Abstract
Astrocytes (ACs) are the most abundant cells in the central nervous system. Retinal ACs play an important role in maintaining the integrity of retinal neurovascular function, and their dysfunction contributes to the pathogenesis of various eye diseases including diabetic retinopathy. Cytochrome P450 1B1 (CYP1B1) expression in the neurovascular structures of the central nervous system including ACs has been reported. We previously showed that CYP1B1 expression is a key regulator of redox homeostasis in retinal vascular cells. Its deficiency in mice resulted in increased oxidative stress and attenuation of angiogenesis in vivo and proangiogenic activity of retinal vascular cells in vitro. Here, using retinal ACs prepared from wild-type (Cyp1b1+/+) and Cyp1b1-deficient (Cyp1b1−/−) mice, we determined the impact of Cyp1b1 expression on retinal AC function. We showed that Cyp1b1−/− retinal ACs were more proliferative and migratory. These cells also produced increased amounts of fibronectin and its receptors, αvβ3- and α5β1-integrin. These results were consistent with the increased adhesive properties of Cyp1b1−/− ACs and their lack of ability to form a network in Matrigel. This was reversed by reexpression of Cyp1b1 in Cyp1b1−/− ACs. Although no significant changes were observed in Akt/SRC/MAPK signaling pathways, production of inflammatory mediators bone morphogenetic protein-7 (BMP-7) and monocyte chemoattractant protein-1 (MCP-1) was decreased in Cyp1b1−/− ACs. Cyp1b1−/− ACs also showed increased levels of connexin 43 phosphorylation and cluster of differentiation 38 expression when challenged with H2O2. These results are consistent with increased proliferation and diminished oxidative stress in Cyp1b1−/− cells. Thus, Cyp1b1 expression in ACs plays an important role in retinal neurovascular homeostasis.
Keywords: cell adhesion, extracellular matrix proteins, inflammation, oxidative stress, thrombospondins
INTRODUCTION
Cytochrome P450 (CYP) enzymes are membrane-bound, heme-containing terminal oxidases involved in metabolic activation and detoxification of many compounds. CYP450s are expressed in the vasculature and utilize endogenous substrates, such as arachidonic acid, to generate intracellular second messengers that modulate vascular tone, blood flow, and angiogenesis (10, 11, 14). Cytochrome P450 1B1 (CYP1B1) belongs to the family of CYP450s that catalyzes NADPH-supported monooxygenation of diverse xenobiotics and endogenous molecules (28, 35). Its expression is conserved in the early embryo across many species during the development of ocular tissues, hindbrain, and neural crest (5). CYP1B1 gene mutations are a risk factor for development of primary congenital glaucoma. However, the molecular and cellular mechanisms by which CYP1B1 expression influences the development and maintenance of ocular function need further investigation.
We previously showed that Cyp1b1 expression plays a significant role in retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy (OIR). Mice deficient in Cyp1b1 (Cyp1b1−/−) failed to undergo neovascularization during OIR. We also showed that lack of Cyp1b1 in retinal vascular cells was associated with increased oxidative stress, sustained activation of NF-κB, and upregulation of thrombospondin-2 (TSP2), an endogenous inhibitor of angiogenesis. Thus, Cyp1b1 expression in retinal vascular cells regulates their proangiogenic function, significantly affecting angiogenesis. How Cyp1b1 expression affects retinal astrocyte (AC) function remains largely unknown.
Astrocytes, as a major cellular component of the retinal vasculature, have a prominent role in the retinal neurovascular system by modulating angiogenesis, neurogenesis, and maintenance of the neuroretina function. Astrocytes play a key role in endothelial cell (EC) differentiation and establishment and maintenance of the blood-retina barrier functions (4, 16, 22, 26). They enter the developing retina from the brain along the developing optic nerve, laying down the scaffold that guides retinal vascularization, with expression of fibronectin playing a key role (15, 31, 33). In animals with only partially vascularized retinas, such as rabbit and horse, retinal ACs are not found in the avascular regions of the retina (30, 31, 33). In addition, AC hypoxic response is essential for the retinal pathological neovascularization (39). These findings demonstrate the importance of retinal ACs during retinal vascular development and neovascularization.
Astrocyte dysfunction could contribute to various pathologies including diabetic retinopathy and neurological disorders (13). CYP1B1 expression in ACs from human brain cortex and its inducibility in various astrocytoma cell lines have been reported (21). Furthermore, recent studies have linked altered CYP1B1 expression to the development and progression of astrocyte/glial tumors (1). However, expression of Cyp1b1 in retinal ACs and its impact on their function remain to be explored. Here we isolated retinal ACs from Cyp1b1+/+ and Cyp1b1−/− mice as previously described by us (29). We showed that Cyp1b1 is constitutively expressed in retinal ACs. The Cyp1b1−/− cells exhibited alterations in their rate of proliferation, migration, and adhesion and failed to undergo network formation in Matrigel. In addition, Cyp1b1−/− ACs expressed significantly lower levels of proinflammatory mediators but showed increased levels of phosphorylated connexin 43. Cyp1b1−/− ACs were also resistant to H2O2-mediated oxidative stress likely through increased cluster of differentiation 38 (CD38) expression. These results are consistent with increased proliferation of Cyp1b1−/− retinal ACs and their reduced proinflammatory phenotype. Together, our results suggest that the Cyp1b1 expression in ACs is essential for appropriate retinal neurovascular function.
MATERIALS AND METHODS
Experimental animals.
All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health. ImmortoMouse mice expressing a temperature-sensitive simian virus 40 (SV40) large T antigen (Charles River Laboratories, Wilmington, MA) were backcrossed into C57BL/6J mice in our laboratory and further crossed with Cyp1b1−/− mice generated in a C57BL/6J background. The Immorto-Cyp1b1−/− mice were identified by PCR analysis of DNA isolated from tail biopsies. The PCR primer sequences were as follows: Immorto forward: 5′-CCTCTGAGCTATTCCAGAAGTAGTG-3′, Immorto reverse: 5′-TTAGAGCTT TAAATCTCTGTAGGTAG-3′; neomycin forward: 5′-TTGGGTGGAGAGGCTATTCGG CTATGA-3′, neomycin reverse: 5′-GGCGCGAGCCCCTGATGCTC-3′; and Cyp1b1 forward: 5′-CTGAGTTGGACCAGGTTGTGG-3′; Cyp1b1 reverse: 5′-CATGGATTCTAAA CGACTAGG-3′. Both male and female mice were used in the studies presented here.
Isolation and culture of Cyp1b1−/− retinal ACs.
Retinal ACs were isolated from mouse retina by collecting retinas from one litter of 4-wk-old (n = 6–7) mice using a dissecting microscope. Retinas (n = 12–14) were rinsed with serum-free Dulbecco’s modified Eagle’s medium (DMEM; D-5523; Sigma, St. Louis, MO), pooled in a 60-mm dish, minced, and digested for 45 min with collagenase type I (1 mg/ml; LS-004194; Worthington, Lakewood, NJ) in serum-free DMEM at 37°C. Cells were rinsed in DMEM containing 10% fetal bovine serum (FBS) and centrifuged for 5 min at 400 g. Digested cells were rinsed again in DMEM containing 10% FBS and filtered through a double layer of sterile 40-μm nylon mesh (Sefar America, Fisher Scientific, Hanover Park, IL). Cells were centrifuged for 5 min at 400 g, and medium was aspirated. Cells were washed twice with DMEM containing 10% FBS, resuspended in 1 ml of DMEM containing 10% FBS in a 1.5-ml microfuge tube, incubated with rat-anti-mouse CD31 (Mec13.3, 553370; BD Biosciences, Bedford, MA) coated with sheep anti-rat magnetic beads (Invitrogen, Carlsbad, CA), and gently rocked for 1 h at 4°C. Using a Dynal magnetic tube holder (Invitrogen), cells not bound to magnetic beads were collected and washed in DMEM containing 10% FBS. Cells were plated in growth medium in a single well of a 24-well plate coated with human fibronectin (2 μg/ml in serum-free DMEM, CB-40008; BD Biosciences) and incubated at 33°C with 5% CO2. The cells bound to magnetic beads are generally used to remove retinal ECs as we described (34). Retinal ACs were grown in DMEM containing 10% FBS, 2 mM l-glutamine, 2 mM sodium pyruvate, 20 mM HEPES, 1% nonessential amino acids, 100 μg/ml streptomycin, 100 U/ml penicillin, freshly added heparin at 55 U/ml (H-3149-250KU; Sigma), endothelial growth supplement at 100 μg/ml (E-2759; Sigma), and murine recombinant interferon-γ (485-MI-100; R&D Systems, Minneapolis, MN) at 44 U/ml. Cells were maintained at 33°C with 5% CO2. Cells were progressively passed to larger plates, maintained, and propagated in 1% gelatin-coated 60-mm dishes (G-8150; Sigma). For all experiments, cells were used at 70–80% confluence unless stated otherwise, and all experiments were performed in triplicate and repeated at least three times using different cell isolations.
FACS analysis.
Flow cytometry was used to assess the expression of astrocyte markers and integrins in Cyp1b1+/+ and Cyp1b1−/− ACs as described previously (29). Cyp1b1+/+ and Cyp1b1−/− AC cultured plates were rinsed with phosphate-buffered saline (PBS) containing 0.04% EDTA and incubated with 1.5 ml of cell dissociation solution [Tris-buffered saline (TBS; 20 mM Tris·HCl and 150 mM NaCl; pH 7.6) containing 2 mM EDTA and 0.05% BSA]. Cells were rinsed off from the plates with DMEM containing 10% FBS, washed once with 5 ml of TBS, and blocked in 0.5 ml of TBS with 1% goat serum for 20 min on ice. Cells were centrifuged for 5 min at 400 g and medium aspirated, and the cell pellet was resuspended in 0.5 ml of TBS with 1% BSA containing an appropriate dilution of primary antibody as recommended by the supplier and incubated on ice for 30 min. The following antibodies were used: rabbit anti-glial fibrillary acidic protein (GFAP, Z-0334; Dako, Fisher Scientific), rat anti-PDGF receptor-α (PDGFRα, 14140182; eBioscience, Fisher Scientific), rabbit anti-neuroglia proteoglycan 2 (NG2, AB-5320; Millipore, Temecula, CA), mouse anti-β1-integrin (MAB-2000; BD Biosciences), mouse anti-β3-integrin (MAB-1957; BD Biosciences), rat anti-β4-integrin (553745; BD Biosciences), goat anti-β5-integrin (SC-5401; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-β8-integrin (SC-25714; Santa Cruz Biotechnology), Armenian hamster anti-α1-integrin (555001; BD Biosciences), rabbit anti-α2-integrin (558295; BD Biosciences), rabbit anti-α3-integrin (AB-1920; Millipore), rabbit anti-α4-integrin (AB-1924; Millipore), rabbit anti-α5-integrin (AB-1921; Millipore), rat anti-α6-integrin (MAB-1378; Millipore), rat anti-α7-integrin (MAB-3518; R&D Systems), rat anti-αv-integrin (MAB-1930; Millipore), mouse anti-α5β1-integrin (MAB-1999; Millipore), mouse anti-αvβ3-integrin (MAB-1976Z; Millipore), Armenian hamster anti-ICAM-1 (SC-1511; Santa Cruz Biotechnology), rat anti-ICAM-2 (553326; BD Biosciences), rat anti-VCAM-1 (CBL-1300; BD Biosciences), rat anti-endoglin (CBL-1358; BD Biosciences), rat anti-VEGF receptor 1 (VEGFR1, MAB-471; R&D Systems), and rat anti-VEGFR2 (MAB-443; R&D Systems). Following incubation, cells were washed twice with TBS containing 1% BSA and then incubated with appropriate FITC-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) prepared in TBS with 1% BSA for 30 min on ice. The stained cells were washed twice with TBS containing 1% BSA, resuspended in 0.5 ml of TBS with 1% BSA, and analyzed by FACScan Calibur flow cytometer (Becton Dickinson, San Jose, CA). The representative mean fluorescence intensities are shown in each histogram.
Cell proliferation assays.
Cell proliferation assay was performed by counting the number of cells every other day for 2 wk. Cells (2 × 104) were plated on multiple sets of gelatin-coated 60-mm tissue culture dishes. The number of cells was determined by counting using a hemocytometer every other day, on days not fed, in triplicate. The rate of DNA synthesis was also measured using Click-iT EdU Alexa Fluor 488 kit as recommended by the supplier (C-10425; Invitrogen). The assay measures incorporation of 5-ethynyl-2′-deoxyuridine (EdU), a nucleoside analog of thymidine, during cell proliferation. Cyp1b1+/+ and Cyp1b1−/− ACs were plated at 5 × 105 cells on 60-mm tissue culture dishes and were incubated with 10 μM EdU in growth medium for 2 h at 33°C. DNA synthesis was analyzed by measuring incorporated EdU using the FACScan Calibur flow cytometer (Becton Dickinson). Ten thousand cells were analyzed for each sample, and three independent experiments were performed with two different isolations of ACs. The mean percentage of EdU-positive cells was determined for each genotype.
Apoptosis assays.
The level of apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP terminal nick end labeling (TUNEL) staining. Cells were cultured on fibronectin-coated chamber slides (2 µg/ml in plain DMEM) and fed with growth medium overnight. A group of cells were also challenged with H2O2 (2 mM, H-325; Fisher Scientific) or staurosporine (100 nM, S-4400; Sigma) for 24 h. Following incubation, cells were washed twice with PBS, fixed with 4% paraformaldehyde for 20 min, and washed with PBS twice. Cells were permeabilized with 0.5% Triton X-100 in PBS for 10 min. Apoptosis was assessed using Click-iT TUNEL Alexa Fluor Imaging Assay as recommended by the supplier (C-10246; Invitrogen). Positive cells were counted under fluorescence microscope, and the percentage of apoptotic cells relative to the total number of cells per 10 high-power fields (×400) was determined. Alternatively, the levels of apoptosis were determined by measuring caspase 3/7 activity using the Caspase-Glo 3/7 assay as recommended by the suppliers (G-8090; Promega, Madison, WI) under basal and challenged (100 nM staurosporine for 24 h) conditions.
Scratch wound assays.
Cells (6 × 105) were plated in 60-mm tissue culture plates and allowed to reach confluence (2–3 days). Cell monolayers were wounded with a 1-ml micropipette tip, rinsed with DMEM containing 10% FBS twice, and fed with growth medium containing 1 μM 5-fluorouracil (F-6627; Sigma) to exclude potential contribution of cell proliferation to wound closure. The wound closure was monitored and photographed at 0, 24, and 48 h using a phase microscope in digital format. For quantitative assessment, the distances migrated as percentage of total distance were determined.
Transwell migration assays.
Migration was also determined using Transwell assays. Transwell filters (8 µm, 07200150; Fisher Scientific) were coated with 2 μg/ml fibronectin in PBS and incubated overnight at 4°C. The bottom of the Transwell was rinsed with PBS and blocked with 2% BSA in PBS for 1 h at room temperature. The Transwell was rinsed with PBS, 500 μl of serum-free DMEM were added to the bottom of each well, and 1 × 105 cells in 100 μl of serum-free medium were added to the top of the Transwell membrane. Following 4 h in a 33°C tissue culture incubator, the cells and medium were aspirated, and the upper side of the membrane was wiped with a cotton swab. The cells that had migrated through the membrane and attached to the bottom were fixed with 4% paraformaldehyde (15710; Electron Microscopy Sciences, Hatfield, PA), stained with hematoxylin-eosin (HHS32-HT110132; Sigma), and mounted on a slide. Ten high-power fields (×200) of cells were counted for each condition, and the mean and standard error of the means were determined.
Cell adhesion assays.
Retinal AC adhesion to various extracellular matrix (ECM) proteins was determined as previously described (24). Briefly, 96-well plates (Maxisorb, Nunc, 12565136; Fisher Scientific) were coated with various concentrations of fibronectin (CB-40008), collagen I (CB-40243), collagen IV (CB-40233; all from BD Biosciences), and vitronectin (2349-VN-100; R&D Systems) prepared in TBS with 2 mM Ca2+and 2 mM Mg2+ (Ca/Mg) overnight at 4°C. The next day the plates were rinsed four times with TBS containing Ca/Mg and blocked for 1 h with 200 µl of 1% BSA prepared in TBS with Ca/Mg for at least 1 h at room temperature. Cells were removed using cell dissociation solution (C-5914; Sigma), washed once with TBS, and resuspended in cell-binding buffer (25 mM HEPES and 150 mM NaCl containing 4 mg/ml of BSA; pH 7.4) at 5 × 105 cells/ml. Blocking solution was then removed from the coated plates and washed with TBS Ca/Mg once. Each well received 50 µl of TBS with Ca/Mg and 50 µl of cell suspension in triplicate. Cells were then allowed to adhere for 90 min at 37°C, and nonadherent cells were removed by gently washing the wells with 200 µl of TBS with Ca/Mg until no cells were left in control wells coated with BSA. The number of adherent cells in each well was quantified by measuring the levels of intracellular acid phosphatase. Cells were lysed in 100 µl of lysis buffer [50 mM sodium acetate pH 5.0, 1% Triton X-100, and 4 mg/ml p-nitrophenyl phosphate (P-4744; Sigma)] and incubated at 4°C overnight. The reaction was neutralized by adding 50 µl of 1 M NaOH, and the absorbance was determined at 405 nm using a microplate reader (ThermoMax; Molecular Devices, Fisher Scientific).
Immunofluorescence analysis.
Cells (5 × 104) were plated on fibronectin-coated glass chamber slides until near confluence (1–2 days), washed in 1X MES (M-5287; Sigma), fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 12 min on ice, and blocked with 1% ovalbumin in TBS at 37°C for 20 min. Slides were washed with PBS and incubated with mouse anti-vinculin (V-4505; Sigma), Alexa Fluor 488 phalloidin (A-12379; Thermo Fisher Scientific), rabbit anti-vimentin (ab-92547; Abcam, Cambridge, MA), and rabbit anti-connexin 43 (C-6219; Sigma) in TBS containing 1% ovalbumin at 37°C for 30 min. After washing with PBS, cells were incubated with appropriate Cy3-conjugated secondary antibody (1:500 dilution in TBS containing 1% ovalbumin; Jackson ImmunoResearch) at 37°C for 30 min. Cells were washed with PBS twice and analyzed using a fluorescence microscope (Carl Zeiss Optical), and images were captured in digital format.
Western blot analysis.
Cells (6 × 105) were plated on 60-mm culture dishes and allowed to reach ~90% confluence. The cells were then rinsed once with serum-free DMEM and incubated with growth medium without serum for 2 days. Conditioned medium was collected and centrifuged to remove cell debris. The cells were also lysed in 0.1 ml of lysis buffer [50 mM HEPES pH 7.5, 100 mM NaCl, 0.1 mM EDTA, 1 mM CaCl2, 1 mM MgCl2, 1% Triton X-100, 1% Nonidet P-40, and protease inhibitor cocktail (Roche Biochemicals, Mannheim, Germany)]. Samples were adjusted for protein content, mixed with appropriate volume of 6× SDS-sample buffer, and analyzed by SDS-PAGE (4–20% Tris-glycine gels; Invitrogen). Proteins were transferred to nitrocellulose membrane and incubated in blocking buffer (0.05% Tween 20 and 5% skim milk in TBS) for 1 h at room temperature. The following antibodies were used at dilutions recommended by the supplier: rabbit anti-fibronectin (F-3648; Sigma), rat anti-periostin (MAB-3548; R&D Systems), goat anti-osteopontin (AF-808; R&D Systems), mouse anti-TSP1 (MS-421P clone A6.1; NeoMarkers, Fremont, CA), mouse anti-TSP2 (611150; BD Biosciences), mouse anti-β-actin (MAB-515739; Thermo Fisher Scientific), rabbit anti-nuclear factor erythroid 2-related factor 2 (Nrf2, SC-13032; Santa Cruz Biotechnology), rabbit anti-peroxiredoxin-2 (Prx2, YIF-LF-PA-0091; Enzo Life Sciences, Farmingdale, NY), rabbit anti-heme oxygenase-1 (HO-1, 5853; Cell Signaling Technology), rabbit anti-phospho-JNK (AF-1205; R&D Systems), rabbit anti-JNK (AF-1387; R&D Systems), rabbit anti-phospho-Akt (9271; Cell Signaling Technology), rabbit anti-Akt (9272; Cell Signaling Technology), mouse anti-phospho-Erk1/2 (9106; Cell Signaling Technology), mouse anti-Erk1/2 (9102; Cell Signaling Technology), rabbit-anti-phospho-MAPK p38 (p38, 9211; Cell Signaling Technology), rabbit anti-p38 (9212; Cell Signaling Technology), rabbit anti-phospho-Src (2101; Cell Signaling Technology), and rabbit anti-Src (2123p; Cell Signaling Technology). The anti-Cyp1b1 antibody was a rabbit polyclonal antibody obtained from Dr. Colin Jefcoate (University of Wisconsin). Membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) for 1 h at room temperature, and the protein bands were visualized according to the chemiluminescence procedure (chemiluminescence reagent; GE Biosciences, Fisher Scientific). The same blots were first probed with phosphoantibodies, stripped and probed for total protein, and subsequently probed with mouse anti-β-actin antibody (Thermo Fisher Scientific) for loading control. The mean band intensities were measured densitometrically using ImageJ software (National Institutes of Health, Bethesda, MD).
Matrigel-mediated morphogenesis assays.
Tissue culture plates (35 mm) were coated with 0.5 ml of Matrigel (10 mg/ml, CB-40230; Corning) and allowed to harden by incubating at 37°C for at least 30 min. Cells were removed by trypsin-EDTA, washed with DMEM containing 10% FBS, and resuspended at 2 × 105 cells/ml in growth medium without FBS. Cells (2 × 105) in 2 ml were applied to the Matrigel-coated plates, incubated at 33°C, and photographed after 18 h using a Nikon microscope in a digital format. For quantitative assessment of the data, the mean numbers of branch points were determined by counting the number of branch points in five high-power fields (×100). Longer incubation times did not further improve the network formation.
Adenovirus construction and transduction of retinal ACs.
Replication-deficient adenovirus expressing mouse full-length Cyp1b1 (accession no. NM_009994), under the control of the cytomegalovirus (CMV) promoter, were generated using the pAdTrack-CMV vector and AdEasy System (Agilent/Stratagene, La Jolla, CA) as previously described (17). Retinal ACs were plated in 60-mm dishes and were 80% confluent by the next day. Cells were incubated with either recombinant adenovirus (5–50 plaque-forming units per cell) expressing Cyp1b1 sense cDNA or vector control in 2 ml of Opti-MEM and 10 μl of Lipofectin (Invitrogen) for 24 h in a tissue culture incubator. After incubation, cells were fed with growth medium and incubated for 2 days. Cell lysates were prepared and evaluated for Cyp1b1 expression by Western blot analysis.
VEGF measurements.
The amount of VEGF secreted by Cyp1b1+/+ and Cyp1b1−/− retinal ACs was determined using Mouse VEGF Immunoassay kit (R&D Systems). Cells (6 × 105) were plated on 60-mm tissue culture dishes and allowed to reach ~90% confluence. The cells were then rinsed once with serum-free DMEM and incubated with 2 ml of growth medium without serum for 2 days. The conditioned medium was centrifuged to remove cell debris and stored at −80°C. The cells were also lysed in PBS and sonicated, and cleared lysates were stored at −80°C. The secreted VEGF in conditioned medium and cell lysates were analyzed according to the manufacturer’s instructions. The amount of VEGF was determined using a standard curve generated with known amounts of VEGF in the same experiment.
RNA purification and real-time quantitative PCR analysis.
Total RNA from retinal ACs was extracted using mirVana PARIS kit (Invitrogen). The cDNA synthesis was performed from 1 µg of total RNA using Sprint RT Complete-Double PrePrimed kit (Clontech, Mountain View, CA). One microliter of each cDNA (dilution 1∶10) was used as template in quantitative PCR (qPCR) assays, performed in triplicate of three biological replicates on MasterCycler RealPlex (Eppendorf, Fisher Scientific) using SYBR Green qPCR Premix (Clontech). Amplification parameters were as follows: 95°C for 2 min; 40 cycles of amplification (95°C for 15 s, 60°C for 40 s); dissociation curve step (95°C for 15 s, 60°C for 15 s, 95°C for 15 s). Primer sequences used were as follows: Cyp1b1, 5′-CCAGG CGTCGCACTTGTACT-3′ (forward) and 5′-TGGAAAACGTCGCCAT AGC-3′ (reverse); CD38, 5′-AAGATGTT CACCCTGGAGGA-3′ (forward) and 5′-ACTCCAATGTGGGCAAGAGA-3′ (reverse); VEGF all, 5′-GGAGAGCAGAAGTCCCA TGA-3′ (forward) and 5′-ACTCCAGGGCTTCATCGTTA-3′ (reverse); VEGF 164, 5′-GCAGCTTGAGTTAAAC GAACG-3′ (forward) and 5′-GGTTCCCGAAA CCCTGAG-3′ (reverse); VEGF 164b, 5′-GTTTGTCCAAGATCCGCAGAC-3′ (forward) and 5′-GAGAGGTCT GCAAGTACGTTC-3′ (reverse); VEGF 120, 5′-ATCTTC AAGCCGTCCTGTGTGC-3′ (forward) and 5′-TTGGCTTGTCACATTTTT CTGG-3′ (reverse); bone morphogenetic protein-7 (BMP-7), 5′-GG GCTGGTTGGTGTTTGA-3′ (forward) and 5′-GATGCTCTGCCCATCCAG-3′ (reverse); monocyte chemoattractant protein-1 (MCP-1), 5′-GTCTGTGCTGACCCCAAGAAG-3′ (forward) and 5′-TGG TTCCGATCCAGGTTTTTA-3′ (reverse); inducible nitric oxide synthase (iNOS), 5′-GGC AGCCTGTGAGACCTTTG-3′ (forward) and 5′-CATTGGAAGTGAAGCGTTTCG-3′ (reverse); neuronal nitric oxide synthase (nNOS), 5′-TCG GCTGTGCTTTGATGGA-3′ (forward) and 5′-TTGAA TCGGACCTTGTAGCTCTTC-3′ (reverse); IL-1β, 5′-GTTCCCATTAGACAACTGCACT ACA-3′ (forward) and 5′-CCGACAGCACGAGGCTTTT-3′ (reverse); IL-6, 5′-CAACC ACGGCCTTCCCTACT-3′ (forward) and 5′-TTGGGAGTGGTATCCTCTGTGA-3′ (reverse); INF-γ, 5′-CCACA TTGCTCAGTTCAAGTCTCT-3′ (forward) and 5′-CAC CCTTGGCCTTTTTGAAG-3′ (reverse); and TNF-α, 5′-ACCGTCAGCCGATTTGCTAT-3′ (forward) and 5′-TT GACGGCAGAGAGGAGGTT-3′ (reverse). Standard curves were generated from known quantities for each of the target genes of linearized plasmid DNA. Ten times dilution series were used for each known target, which were amplified using SYBR Green qPCR. The linear regression line for nanograms of DNA was determined from relative fluorescence units (RFU) at a threshold fluorescence value (Ct) to quantify gene targets from cell extracts by comparing the RFU at the Ct to the standard curve, normalized by the simultaneous amplification of 60S ribosomal protein L13a (RpL13a), a housekeeping gene. Primer sequences for RpL13a were 5′-TCTCAAGGTTGTTCG GCTGAA-3′ (forward) and 5′-CCAGAC GCCCCAGGTA-3′ (reverse).
Determination of oxidative stress.
Intracellular reactive oxygen species (ROS) level was determined by dihydroethidium (DHE, D-11347; Invitrogen) staining. For DHE staining, cells were plated on fibronectin-coated (2 µg/ml) chamber slides (3 × 104 cells) and incubated for 1 day. To induce oxidative stress, cells were incubated with 2 mM H2O2 in growth medium for 24 h. Cells were then incubated with 5 µM DHE at 33°C for 20 min. Following incubation, cells were fed with normal growth medium without DHE for 30 min and rinsed with PBS. Fluorescence images were captured using a Zeiss microscope (Carl Zeiss) with digital format (×200). For the quantitative assessment, fluorescence intensities (100 cells per cell type) were analyzed using ImageJ software. Mean fluorescence intensities of Cyp1b1+/+ control cells were regarded as “1,” and relative intensities of Cyp1b1−/− cells were normalized to control cells (means ± SE).
Statistical analysis.
Statistical differences between control and treated samples were evaluated with Student’s unpaired t-test (2-tailed) or one-way ANOVA with Bonferroni posttest for multiple comparisons. P ≤ 0.05 was considered significant. Data are presented as means ± SE from cells with n ≥ 3 (as indicated in figure legends). All data analysis was done in GraphPad Prism or Microsoft Excel.
RESULTS
Cyp1b1 is expressed in retinal ACs.
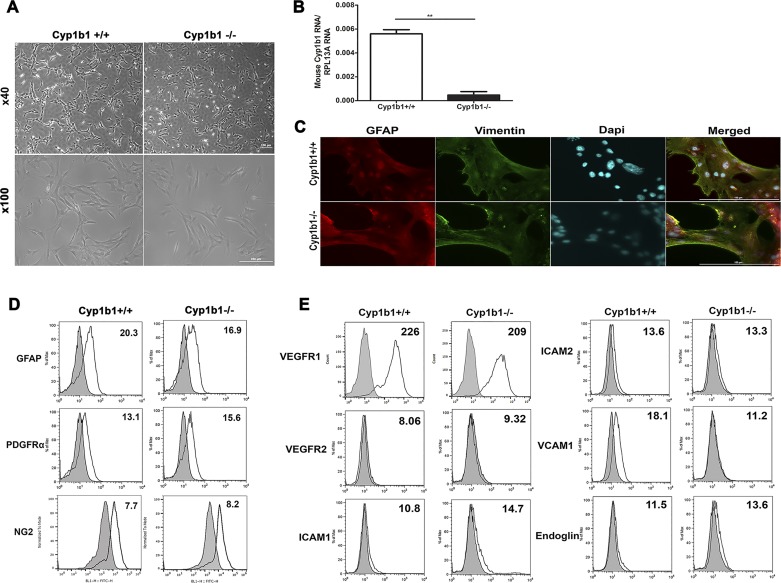
Astrocytes play a vital role in the development, organization, integrity, and function of retinal vasculature and its communication with retinal neurons. They also protect neurons from various toxicities and neurodegenerative diseases. Here we evaluated how expression of Cyp1b1 affects retinal AC function. Retinal ACs, isolated from Cyp1b1+/+ and Cyp1b1−/− mice, showed a similar flattened stellate morphology when cultured on gelatin-coated plates (Fig. 1A). We assessed the expression of Cyp1b1 in these cells by qPCR analysis of cDNA prepared from Cyp1b1+/+ and Cyp1b1−/− retinal ACs (Fig. 1B). Please note that background expression of Cyp1b1 in null cells was as expected. We had previously isolated ACs from mouse retina and confirmed their identity as ACs and their purity by immunofluorescence (29). Here we also examined AC marker expression to elucidate whether Cyp1b1 expression could affect AC characteristics. Cyp1b1+/+ and Cyp1b1−/− ACs were positive for PDGFRα, GFAP, NG2, vimentin, and paired-box protein Pax-2 (Pax2; Fig. 1, C and D). Thus, lack of Cyp1b1 minimally affected the morphology and expression of AC markers. Greater than 98% of ACs were positive for Pax2/vimentin staining (Fig. 1C). We also analyzed the expression of other cell adhesion markers and growth factor receptors in Cyp1b1+/+ and Cyp1b1−/− retinal ACs by flow cytometry (Fig. 1E). The expression of ICAM-1, ICAM-2, VEGFR2, and endoglin was limited in Cyp1b1−/− ACs, but they expressed significant amounts of VEGFR1, with modest VCAM-1 expression compared with Cyp1b1+/+ cells.
Fig. 1.
Isolation of mouse retinal astrocytes (ACs). Wild-type (Cyp1b1+/+) and cytochrome P450 1B1-deficient (Cyp1b1−/−) retinal ACs were isolated and cultured on gelatin-coated 60-mm dishes. A: cells were photographed in digital format at ×40 and ×100 magnification. Scale bars = 250 µm. Please note that there are no differences in retinal AC morphology regardless of Cyp1b1 status. B: expression of Cyp1b1 mRNA determined by quantitative PCR (**P < 0.01; n = 3). C: indirect immunofluorescence staining using an anti-vimentin and anti-paired-box protein Pax-2 (Pax2) antibody was performed as described in materials and methods. DAPI was used to stain cell nuclei. Greater than 98% of cells were positive for vimentin and Pax2 AC markers (n = 3; scale bars = 200 µm). D: Cyp1b1+/+ and Cyp1b1−/− ACs were examined for expression of other AC markers including glial fibrillary acidic protein (GFAP), PDGF receptor-α (PDGFRα), and neuroglia proteoglycan 2 (NG2) by flow cytometry. E: expression of VEGF receptor 1 (VEGFR1), VEGFR2, ICAM-1, ICAM-2, VCAM-1, and endoglin was also determined by flow cytometry. The representative mean fluorescence intensities are indicated in each histogram. Max, maximum; RpL13A, 60S ribosomal protein L13a.
Cyp1b1−/− ACs exhibited enhanced proliferation.
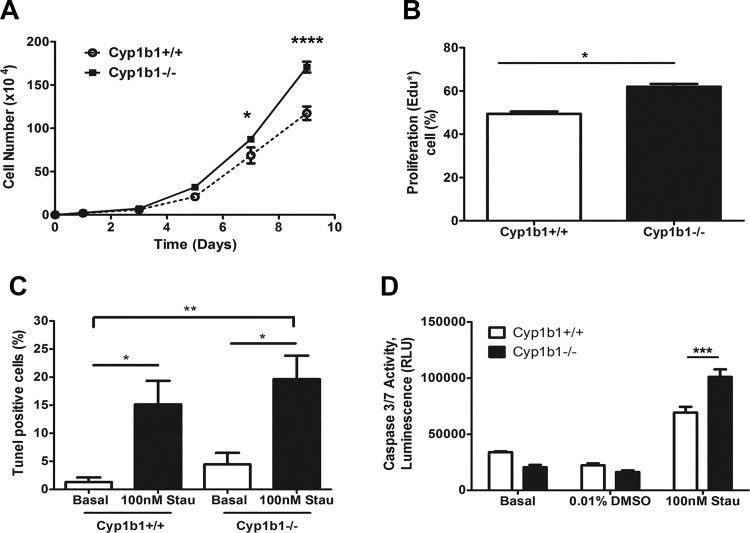
The effects of Cyp1b1 expression on cell proliferation was determined by counting the number of cells over a 2-wk period in culture. Cyp1b1−/− retinal ACs showed a modest but significant increase in proliferation compared with Cyp1b1+/+ cells (Fig. 2A). Next, we determined whether the increased proliferation was due to enhanced DNA synthesis. The percentage of cells undergoing active DNA synthesis was determined by EdU labeling. Cyp1b1−/− ACs displayed increased levels of DNA synthesis compared with Cyp1b1+/+ cells (Fig. 2B). We next determined whether Cyp1b1 expression affects the level of apoptosis. TUNEL staining was performed to assess apoptosis of retinal ACs under basal and challenged conditions. No significant differences in apoptosis levels under basal or challenged [H2O2 (not shown) or staurosporine (Fig. 2C)] conditions were noted. Similar results for the basal level of apoptosis were also observed using the caspase 3/7 activity measurements. However, Cyp1b1−/− cells were significantly more sensitive to staurosporine treatment compared with Cyp1b1+/+ cells (Fig. 2D). Thus, lack of Cyp1b1 in retinal ACs has minimal effects on the basal rate of apoptosis.
Fig. 2.
Cytochrome P450 1B1-deficient (Cyp1b1−/−) retinal astrocytes (ACs) proliferate at a faster rate. A: the rate of cell proliferation was determined by counting the number of cells for 2 wk. Cyp1b1−/− ACs showed an increase in the rate of proliferation compared with Cyp1b1+/+ ACs (*P < 0.05, ****P < 0.0001; n = 3). B: the rate of DNA synthesis was determined using Click-iT 5-ethynyl-2′-deoxyuridine (EdU) labeling. Cyp1b1−/− ACs displayed an enhanced rate of DNA synthesis compared with Cyp1b1+/+ ACs (*P < 0.05; n = 3). C: the degree of apoptosis was determined by terminal deoxynucleotidyl transferase dUTP terminal nick end labeling (TUNEL) staining. Please note that there are no significant differences in the basal levels of apoptosis; staurosporine (Stau) treatments caused a significant increase in the rate of apoptosis compared with untreated cells, but it was not significantly different in Cyp1b1+/+ cells compared with Cyp1b1−/− cells (P > 0.05; *P < 0.05, **P < 0.01; n = 3). D: a similar basal level of apoptosis was also observed in Cyp1b1+/+ and Cyp1b1−/− cells using caspase 3/7 activity measurements. However, the increased rate of apoptosis in response to staurosporine was significant in Cyp1b1−/− cells compared with Cyp1b1+/+ cells (P > 0.05, ***P < 0.001; n = 3). RLU, relative light units.
Cyp1b1−/− ACs are more migratory.
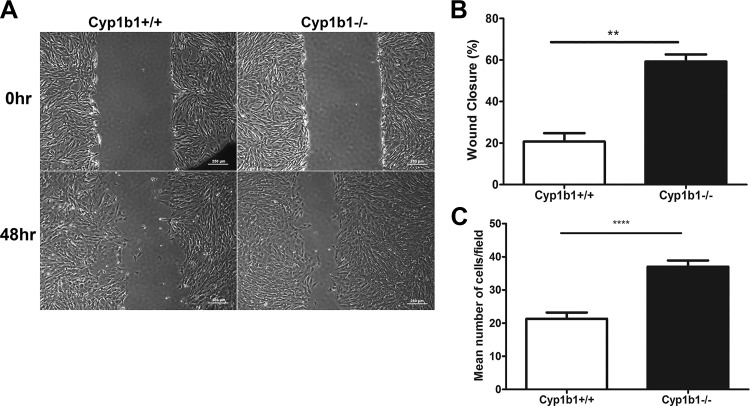
Development of the retinal vasculature proceeds by an invasion of migrating ACs from the optic disk into the retinal periphery. This lays down a fibronectin-rich scaffold for the recruitment of both ECs and pericytes (PCs) into the retina (33, 38). The migratory characteristics of Cyp1b1+/+ and Cyp1b1−/− ACs were determined by scratch wound assays. Cyp1b1−/− ACs exhibited accelerated wound closure compared with Cyp1b1+/+ cells (Fig. 3, A and B). Similar results were observed using a Transwell migration assay, with Cyp1b1−/− ACs demonstrating a twofold increase in the number of cells migrated through the membrane compared with Cyp1b1+/+ cells (Fig. 3C). Thus, Cy1b1 expression affects retinal AC migration.
Fig. 3.
Cytochrome P450 1B1-deficient (Cyp1b1−/−) astrocytes (ACs) are more migratory. Cell migration was assessed using scratch wound and Transwell migration assays as detailed in materials and methods. A: confluent monolayers of Cyp1b1+/+ and Cyp1b1−/− retinal ACs were wounded, wound closure was monitored using a phase microscope, and images were captured in digital format (scale bars = 250 µm). B: quantitative assessment of wound closure (**P < 0.01; n = 3). C: Cyp1b1−/− retinal ACs also migrated faster than Cyp1b1+/+ ACs in Transwell migration assays (****P < 0.0001; n = 4).
Cyp1b1−/− ACs are more adhesive.
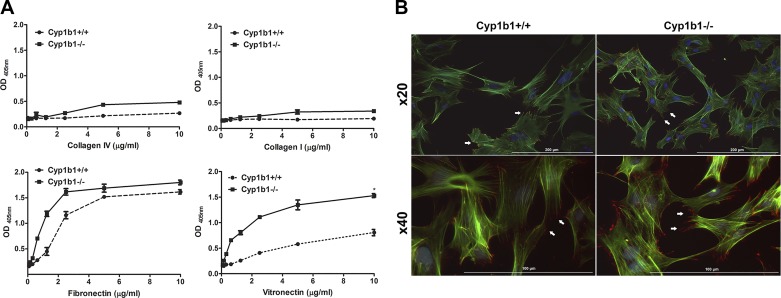
Altered migration in Cyp1b1−/− ACs could be attributed to altered cell adhesion. We next evaluated AC ability to adhere to various ECM proteins, including fibronectin, collagen I, collagen IV, and vitronectin. Cyp1b1−/− ACs showed a significant increase in adhesion on fibronectin and vitronectin compared with Cyp1b1+/+ ACs (Fig. 4A). However, minimal changes in adhesion to collagen I and collagen IV were observed. To confirm the change in migratory and adhesive properties of Cyp1b1−/− cells, the formation of actin stress fibers and distribution of focal adhesions were assessed by indirect immunofluorescence staining using phalloidin and anti-vinculin. An increase in the number of focal adhesions at the periphery and actin stress fibers was observed in Cyp1b1−/− compared with Cyp1b1+/+ ACs (Fig. 4B).
Fig. 4.
Cytochrome P450 1B1-deficient (Cyp1b1−/−) retinal astrocytes (ACs) are more adherent. The adhesion properties of retinal ACs were determined by plating them on various extracellular matrix proteins. A: adhesion of Cyp1b1+/+ and Cyp1b1−/− ACs to collagen IV, collagen I, fibronectin, and vitronectin was determined as described in materials and methods. Please note an increase in adhesion of Cyp1b1−/− ACs on fibronectin and vitronectin (*P < 0.05; n = 3). B: localization of F-actin and vinculin in Cyp1b1+/+ and Cyp1b1−/− retinal ACs determined by immunofluorescence staining. Please note increased levels of actin stress fibers and peripheral focal adhesions in Cyp1b1−/− ACs (white arrows). Higher magnifications are shown in panels at bottom. Scale bars = 200 µm (panels at top) and 100 µm (panels at bottom). OD405nm, optical density at 405 nm.
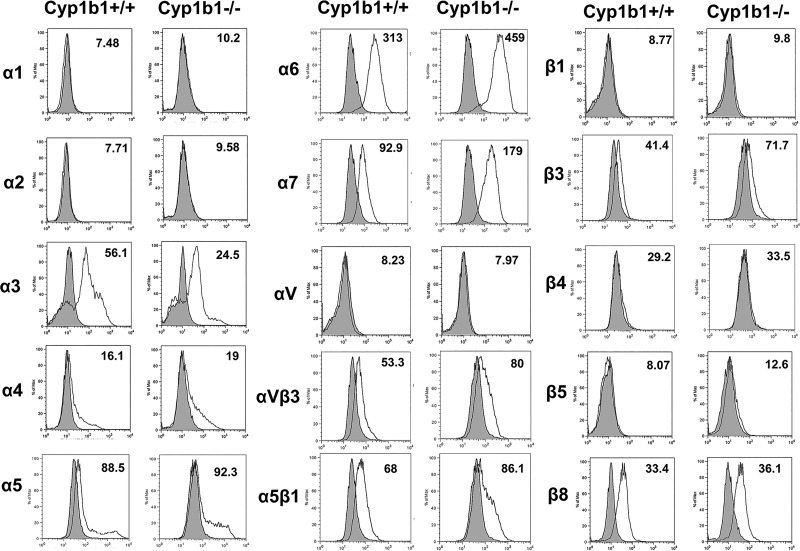
Cell adhesion and migration require binding to the ECM proteins through specific cell surface integrins. To determine whether the alterations observed in adhesion and migration of Cyp1b1−/− ACs were mediated through changes in integrin expression, integrin expression was analyzed by FACS analysis. Cyp1b1+/+ and Cyp1b1−/− ACs exhibited similar levels of α1-, α2-, α4-, α5-, αv-, β1-, β4-, β5-, and β8-integrin. However, Cyp1b1−/− cells exhibited a modest increase in α6-, α7-, β3-, αvβ3-, and α5β1-integrin compared with Cyp1b1+/+. In addition, the level of α3-integrin was decreased in Cyp1b1−/− cells compared with Cyp1b1+/+ cells (Fig. 5). The increased levels of αvβ3- and α5β1-integrin are consistent with the increased adhesion observed in Cyp1b1−/− cells plated on fibronectin and vitronectin.
Fig. 5.
Altered expression of integrins in cytochrome P450 1B1-deficient (Cyp1b1−/−) astrocytes (ACs). Expression of integrins was determined by fluorescence-activated cell sorting analysis of Cyp1b1+/+ and Cyp1b1−/− retinal ACs using specific antibodies as detailed in materials and methods. The numbers in the histograms show mean fluorescence intensity of the representative samples. Cyp1b1−/− ACs were found to express higher levels of α6-, α7-, αvβ3-, and α5β1-integrin compared with Cyp1b1+/+ ACs. Max, maximum.
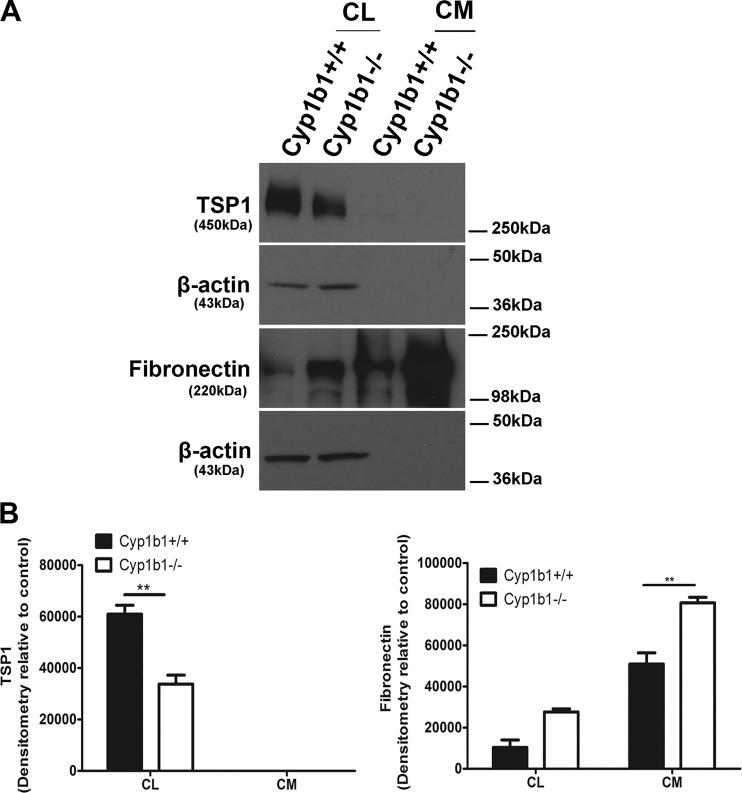
Altered production of different ECM proteins can influence various cellular functions, including providing support, regulating the dynamic behavior of a cell, wound closure, and angiogenesis. We next examined the level of various ECM proteins including fibronectin, secreted protein acidic and rich in cysteine (SPARC), TSP1, TSP2, tenascin C, periostin, and osteopontin by Western blot analysis of conditioned medium and cell lysates (Fig. 6). Although retinal ACs expressed a significant amount of TSP1, no detectable level of TSP2 was observed in these cells, regardless of Cyp1b1 status. Cyp1b1−/− ACs showed decreased amounts of TSP1 in cell lysates, whereas a significant increase in the fibronectin level was observed in cell lysates and conditioned medium (Fig. 6, A and B). The levels of SPARC, tenascin C, osteopontin, and periostin produced by these cells were below the limit of detection regardless of the Cyp1b1 status.
Fig. 6.
Altered production of extracellular matrix proteins in retinal astrocytes (ACs). The conditioned medium (CM) and cell lysates (CL) were collected for analysis of extracellular matrix proteins by Western blot as described in materials and methods. The expression of fibronectin, tenascin C, thrombospondin-1 (TSP1), TSP2, secreted protein acidic and rich in cysteine (SPARC), periostin, and osteopontin was determined using specific antibodies. β‐Actin was used as a loading control for cell lysates. Please note a decrease in the level of TSP1 in cell lysates and an increase in the levels of fibronectin in cell lysates and conditioned medium prepared from cytochrome P450 1B1-deficient (Cyp1b1−/−) ACs compared with Cyp1b1+/+ cells. No TSP1 was detected in the conditioned medium collected from these cells. The expressions of periostin, osteopontin, SPARC, tenascin C, and TSP2 were not detected (**P < 0.01; n = 3).
Lack of Cyp1b1 attenuates morphogenesis of retinal ACs.
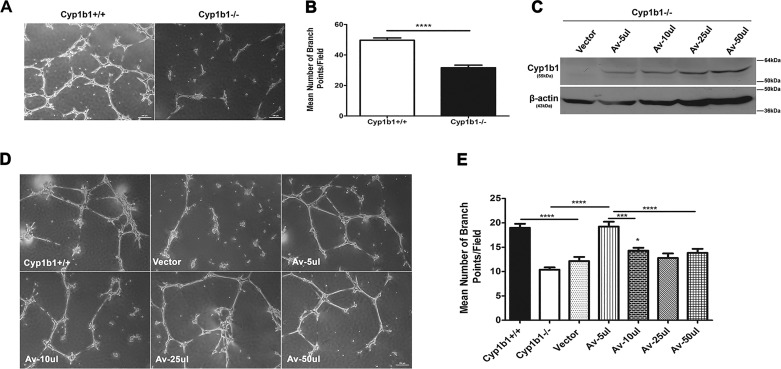
Prior to retinal vascularization, retinal ACs create a fibronectin-rich scaffold-like network that is utilized by ECs to establish the primary retinal vascular network. Thus, appropriate expression and organization of fibronectin are important in adhesion and migration of retinal ACs and retinal vascularization (37). We observed a significant increase in the level of fibronectin produced by Cyp1b1−/− retinal ACs, which may impact their ability to lay a proper scaffold for EC migration and retinal vascularization. We have previously shown that like retinal ECs, retinal ACs organize into a three-dimensional-like network when plated on Matrigel (29). We next asked whether lack of Cyp1b1 affects retinal ACs’ organization into a network on Matrigel. Figure 7A shows that Cyp1b1+/+ ACs readily organized on Matrigel forming a network. In contrast, this ability was compromised in Cyp1b1−/− ACs (Fig. 7A). A quantitative assessment of the data is shown in Fig. 7B. These results are consistent with the altered migration and/or adhesion properties observed in Cyp1b1−/− ACs.
Fig. 7.
Attenuation of cytochrome P450 1B1-deficient (Cyp1b1−/−) retinal astrocyte (AC) network formation in Matrigel and its restoration by reexpression of Cyp1b1. The ability of retinal ACs to undergo network formation on Matrigel was assessed by plating ACs on Matrigel as detailed in materials and methods. A: although Cyp1b1+/+ retinal ACs organized and formed a network on Matrigel, Cyp1b1−/− ACs failed to undergo network formation (×40). B: quantitative assessment of data. Mean number of branch points per five high-power fields (×100) was determined (****P < 0.0001; n = 4). C: reexpression of Cyp1b1 in Cyp1b1−/− retinal ACs using an adenovirus expression system. Western blot analysis of whole cell lysates from Cyp1b1−/− ACs infected with the adenovirus expressing empty vector (Vector) or mouse Cyp1b1 cDNA at different virus inputs (Av). β-Actin was used as a loading control. Please note lack of Cyp1b1 in vector control and increasing amounts of Cyp1b1 with increasing viral input. D: expression of Cyp1b1 restores the morphogenesis defect observed in Cyp1b1−/− ACs. Retinal ACs infected with adenovirus expressing empty vector or Cyp1b1 were plated in Matrigel. E: quantitative assessment of data as above. Please note significant restoration of morphogenesis in Cyp1b1−/− ACs expressing Cyp1b1 (Av-5 or 10 µl; *P < 0.05, ***P < 0.001, ****P < 0.0001; n = 3). Scale bars = 250 µm.
To demonstrate that the changes in cellular functions described above are due specifically to lack of Cyp1b1 expression, we restored expression of Cyp1b1 in Cyp1b1−/− ACs. Adenovirus expressing mouse Cyp1b1 or empty vector was used to infect Cyp1b1−/− ACs. Figure 7C shows that the virus effectively expressed Cyp1b1 in ACs in a virus input-dependent manner. Figure 7D shows that restoration of Cyp1b1 expression in Cyp1b1−/− cells significantly improved their ability to form a three-dimensional network compared with Cyp1b1−/− vector control cells (Fig. 7D). A quantitative determination of the data is shown in Fig. 7E. Thus, Cyp1b1 expression affects the ability of ACs to undergo morphogenesis and form a network.
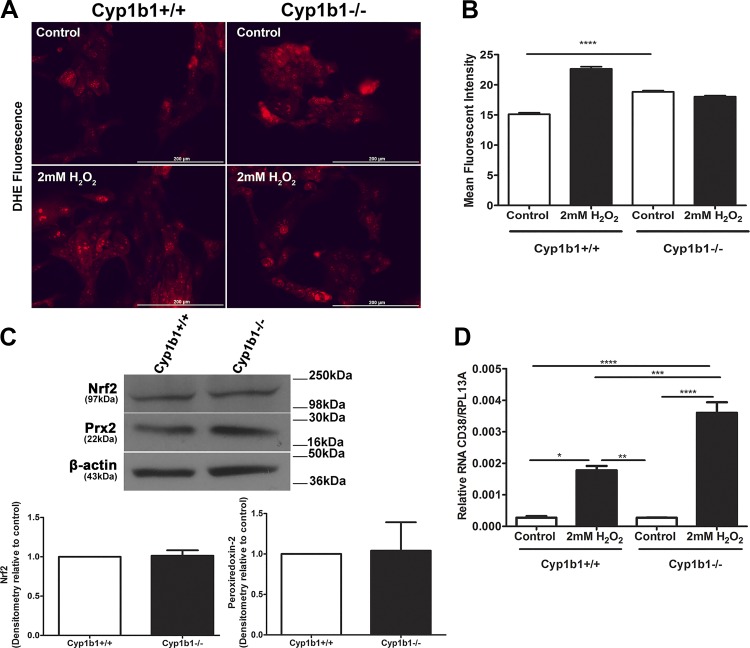
Retinal ACs exhibited changes in ROS generation.
Many vascular diseases and dysfunctions are associated with alterations in the cellular redox state. We have shown that deficiency in Cyp1b1 increases oxidative stress in the retina and retinal ECs and PCs, which can be reversed using the antioxidant N-acetyl-cysteine (18, 24, 36). We next determined the levels of ROS in Cyp1b1+/+ and Cyp1b1−/− ACs using DHE staining under both basal and stress conditions. Figure 8A shows differences in fluorescence intensity of both cell types at basal levels (control), which were significant. We also observed that under H2O2-challenged conditions, Cyp1b1−/− ACs did not show a further increase in ROS level compared with untreated cells (control). In contrast, incubation of Cyp1b1+/+ ACs with H2O2 resulted in a significant increase in ROS levels (Fig. 8, A and B). This suggested that the retinal ACs are tolerant of ROS and the absence of Cyp1b1, unlike in retinal ECs and PCs, does not result in further increased oxidative stress in retinal ACs. This may be attributed to the enhanced ROS scavenging capacity of these cells, which express the oxidative stress-stimulated transcription factor Nrf2 and its downstream target gene Prx2 (Fig. 8C). However, we were unable to detect HO-1, another Nrf2 target.
Fig. 8.
Increased basal reactive oxygen species (ROS) levels and resistance to H2O2-mediated ROS production in cytochrome P450 1B1-deficient (Cyp1b1−/−) retinal astrocytes (ACs). A: the level of oxidative stress (ROS) in retinal ACs was assessed by dihydroethidium (DHE) staining under basal or challenged conditions (2 mM H2O2). B: quantitative assessment of data. Please note increased ROS levels in Cyp1b1−/− ACs compared with Cyp1b1+/+ ACs at basal level. Although a significant increase in ROS levels was observed in Cyp1b1+/+ ACs incubated with H2O2, no significant increase in ROS level was noted in Cyp1b1−/− cells incubated with H2O2 (****P < 0.0001; n = 4). C: expression of antioxidant genes in Cyp1b1+/+ and Cyp1b1−/− retinal ACs. We observed no significant differences in the levels of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target gene peroxiredoxin-2 (Prx2). D: enhanced expression of cluster of differentiation 38 (CD38) in Cyp1b1−/− retinal ACs incubated with H2O2. The level of CD38 mRNA was determined by quantitative PCR analysis of RNA prepared from Cyp1b1+/+ and Cyp1b1−/− retinal ACs incubated with or without H2O2. Please note that the basal expression of CD38 is similar in these cells regardless of Cyp1b1 expression. However, a significant increase in the levels of CD38 was observed with incubation with H2O2 that was more dramatic in Cyp1b1−/− ACs (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n = 3). Scale bars = 250 µm. RpL13A, 60S ribosomal protein L13a.
We next explored the reason for the higher capacity of Cyp1b1−/− ACs to tolerate oxidative stress. Expression of CD38 in ACs plays an important role in their antioxidant capacity and survival when challenged with H2O2 (20). CD38 is a multifunctional ectoenzyme that metabolizes NAD+ producing cyclic ADP ribose, a potent agonist of ryanodine receptors and a modulator of intracellular calcium concentration. CD38 also converts cyclic ADP ribose to ADP ribose, with important roles in survival of ACs and microglial cells (19, 20). At basal levels, CD38 expression was similar in Cyp1b+/+ and Cyp1b1−/− ACs. Although CD38 expression was increased when ACs were incubated with H2O2, as previously demonstrated (20), this increase was significantly higher in Cyp1b1−/− compared with Cyp1b1+/+ ACs when challenged with H2O2 (Fig. 8D). Thus, higher levels of CD38 in Cyp1b1−/− ACs could contribute to their resistance to H2O2-mediated ROS production and oxidative stress.
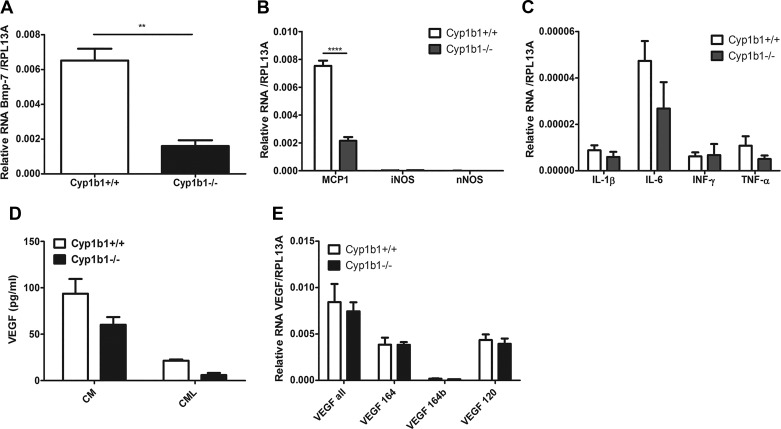
Increased oxidative stress generally leads to activation of NF-κB and production of inflammatory mediators. We determined the expression of inflammatory mediators in Cyp1b1+/+ and Cyp1b1−/− ACs by qPCR analysis (Fig. 9). We observed a significant decrease in the expression of BMP-7 and MCP-1 in Cyp1b1−/− ACs (Fig. 9, A and B), whereas the expression of IL-1β, IL-6, INF-γ, and TNF-α was not significantly affected (Fig. 9C). Expression of iNOS and nNOS was undetectable in these cells (Fig. 9B).
Fig. 9.
Cytochrome P450 1B1-deficient (Cyp1b1−/−) retinal astrocytes (ACs) express reduced levels of inflammatory mediators. A–C: expression levels of various inflammatory factors assessed by real-time quantitative (q)PCR analysis: bone morphogenetic protein-7 (BMP-7) (A); monocyte chemoattractant protein-1 (MCP-1), inducible nitric oxide synthase (iNOS), and neuronal nitric oxide synthase (nNOS) (B); and IL-1β, IL-6, INF-γ, and TNF-α (C). D: VEGF-A amounts determined by ELISA in both conditioned medium (CM) and AC lysates (CML). E: expression levels of various VEGF-A isoforms determined by real-time qPCR. Please note a significant decrease in the levels of BMP-7 and MCP-1 (**P < 0.01, ****P < 0.0001; n = 3). RpL13A, 60S ribosomal protein L13a.
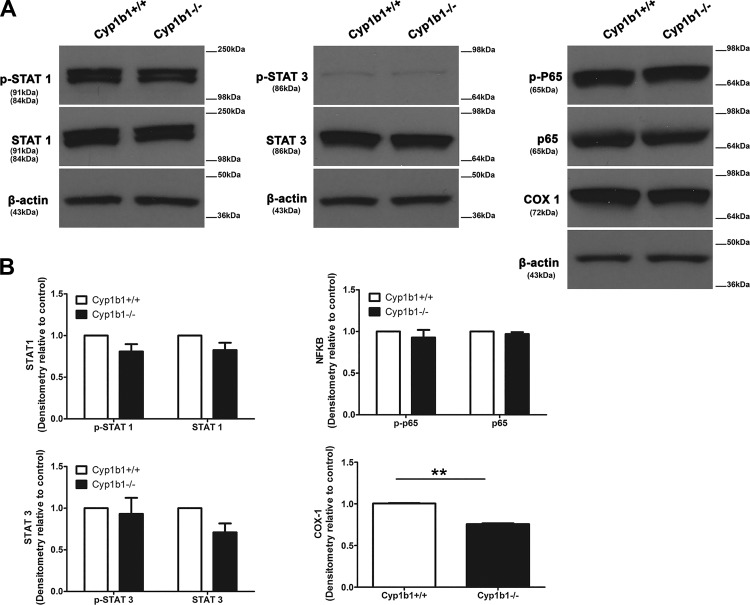
Vascular endothelial growth factor is a well-recognized inducer of angiogenesis and inflammation. Astrocytes in the vasculature secrete VEGF to promote vessel-stabilizing functions in the retinal vasculature (32). In addition, hypoxia-mediated VEGF production by ACs is essential for retinal neovascularization (39). We assessed whether secretion of VEGF into the medium was altered in the absence of Cyp1b1. Although a decrease in VEGF levels was observed in Cyp1b1−/− ACs compared with Cyp1b1+/+ ACs, it was not significant (Fig. 9D). A limited amount of VEGF was cell associated. VEGF-A isoform mRNA levels were also not significantly affected by Cyp1b1 expression (Fig. 9D). We also observed similar levels of total and active STAT1, STAT3, and NF-κB p65 subunit (p65). However, cyclooxygenase-1 (COX-1) expression was significantly decreased in Cyp1b1−/− ACs (Fig. 10). These results are consistent with the reduced inflammatory nature of Cyp1b1−/− ACs.
Fig. 10.
Expression of various inflammatory transcriptional regulatory factors is not altered by cytochrome P450 1B1 (Cyp1b1) expression. A: the expressions of Stat1, phospho (p)-Stat1, Stat3, p-Stat3, NF-κB p65 subunit (p65), p-p65, and cyclooxygenase-1 (Cox-1) were determined using lysates prepared from Cyp1b1+/+ and Cyp1b1−/− retinal astrocytes (ACs) by Western blot analysis using specific antibodies. β-Actin was used as a loading control. B: quantitative assessment of the data. Please note a significant decrease in the levels of Cox-1 in Cyp1b1−/− ACs (**P < 0.01; n = 3).
Alterations in intracellular signaling pathways of Cyp1b1−/− ACs.
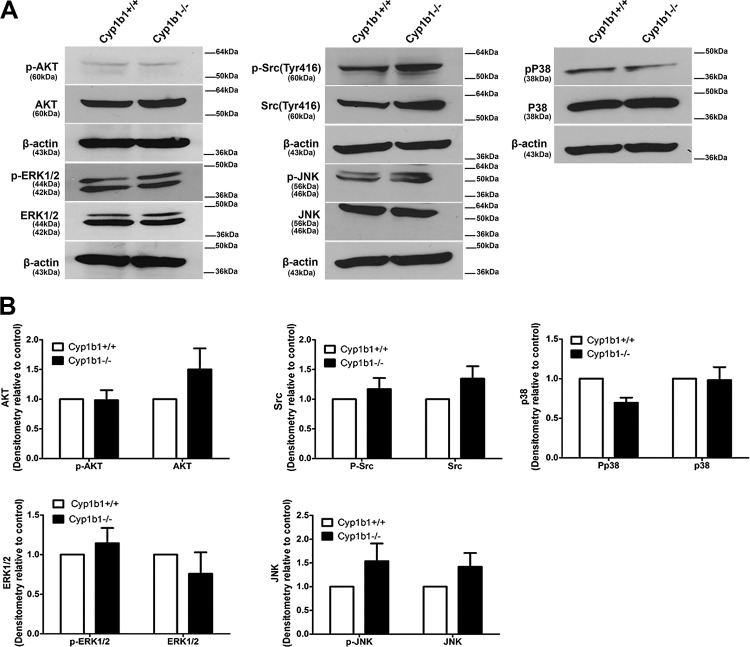
Mitogen activated protein kinase (MAPK), Akt, and SRC signaling pathways regulate a diverse range of processes including cell proliferation, adhesion, cellular stress, inflammation, and apoptosis. Here we assessed whether these signaling pathways were impacted in Cyp1b1−/− ACs. We observed similar phosphorylation in AKT, ERK1/2, SRC, and total p38 in both cell types. Although there was an increase in total AKT, SRC, and JNK in Cyp1b1−/− ACs, this change was not significant (Fig. 11A). Figure 11B represents the quantitative evaluation of the data.
Fig. 11.
Cytochrome P450 1B1 (Cyp1b1) deficiency does not affect protein kinase B (AKT)/SRC/MAPK signaling pathways in retinal astrocytes (ACs). A: the expression levels of Akt, phospho (p)-Akt, Erk1/2, p-Erk1/2, Src, p-Src, Jnk, p-Jnk, MAPK p38 (p38), and p-p38 were determined by Western blot analysis of lysates prepared from Cyp1b1+/+ and Cyp1b1−/− retinal ACs. β-Actin was used as a loading control. B: quantitative assessment of the data. Please note that no significant differences were observed between Cyp1b1+/+ and Cyp1b1−/− ACs.
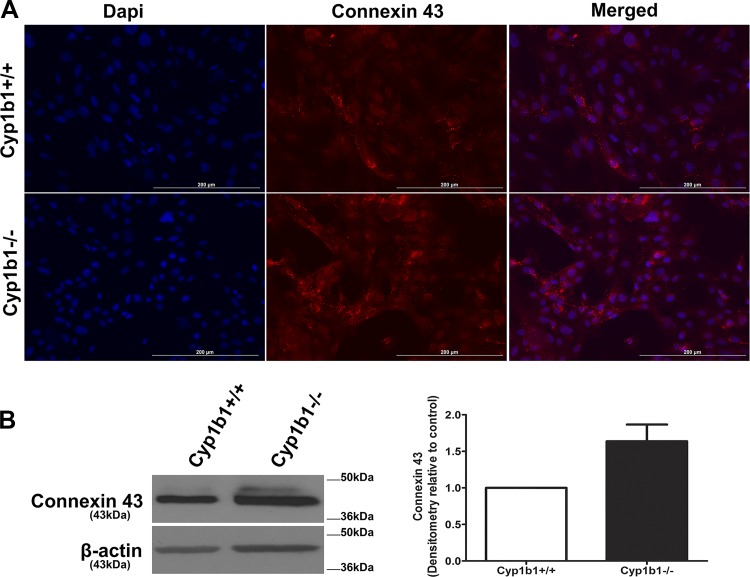
Connexin 43 is a prominent gap junction protein expressed in retinal ACs. Its upregulation and hemichannel’s opening with injury and disease are implicated in glial cell activation, edema, and loss of vascular integrity and neuronal death (6). Astrocytes respond to damage in the central nervous system with increased connexin 43 expression, proliferation, and migration. We next asked whether Cyp1b1 expression affects gap junctional organization and connexin 43 expression and/or phosphorylation levels. Figure 12A shows localization of connexin 43 in Cyp1b1+/+ and Cyp1b1−/− ACs. We observed an increased punctate staining, typical of the connexin 43 hemichannel’s staining, in Cyp1b1−/− ACs compared with Cyp1b1+/+ cells. This was concomitant with significantly increased levels of connexin 43 protein and phosphorylation in Cyp1b1−/− ACs (Fig. 12B). These results are consistent with increased proliferation and migration and reduced inflammatory phenotype of Cyp1b1−/− ACs.
Fig. 12.
Altered expression, localization, and phosphorylation of connexin 43 in cytochrome P450 1B1-deficient (Cyp1b1−/−) retinal astrocytes (ACs). A: immunofluorescence staining of Cyp1b1+/+ and Cyp1b1−/− retinal ACs for connexin 43. Please note enhanced connexin 43 staining in Cyp1b1−/− ACs. B: Western blot analysis of connexin 43 protein levels in lysates prepared from Cyp1b1+/+ and Cyp1b1−/− retinal ACs. Please note a significant increase in the level and phosphorylated form of connexin 43 in Cyp1b1−/− retinal ACs (n = 3). Scale bars = 200 µm.
DISCUSSION
ACs have an important role in retinal vascular development, providing physical support and nutrients for neurons in the central nervous system (13, 15). Astrocytes under inflammatory and/or oxidative conditions express higher levels of Cyp1b1, which is relieved using the antioxidant N-acetyl-cysteine (21). Unfortunately, the activity of Cyp1b1 in retinal ACs and its dysfunction in retinal neurovascular defects remain largely unknown. The main objective of this study was to assess the impact of Cyp1b1 expression on retinal AC function. Here we showed that Cyp1b1 is constitutively expressed in retinal ACs and lack of Cyp1b1 was associated with enhanced proliferation and migration of ACs. Cyp1b1−/− ACs also exhibited increased adhesion to ECM proteins. This was concomitant with decreased levels of TSP1 and increased levels of fibronectin and its receptors, αvβ3- and α5β1-integrin. In addition, Cyp1b1−/− ACs showed increased resistance to oxidative stress, expressed higher levels of CD38 when challenged with H2O2, and exhibited compromised network formation in Matrigel. Reexpression of Cyp1b1 in Cyp1b1−/− ACs was sufficient to restore network formation in Matrigel. We also observed decreased levels of inflammatory mediators BMP-7 and MCP-1 in Cyp1b1−/− ACs. Although the AKT/SRC/MAPK signaling pathways were not significantly affected by Cyp1b1 expression, we observed a significant increase in the level and phosphorylation of connexin 43 in Cyp1b1−/− ACs. Increased connexin 43 phosphorylation and decreased MCP-1 levels are consistent with increased Cyp1b1−/− AC proliferation. Together, our results suggest that Cyp1b1 expression and/or activity are important in modulation of AC proliferation and migration as well as morphogenesis and formation of a proper fibronectin network that supports retinal neurovascular function.
The immense functional diversity of ACs suggests the existence of heterogeneous AC populations (2, 40). The multiple markers used to identify ACs are neither specific nor stable in their expression. However, we observed expression of classical AC markers PDGFRα, GFAP, Pax2, and vimentin. Immunofluorescence staining revealed uniform, but relatively low expression of GFAP in these cells (not shown), which may partially be due to low binding affinity of the antibody in this method or due to the fact that increased GFAP immunoreactivity is usually viewed as an indicator of gliosis or a relatively slow-developing correlate of neural damage (9, 25). Uniform vimentin, S100 calcium-binding protein B (S100B), and Pax2 staining of ACs (29) indicated that >98% of the cells were positive for these markers.
Cyp1b1−/− ACs exhibited an increased proliferation with no significant changes in the level of apoptosis. These cells were also more migratory. These changes may be in part due to altered adhesion to ECM proteins and/or their altered production. The lack of Cyp1b1 in ACs resulted in decreased levels of cell-associated TSP1. In contrast, the levels of cell-associated and secreted fibronectin were significantly increased in Cyp1b1−/− ACs. Our previous studies showed that loss of Cyp1b1 in ECs and PCs resulted in increased expression of TSP2, a TSP family member closely related to TSP1 (24, 36). Although we were unable to detect TSP2 protein in retinal ACs, the TSP2 mRNA level was increased in Cyp1b1−/− ACs compared with Cyp1b1+/+ cells (not shown). The low level of TSP2 in Cyp1b1−/− ACs compared with Cyp1b1−/− ECs and PCs with high levels of oxidative stress is consistent with their tolerant nature toward oxidative stress (24, 36). The increased adhesion of Cyp1b1−/− ACs on fibronectin and vitronectin observed here is consistent with the increased expression of α5β1- and αvβ3-integrin, the major receptors for these ECM proteins, in these cells. Furthermore, the decreased expression of MCP-1 and increased expression of connexin 43 in Cyp1b1−/− cells are consistent with their increased proliferation and migration (6).
Retinal ACs are a major source of fibronectin, laying down a fibronectin-rich scaffolding essential for retinal vascularization (37). We previously showed that retinal ACs form a network when plated on Matrigel (29). The observed alterations in adhesion and migration of Cyp1b1−/− ACs and their increased fibronectin production suggested that their ability to organize into a network could be compromised. We observed that Cyp1b1−/− retinal AC ability to form a network on Matrigel was significantly reduced. We showed that restoration of Cyp1b1 expression was sufficient to restore the ability of Cyp1b1−/− ACs to form a network when plated on Matrigel, likely through restoration of their normal adhesive and migratory properties. Thus, expression of Cyp1b1 plays a key role in modulation of adhesive and migratory properties of ACs and their ability to undergo morphogenesis.
Reactive oxygen species have an important role as intracellular mediators of VEGF signaling in vitro and angiogenesis in vivo. However, when ROS are generated in high concentrations, they cause tissue injury and cell death and are considered as risk factors for a variety of vascular diseases. We have previously shown increased levels of superoxide and 4-hydroxynonenal staining in Cyp1b1−/− ECs and PCs compared with wild-type cells (24, 36). Here we observed significantly higher levels of ROS in Cyp1b1−/− compared with Cyp1b1+/+ ACs, although we observed a significant increase in ROS levels when Cyp1b1+/+ ACs were incubated with H2O2 but no significant change in ROS levels in Cyp1b1−/− ACs incubated with H2O2. We also observed no significant differences in the levels of Nrf2 and its downstream effector peroxiredoxin-2 in these cells. The reason for the lack of increase in ROS levels when Cyp1b1−/− ACs are incubated with H2O2 is not clear. However, the expression of CD38 in ACs plays a significant role in the antioxidant capacity and survival of ACs in response to H2O2 (20). Incubation of ACs with H2O2 increases CD38 expression, and we observed a more significant increase in CD38 expression in Cyp1b1−/− ACs. This is consistent with reduced levels of ROS and oxidative stress in these cells when incubated with H2O2 (19, 20). However, whether the tolerance of Cyp1b1−/− ACs to H2O2 is a direct result of CD38 upregulation in ACs or whether increased levels of intracellular glutamate play a role in increased CD38 levels (3) needs further investigation.
The increases in oxidative stress result in enhanced NF-κB activation and production of inflammatory mediators. We previously showed that Cyp1b1−/− retinal ECs and PCs exhibit increased oxidative stress and sustained activation of NF-κB along with a proinflammatory characteristics (23, 24). Cyp1b1−/− retinal ACs expressed significantly lower amounts of MCP-1 and BMP-7, whereas expression of other inflammatory cytokines, chemokines, and mediators was not affected. These results are consistent with the reduced expression of inflammatory cell adhesion molecules ICAM-1 and VCAM-1 in Cyp1b1−/− ACs observed here. Astrocyte-derived MCP-1/C-C motif chemokine 2 (CCL2) and BMP-7 could activate microglia through their interactions with their receptors and elicit neuroinflammation and gliosis (7, 8, 41). In addition, attenuation of MCP-1/CCL2 signaling in C-C chemokine receptor type 2-deficient (CCR2−/−) mice is shown to increase AC proliferation during brain injury and reduce scar formation due to diminished ECM deposition (12). Aryl hydrocarbon receptor (Ahr) also represses the expression MCP-1/CCL2 as well as other chemokines in ACs and diminishes central nervous system inflammation (27). Cyp1b1 is also a target of Ahr, and activation of Ahr in ACs results in increased Cyp1b1 expression (27). How lack of Cyp1b1 affects Ahr expression/activity and whether these changes in Ahr contribute to an inflammatory phenotype remain the subjects of future investigation. The reduced production of inflammatory mediators in Cyp1b1−/− ACs was also consistent with no significant changes in expression and/or activation of inflammatory transcription factors including STAT1, STAT3, and NF-κB, whereas COX-1 levels were decreased.
In summary, we showed that Cyp1b1 expression in retinal ACs is essential for their appropriate proliferation, migration, and adhesion. Cyp1b1−/− ACs exhibited decreased network formation in Matrigel that was restored by reexpression of Cyp1b1 in these cells. In addition, the altered expression and activity of CD38 and connexin 43 may further contribute to changes observed with Cyp1b1 expression. Collectively, our results support an important role for Cyp1b1 expression in modulation of retinal AC function.
GRANTS
This work was supported by an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, Retina Research Foundation, National Eye Institute Grant P30-EY-016665, National Cancer Institute Grant P30-CA-014520, US Environmental Protection Agency Grant EPA 83573701, and National Eye Institute Grants R24-EY-022883 and R01-EY-026078. C. M. Sorenson is supported by the Retina Research Foundation/Daniel M. Albert Chair. N. Sheibani is a recipient of the Research to Prevent Blindness Stein Innovation Award. J. Falero-Perez is supported by National Institute of Environmental Health Sciences T32 Training Grant T32-ES-007015.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.F.-P., C.M.S., and N.S. conceived and designed research; J.F.-P. and C.M.S. performed experiments; J.F.-P. and N.S. analyzed data; J.F.-P., C.M.S., and N.S. interpreted results of experiments; J.F.-P. prepared figures; J.F.-P. drafted manuscript; J.F.-P., C.M.S., and N.S. edited and revised manuscript; J.F.-P., C.M.S., and N.S. approved final version of manuscript.
REFERENCES
- 1.Barnett JA, Urbauer DL, Murray GI, Fuller GN, Heimberger AB. Cytochrome P450 1B1 expression in glial cell tumors: an immunotherapeutic target. Clin Cancer Res 13: 3559–3567, 2007. doi: 10.1158/1078-0432.CCR-06-2430. [DOI] [PubMed] [Google Scholar]
- 2.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol 7: a020362, 2015. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruzzone S, Verderio C, Schenk U, Fedele E, Zocchi E, Matteoli M, De Flora A. Glutamate-mediated overexpression of CD38 in astrocytes cultured with neurones. J Neurochem 89: 264–272, 2004. doi: 10.1111/j.1471-4159.2003.02326.x. [DOI] [PubMed] [Google Scholar]
- 4.Chan-Ling T, Stone J. Degeneration of astrocytes in feline retinopathy of prematurity causes failure of the blood-retinal barrier. Invest Ophthalmol Vis Sci 33: 2148–2159, 1992. [PubMed] [Google Scholar]
- 5.Choudhary D, Jansson I, Rezaul K, Han DK, Sarfarazi M, Schenkman JB. Cyp1b1 protein in the mouse eye during development: an immunohistochemical study. Drug Metab Dispos 35: 987–994, 2007. doi: 10.1124/dmd.106.014282. [DOI] [PubMed] [Google Scholar]
- 6.Danesh-Meyer HV, Zhang J, Acosta ML, Rupenthal ID, Green CR. Connexin43 in retinal injury and disease. Prog Retin Eye Res 51: 41–68, 2016. doi: 10.1016/j.preteyeres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Dharmarajan S, Fisk DL, Sorenson CM, Sheibani N, Belecky-Adams TL. Microglia activation is essential for BMP7-mediated retinal reactive gliosis. J Neuroinflammation 14: 76, 2017. doi: 10.1186/s12974-017-0855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dharmarajan S, Gurel Z, Wang S, Sorenson CM, Sheibani N, Belecky-Adams TL. Bone morphogenetic protein 7 regulates reactive gliosis in retinal astrocytes and Müller glia. Mol Vis 20: 1085–1108, 2014. [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson PA, Fisher SK, Guérin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Müller cells after retinal detachment. Exp Eye Res 44: 37–48, 1987. doi: 10.1016/S0014-4835(87)80023-4. [DOI] [PubMed] [Google Scholar]
- 10.Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent, nitric oxide-mediated vasodilatation in patients with coronary artery disease. Circulation 109: 178–183, 2004. doi: 10.1161/01.CIR.0000105763.51286.7F. [DOI] [PubMed] [Google Scholar]
- 11.Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med 18: 20–25, 2008. doi: 10.1016/j.tcm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Frik J, Merl-Pham J, Plesnila N, Mattugini N, Kjell J, Kraska J, Gómez RM, Hauck SM, Sirko S, Götz M. Cross-talk between monocyte invasion and astrocyte proliferation regulates scarring in brain injury. EMBO Rep 19: e45294, 2018. doi: 10.15252/embr.201745294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fruttiger M, Calver AR, Krüger WH, Mudhar HS, Michalovich D, Takakura N, Nishikawa S, Richardson WD. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron 17: 1117–1131, 1996. doi: 10.1016/S0896-6273(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 14.Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol 546: 307–314, 2003. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang B, Behzadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on endothelial cell differentiation. Glia 15: 1–10, 1995. doi: 10.1002/glia.440150102. [DOI] [PubMed] [Google Scholar]
- 16.Jiang B, Liou GI, Behzadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on fibronectin expression. J Cell Sci 107: 2499–2508, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. Am J Physiol Cell Physiol 292: C2070–C2083, 2007. doi: 10.1152/ajpcell.00489.2006. [DOI] [PubMed] [Google Scholar]
- 18.Kondo S, Tang Y, Scheef EA, Sheibani N, Sorenson CM. Attenuation of retinal endothelial cell migration and capillary morphogenesis in the absence of bcl-2. Am J Physiol Cell Physiol 294: C1521–C1530, 2008. doi: 10.1152/ajpcell.90633.2007. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Jiang J, Wang L, Nie H, Xia W, Liu J, Ying W. CD38 is a key enzyme for the survival of mouse microglial BV2 cells. Biochem Biophys Res Commun 418: 714–719, 2012. doi: 10.1016/j.bbrc.2012.01.084. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Wu D, Ding X, Ying W. CD38 plays key roles in both antioxidation and cell survival of H2O2-treated primary rodent astrocytes. Int J Physiol Pathophysiol Pharmacol 6: 102–108, 2014. [PMC free article] [PubMed] [Google Scholar]
- 21.Malaplate-Armand C, Ferrari L, Masson C, Siest G, Batt AM. Astroglial CYP1B1 up-regulation in inflammatory/oxidative toxic conditions: IL-1β effect and protection by N-acetylcysteine. Toxicol Lett 138: 243–251, 2003. doi: 10.1016/S0378-4274(02)00417-4. [DOI] [PubMed] [Google Scholar]
- 22.Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci 21: 1538–1547, 2001. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palenski TL, Gurel Z, Sorenson CM, Hankenson KD, Sheibani N. Cyp1B1 expression promotes angiogenesis by suppressing NF-κB activity. Am J Physiol Cell Physiol 305: C1170–C1184, 2013. doi: 10.1152/ajpcell.00139.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palenski TL, Sorenson CM, Jefcoate CR, Sheibani N. Lack of Cyp1b1 promotes the proliferative and migratory phenotype of perivascular supporting cells. Lab Invest 93: 646–662, 2013. doi: 10.1038/labinvest.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia 50: 427–434, 2005. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 26.Pekny M, Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta 1862: 483–491, 2016. doi: 10.1016/j.bbadis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22: 586–597, 2016. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savas U, Bhattacharyya KK, Christou M, Alexander DL, Jefcoate CR. Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. J Biol Chem 269: 14905–14911, 1994. [PubMed] [Google Scholar]
- 29.Scheef E, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal astrocytes. Mol Vis 11: 613–624, 2005. [PubMed] [Google Scholar]
- 30.Schnitzer J. Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia 1: 74–89, 1988. doi: 10.1002/glia.440010109. [DOI] [PubMed] [Google Scholar]
- 31.Schnitzer J. Retinal astrocytes: their restriction to vascularized parts of the mammalian retina. Neurosci Lett 78: 29–34, 1987. doi: 10.1016/0304-3940(87)90556-8. [DOI] [PubMed] [Google Scholar]
- 32.Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One 5: e11863, 2010. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone J, Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol 255: 35–49, 1987. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- 34.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis 9: 171–178, 2003. [PubMed] [Google Scholar]
- 35.Sutter TR, Tang YM, Hayes CL, Wo YY, Jabs EW, Li X, Yin H, Cody CW, Greenlee WF. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem 269: 13092–13099, 1994. [PubMed] [Google Scholar]
- 36.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood 113: 744–754, 2009. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uemura A, Kusuhara S, Wiegand SJ, Yu RT, Nishikawa S. Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J Clin Invest 116: 369–377, 2006. doi: 10.1172/JCI25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature 332: 834–837, 1988. doi: 10.1038/332834a0. [DOI] [PubMed] [Google Scholar]
- 39.Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 58: 1177–1185, 2010. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkin GP, Marriott DR, Cholewinski AJ. Astrocyte heterogeneity. Trends Neurosci 13: 43–46, 1990. doi: 10.1016/0166-2236(90)90065-I. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Dong H, Qian Q, Zhang X, Wang Y, Jin W, Qian Y. Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav Brain Res 332: 145–153, 2017. doi: 10.1016/j.bbr.2017.05.066. [DOI] [PubMed] [Google Scholar]