration ([SCN−]ex). A–E: families of whole cell currents were recorded in the same cell in the presence of 0 µM (A), 50 µM (B), 100 µM (C), 250 µM (D), or 500 µM SCN− (E). The pipette and bath solutions contained 140 mM Cl− and 145.6 mM Cl−, respectively. Currents were evoked from a holding potential of 0 mV by a series of voltage steps in the range of −120 mV to +50 mV. The interval between the start of voltage steps was 3 s. The horizontal trace to the left of each family of currents represents the zero-current level. F: dependence of instantaneous current amplitude on [SCN−]ex. Currents were normalized by membrane capacitance, and membrane voltage was corrected for liquid junction potentials and the voltage drop across series resistance (Rs). Symbols represent means and bidirectional error bars represent SE (n = 5–7 cells from 3 C57BL/6J mice); where not visible, the error bars are smaller than the symbols. The mean current at each SCN− concentration is significantly larger than control at all voltages in the ranges of −100 mV to −30 mV and +20 mV to +50 mV (P < 0.05, two-way ANOVA followed by Tukey’s multiple-comparisons test). G: dependence of tail current amplitude on [SCN−]ex. Currents are normalized by membrane capacitance, and voltages represent the membrane potential before the initiation of tail currents by returning to the holding potential. Symbols represent means and bidirectional error bars represent SE (n = 5–7 cells from 3 C57BL/6J mice). The mean tail current at each SCN− concentration is significantly larger compared with control (P < 0.05) for pre-pulses to voltages in the ranges of −120 mV to −30 mV and +20 mV to +50 mV. H: dependence of conductance on [SCN−]ex. Slope conductance was calculated from instantaneous currents in cells individually at each concentration. Symbols represent means and error bars represent SE (n = 5–7 cells from 3 C57BL/6J mice). The mean conductance at each concentration is significantly different for all comparisons (P < 0.01, one-way ANOVA followed by Tukey’s multiple comparisons test), except for 50 µM vs. 100 µM.
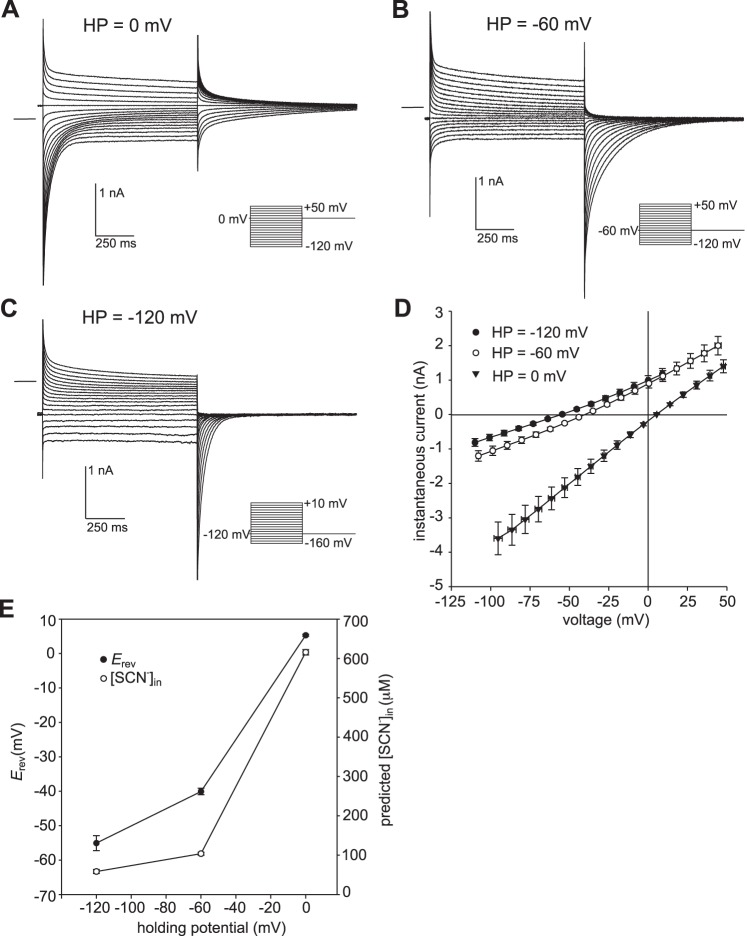
Dependence of currents in 500 µM external SCN− on holding potential.
Previously, we showed that in the presence of 10 mM or 140 mM external SCN−, the amplitude, kinetics, and reversal potential of whole cell currents in mouse RPE cells depended on the holding potential (7). These effects were also observed when the external SCN− concentration was reduced to 500 µM. Figure 2 shows representative families of currents evoked by a series of voltage steps from holding potentials of 0 mV (Fig. 2A), −60 mV (Fig. 2B), and −120 mV (Fig. 2C) in the same RPE cell bathed in 500 µM SCN−. As the holding potential was made more negative, the amplitude of transient inward currents decreased, whereas inward tail currents following the offset of depolarizing voltage steps grew in amplitude and decayed faster. Note that at a holding potential of −120 mV, transient inward currents were virtually eliminated, indicating that little SCN− had accumulated intracellularly at this membrane voltage. In addition to current amplitude and kinetics, Erev was also greatly affected by changes in holding potential. Figure 2D summarizes the results of this and similar experiments obtained in six other cells and depicts I-V plots of instantaneous currents evoked from holding potentials of 0 mV, −60 mV, and −120 mV. As shown in Fig. 2E (closed circles), Erev averaged +5.3 ± 0.3 mV at HP = 0 mV (n = 7), −40.1 ± 0.9 mV at HP = −60 mV (n = 7), and −55.1 ± 2.2 mV at HP = −120 mV (n = 7). Although the Erev values at the three holding potentials were significantly different from one another (P < 0.0001, two-way ANOVA followed by Tukey’s multiple comparisons test), the change in Erev was much larger when HP was hyperpolarized from 0 mV to −60 mV (∼45 mV) than it was when it was hyperpolarized from −60 mV to −120 mV (∼15 mV). If we assume that [SCN−]in approaches electrochemical equilibrium in the steady state, as we have previously shown for the condition where the external SCN− concentration ([SCN−]ex) = 10 mM (7), then [SCN−]in can be calculated from Erev using the Nernst equation:
Fig. 2.
The effect of holding potential on currents produced in the presence of 500 µM external thiocyanate (SCN−). A–C: families of whole cell currents evoked in the same retinal pigment epithelial (RPE) cell from a holding potential of 0 mV (A), −60 mV (B), or −120 mV (C). The zero-current level is shown by the horizontal line to the left of each family of currents. The interval between the start of voltage steps was 7 s for holding potential (HP) = −120 mV and 3 s for HP = −60 mV and HP = 0 mV. The pipette and bath solutions contained 140 mM Cl− and 145.6 mM Cl−, respectively. D: summary of similar experiments (repeated measures in 7 cells from 2 C57BL/6J mice) showing current-voltage (I-V) plots of instantaneous current obtained from holding potentials of −120 mV (●), −60 mV (○), and 0 mV (▼). Symbols represent means and bidirectional error bars represent SE. Voltages are corrected for liquid junction potentials and the voltage drop across series resistance (Rs). Membrane potential (Vm) corrected for voltage errors averaged −110.0 ± 0.8 mV, −53.1 ± 0.2 mV, and +5.4 ± 0.1 mV at holding potentials of −120 mV, −60 mV, and 0 mV, respectively. The mean current amplitudes for the three holding potentials are significantly different from each other at all voltages, except for HP = −120 mV vs. HP = −60 mV at +10 mV (P < 0.05, two-way ANOVA followed by Tukey’s multiple-comparison test). E: dependence of reversal potential (Erev, ●, left y-axis) and intracellular SCN− concentration predicted for passive distribution (○, right y-axis) on holding potential. Symbols represent means and error bars represent SE (n = 7; P < 0.005, two-way ANOVA followed by Tukey’s multiple-comparison test).
| (3) |
The values for [SCN−]in expected for passive distribution when [SCN−]ex = 500 µM are 617 ± 7 µM, 104 ± 4 µM, and 58 ± 5 µM at holding potentials of 0 mV, −60 mV, and −120 mV, respectively (Fig. 2E, open circles). The actual values for [SCN−]in could be different if secondary active transport mechanisms for SCN− were functional under our recording conditions.
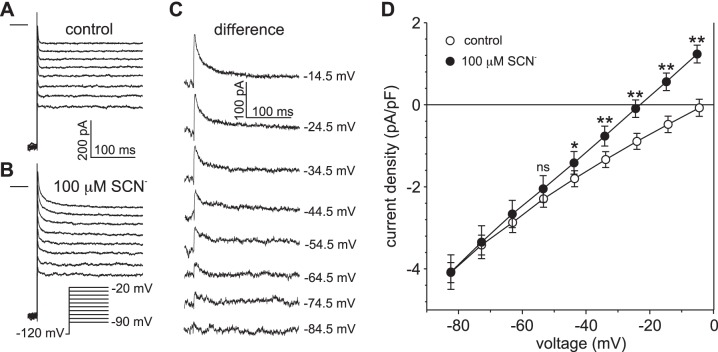
Voltage dependence of SCN− influx under the condition of low intracellular [SCN−].
To determine the extent to which SCN− influx occurs at negative membrane potentials when intracellular SCN− accumulation is minimal, we held the membrane potential at −120 mV and evoked currents by a series of depolarizing voltage steps in the absence and presence of 100 µM external SCN−. Figure 3 shows that currents were essentially time independent under control conditions (Fig. 3A) but that, in the presence of 100 µM external SCN−, currents were larger in amplitude and exhibited time-dependent decay (Fig. 3B). These effects of SCN− can be more easily seen in the family of difference currents depicted in Fig. 3C, which shows that transient SCN− currents were produced by voltage steps to −74.5 mV and more positive potentials and that the amplitude of transient current increased in proportion to the size of the depolarizing voltage step. Figure 3D summarizes the results of this and four similar experiments and plots the mean peak current as a function of voltage in the absence (open circles) and presence (closed circles) of 100 µM SCN−. Overall, the amplitude of the peak current was significantly larger in the presence than in the absence of 100 µM SCN− when the voltage was stepped to −44.5 mV (P < 0.05) and more positive potentials (P < 0.01, two-way ANOVA followed by Sidak’s multiple-comparison test). The results indicate that intracellular SCN− influx occurs at negative membrane potentials near the basolateral resting membrane potential of −55 mV (14, 21) when SCN− is present externally at a physiologically relevant concentration.
Fig. 3.
Determination of the voltage threshold for SCN− influx in retinal pigment epithelial (RPE) cells exposed to 100 µM external thiocyanate (SCN−). A and B: families of whole cell currents recorded in the same RPE cell in the absence (A) and presence (B) of 100 µM external SCN−. Currents were evoked by a series of voltage steps from a holding potential of −120 mV. The horizontal lines to the left of the current families represent the zero-current potential. The pipette and bath solutions contained 140 mM Cl− and 145.6 mM Cl−, respectively. C: family of SCN−-dependent currents obtained by taking the difference between the current traces in B from those in A. The value at the right of each current trace represents the amplitude of the voltage step, corrected for liquid junction potentials. D: current-voltage (I-V) relationships of peak current density obtained in the absence (○) and presence (●) of 100 µM external SCN−. Peak currents were measured 6–12 ms after the onset of the voltage steps. Symbols represent means and error bars represent SE; n = 5 cells from 3 C57BL/6J mice. *P < 0.05 compared with control; **P < 0.01 compared with control; ns, not significant, P > 0.05 (two-way ANOVA followed by Sidak’s multiple-comparisons test).
Relative permeability for SCN−.
We used a modified Goldman-Hodgkin-Katz equation (Eq. 2) to calculate PSCN/PCl from Erev. To minimize the influence of [SCN−]in, we considered Erev values measured at a holding potential of −120 mV (Fig. 2E). For seven cells bathed in 500 µM SCN−, we obtained an average PSCN/PCl value of 2,269 ± 213 (mean ± SE; range 1,505–2,900), which should be considered as a lower limit for the actual PSCN/PCl value due to the fact that [SCN−]in was likely low but significant. This result provides further support to our previous conclusion that the RPE cell membrane is endowed with an extraordinarily large relative permeability for SCN− (7).
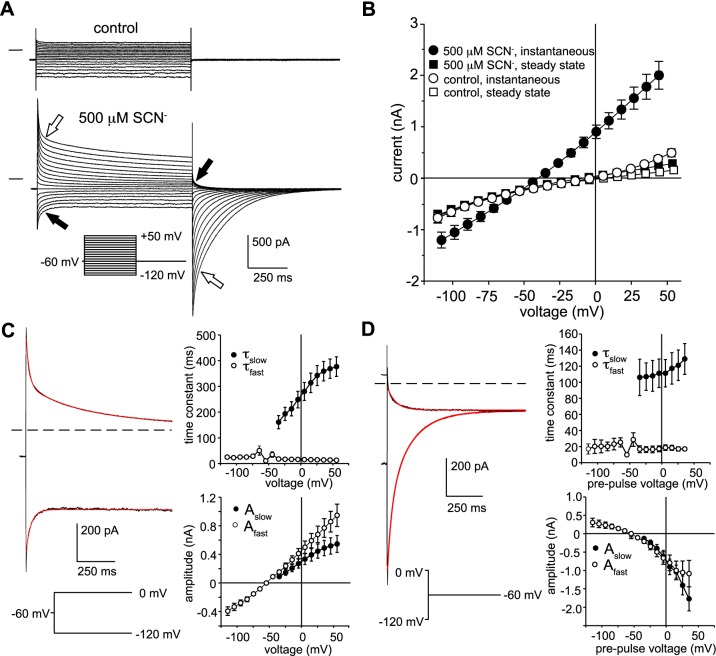
Kinetics of transient currents.
We investigated the time-dependent decay of current in 500 µM external SCN− in more detail. Figure 4A shows families of currents evoked from a holding potential of −60 mV by voltage steps in the range of −120 mV to +50 mV in the absence (top) and presence (bottom) of 500 µM external SCN−. Instantaneous currents were significantly larger in the presence than in the absence of SCN−, but by the end of the 1-s voltage steps, currents had declined to reach amplitudes approaching those of control currents (Fig. 4B). The decay in inward current could be adequately fitted by a single exponential function (Fig. 4C, left, bottom red trace); the decay in outward currents, on the other hand, exhibited fast and slow components (Fig. 4C, left, top red trace), the latter contributing ∼40% of the current amplitude (Fig. 4C, bottom right). The time constant of the fast component (τfast) was essentially voltage independent, whereas the time constant of the slow component (τslow) increased as the voltage step was made more positive (Fig. 4C, top right). We also found that the decay in tail currents following the termination of depolarizing voltage steps could be fitted by a double-exponential function (Fig. 4D, left, bottom red trace) whose fast and slow components contributed about equally to amplitude (Fig. 4D, bottom right) and whose time constants were voltage independent (Fig. 4D, top right). The two components to the time-dependent decay in currents may reflect the accumulation or depletion of SCN− taking place in two cellular compartments: a relatively small extracellular compartment corresponding to basal membrane infoldings wherein flux-associated SCN− concentration changes occur rapidly and a larger compartment consisting of the cytoplasm where SCN− concentration changes develop more slowly (7).
Fig. 4.
Time dependence of currents in the presence of 500 µM external thiocyanate (SCN−). A: families of currents recorded in the same mouse retinal pigment epithelial (RPE) cell in the absence (top) and presence (bottom) of 500 µM SCN−. The membrane potential was held at −60 mV between voltage steps in the range of −120 mV to +50 mV. Open block arrows point to the outward current and inward tail current produced by a voltage step to +50 mV and the closed block arrows indicate the inward current and outward tail current produced by a voltage step to −120 mV. B: voltage dependence of instantaneous and steady-state currents elicited from a holding potential of −60 mV in the absence and presence of 500 µM external SCN−. Instantaneous currents were measured 10 ms after the onset of the voltage steps (●), and steady-state currents were measured 10 ms before their termination (■). Symbols represent means and the bidirectional error bars represent SE; n = 7 cells from two C57BL/6J mice. C: kinetics of the time-dependent decay in currents in 500 µM SCN−. Left: time course of current decay during voltage steps from a holding potential of −60 to 0 mV (top), fitted by a double-exponential function (red trace) or to −120 mV (bottom), and fitted by a single exponential function (red trace). Right: voltage dependence of the time constant (τ; top) and amplitude (A; bottom) of the fast and slow components of the transient current obtained from single or double-exponential fits. Data shown represent means ± SE; n = 7 cells from 2 C57BL/6J mice). D: kinetics of the time-dependent decay in tail currents in 500 µM SCN−. Left: time course of tail current decay at −60 mV following the offset of voltage steps to −120 mV (top) fitted by a single-exponential function (red trace) or to 0 mV (bottom), fitted by a double-exponential function (red trace). Right: voltage dependence of the time constant (top) and amplitude (bottom) of the fast and slow components of the tail current obtained from single or double-exponential fits. Data shown represent means ± SE; n = 5–7 cells from 2 C57BL/6J mice.
Time-dependent decay in currents is a consequence of changes in Erev.
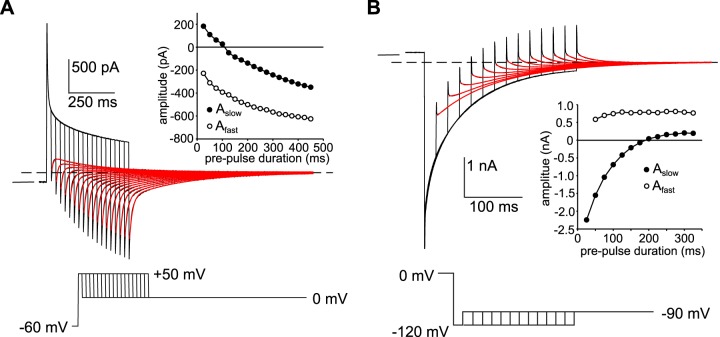
In our previous study, we obtained evidence that the time-dependent decay of whole cell current in mouse RPE cells exposed to 10 mM SCN− results largely from the accumulation or depletion of intracellular SCN− and the consequent decrease in driving force (Vm – ESCN) (7). To determine whether the decay in outward current that occurs in the presence of 500 µM external SCN− is associated with a change in driving force, we varied the duration of depolarizing voltage steps to +50 mV from a holding potential of −60 mV and monitored tail currents at 0 mV (Fig. 5A). The decay in the tail current at 0 mV was biphasic and could be fitted by a double-exponential function (red traces), revealing two components. The polarity of the slow component was outward for depolarizing steps of short duration but inward for steps of longer duration (Fig. 5A, inset, closed circles). The fast component, on the other hand, was inward for all durations, with its amplitude increasing as the duration of the depolarizing voltage step was increased (Fig. 5A, inset, open circles). A straightforward explanation for the reversal in polarity of the slow component of the tail current is that when the membrane potential was stepped from −60 mV to +50 mV, it took ∼100 ms for the influx of SCN− to elevate [SCN−]in to 500 µM (ESCN = Vm = 0 mV); at earlier times, [SCN−]in was <500 µM, and SCN− influx occurred when the membrane potential was stepped to 0 mV (producing outward tail currents), whereas at later times [SCN−]in was >500 µM, and consequently SCN− efflux occurred at 0 mV (producing inward tail currents). The basis for the growth in the inward fast component of the tail currents is not clear but might be explained by an increase in the rate of SCN− efflux into a small diffusion-limited space such as basal membrane infoldings, as [SCN−]in increased with progressively longer depolarizing voltage steps.
Fig. 5.
Time-dependent decay in currents is associated with changes in thiocyanate equilibrium potential (ESCN). A: tail currents recorded in a C57BL/6J mouse retinal pigment epithelial (RPE) cell in the presence of 500 µM external thiocyanate (SCN−) elicited by stepping the membrane voltage to 0 mV after depolarizing the membrane potential to +50 mV for different durations from a holding potential of −60 mV. The horizontal interrupted line marks the steady-state current at 0 mV and the short horizontal unbroken line the zero-current potential. The pipette and bath solutions contained 140 mM Cl− and 145.6 mM Cl−, respectively. The red traces are best fits of the tail currents to a double-exponential function. Tail currents following brief depolarizations had a fast inward component followed by a slow outward component. Inset: amplitudes of the slow and fast components of tail current plotted as a function of the duration of the voltage step to +50 mV. The slow component of tail currents was outward for depolarizing voltage steps of short duration and inward for voltage steps of longer duration. The reversal in tail current polarity indicates that the time-dependent decay in outward current is associated with the intracellular accumulation of SCN−. Results are representative of experiments in 6 cells from 2 C57BL/6J mice. B: tail currents recorded in a C57BL/6J mouse RPE cell in the presence of 500 µM external SCN− elicited by stepping the membrane voltage to −90 mV after hyperpolarizing the membrane potential to −120 mV for different durations from a holding potential of 0 mV. The pipette and bath solutions contained 140 mM Cl− and 145.6 mM Cl−, respectively. The horizontal interrupted line marks the steady-state current at −90 mV and the short horizontal unbroken line the zero-current potential. The red traces are best fits of the tail currents to a double-exponential function. Inset: amplitudes of the slow and fast components of tail current plotted as a function of the duration of the voltage step to −120 mV. The slow component was inward for hyperpolarizing voltage steps of short duration and outward for voltage steps of long duration. The reversal in tail current polarity indicates that the time-dependent decay in inward current is associated with the depletion of intracellular SCN−. Results are representative of experiments in 7 cells from 2 C57BL/6J mice.
We used a similar strategy to investigate the time-dependent decay of inward current. Figure 5B shows the results of an experiment in which we held the membrane voltage at 0 mV and monitored tail currents at −90 mV following hyperpolarizing voltage steps to −120 mV of various durations. The decay in the tail current at −90 mV was biphasic and could be fitted by a double-exponential function (Fig. 5B, red traces), again revealing two components. The slow component of the tail current was inward following a 25-ms hyperpolarizing voltage step, and as the duration of the hyperpolarizing voltage step was increased its amplitude decreased until it reversed polarity (Fig. 5B, inset, closed circles). The polarity of the fast component, on the other hand, was outward for hyperpolarizing steps of all durations (Fig. 5B, inset, open circles). The observation that the slow component of the tail current decreased in amplitude and then reversed polarity is consistent with the idea that the inward current evoked by the hyperpolarizing voltage step reflects the efflux of SCN− that had accumulated intracellularly at the holding potential of 0 mV and that its time-dependent decay results from a gradual depletion of intracellular SCN− and consequent decrease in driving force for SCN− efflux. The basis for the fast component of the tail currents at −90 mV is not clear, but it might reflect the influx of SCN− that had accumulated within basal membrane infoldings during the hyperpolarizing voltage step to −120 mV.
Responses to rapid changes in extracellular [SCN−].
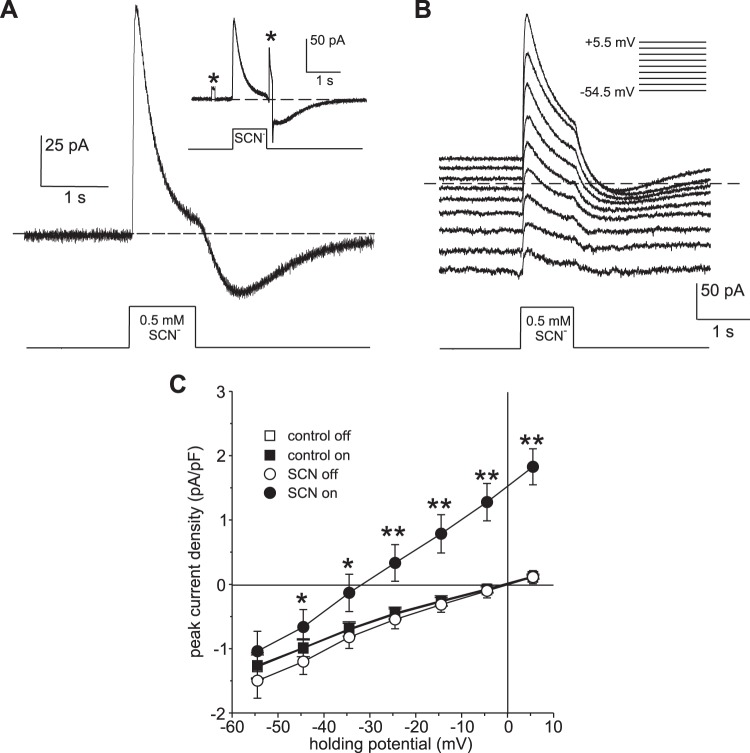
As an alternative approach to evoking SCN− currents, we rapidly changed the external [SCN−] in the vicinity of isolated RPE cells by pressure-ejecting SCN− from a second pipette while holding the membrane potential constant. Figure 6A shows the time course of changes in the whole cell current of an RPE cell at a holding potential of 0 mV in response to the pressure ejection of 500 µM SCN− for 1 s. The local application of SCN− evoked a rapid increase in outward current, which reached a peak amplitude of 113 pA before declining toward baseline. Following the offset of pressure ejection and washout of SCN− from the bath, an inward current grew and then declined toward baseline. The inward current cannot be attributed to Na+ influx because the bathing solution used in this experiment was Na+ free. To determine whether the decline in current during exposure to SCN− is associated with a decrease in membrane conductance, we applied +10 mV voltage steps before and immediately following the pressure ejection of SCN−. As can be seen from the inset of Fig. 6A, the change in current produced by the voltage step (marked by asterisks) increased more than twofold after SCN− exposure, indicating that the decay in outward current was associated with an increase rather than a decrease in membrane conductance. The decline in outward current in the continued presence of external SCN− and the appearance of a transient inward current following SCN− washout are consistent with the time-dependent accumulation and depletion of intracellular SCN−, respectively.
Fig. 6.
Pressure ejection of thiocyanate (SCN−) into the local extracellular environment of retinal pigment epithelial (RPE) cells elicits transient outward currents. A: representative whole cell current response of a C57BL/6J mouse RPE cell voltage clamped to 0 mV to the pressure ejection of 500 µM NaSCN (in 140 mM NMDG-Cl bath solution) via a second pipette to the cell exterior. The horizontal interrupted line marks the zero-current level, and the onset and offset of the pressure step applied to the back of the second pipette are indicated below. The recording chamber was constantly superfused with Na+-free bath solution (NMDG substitution). The local application of SCN− elicited an instantaneous outward current that gave way to a slower decline, consistent with rapid SCN− influx, followed by a decrease in the influx rate as SCN− accumulated intracellularly. Upon termination of the pressure ejection and washout of SCN− from the cell exterior, an inward current developed and then declined toward baseline. Because the bath solution was Na+-free, the inward current cannot be attributed to inward Na+ movement but rather likely resulted from the efflux of SCN− that had accumulated intracellularly. Inset: changes in current in the same cell evoked by voltage steps from 0 mV to +10 mV for 100 ms before and shortly after the pressure ejection of SCN−. Asterisks mark changes in current evoked by the voltage steps. The fact that the second voltage-evoked current change was larger than the first indicates that the membrane conductance had increased during exposure to SCN−. B: voltage dependence of the current response to pressure ejection of 500 µM NaSCN (in 140 mM NaCl bath solution). A series of responses were obtained from the same RPE cell at holding potentials ranging from −54.5 mV to +5.5 mV (liquid junction potentials corrected), as indicated by the inset. The horizontal interrupted line marks the zero-current level. The recording chamber was constantly perfused with NaCl bath solution. The amplitude of the SCN−-evoked current decreased with increasing hyperpolarization. C: current-voltage (I-V) relationship of the current evoked by the local application of 500 µM SCN− by pressure ejection. Symbols represent the mean current density of baseline (○) and peak SCN− currents (●) obtained in 5 C57BL/6J mouse RPE cells (3 animals) bathed in 140 mM NaCl solution, to which 500 µM SCN− was applied by pressure ejection; error bars indicate the SE (*P < 0.05; **P < 0.002; two-way ANOVA followed by Sidak’s multiple comparisons test). Also shown are control I-V relationships of currents measured before (control off; □) and during (control on; ■) pressure ejection of solution containing 0 SCN− (n = 6 cells from 1 C57BL/6J mouse). Symbols representing data from these control experiments overlap, reflecting no significant difference.
Figure 6B shows a family of currents obtained in a different cell produced by the pressure ejection of 500 µM SCN− while holding the membrane potential at various voltages. The application of SCN− produced a transient outward current relative to the baseline current at all voltages tested, with the amplitude of the transient current decreasing as the holding potential was made more negative. The results of this and similar experiments obtained in four other cells are summarized in Fig. 6C, which plots the average amplitude of the baseline (open circles) and peak SCN− current (closed circles) as a function of holding potential. The I-V plots show that SCN− produced an increase in slope conductance and a negative shift in Erev from an average of −0.5 ± 4.5 mV to −31.5 ± 6.1 mV (± SE). In control experiments, pressure ejection of SCN−-free solution had no significant effect on currents over this same voltage range (Fig. 6C, open and closed squares). Using the Goldman-Hodgkin-Katz equation (Eq. 2), we calculated PSCN/PCl for each cell from Erev measured from the I-V plot of peak SCN− currents and obtained an average value for PSCN/PCl of 742 ± 157. This value should be considered an underestimate of the actual PSCN/PCl value, as some SCN− likely accumulated intracellularly before the peak current was reached.
Voltage-dependent changes in intracellular [SCN−].
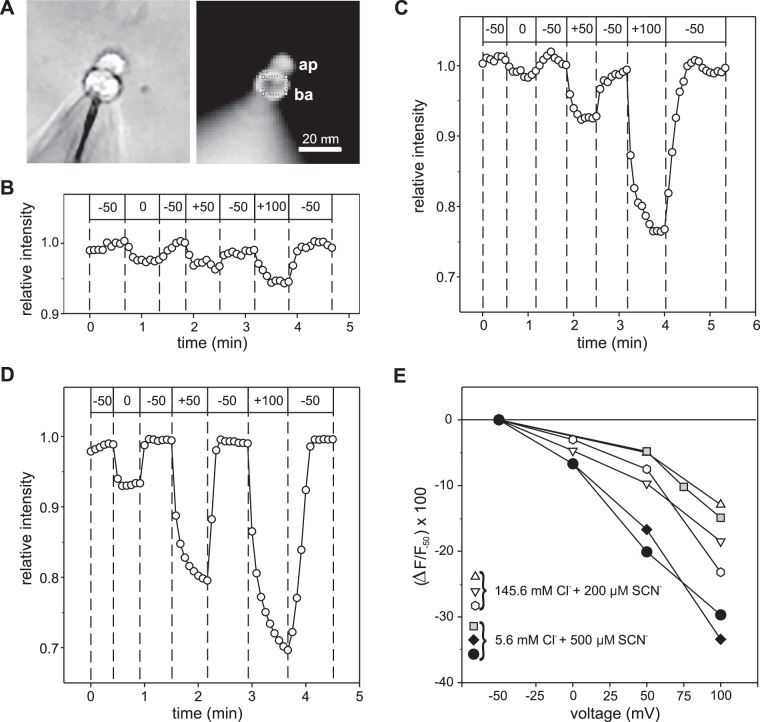
We next investigated voltage-dependent fluxes of SCN− into and out of isolated BALB/c mouse RPE cells under the whole cell voltage clamp condition by monitoring the emission fluorescence of the anion-sensitive dye MQAE, which was loaded into the cytoplasm via the patch pipette. RPE cells from albino BALB/c mice were used in these experiments to avoid the absorption of excitation and emission light by melanin, which is abundant in the melanosomes of C57BL/6J mouse RPE cells. In separate experiments, we confirmed that RPE cells from BALB/c mice exposed to submillimolar concentrations of external SCN− exhibited transient voltage-dependent currents that were indistinguishable from those obtained in RPE cells from C57BL/6J mice (data not shown). Figure 7A shows bright-field (left) and MQAE fluorescence emission images of an isolated BALB/c mouse RPE cell recorded in the whole cell configuration with the patch-pipette solution containing 5 mM Cl− and 1 mM MQAE. Figure 7B shows the time course of voltage-dependent changes in relative MQAE fluorescence intensity in the basolateral pole of this cell with the bathing solution containing 145.6 mM Cl− but no SCN−. Membrane depolarization from −50 mV to 0 mV, +50 mV, and +100 mV produced reversible quenching of MQAE fluorescence indicative of Cl− influx and increases in cytoplasmic Cl− concentration. When depolarizing voltage steps were applied in the same cell after the addition of 200 µM SCN− to the bathing solution, substantially larger decreases in relative fluorescence intensity were obtained in response to depolarizations to +50 mV and +100 mV (Fig. 7C).
Fig. 7.
Changes in membrane voltage produce rapid changes in cytoplasmic thiocyanate concentration. A: representative photomicrographs of a BALB/c mouse retinal pigment epithelium (RPE) cell loaded with N-(ethoxycarbonylmethyl)-6-methoxyquinolium bromide (MQAE) imaged with bright-field (left) and fluorescence (right) optics. Patch pipette used for voltage-clamping membrane potential (Vm) and loading the cell with 0.5 mM MQAE can be seen at the bottom left. The circle superimposed on the basolateral pole (right) indicates the region of interest (ROI) from which the MQAE emission fluorescence was measured in the experiments depicted in B and C. ap, apical pole; ba, basolateral pole. B: effect of changes in membrane voltage on relative MQAE fluorescence intensity in a BALB/c mouse RPE cell in the absence of external SCN−. The bath solution contained 145.6 mM Cl−, and the Cs+-gluconate-based pipette solution contained 5 mM Cl− and 0.5 mM MQAE. The command potentials issued by the voltage clamp amplifier are indicated by the values (mV) in the boxes at the top. Depolarization of Vm from −50 mV to 0 mM, +50 mV, and +100 mV produced reversible and proportional decreases in relative fluorescence, indicating MQAE quenching due to Cl− influx. C: effect of changes in membrane voltage on relative MQAE fluorescence intensity in the same cell after the addition of 200 µM SCN− to the bathing solution. MQAE quenching produced by the depolarizing voltage steps to +50 mV and +100 mV was significantly larger in the presence than in the absence (B) of SCN−, reflecting a relatively high rate of conductive SCN− flux compared with conductive Cl− flux despite its much lower concentration in the bath (0.2 mM SCN− vs. 145.6 mM Cl−). D: voltage-dependent quenching of MQAE fluorescence by SCN− influx under the condition of low external Cl− concentration. Time course of relative fluorescence intensity measured in a different BALB/c mouse RPE cell with 5.6 mM Cl−, 140 mM isethionate, and 500 µM SCN− in the bathing solution and a NMDG-gluconate based pipette solution containing 5 mM Cl− and 1 mM MQAE. E: summary of voltage-dependent MQAE fluorescence quenching experiments performed under conditions of low and high external Cl− concentrations in 6 cells from 3 BALB/c mice. Each symbol shape represents a different cell, and symbols with the same fill (white, black, or gray) indicate cells from the same mouse. Data are expressed as the percent decrease in fluorescence intensity (∆F) at the test voltage relative to the fluorescence intensity at −50 mV (F−50). The concentrations of external Cl− and SCN− for each experiment are as indicated.
To minimize the contribution of Cl− influx to MQAE fluorescence quenching, we performed additional experiments in which most (all but 5.6 mM) of the Cl− in the bathing solution was replaced with the impermeant anion isethionate. In separate experiments using C57BL/6 mouse RPE cells, we found that replacement of 140 mM Cl− with isethionate or methanesulfonate decreased the whole cell current at 0 mV from 52.4 ± 10.9 pA to −0.1 ± 5.1 pA and the current at +50 mV from 143.4 ± 20.0 pA to 40.6 ± 9.7 pA (n = 6; P < 0.005, two-way paired t-test). Figure 7D depicts the time course of voltage-induced changes in relative fluorescence intensity in a BALB/c mouse RPE cell loaded with 500 µM MQAE and bathed in low Cl− solution containing 500 µM SCN− and shows that the degree of MQAE fluorescence quenching was proportional to the size of the depolarizing voltage step. Of note is that the kinetics of fluorescence quenching during membrane depolarization and recovery of fluorescence during repolarization were also voltage dependent, with the changes being complete within 10 s for the voltage step to 0 mV but taking >25 s for the voltage step to +100 mV. Figure 7E summarizes the results obtained in six cells from three mice superfused with solutions containing 200 µM or 500 µM SCN− and high or low external Cl− and expresses the percent change in fluorescence relative to the baseline fluorescence at −50 mV. Overall, the voltage-dependent decrease in MQAE fluorescence was proportional to degree of depolarization. The relative change in MQAE fluorescence averaged −5.3 ± 1.0% for voltage steps to 0 mV (means ± SE; n = 4), −10.6 ± 2.9% for voltage steps to +50 mV (n = 6), and −22.1 ± 3.7% for voltage steps to +100 mV (n = 6; P < 0.05 for all comparisons, except for voltage steps to 0 mV vs. voltage steps to +50 mV, mixed-effects model followed by Tukey’s multiple comparisons test). The variation in the responses of the cells may reflect differences in cell volume, SCN− conductance, or MQAE fluorescence quenching by immobile intracellular anions. Although Cl− flux across the cell membranes may have contributed to the voltage-dependent MQAE fluorescence quenching and its recovery, especially in experiments in which the extracellular Cl− concentration was high, the fluxes of SCN− into and out of the cell were likely the major factor. We conclude that conductive fluxes of SCN− across the RPE cell membranes elicited by voltage steps cause changes in intracellular SCN− concentration on a time scale of seconds. These results provide strong support for the voltage-dependent intracellular SCN− accumulation/depletion hypothesis developed on the basis of our electrophysiological data.
DISCUSSION
Previously, we showed that exposure of isolated mouse RPE cells to 10 mM external SCN− produced large transient currents whose time-dependent decay is consistent with intracellular SCN− accumulation/depletion (7). We also determined from Erev measurements that the relative permeability for SCN− (PSCN/PCl) is at least 500. In the present study, we show by several different approaches that substantial SCN− fluxes occur across the RPE cell membranes in the presence of external SCN− in submillimolar concentrations and that these fluxes are sufficiently large to alter the intracellular concentration of SCN− on a time scale of seconds.
Electrophysiological responses of RPE cells to submillimolar extracellular SCN−.
We found that step changes in the membrane potential of mouse RPE cells exposed to submillimolar concentrations of SCN− produced transient currents and tail currents whose amplitudes were concentration dependent (Fig. 1). Remarkably, these SCN−-induced currents were evident at concentrations of 50 µM and 100 µM (Figs. 1 and 3), which fall within the range of SCN− levels reported for the plasma of normal individuals (22, 23).
As we have described previously (7), the time-dependent decay of whole cell currents in external SCN− is consistent with a scenario in which SCN− flux across the RPE cell membranes causes the intracellular SCN− concentration to rapidly approach electrochemical equilibrium following changes in membrane voltage. This accumulation/depletion model is supported by two observations in the present study. 1) Erev of instantaneous currents in 500 µM external SCN− was within several millivolts of the holding potential when the membrane was held at 0 mV or −60 mV (Fig. 2), consistent with [SCN−]in approaching electrochemical equilibrium in the steady state; and 2) tail currents reversed polarity as the duration of hyperpolarizing or depolarizing voltage steps was increased, consistent with thiocyanate equilibrium potential (ESCN) changing as the decay in inward or outward current progressed (Fig. 5). In addition to evoking currents by stepping the membrane voltage at constant [SCN−]ex, we elicited currents by rapidly changing the local external SCN− concentration by pressure ejection of 500 µM SCN− via a second pipette while holding the membrane voltage constant. Currents evoked by this protocol also exhibited time-dependent decay, consistent with intracellular SCN− accumulation.
We estimated PSCN/PCl from Erev values measured under two different protocols designed to minimize [SCN−]in: 1) instantaneous currents evoked by voltage steps from a holding potential of −120 mV in the presence of 500 µM SCN− (Fig. 3D), which yielded a PSCN/PCl value of ∼2,000 and 2) peak currents produced by the pressure ejection of 500 µM SCN− at various holding potentials (Fig. 6C), which gave a PSCN/PCl value of ∼750. Because intracellular SCN− has a depolarizing influence on Erev, these estimated PSCN/PCl values likely underestimate the actual PSCN/PCl value. These results provide additional support to the conclusion made previously by us that the anion conductance of the RPE cell membrane has an extraordinarily large relative permeability for SCN−.
Fluorescence imaging of voltage-dependent changes in intracellular [SCN−].
As an alternative approach to assessing voltage-dependent SCN− fluxes across RPE cell membranes by current measurements, we loaded RPE cells with the anion-sensitive dye MQAE via a patch pipette and monitored changes in its emission fluorescence resulting from voltage-clamped changes in membrane potential. MQAE is collisionally quenched by halide ions, resulting in a concentration-dependent decrease in fluorescence. The efficiency of the quenching process is about twofold greater for SCN− than it is for Cl−, at least in free solution (25). To minimize MQAE fluorescence quenching by Cl−, we dialyzed cells with solutions containing 5 mM Cl− and, in some experiments, decreased the external Cl− concentration to 5.6 mM as well. We found that in the presence of 200 or 500 µM external SCN−, cytoplasmic MQAE fluorescence intensity decreased reversibly when the RPE cell membrane potential was depolarized from a holding potential of −50 mV. The size of the responses varied among cells (Fig. 7E), possibly due to differences in cell volume, SCN− conductance, or quenching by fixed cytoplasmic anions, but overall, the extent and rate of fluorescence quenching was proportional to the size of the membrane depolarization, consistent with voltage-dependent SCN− influx and intracellular accumulation. Taken together, our results provide strong support for the idea that the mechanism underlying the time-dependent decay in voltage-clamped currents observed in the presence of a submillimolar concentration of SCN− in the external solution is the accumulation or depletion of SCN− in the cytoplasm.
Physiological significance.
In common with other epithelial cells, RPE cells are morphologically and functionally polarized, with an asymmetric distribution of transporters and ion channels in its apical and basolateral membranes. Previously, we measured macroscopic currents in excised membrane patches from isolated mouse RPE cells to show that the basolateral membrane accounts for a significant fraction of the whole cell SCN− conductance (7). In situ, the RPE basolateral membrane faces the choriocapillaris and is exposed to SCN− circulating in the blood. Studies in living rat showed that the injection of SCN− into the systemic circulation produced a negative-going change in the transocular potential (19), which was hypothesized to have originated as a hyperpolarization of the RPE basolateral membrane potential (Vba) arising from a diffusion potential across the basolateral membrane anion conductance. The results of our present study provide support for such a mechanism by showing that the application of 500 µM SCN− to the outside of mouse RPE cells generates a hyperpolarizing current at −55 mV, a value close to the RPE basolateral membrane resting potential (14, 21).
SCN− is derived from cyanogenic foods and its level in the plasma typically is in the range from 25 to 100 µM but can exceed 150 µM in heavy tobacco smokers (22, 23). Our results indicate that at these concentrations SCN− readily crosses the RPE basolateral membrane and, in the absence of SCN− transport activity by other mechanisms, may reach an intracellular concentration dictated by Vba. In mammalian RPE, Vba is generally in the range of −55 to −60 mV (14, 21). According to the Nernst equation, the intracellular SCN− concentration at electrochemical equilibrium would be about 10-fold lower than that in the plasma, in the range of 2.5 to 15 µM. Given that lysosomes have a high membrane permeability for SCN− (16) and a lumen-positive membrane potential (27), it seems likely that SCN− would accumulate in the lumen of RPE lysosomes. Recently, it was shown that myeloperoxidase (MPO), a heme peroxidase secreted by neutrophils, is taken up by cultured RPE and delivered to its lysosomes (29). Extracellularly, MPO catalyzes the oxidation of Cl− by H2O2 to form HOCl, a potent bactericide, but in the presence of SCN−, its preferred substrate, MPO generates HOSCN (28). In inflammatory conditions of the retina, such as uveitis, MPO taken up into RPE lysosomes could catalyze the production of HOSCN from SCN− and H2O2, which potentially could impair lysosomal function by oxidizing thiol groups of key proteins (2).
SCN− is actively transported by a number of epithelia. For example, tracheal epithelial cells secrete concentrated SCN− into the lumen of airways by a mechanism involving the uptake of SCN− at the basolateral membrane by the sodium-iodide symporter (NIS/SLC5A5) (11), a secondary active transporter that couples the influx of two sodium ions and one thiocyanate ion (10). On the other hand, the choroid plexus actively transports SCN− out of the cerebral spinal fluid (CSF) via a mechanism that is blocked by ClO4− (26), an NIS inhibitor (10), resulting in a 10-fold lower concentration of SCN− in the CSF than in plasma (6). It was reported that iodide is transported out of the vitreous humor of rabbits by a ClO4−-sensitive mechanism (3), suggesting the possible expression of NIS in the RPE. If NIS were functional in isolated mouse RPE cells, then the influx of SCN− via this electrogenic transporter may have generated an inward current and contributed to the intracellular accumulation of SCN− in our experiments. However, as NIS-dependent SCN− influx would lead to underestimations of the amplitude of current through the SCN− conductance and PSCN/PCl, activity of this SCN− transporter would not alter our interpretations of the present results. Additional experiments are needed to determine whether NIS is expressed in the RPE and the extent to which it operates in conjunction with the SCN−-selective conductance to mediate the transport of SCN− between the outer retina and the choroidal blood supply.
GRANTS
This work was supported by National Institutes of Health Grant EY-08850, Core Grant EY-07703, and an unrestricted grant from Research to Prevent Blindness.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.C., C.B., and B.A.H. conceived and designed research; X.C., C.B., and B.A.H. performed experiments; X.C. and B.A.H. analyzed data; X.C. and B.A.H. interpreted results of experiments; B.A.H. prepared figures; B.A.H. drafted manuscript; X.C., C.B., and B.A.H. edited and revised manuscript; X.C., C.B., and B.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bikash Pattnaik for contributions leading to design of this study and Donald Puro for helpful discussions.
REFERENCES
- 1.Arai A, Silberg J, Kessler M, Lynch G. Effect of thiocyanate on AMPA receptor mediated responses in excised patches and hippocampal slices. Neuroscience 66: 815–827, 1995. doi: 10.1016/0306-4522(94)00616-D. [DOI] [PubMed] [Google Scholar]
- 2.Aune TM, Thomas EL. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry 17: 1005–1010, 1978. doi: 10.1021/bi00599a010. [DOI] [PubMed] [Google Scholar]
- 3.Becker B. The turnover of iodide in the rabbit eye. Arch Ophthalmol 65: 832–836, 1961. doi: 10.1001/archopht.1961.01840020834017. [DOI] [PubMed] [Google Scholar]
- 4.Blaug S, Quinn R, Quong J, Jalickee S, Miller SS. Retinal pigment epithelial function: a role for CFTR? Doc Ophthalmol 106: 43–50, 2003. doi: 10.1023/A:1022514031645. [DOI] [PubMed] [Google Scholar]
- 5.Bösl MR, Stein V, Hübner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J 20: 1289–1299, 2001. doi: 10.1093/emboj/20.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boxer GE, Rickards JC. Determination of thiocyanate in body fluids. Arch Biochem Biophys 39: 292–300, 1952. doi: 10.1016/0003-9861(52)90338-X. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Pattnaik BR, Hughes BA. Mouse retinal pigment epithelial cells exhibit a thiocyanate-selective conductance. Am J Physiol Cell Physiol 315: C457–C473, 2018. doi: 10.1152/ajpcell.00231.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauner K, Möbus C, Frings S, Möhrlen F. Targeted expression of anoctamin calcium-activated chloride channels in rod photoreceptor terminals of the rodent retina. Invest Ophthalmol Vis Sci 54: 3126–3136, 2013. doi: 10.1167/iovs.13-11711. [DOI] [PubMed] [Google Scholar]
- 9.Erdoǧan MF. Thiocyanate overload and thyroid disease. Biofactors 19: 107–111, 2003. doi: 10.1002/biof.5520190302. [DOI] [PubMed] [Google Scholar]
- 10.Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J Biol Chem 272: 27230–27238, 1997. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 11.Fragoso MA, Fernandez V, Forteza R, Randell SH, Salathe M, Conner GE. Transcellular thiocyanate transport by human airway epithelia. J Physiol 561: 183–194, 2004. doi: 10.1113/jphysiol.2004.071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto K, Esplin DW. Excitatory effects of thiocyanate on spinal cord. J Pharmacol Exp Ther 133: 129–136, 1961. [PubMed] [Google Scholar]
- 13.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, 2001. [Google Scholar]
- 14.Joseph DP, Miller SS. Apical and basal membrane ion transport mechanisms in bovine retinal pigment epithelium. J Physiol 435: 439–463, 1991. doi: 10.1113/jphysiol.1991.sp018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keckeis S, Reichhart N, Roubeix C, Strauß O. Anoctamin2 (TMEM16B) forms the Ca2+-activated Cl− channel in the retinal pigment epithelium. Exp Eye Res 154: 139–150, 2017. doi: 10.1016/j.exer.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Klemm AR, Pell KL, Anderson LM, Andrew CL, Lloyd JB. Lysosome membrane permeability to anions. Biochim Biophys Acta 1373: 17–26, 1998. doi: 10.1016/S0005-2736(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 17.Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci USA 97: 12758–12763, 2000. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 157: 1380–1392, 2014. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pautler EL, Noell WK. The slow ERG potentials in hereditary retinal dystrophy of albino and pigmented rats. Exp Eye Res 22: 493–503, 1976. doi: 10.1016/0014-4835(76)90187-1. [DOI] [PubMed] [Google Scholar]
- 20.Pollay M, Kaplan R. Transependymal transport of thiocyanate. J Neurobiol 3: 339–346, 1972. doi: 10.1002/neu.480030407. [DOI] [PubMed] [Google Scholar]
- 21.Quinn RH, Miller SS. Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci 33: 3513–3527, 1992. [PubMed] [Google Scholar]
- 22.Robertson AS, Burge PS, Cockrill BL. A study of serum thiocyanate concentrations in office workers as a means of validating smoking histories and assessing passive exposure to cigarette smoke. Br J Ind Med 44: 351–354, 1987. doi: 10.1136/oem.44.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saloojee Y, Vesey CJ, Cole PV, Russell MA. Carboxyhaemoglobin and plasma thiocyanate: complementary indicators of smoking behaviour? Thorax 37: 521–525, 1982. doi: 10.1136/thx.37.7.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Haeringen NJ, Ensink FT, Glasius E. The peroxidase-thiocyanate-hydrogenperoxide system in tear fluid and saliva of different species. Exp Eye Res 28: 343–347, 1979. doi: 10.1016/0014-4835(79)90095-2. [DOI] [PubMed] [Google Scholar]
- 25.Verkman AS, Sellers MC, Chao AC, Leung T, Ketcham R. Synthesis and characterization of improved chloride-sensitive fluorescent indicators for biological applications. Anal Biochem 178: 355–361, 1989. doi: 10.1016/0003-2697(89)90652-0. [DOI] [PubMed] [Google Scholar]
- 26.Wright EM. Active transport of iodide and other anions across the choroid plexus. J Physiol 240: 535–566, 1974. doi: 10.1113/jphysiol.1974.sp010622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol 77: 57–80, 2015. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Szép S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci USA 106: 20515–20519, 2009. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yogalingam G, Lee AR, Mackenzie DS, Maures TJ, Rafalko A, Prill H, Berguig GY, Hague C, Christianson T, Bell SM, LeBowitz JH. Cellular uptake and delivery of myeloperoxidase to lysosomes promote lipofuscin degradation and lysosomal stress in retinal cells. J Biol Chem 292: 4255–4265, 2017. doi: 10.1074/jbc.M116.739441. [DOI] [PMC free article] [PubMed] [Google Scholar]