Abstract
The attachment of O-linked β-N-acetylglucosamine (O-GlcNAc) to the serine and threonine residues of proteins in distinct cellular compartments is increasingly recognized as an important mechanism regulating cellular function. Importantly, the O-GlcNAc modification of mitochondrial proteins has been identified as a potential mechanism to modulate metabolism under stress with both potentially beneficial and detrimental effects. This suggests that temporal and dose-dependent changes in O-GlcNAcylation may have different effects on mitochondrial function. In the current study, we found that acutely augmenting O-GlcNAc levels by inhibiting O-GlcNAcase with Thiamet-G for up to 6 h resulted in a time-dependent decrease in cellular bioenergetics and decreased mitochondrial complex I, II, and IV activities. Under these conditions, mitochondrial number was unchanged, whereas an increase in the protein levels of the subunits of several electron transport complex proteins was observed. However, the observed bioenergetic changes appeared not to be due to direct increased O-GlcNAc modification of complex subunit proteins. Increases in O-GlcNAc were also associated with an accumulation of mitochondrial ubiquitinated proteins; phosphatase and tensin homolog induced kinase 1 (PINK1) and p62 protein levels were also significantly increased. Interestingly, the increase in O-GlcNAc levels was associated with a decrease in the protein levels of the mitochondrial Lon protease homolog 1 (LonP1), which is known to target complex IV subunits and PINK1, in addition to other mitochondrial proteins. These data suggest that impaired bioenergetics associated with short-term increases in O-GlcNAc levels could be due to impaired, LonP1-dependent, mitochondrial complex protein turnover.
Keywords: bioenergetics, LonP1, mitochondria, protein O-GlcNAcylation, protein turnover, Thiamet-G
INTRODUCTION
The attachment of an O-linked N-acetylglucosamine (O-GlcNAc) moiety to serine/threonine residues of proteins is increasingly recognized as an important regulatory posttranslational modification, which plays a pivotal role in protein activation, signal transduction, receptor binding and turnover, transcriptional regulation, and cell survival (28). Since its identification in 1984 (48), the majority of studies have focused on the O-GlcNAcylation of nuclear and cytoplasmic proteins; however, the presence of a mitochondrial isoform of O-GlcNAc transferase (mOGT) raised the possibility that mitochondrial proteins could also be O-GlcNAcylated (15). On the other hand, initially it was reported that O-GlcNAcylation levels of mitochondrial proteins were very low possibly due to limited availability of UDP-GlcNAc (29). The apparent absence of a mitochondrial O-GlcNAcase (OGA), which removes O-GlcNAc from proteins, also placed the possibility of an O-GlcNAc cycle in the mitochondria in doubt unless an alternative mechanism for removing O-GlcNAcylated proteins could be identified.
Despite the uncertainty as to how mitochondrial proteins were O-GlcNAcylated, a growing number of studies suggested that changes in cellular O-GlcNAc levels affected mitochondrial function. For example, hyperglycemia increased O-GlcNAcylation of components of the electron transport chain complexes, including cyclooxygenase 1 (COX1) and NDUFA9, which was associated with impaired mitochondrial function (19); moreover, increasing OGA expression decreased O-GlcNAcylation of COX1 and NDUFA and improved mitochondrial function (19). In addition, it was shown that VDAC2 was O-GlcNAcylated and, importantly, that cells lacking VDAC2 were protected against O-GlcNAc-dependent mitochondrial dysfunction and apoptosis (37). Moreover, both suppression and augmentation of protein O-GlcNAcylation caused mitochondrial dysfunction suggesting an optimal level appears to be required for normal metabolism (46). The cytoprotective effects that have been observed with acute increases in O-GlcNAcylation have also been linked to preservation of mitochondrial function. For example, genetic or pharmacological approaches to increase O-GlcNAc levels reduced ischemia/hypoxia-mediated mitochondrial permeability transition pore formation through inhibition of both calcium overload and reactive oxygen species generation (34). Increasing O-GlcNAc levels also attenuated the hydrogen peroxide-induced loss of mitochondrial membrane potential, which has been attributed in part to O-GlcNAcylation of VDAC (21) and increased mitochondrial translocation of the prosurvival protein B-cell lymphoma (Bcl-2) (5).
In the past few years, greater evidence supporting an O-GlcNAc cycle in mitochondria has been reported, including that the inner mitochondrial membrane transporter pyrimidine nucleotide carrier transports UDP-GlcNAc from the cytosol into the mitochondrion, and that mitochondria exhibit an O-GlcNAcase activity (30). Additional studies demonstrated that components of a number of mitochondrial metabolic pathways including oxidative phosphorylation, the TCA cycle, and fatty acid oxidation are O-GlcNAcylated (31). Nevertheless, the effects of O-GlcNAcylation on mitochondrial function remain unclear with some studies suggesting that the adverse effects of hyperglycemia are not mediated by increased O-GlcNAc levels (8), and yet others have reported that pharmacologically increasing O-GlcNAc levels increased oxygen consumption in cardiac mitochondria (31). These data combined with earlier conflicting studies discussed above suggested that the effects of increased O-GlcNAcylation on mitochondrial function likely depends on the duration and intensity of the treatments such as hyperglycemia or the O-GlcNAcase inhibitor Thiamet-G (ThG). Therefore, the goal of this study was to determine the effects of increasing O-GlcNAc levels with low concentrations of ThG over a period of 1–6 h. We found that a two- to threefold increase in cellular O-GlcNAc levels for up to 6 h resulted in impaired mitochondrial bioenergetics and decreased activities of mitochondrial complexes I, II, and IV, which were associated with decreased expression in the mitochondrial protease Lon protease homolog 1 (LonP1).
MATERIALS AND METHODS
Cell culture.
AC16 cells (9) were cultured in DMEM (Corning) supplemented with 12.5% fetal bovine serum (Atlanta Biologicals) and 2% antibiotic/antimycotic (Atlanta Bologicals) and used up to passage 13. The standard medium for AC16 cell culture is DMEM/F-12 (Life Technologies), which includes a 17.5 mM glucose concentration for optimal growth conditions. Since high-glucose concentrations are known to augment O-GlcNAc levels (14, 52), the glucose concentration was changed to 5 mM 16 h before all experiments and remained at that level for the duration of the studies. Due to the variation in glucose concentrations in different cell culture media, we have used this protocol to standardize experimental conditions across all cell types. We have not observed any adverse outcomes of using this lower glucose concentration for the duration of the studies.
Experimental design.
To increase overall O-GlcNAc levels, cells were treated with ThG (SD ChemMolecules) a highly selective inhibitor of OGA, which leads to rapid increases in overall protein O-GlcNAcylation (58). In the initial series of experiments, cells were treated with ThG at 1 or 5 µM for up to 6 h followed by assessment of bioenergetics in intact cells, as described below. These time points and concentrations were chosen based on our early studies that showed robust increases in O-GlcNAc at 1 µM within 1 h (61). Due to minimal effects of 1 µM ThG on bioenergetics at any time point, subsequent studies on complex activities were performed at 6 h following treatment with 1, 3, or 5 µM ThG.
Immunoblotting.
Lysates were prepared in 600 µl buffer containing 1 ml Tissue Protein Extraction Reagent T-PER (ThermoFisher Scientific) and 4 µl, 0.5 M EDTA, pH 8.0; 5 µl each of Protease Inhibitor Cocktail #1, #2, and #3 (Sigma); and 10% Nonidet P-40 (Sigma). All samples were prepared at 25 µg/ml in 20 µl of lysate/6× sample buffer (375 mM Tris·HCl pH 6.8, 6% SDS, 48% glycerol, 9% 2-mercaptoethanol, and 0.03% bromophenol blue) and separated by electrophoresis with 7.5 and 12% polyacrylamide gels at 120 V for 90 min, followed by transfer of proteins to the polyvinylidene fluoride membrane at 100 V (Immobilon, Millipore). Ponceau S staining was used to ensure equal protein loading for the O-GlcNAc immunoblots. Cellular O-GlcNAc was measured with anti-O-GlcNAc antibody CTD 110.6 [University of Alabama at Birmingham (UAB) Epitope Recognition and Immunoreagent Core] at a primary dilution of 1:20,000 in PBS/1% casein (Bio-Rad) whereas mitochondrial O-GlcNAc levels were measured using anti-O-GlcNAc antibody CTD 110.6 (no. MABS1254; Millipore) at a primary dilution of 1:5,000 in TBS-T 2% bovine serum albumin. Preliminary studies in whole cell lysates demonstrated that both antibodies exhibited similar patterns of O-GlcNAcylated proteins (data not shown); however, the UAB core antibody was more sensitive and therefore was used in the first series of experiments. However, when comparing the two antibodies in mitochondrial extracts, we found that the Millipore antibody (MABS1254) recognized a wider range of proteins particularly in the lower molecular weight range; consequently, we used this antibody for all studies involving mitochondrial extracts.
O-GlcNAc blots were developed using horseradish peroxidase-conjugated secondary (anti-mouse IgM) at a dilution of 1:20,000 (401225; Calbiochem), and chemiluminescent substrate (Perkin Elmer). For O-GlcNAc immunoblots, overall cellular O-GlcNAc levels were assessed by total lane densitometric analysis and normalized to β-actin and the untreated control group. OGT (DM-17 O-6246; Sigma) and OGA (no. ab124807; Abcam) antibodies were used to assess changes in levels of these enzymes in response to ThG treatment. Antibody for β-actin (no. ab8227; Abcam) was used as a loading control for whole-cell protein. Peroxisome proliferator-activated receptor γ-coactivator 1-α (PGC1-α; SC-13067; Santa Cruz Biotechnology) and mitochondrial transcription factor A (TFAM; no. 8076; Cell Signaling) antibodies were used to assess proteins responsible for mitochondrial biogenesis. Membranes were incubated in OXPHOS antibody cocktail (no. ab110413; Abcam) to assess the levels of electron transport chain subunits in response to changing O-GlcNAc via ThG. MTCO2 (no. ab156031; Abcam), LonP1 (Abcam, no. ab103809), PINK1 (no. ab23707; Abcam), VDAC (no. ab14734; Abcam), p62 (PW9860-0025; EnzoLifeSciences), and Parkin (SC-30130; Santa Cruz Biotechnology) antibodies were used to assess the levels of a complex IV subunit, a mitochondrial protease, and proteins involved in autophagy and mitophagy. Ubiquitin 6c1 antibody (SC-47721; Santa Cruz Biotechnology) was a generous gift from Dr. Scott Wilson, Department of Neuroscience, UAB.
Cellular bioenergetics and complex activity measurements.
Cellular bioenergetics were measured using an XF96 Extracellular Flux Analyzer (Agilent Seahorse Bioscience, North Billerica, MA) (17). AC16 cells were seeded onto XF96 plates at a density of 10,000 cells/well ~18 h before ThG treatment to allow cell for cell adhesion. Data were normalized to the amount of protein in individual wells as determined by DC protein assay (no. 5000116; Bio-Rad). Before bioenergetics assay (~1 h), cells were incubated in XF DMEM media (DMEM with 1 mM pyruvate, 5.5 mM d-glucose, and 4 mM l-glutamine, pH 7.4 at 37°C). As previously described, the oxygen consumption rate (OCR) was measured over a period of 70 min during which sequential injections of 1 µg/ml oligomycin, 1 µM FCCP, and 10 µM antimycin A were performed (11). Basal OCR was calculated by subtracting the OCR following antimycin injection from the OCR before oligomycin injection. Maximal OCR was calculated by subtracting the OCR following antimycin injection from the OCR following FCCP injection. We have found that antimycin A alone is sufficient to inhibit all mitochondrial oxygen consumption and that the addition of complex I or complex II inhibitors does not decrease oxygen consumption further (data not shown). Reserve capacity, which represents the ability of the cell to perform work under a stress or demand, was calculated by subtracting the OCR before oligomycin from the OCR following FCCP injection as described in detail elsewhere (16). ATP-linked OCR was calculated by subtracting OCR postoligomycin from the OCR before oligomycin (16). As these studies were performed in intact cells, as opposed to isolated mitochondria, there were also nonmitochondrial sources of oxygen consumption. Therefore, proton leak was calculated by subtracting the OCR following antimycin injection from the OCR following oligomycin injection (16). As we and others have noted, using this approach, there is a slight underestimation of ATP-linked OCR and overestimation of proton leak because of the increased membrane potential caused by oligomycin (4, 11). It should also be noted that the rate of proton leak also includes contributions to the movement of other cations such as calcium across the inner membrane, which also decrease the proton gradient. Nonmitochondrial OCR was defined as the OCR following antimycin injection, which blocks electron transfer to cytochrome-c oxidase.
To determine the effects of ThG on individual complex activities, cells were incubated in MAS buffer (70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, and 1 mM EGTA, pH 7.2) for ~10 min followed by treatment with a plasma membrane permeabilizer reagent (2 nM, XF-PMP; 12504-100; Seahorse bioscience), as previously described (41, 42). To measure complex I substrate linked activity, pyruvate (5 mM), malate (2.5 mM), and ADP (1 mM) were added, followed by complex I inhibitor rotenone (1 µM); complex I substrate linked activity was calculated by subtracting the postrotenone OCR from the postpyruvate/malate OCR. Complex II substrate-linked activity was measured in the same cells as complex I by adding 10 mM succinate and 1 mM ADP followed by 10 µM antimycin A; the rate of complex II substrate linked activity was calculated by subtracting the postantimycin A OCR from the OCR postsuccinate. The presence of rotenone limited any potentially confounding effects of reverse electron transport that might occur following the addition of antimycin A. In separate experiments, complex IV substrate linked activity was measured with the addition of 0.5 mM N,N,N′,N′-tetramethyl-p-phenylenediamine, 2 mM ascorbate, and 1 mM ADP, followed by 1 µg/ml oligomycin, 1 µM FCCP, and 20 mM azide. Subtracting OCR following azide injection from OCR following N,N,N′,N'-tetramethyl-p-phenylenediamine/ascorbate injection was used to calculate complex IV substrate linked activity.
Citrate synthase activity assay.
Cells were plated at 250 k/well on six-well plates. Cells were scraped into 200 µl of T-PER lysis buffer. Activity was measured using a Beckman Coulter DU800 spectrophotometer. 50 µl of 100 mM Tris (pH 8.0 at 37°C) with 0.1% Triton X-100 was added to the cuvette followed by 25 µl of sample, 10 µl of 10 mM acetyl-CoA, 10 µl 5,5′-dithiobis(2,4-nitrobenzoic acid), and 10 µl of 20 mM oxaloacetate. Activity was measured for 4 min, and the rate was calculated from the linear range and normalized to total protein.
Relative mitochondrial DNA copy number.
DNA was extracted from cells using a QIAamp DNA Mini Kit per manufacturer’s protocol. Quantitative real-time PCR was performed by using a SYBR Green master mix (Life Tech) in an ABI 7500 qRT-PCR system. The primer sequences used for mitochondrial DNA damage (mtDNA) were mtDNA-forward (F) (5′-CCC CAC AAA CCC CAT TAC TAA ACC CA-3′) and mtDNA-reverse (R) (5′-TTT CAT CAT GCG GAG ATG TTG GAT GG-3′). The primer sequences for the nuclear DNA were 18S-F (5′-TAGAGGGACAAGTGGCGTTC-3′) and 18S-R (5′-CGCTGAGCCAGTCAGTGT-3′). Cycling conditions were as follows: 94°C for 15 s, followed by 40 cycles at 94°C for 15 s, 60°C for 1 min and 60°C for 1 min. The mtDNA copy number was normalized to the 18S copy number.
mtDNA assay.
mtDNA was evaluated by modified quantitative PCR method, as described previously (7). Briefly, total DNA was extracted and used as a PCR sample. The primer sequences used for mtDNA long segment (16 kb) were mtLong-F (5-TGA GGC CAA ATA TCA TTC TGA GGG GC-3) and mtLong-R (5-TTT CAT CAT GCG GAG ATG TTG GAT GG-3). The primer sequences for mtDNA short (80 bp) segment were mtShortF (5-CCC CAC AAA CCC CAT TAC TAA ACC CA-3) and mtShortR (5-TTT CAT CAT GCG GAG ATG TTG GAT GG-3). The mtDNA long segment and the short segment were amplified using AccuPrime Taq DNA Polymerase High Fidelity kit (Life Tech) and separated by agarose gel electrophoresis, respectively. mtDNA long PCR condition was as follows: 94°C for 11 s, followed by 27 cycles of denaturation at 94°C for 15 s, annealing and extension at 67°C for 12 min, and final extension at 72°C for 10 min. mtDNA Short PCR condition was as follows: 94°C for 5 s, followed by 19 cycles of denaturation at 94°C for 25 s, annealing at 60°C for 45 s, extension at 72°C for 1 min, and final extension at 72°C for 10 min. The gels were stained by ethidium bromide and visualized with Alpha Imager, and densitometry analysis was performed using NIH ImageJ software. Lesion frequency per 16 kb of mtDNA was calculated by the following equation (22):
Isolation of mitochondria.
Mitochondria were isolated using the Mitochondria Isolation Kit for Cultured Cells (no. ab110170; Abcam). The mitochondrial isolation was performed by first incubating cells in ThG at 1 µM and 5 µM for 6 h. Following treatment with ThG, cells were harvested in 1× PBS and cell lysates were frozen and thawed to weaken membranes and mitochondria isolated, as per manufacturer’s protocol. Briefly, samples were resuspended in chilled reagents A and B, followed by rupture with a dounce homogenizer and differential centrifugation, which separated the mitochondria pellet away from the remaining postmitochondrial fraction. The mitochondria pellet was then resuspended in reagent C supplemented with protease inhibitors (P8340, P5726, and P0044; Sigma). Aliquots were frozen at −20°C and stored for subsequent protein assays and immunoblot analysis. All mitochondria fractions were separated by electrophoresis and probed for O-GlcNAc on 12% gels using a different O-GlcNAc antibody (no. MABS1254; Millipore) equipped to stain for O-GlcNAc residues <50 kDa, unlike the UAB core antibody used to assess whole cell lysates.
Cytochrome-c oxidase immunoprecipitation.
Cytochrome-c oxidase was immunoprecipitated from mitochondrial pellets using a total complex IV antibody irreversibly cross linked to protein G-agarose beads (no. ab109801; Abcam), as previously described (35). Samples were immunoprecipitated overnight at 4°C by mixing. After mixing, the beads were collected by centrifugation for 1 min at 1,000 g, and the beads were washed three times with a buffer containing 1× PBS and 1 mM n-dodecyl-β-d-maltopyranoside (0.05% wt/vol lauryl matoside) for 10 min while being gently mixed. For SDS-PAGE, proteins bound to complex IV were eluted from the beads by mixing the beads in 6× Laemmli sample buffer for 10 min at room temperature and centrifuged at 1,000 g, twice. The combined supernatant was then loaded in a 12% SDS-PAGE gel.
Statistical analysis.
All immunoblotting data are representative of a minimum of three independent experiments. Seahorse data represents experiments done with an n ≥ 3 independent samples. NIH ImageJ was used for measuring and analysis of blot densitometry. To compare data containing three or more groups, we used one-way ANOVA followed by post hoc analysis. P < 0.05 was considered significant, different from untreated control. Data are presented as means ± SE.
RESULTS
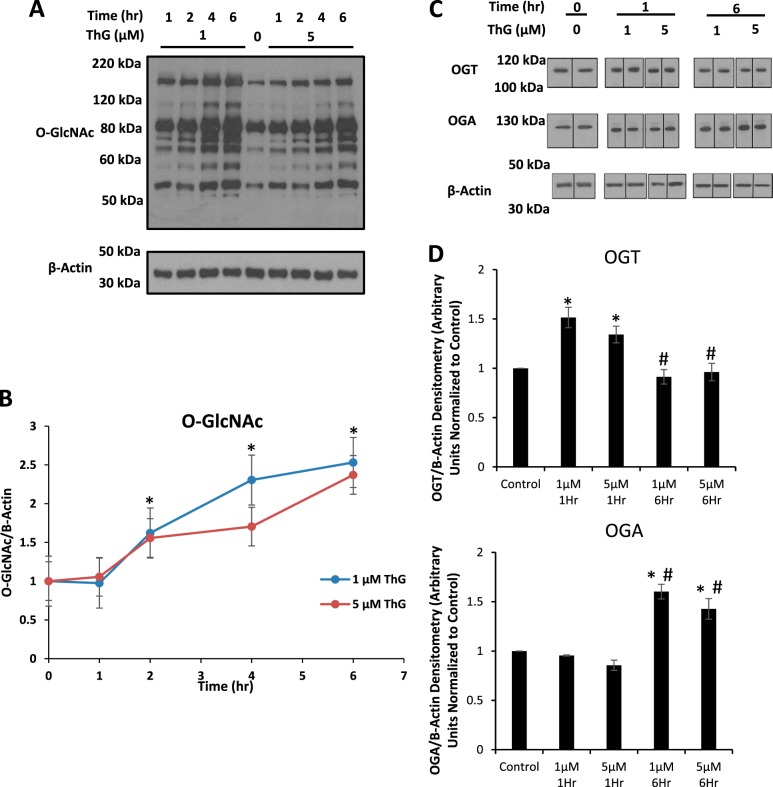
Cells were treated with either 1 or 5 µM ThG for 1, 2, 4, and 6 h, and protein O-GlcNAcylation was measured by Western blotting. Both doses of ThG increased total whole cell O-GlcNAc levels in a time-dependent manner to similar levels (Fig. 1, A and B), consistent with earlier studies in neonatal rat cardiomyocytes (61). With respect to OGT and OGA protein levels, we found a ThG-dependent transient increase for OGT at 1 h and an increase in OGA at 6 h (Fig. 1, C and D). The rise in OGA protein levels in response to ThG is consistent with other reports and interpreted as a homeostatic mechanism to try and lower cellular O-GlcNAc levels (53, 60). The transcription regulation of OGT is not well understood; consequently, the reason for the changes in OGT protein over time is not known.
Fig. 1.
Time- and dose-dependent effects of Thiamet-G (ThG) protein O-linked β-N-acetylglucosamine (O-GlcNAc)ylation in AC16 cells. AC16 cardiomyocytes below passage 13 were used for experiments. A: immunoblot analysis of protein O-GlcNAcylation in AC16 cells exposed to ThG (1 and 5 µM) over 0–6 h. B: quantification of whole lane densitometry representative of O-GlcNAc relative to β-actin from A. C: immunoblot analysis of O-GlcNAcase (OGA) and O-GlcNAc transferase (OGT) in AC16 cells exposed to 1 and 5 µM ThG for 1- and 6-h time points. D: densitometric analysis of immunoblots in C OGA/OGT relative to β-actin. Data are means ± SE; n = 3 replicates per sample. *P < 0.05, different from untreated control. #P < 0.05, different from same dose at different time point.
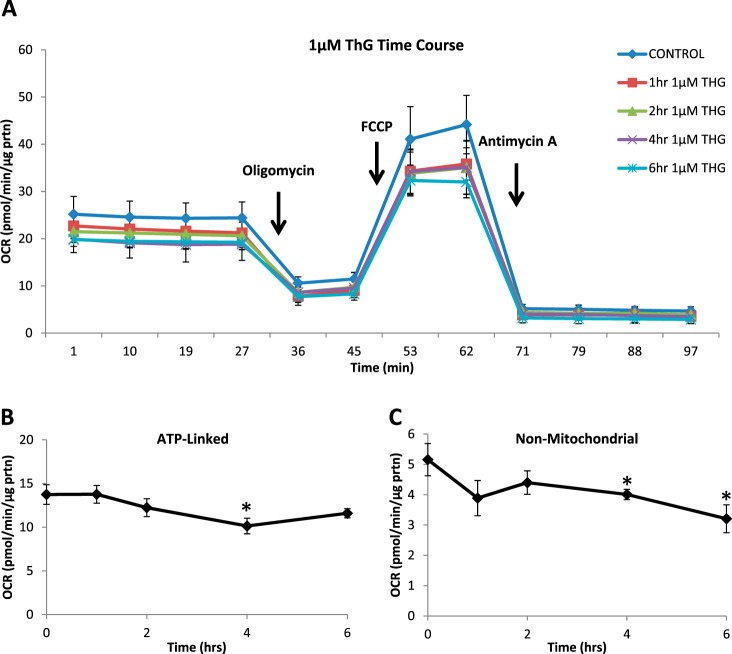
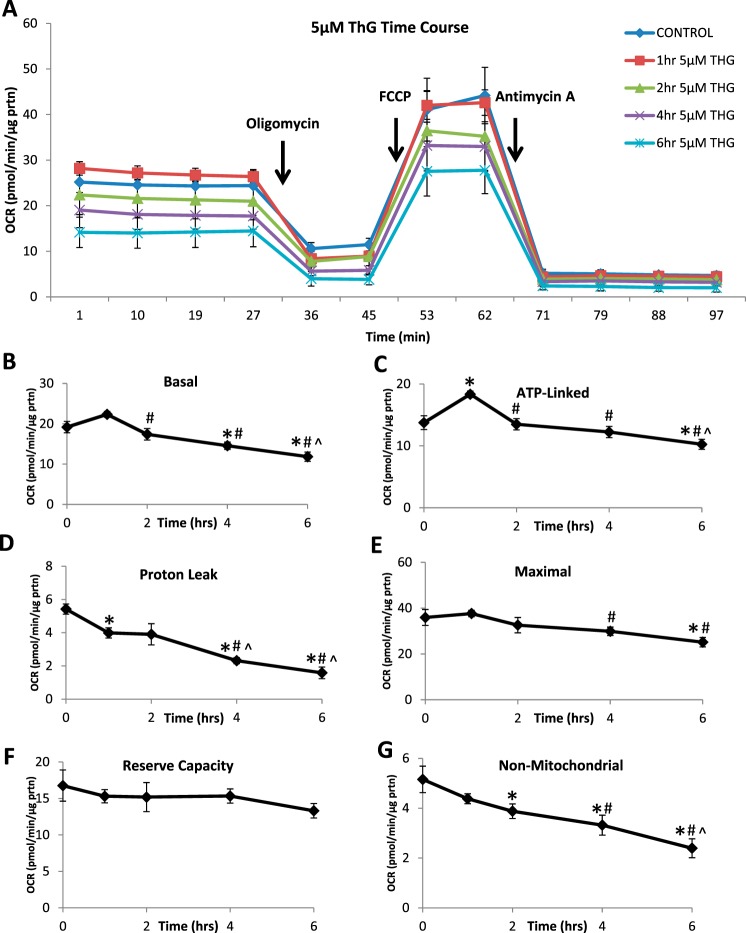
Cellular bioenergetics were measured over 6 h at 1 and 5 μM ThG and compared with untreated controls (Figs. 2 and 3). There were no significant changes in basal, proton leak, maximal or reserve capacity OCR at any time point with 1 µM ThG (data not shown). At 1 µM ThG, ATP-linked OCR, determined after the addition of oligomycin was decreased at 4 h but recovered by 6 h (Fig. 2B). Nonmitochondrial OCR was also decreased as time of ThG exposure increased (Fig. 2C). In contrast, at 5 µM ThG there was a time-dependent decrease in basal OCR (Fig. 3, A and B) as well as significant decreases in proton leak, maximal, and nonmitochondrial OCR (Fig. 3, D, E, and G). There was a transient increase in ATP linked OCR at 1 h with 5 µM ThG, followed by a progressive decrease, which reached significance at 6 h (Fig. 3C). Interestingly, reserve capacity was maintained over the time course of exposure for both concentrations (Fig. 3F).
Fig. 2.
Effect of low-dose Thiamet-G (ThG) treatment (1 µM) on mitochondrial function over time. Oxygen consumption rate (OCR) was measured following 1 µM ThG treatment over increasing time periods. A mitochondrial stress test was performed by establishing basal OCR followed by sequential injections of 1 µg/ml oligomycin, 1 µM FCCP, and 10 µM antimycin A. A: representative OCR traces of mitochondrial stress test. B and C: indexes of mitochondrial function: ATP-linked (B) and nonmitochondrial (C) OCR were calculated. Data are means ± SE; n = 3–5 replicates per sample. *P < 0.05, different from untreated control.
Fig. 3.
Effect of high-dose Thiamet-G (ThG) treatment (5 µM) on mitochondrial function over time. Oxygen consumption rate (OCR) of mitochondria was measured following 5 µM ThG treatment over increasing time periods. A mitochondrial stress test was performed by establishing basal OCR followed by sequential injections of 1 µg/ml oligomycin, 1 µM FCCP, and 10 µM antimycin A. A: representative OCR traces of mitochondrial stress test. B–F: indexes of mitochondrial function: basal (B), ATP-linked (C), proton leak (D), maximal (E), reserve capacity (F), and nonmitochondrial (D) OCR were calculated. Data are means ± SE; n = 3–5 replicates per sample. *P < 0.05, different from untreated control. #P < 0.05, different from 1-h time point. ^P < 0.05, different from 2-h time point.
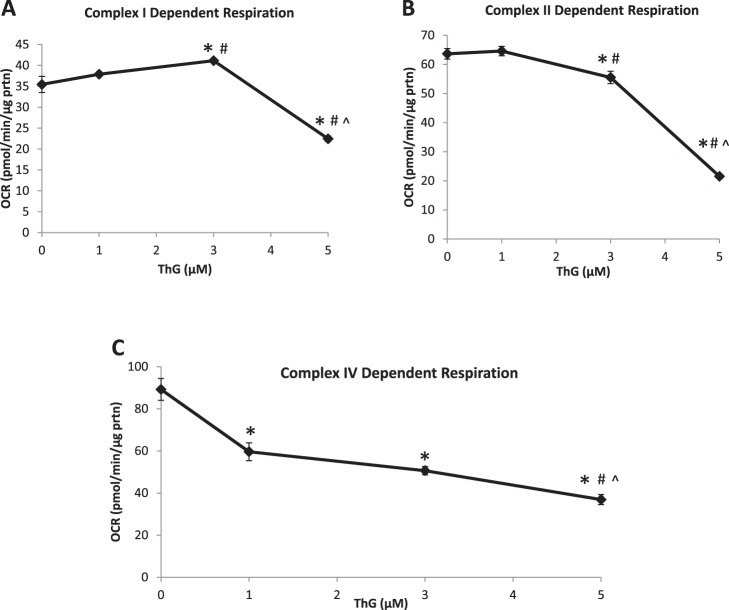
To determine whether the changes in mitochondrial bioenergetics could be attributed to deficits in the activity of the mitochondrial electron transport chain complexes, we measured complex I-, II-, and IV-mediated activity in permeabilized cells at 6 h following treatment with 1, 3, and 5 µM ThG. As shown in Fig. 4, the activities of complexes I and II were significantly decreased with only 5 µM ThG (Fig. 4, A and B). In contrast, complex IV (Fig. 4C) was more sensitive with a significant decrease in activity detected at 1 µM ThG and a further decrease at 5 µM. To determine whether changes in O2 consumption via reverse electron transport through complex I might influence the results following addition of rotenone and antimycin A, we also calculated complex I and II activities without subtraction of the rates following addition of their respective inhibitors. The response of both complexes to ThG treatment was almost identical to that shown in Fig. 4, A and B (data not shown). Consequently, possible increases in O2 consumption from other sources did not alter the dose-dependent effects of ThG on complex I- and II-dependent activity.
Fig. 4.
Mitochondrial complex activity in permeabilized cardiomyocytes following Thiamet-G (ThG). AC16 cells (10 × 106/well) were plated on 96-well plates and treated with increasing doses of ThG (1, 3, and 5 µM) for 6 h. Cells were placed in MAS buffer for the permeabilization assay. First basal oxygen consumption rate (OCR) was established, and then, 5 mM pyruvate, 2.5 mM malate, and 1 mM ADP were injected; followed by 1 µM rotenone, 10 mM succinate, and 1 mM ADP; and then 10 µM antimycin A. A: indexes of increasing ThG concentration on complex I-dependent activity were calculated. B and C: complex II-dependent activity was determined (B) and complex IV dependent activity (C). Data are means ± SE; n = 3–5 replicates per sample. *P < 0.05, different from untreated control. #P < 0.05, different from 1 µM ThG treatment. ^P < 0.05, different from 3 µM ThG treatment.
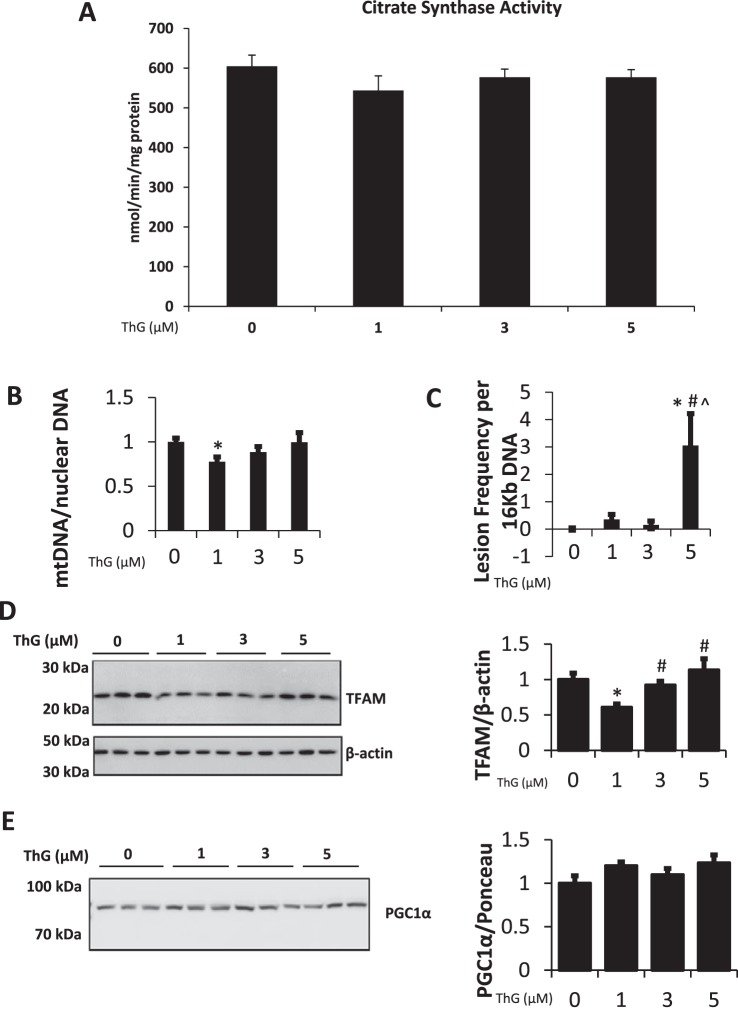
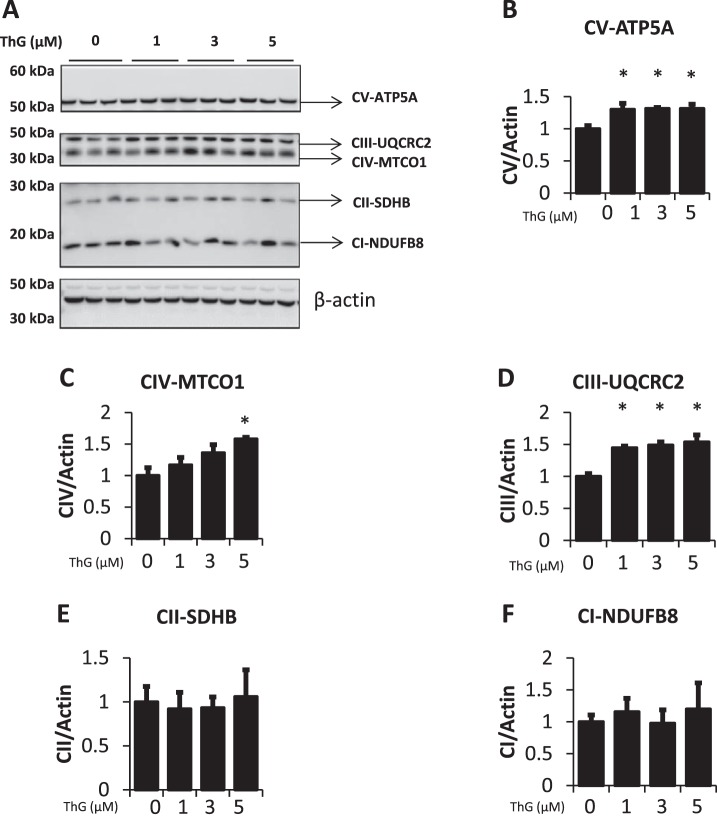
To determine whether the decrease in complex activity in response to ThG treatment was a consequence of reduced mitochondrial mass, we determined the activity of citrate synthase, mtDNA copy number, mtDNA damage, as well as levels of proteins involved in mitochondrial biogenesis. There were no significant changes in citrate synthase activity after 6 h of treatment at any concentration of ThG tested, consistent with no change in mitochondrial mass (Fig. 5A). A decrease in mtDNA copy number was observed at 1 μM ThG, while higher ThG concentrations yielded no change (Fig. 5B). Evidence of mtDNA damage was only seen with 5 µM ThG where it was increased greater than threefold compared with untreated controls (Fig. 5C). There was a decrease in TFAM levels after exposure to 1 µM ThG, which was not present at the higher concentrations. (Fig. 5D). The levels of PGC1-α, the coactivator that works in concert with TFAM, show no significant change at any ThG concentration (Fig. 5E). Decreases in complex activity could be due to reduction in the level of complex subunit proteins. However, we saw either no change or a modest but significant increase in representative subunits of the mitochondrial complexes in response to ThG treatment (Fig. 6A). Representative subunit proteins ATP5A (complex V) and UQCRC2 (complex III) were both increased by ThG treatment (Fig. 6, B and D). Protein amounts of complex II subunit SDHB and complex I subunit NDUFB8 remained relatively unchanged by ThG treatment (Fig. 6, E and F), whereas complex IV subunit MTCO1 increased in a dose-dependent manner following ThG treatment (Fig. 6C).
Fig. 5.
Effect of increasing doses of Thiamet-G (ThG) on mitochondrial DNA and biogenesis proteins. A–C: following treatment for 6 h with increasing doses of ThG citrate synthase activity (A), mitochondrial DNA copy number relative to nuclear DNA (B), and mitochondrial DNA damage were measured (C). D and E: the levels of transcription factor A, mitochondrial (TFAM; D) and peroxisome proliferator-activated receptor γ-coactivator 1-α (PGC1-α; E) were measured via immunoblot analysis and quantified relative to β-actin. Data are means ± SE; n = 3 replicates per sample. *P < 0.05, different from untreated control. #P < 0.05, different from 1 µM ThG treatment. ^P < 0.05, different from 3 µM ThG treatment.
Fig. 6.
Effect of increasing doses of Thiamet-G (ThG) on mitochondrial complex proteins. A: The levels of complex I-V subunits in response to increasing doses of ThG (1, 3, and 5 µM) treatment for 6 h were measured. B–F: quantification of the Western blot images normalized to the untreated control. Data are means ± SE; n = 3 replicates per sample. *P < 0.05, different from untreated control.
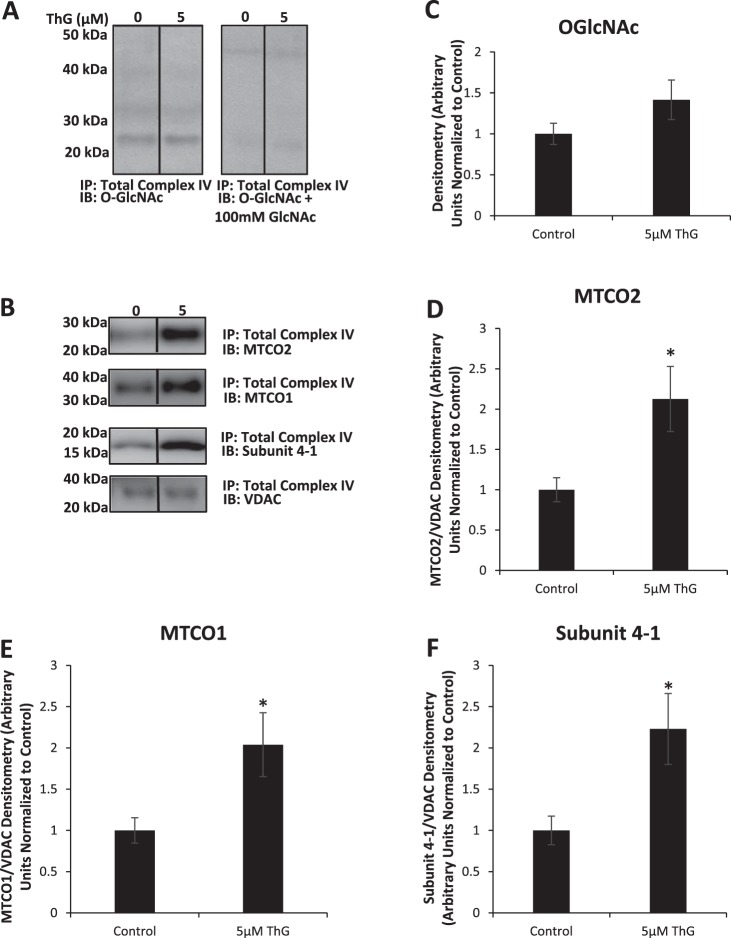
Complex IV activity was the most sensitive to ThG treatment (Fig. 4, A and C); therefore, we examined whether the observed decline in the activity of this complex could be linked to changes in O-GlcNAcylation of complex IV subunits. Following mitochondrial isolation and immunoprecipitation with an antibody against total complex IV, the proteins were subjected to O-GlcNAc immunoblot (Fig. 7). Only a single O-GlcNAc-positive band at ~26 kDa was observed, which was absent in the presence of GlcNAc confirming the specificity of the O-GlcNAc immunoblot (Fig. 7, A and C). This band was present in both untreated and treated groups indicating constitutive modification of a subunit of complex IV by O-GlcNAc; however, there was no significant increase in O-GlcNAc levels following ThG treatment. The complex IV subunit MTCO2 has a molecular mass of 26 kDa and immunoblotting for MTCO2 indicated a protein at the same molecular mass as the O-GlcNAc-positive band (Fig. 7, B and D). Unfortunately, we were unable to successfully immunoprecipitate MTCO2 and consequently cannot be certain that this complex IV subunit is a target for O-GlcNAcylation.
Fig. 7.
Effect of increasing doses of Thiamet-G (ThG) on O-linked β-N-acetylglucosamine (O-GlcNAc) modification of cytochrome c oxidase subunits. Cytochrome c oxidase complex was pulled down to assess any change in O-GlcNAcylation status in response to varying doses of Thiamet-G treatment for 6 h. As noted in materials and methods, the Millipore CTD 110.6 antibody was used in the mitochondrial O-GlcNAc blots shown here. A–F: Western blot analysis of O-GlcNAc with and without preincubation with 100 mM GlcNAc to show specificity for O-GlcNAc immunoblotted (IB) proteins (A) is represented as well as Western blot analysis of MTCO2 (B and D), MTCO1 (B and E), subunit 4-1 (B and F), and VDAC (B) including relative quantification (C) of the Western images normalized to the untreated control. IP, immmunopecipitation. Data are means ± SE; n = 3 replicates per sample. *P < 0.05, different from untreated control.
As we only saw one predominant O-GlcNAc band following immunoprecipitation, we immunoblotted for the additional complex IV subunits MTCO1 and 4-1 to ensure other complex proteins were indeed present. (Fig. 7, B, E, and F). We found that in addition to MTCO2, MTCO1 and subunit 4-1 were also being immunoprecipitated, but there were no O-GlcNAc-positive bands at those molecular weights indicating that they were not targets for O-GlcNAcylation under these conditions. However, even though these subunits were not O-GlcNAcylated, the amount of all three subunit proteins being pulled down was significantly higher following ThG treatment. This is consistent with the effects of ThG treatment on other subunit proteins in whole cell extracts as shown in Fig. 6. The fact that there was a change in total protein levels of MTCO2 with treatment but the O-GlcNAc-positive band at the same molecular weight did not change suggests that it is either not MTCO2 or that the increased MTCO2 protein is not O-GlcNAcylated. Perhaps surprisingly, the mitochondrial outer membrane protein VDAC was also pulled down in this IP; however, the amount of protein was not changed with ThG treatment.
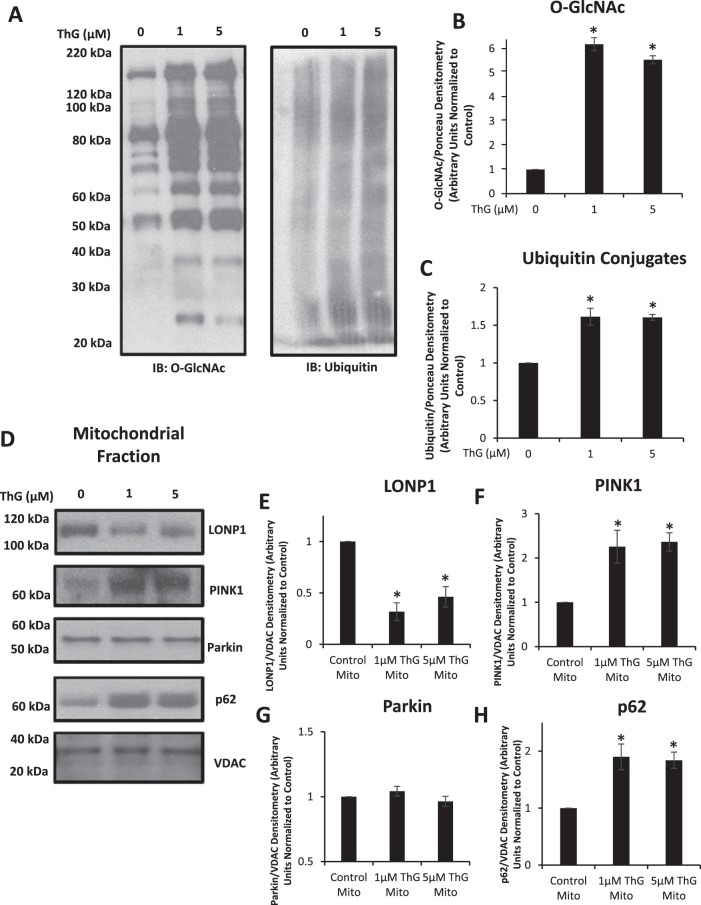
While changes in O-GlcNAcylation of complex IV subunit proteins were not associated with its decreased activity, a consistent observation in both whole mitochondrial extracts (Fig. 6) and following complex IV immunoprecipitation (Fig. 7) was an increase in the amount of subunit proteins following ThG treatment. One possible explanation for this observation is a decrease in mitochondrial protein turnover. Both 1 µM and 5 µM ThG groups markedly increased protein O-GlcNAc levels in mitochondrial extracts, which was associated with an increase in ubiquitinated proteins, supporting the concept of reduced mitochondrial protein turnover (Fig. 8, A–C). It should be noted that the O-GlcNAc blot in Fig. 8A has many more O-GlcNAc-positive bands than in Fig. 7A, consistent with it being from a mitochondrial extract and not following an immunoprecipitation for complex IV. Consequently, the change in O-GlcNAc levels at ~26 kDa is likely due to changes in O-GlcNAc levels of mitochondrial proteins, in addition to those seen in Fig. 7A. Also as noted in materials and methods, a different antibody was used in the mitochondrial O-GlcNAc blots in Figs. 7 and 8 than in whole cell blots shown in Fig. 1.
Fig. 8.
Effect of Thiamet-G (ThG) on ubiquitinated mitochondrial fractions. Mitochondrial fractions were isolated and assessed to measure changes in ubiquitin conjugates, or mitochondrial quality control proteins in response to ThG (1 and 3 µM) treatment for 6 h. The Millipore CTD 110.6 antibody was used in the mitochondrial O-linked β-N-acetylglucosamine (O-GlcNAc) blots shown here. A–C: Western blot analysis of O-GlcNAc (left) ubiquitin conjugates (right) and loading control staining are represented as well as quantification of the Western images normalized to the untreated control (B and C). D–H: Western blot analysis of Lon protease homolog 1 (LonP1), phosphatase and tensin homolog induced kinase 1 (PINK1), Parkin, and p62 are represented (D) as well as relative quantification of the Western images (E–H) normalized to the untreated control. Data are means ± SE; n = 3 replicates per sample. *P < 0.05, different from untreated control.
Protein degradation in the mitochondria is primarily regulated by Lon protease 1 (LonP1), and there was a marked decrease in LonP1 protein at both 1 and 5 µM ThG (Fig. 8, D and E). The reduction in LonP1 was associated with an increase in phosphatase and tensin homolog induced kinase 1 (PINK1), a key initiator of mitophagy (Fig. 8, D and F); however, there was no change in the mitochondrial protein levels of the E3 ubiquitin ligase Parkin (Fig. 8, D and G). The poly-ubiquitin binding protein p62, a scaffolding protein that is decreased when autophagolysosomal degradation is functional, was increased at both doses of ThG treatment (Fig. 8, D and H).
DISCUSSION
Studies have shown that elevated O-GlcNAc levels protect mitochondria from oxidative stress (5, 18, 33, 34), whereas others have reported that O-GlcNAcylation of mitochondrial proteins impairs mitochondrial oxygen consumption (13). In contrast, other studies have suggested that altering O-GlcNAc levels has no effect on mitochondrial oxygen consumption (8). These divergent findings can be attributed at least in part to the differing approaches taken to increase cellular O-GlcNAc levels and the duration of treatment used to increase O-GlcNAc levels. Therefore, the goal of this study was to better understand the acute effects of increased O-GlcNAc levels on mitochondrial function via the use of low concentrations of ThG, a highly selective inhibitor of OGA. We observed a dose- and time-dependent effect with ThG treatment on decreased mitochondrial bioenergetics and reduced activities of complex I-, II-, and IV-dependent activity. We also observed that increased O-GlcNAcylation was associated with the accumulation of several proteins in the oxidative phosphorylation complexes, an increase in ubiquitinated proteins, which was accompanied by a significant decrease in the level of the mitochondrial protease LonP1. LonP1, a key mitochondrial protease regulating matrix and inner membrane protein degradation (1, 2, 26), is known to play an important role in mitochondrial protein quality control (32, 57). Moreover, decreased LonP1 levels have been shown to result in accumulation of oxidized proteins, impair mitochondrial function (3, 50), and increase PINK1 protein levels (47, 51, 63). Consequently, these findings suggest that acute increases in O-GlcNAcylation may lead to impaired mitochondrial oxygen consumption and complex activities due to decreased mitochondrial protein turnover.
We have previously reported that increasing O-GlcNAcylation attenuated the loss of mitochondrial membrane potential in cardiomyocytes subject to ischemia/reperfusion and hydrogen peroxide (5). We found that this was associated with increased recruitment of Bcl-2 to the mitochondria; however, we did not show that this protection was associated with O-GlcNAcylation of mitochondrial proteins (5). Ngoh and colleagues (21, 33) demonstrated that increased O-GlcNAcylation was cardioprotective and this was associated with O-GlcNAcylation of VDAC. They also showed that increased O-GlcNAc levels protected isolated mitochondria from Ca2+ overload. In contrast, Hu and colleagues (19) reported that the adverse effects of hyperglycemia on cardiomyocytes were a consequences of O-GlcNAcylation of mitochondrial proteins such as NDUFA9, nuclear encoded subunit of complex I, and COXI, the mitochondrial encoded subunit of complex IV. On the other hand, Jones and coworkers concluded that the detrimental effects of hyperglycemia on mitochondrial function was due to an increase in osmotic pressure and not an increase in O-GlcNAc levels (8). Conversely, following in vivo treatment with the OGA inhibitor ThG, mitochondria isolated from cardiomyocytes exhibited improved mitochondrial function as indicated by increased rates of mitochondrial oxygen consumption, ATP production, and mitochondrial membrane potential (31). Other studies have reported that sustained changes in O-GlcNAcylation either via genetic manipulation of OGT/OGA or pharmacological approaches led to marked metabolic reprogramming and mitochondrial dysfunction (45).
O-GlcNAcylation is known to regulate numerous transcription pathways, moreover, to ensure quality control mitochondria undergo continuous fission and fusion to maintain a functional mitochondrial network. A common feature of the more recent studies is that the duration of increased O-GlcNAcylation, whether due to hyperglycemia, inhibition of OGA, or OGT overexpression was relatively long, in the range of 24–48 h. Consequently, interpretation of prolonged changes in O-GlcNAcylation could be complicated by adaptive changes. Our findings demonstrated that increasing O-GlcNAc levels in vitro for only 6 h resulted in altered bioenergetics in intact cells as well as decreased activities of complex I, II, and IV in permeabilized cells. This occurred in the absence of mitochondrial loss or changes in indicators of mitochondrial biogenesis (Fig. 5).
While 1 µM ThG had relatively little effect on cellular bioenergetics (Fig. 2), 5 µM ThG decreased basal OCR in a time-dependent manner as well as ATP-linked, proton leak, maximal, and nonmitochondrial OCR (Fig. 3). Reserve capacity, determined by subtracting basal OCR from maximal was unaffected by ThG treatment at either concentration. As discussed in detail by Hill et al. (16), multiple factors can contribute to the changes in each of these parameters. The decrease in basal OCR could be due in part to a reduction in proton leak as well as reduced ATP demand or reduced substrate supply. A reduction in ATP-linked OCR could also be a result of decreased energy demand or low substrate availability, and damage to the electron transport system could also be a potential factor. However, since reserve capacity is unaffected by ThG treatment, this would imply that substrate availability is not limiting and that the electron transport system is not significantly impaired. Consequently, it would appear that under the conditions of these experiments, the most likely explanation of the bioenergetic changes that occur in response to ThG is a combination of decreased energy demand and reduced proton leak. The mechanisms by which this occurs remain to be determined.
Following complex IV immunoprecipitation, we observed an O-GlcNAc-positive band at the molecular weight consistent with that of subunit MTCO2 (Fig. 7); however, the intensity of this band did not increase with ThG treatment and we could not confirm that this band was indeed MTCO2. Mass spectrometry could be used to identify this protein and its specific O-GlcNAc modification site(s), but given the lack of an association between changes in complex activity and O-GlcNAc levels, we did not pursue this line of inquiry. It should be noted however that MTCO2, in addition to other complex subunit proteins, has been shown to be a target for O-GlcNAcylation (31). Although we saw an overall increase in O-GlcNAcylation of mitochondrial proteins, our results suggest that the decline in complex IV activity could not be attributed to changes in O-GlcNAcylation of complex IV subunit proteins. However, we consistently observed that increasing O-GlcNAc levels was associated with higher levels of complex subunit proteins including UQCRC2-CIII, MTCO1-CIV, ATP5A-CV (Fig. 6), and MTCO2 (Fig. 7). In light of the change in subunit protein levels, we estimated complex I-, II-, and IV-specific activities as the ratio of their respective OCR (Fig. 4) normalized to levels of representative subunit proteins (Fig. 6). In all cases the decreases in specific activities remained significant at 5 µM ThG, and in complex IV a decrease in activities remained at 1 µM ThG (data not shown).
Electron transport chain subunit proteins are readily subject to oxidative damage; thus, regulation of mitochondrial protein turnover is critical in maintaining mitochondrial function and requires the tight control of both protein import and protein degradation. Impaired mitochondrial protein turnover, specifically involving subunit proteins, can result in impaired complex activities (44), which have adverse effects on mitochondrial function. The mitochondrial protease LonP1 has been reported to play an important role in degrading complex subunit proteins. For example, increased expression of LonP1 has been shown to reduce amounts of complex IV subunit IVi1 (43), and stress-induced increases in LonP1 transcription have also resulted in degradation of the complex IV subunit I Cox4-1 (12). Conversely, reduced LonP1 is associated with the accumulation of protein aggregates in the mitochondrial matrix, lowered complex I and IV activities, and decreases in ATP content and mitochondria membrane potential (24, 38, 63). Recent studies have shown that decreased levels of LonP1 increased sensitivity to ischemia/reperfusion injury and impaired mitochondrial function (50), further emphasizing the importance of LonP1 in regulating response of mitochondria to stress.
Here we demonstrated for the first time that acutely increasing mitochondrial O-GlcNAc levels resulted in a >50% decrease in LonP1 expression (Fig. 8E), which was associated with accumulation of mitochondrial subunit proteins and impaired complex activity and mitochondrial bioenergetics. Accumulation of damaged proteins has been shown to trigger mitophagy (10, 20, 49, 59), and consistent with that we observed increased PINK1 and p62 and that there was no increase in Parkin protein associated with the mitochondria. The initiation of mitophagy/autophagy is typically associated with increase in mitochondrial Parkin and lower p62. As Parkin is a cytosolic protein that is recruited to the mitochondria, the observed changes are consistent with impaired turnover specifically of mitochondrial proteins. It should also be emphasized that complex IV subunit proteins and PINK1 are known targets of LonP1. In other words, the impaired mitochondrial function following acute increases in whole cell and mitochondrial O-GlcNAc levels is likely due to decreased mitochondrial LonP1 protein levels. While we did not directly measure mitochondrial protein turnover, several reports have related changes in PINK1 and p62 protein levels to early stage indexes of changes in mitochondrial protein turnover (6, 23, 27, 36).
We have focused on the adverse effects of O-GlcNAcylation on mitochondrial function; however, there was some evidence that at shorter durations there could be increased function as seen in the increase in ATP linked oxygen consumption at 1 h of 5 µM ThG (Fig. 3C). In addition, since decreases in complex IV activity are already evident at 1 µM ThG, studies with lower ThG concentrations are warranted. This is potentially of importance as we have previously shown that a lower ThG concentration (0.25 µM) attenuates autophagy in primary neurons (54–56). Overall, these studies have shown that increases in cellular O-GlcNAc levels have relatively rapid effects on mitochondrial function, suggesting that the effects of more prolonged periods of increased O-GlcNAcylation are likely multifactorial and include adaptive responses to these early changes. Further studies, including but not limited to, assessment of complex IV ubiquitination following ThG treatment, are clearly needed to better understand the effects of O-GlcNAcylation on mitochondrial protein turnover and function. Additional studies in primary cells cultures such as isolated adult cardiomyocytes and neurons will be valuable in determining whether the reduction in LonP1 associated with increased O-GlcNAc levels observed here is more broadly applicable.
In conclusion, we have demonstrated that relatively short periods of increased O-GlcNAc levels induced by inhibition of OGA result in impaired mitochondrial bioenergetics and decreased activity of several mitochondrial complexes. However, we were unable to demonstrate that the changes in mitochondrial function were due to direct O-GlcNAcylation of complex subunit proteins. Based on our results it is likely that the decrease in mitochondrial function occurs due to the decrease in LonP1. While we have not identified a specific mechanism by which O-GlcNAcylation reduces LonP1 protein levels, changes in O-GlcNAcylation can directly affect protein turnover/stability and transcription. While we were unable to identify a direct link between increased O-GlcNAc levels and the decrease in LonP1, LonP1 expression has been shown to be regulated by NRF2 and NF-κB (39), two transcription factors that are both O-GlcNAc targets (25, 40, 45, 62). Thus our findings suggest that short-term increases in O-GlcNAc levels result in adverse effects on mitochondrial function via indirectly reducing turnover of complex proteins.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 5T32 HL007918-17 (to J. N. Wright), HL142216 (to J. C. Chatham and J. Zhang, and HL101192 (to J. C. Chatham). This work was also supported by American Diabetes Association Postdoctoral Fellowship 1-16-PDF-024 (to H. E. Collins) and partially supported by University of Alabama at Birmingham AMC21 Reload Multiinvestigator Grant (to J. C. Chatham, J. Zhang, and V. Darley-Usmar) and Nathan Shock Center Grant P30 G050886 (to J. Zhang and V. Darley-Usmar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.C. conceived and designed research; J.N.W., G.A.B., M.S.J., W.W., X.O., and L.Z. performed experiments; J.N.W., G.A.B., M.S.J., W.W., X.O., and L.Z. analyzed data; J.N.W., G.A.B., M.S.J., H.E.C., J.Z., V.D.-U., and J.C.C. interpreted results of experiments; J.N.W. prepared figures; J.N.W. drafted manuscript; J.N.W., G.A.B., M.S.J., H.E.C., J.Z., V.D.-U., and J.C.C. edited and revised manuscript; J.N.W., G.A.B., M.S.J., W.W., X.O., L.Z., H.E.C., J.Z., V.D.-U., and J.C.C. approved final version of manuscript.
REFERENCES
- 1.Bayot A, Gareil M, Rogowska-Wrzesinska A, Roepstorff P, Friguet B, Bulteau AL. Identification of novel oxidized protein substrates and physiological partners of the mitochondrial ATP-dependent Lon-like protease Pim1. J Biol Chem 285: 11445–11457, 2010. doi: 10.1074/jbc.M109.065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender T, Leidhold C, Ruppert T, Franken S, Voos W. The role of protein quality control in mitochondrial protein homeostasis under oxidative stress. Proteomics 10: 1426–1443, 2010. doi: 10.1002/pmic.200800619. [DOI] [PubMed] [Google Scholar]
- 3.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol 4: 674–680, 2002. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 4.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol 294: C1509–C1520, 2008. doi: 10.1152/ajpcell.00456.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Dorn GW 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340: 471–475, 2013. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci 113: 91–98, 1992. doi: 10.1016/0022-510X(92)90270-U. [DOI] [PubMed] [Google Scholar]
- 8.Dassanayaka S, Readnower RD, Salabei JK, Long BW, Aird AL, Zheng YT, Muthusamy S, Facundo HT, Hill BG, Jones SP. High glucose induces mitochondrial dysfunction independently of protein O-GlcNAcylation. Biochem J 467: 115–126, 2015. doi: 10.1042/BJ20141018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson MM, Nesti C, Palenzuela L, Walker WF, Hernandez E, Protas L, Hirano M, Isaac ND. Novel cell lines derived from adult human ventricular cardiomyocytes. J Mol Cell Cardiol 39: 133–147, 2005. doi: 10.1016/j.yjmcc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 393: 547–564, 2012. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic Biol Med 48: 905–914, 2010. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129: 111–122, 2007. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 13.Groves JA, Lee A, Yildirir G, Zachara NE. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones 18: 535–558, 2013. doi: 10.1007/s12192-013-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Y, Ande SR, Mishra S. Altered O-GlcNAc modification and phosphorylation of mitochondrial proteins in myoblast cells exposed to high glucose. Arch Biochem Biophys 505: 98–104, 2011. doi: 10.1016/j.abb.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, Love DC. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys 409: 287–297, 2003. doi: 10.1016/S0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- 16.Hill BG, Benavides GA, Lancaster JR, Ballinger S, Dell’Italia L, Zhang J, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem 393: 1485–1512, 2012. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J 424: 99–107, 2009. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Chen R, Jia P, Fang Y, Liu T, Song N, Xu X, Ji J, Ding X. Augmented O-GlcNAc signaling via glucosamine attenuates oxidative stress and apoptosis following contrast-induced acute kidney injury in rats. Free Radic Biol Med 103: 121–132, 2017. doi: 10.1016/j.freeradbiomed.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Hu Y, Oyeleye MO, Dillmann WH. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem 284: 547–555, 2009. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin SM, Youle RJ. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy 9: 1750–1757, 2013. doi: 10.4161/auto.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marbán E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117: 1172–1182, 2008. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 22.Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, Ischiropoulos H, Ballinger SW. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation 105: 849–854, 2002. doi: 10.1161/hc0702.103977. [DOI] [PubMed] [Google Scholar]
- 23.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524: 309–314, 2015. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HJ, Chung K, Lee H, Lee K, Lim JH, Song J. Downregulation of mitochondrial lon protease impairs mitochondrial function and causes hepatic insulin resistance in human liver SK-HEP-1 cells. Diabetologia 54: 1437–1446, 2011. doi: 10.1007/s00125-011-2074-z. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Liu X, Wang D, Su L, Zhao T, Li Z, Lin C, Zhang Y, Huang B, Lu J, Li X. O-GlcNAcylation of SKN-1 modulates the lifespan and oxidative stress resistance in Caenorhabditis elegans. Sci Rep 7: 43601, 2017. doi: 10.1038/srep43601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lionaki E, Tavernarakis N. Oxidative stress and mitochondrial protein quality control in aging. J Proteomics 92: 181–194, 2013. doi: 10.1016/j.jprot.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Dai C, Fan Y, Guo B, Ren K, Sun T, Wang W. From autophagy to mitophagy: the roles of P62 in neurodegenerative diseases. J Bioenerg Biomembr 49: 413–422, 2017. doi: 10.1007/s10863-017-9727-7. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40: 303–312, 2006. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci 116: 647–654, 2003. doi: 10.1242/jcs.00246. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Banerjee P, Whelan SA, Liu T, Wei AC, Ramirez-Correa G, McComb ME, Costello CE, O’Rourke B, Murphy A, Hart GW. Comparative proteomics reveals dysregulated mitochondrial O-GlcNAcylation in diabetic hearts. J Proteome Res 15: 2254–2264, 2016. doi: 10.1021/acs.jproteome.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Liu T, Wei AC, Banerjee P, O’Rourke B, Hart GW. O-GlcNAcomic profiling identifies widespread O-linked β-N-acetylglucosamine modification (O-GlcNAcylation) in oxidative phosphorylation system regulating cardiac mitochondrial function. J Biol Chem 290: 29141–29153, 2015. doi: 10.1074/jbc.M115.691741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngo JK, Pomatto LC, Davies KJ. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol 1: 258–264, 2013. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol 45: 313–325, 2008. doi: 10.1016/j.yjmcc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids 40: 895–911, 2011. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliva CR, Markert T, Ross LJ, White EL, Rasmussen L, Zhang W, Everts M, Moellering DR, Bailey SM, Suto MJ, Griguer CE. Identification of small molecule inhibitors of human cytochrome c oxidase that target chemoresistant glioma cells. J Biol Chem 291: 24188–24199, 2016. doi: 10.1074/jbc.M116.749978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ordureau A, Heo JM, Duda DM, Paulo JA, Olszewski JL, Yanishevski D, Rinehart J, Schulman BA, Harper JW. Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc Natl Acad Sci USA 112: 6637–6642, 2015. doi: 10.1073/pnas.1506593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palaniappan KK, Hangauer MJ, Smith TJ, Smart BP, Pitcher AA, Cheng EH, Bertozzi CR, Boyce M. A chemical glycoproteomics platform reveals O-GlcNAcylation of mitochondrial voltage-dependent anion channel 2. Cell Rep 5: 546–552, 2013. doi: 10.1016/j.celrep.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peter B, Waddington CL, Oláhová M, Sommerville EW, Hopton S, Pyle A, Champion M, Ohlson M, Siibak T, Chrzanowska-Lightowlers ZM, Taylor RW, Falkenberg M, Lightowlers RN. Defective mitochondrial protease LonP1 can cause classical mitochondrial disease. Hum Mol Genet 27: 1743–1753, 2018. doi: 10.1093/hmg/ddy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinti M, Gibellini L, De Biasi S, Nasi M, Roat E, O’Connor JE, Cossarizza A. Functional characterization of the promoter of the human Lon protease gene. Mitochondrion 11: 200–206, 2011. doi: 10.1016/j.mito.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Ramakrishnan P, Clark PM, Mason DE, Peters EC, Hsieh-Wilson LC, Baltimore D. Activation of the transcriptional function of the NF-κB protein c-Rel by O-GlcNAc glycosylation. Sci Signal 6: ra75, 2013. doi: 10.1126/scisignal.2004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redmann M, Benavides GA, Wani WY, Berryhill TF, Ouyang X, Johnson MS, Ravi S, Mitra K, Barnes S, Darley-Usmar VM, Zhang J. Methods for assessing mitochondrial quality control mechanisms and cellular consequences in cell culture. Redox Biol 17: 59–69, 2018. doi: 10.1016/j.redox.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salabei JK, Gibb AA, Hill BG. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat Protoc 9: 421–438, 2014. doi: 10.1038/nprot.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sepuri NBV, Angireddy R, Srinivasan S, Guha M, Spear J, Lu B, Anandatheerthavarada HK, Suzuki CK, Avadhani NG. Mitochondrial LON protease-dependent degradation of cytochrome c oxidase subunits under hypoxia and myocardial ischemia. Biochim Biophys Acta Bioenerg 1858: 519–528, 2017. doi: 10.1016/j.bbabio.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stiburek L, Cesnekova J, Kostkova O, Fornuskova D, Vinsova K, Wenchich L, Houstek J, Zeman J. YME1L controls the accumulation of respiratory chain subunits and is required for apoptotic resistance, cristae morphogenesis, and cell proliferation. Mol Biol Cell 23: 1010–1023, 2012. doi: 10.1091/mbc.e11-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan EP, McGreal SR, Graw S, Tessman R, Koppel SJ, Dhakal P, Zhang Z, Machacek M, Zachara NE, Koestler DC, Peterson KR, Thyfault JP, Swerdlow RH, Krishnamurthy P, DiTacchio L, Apte U, Slawson C. Sustained O-GlcNAcylation reprograms mitochondrial function to regulate energy metabolism. J Biol Chem 292: 14940–14962, 2017. doi: 10.1074/jbc.M117.797944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan EP, Villar MT, e L, Lu J, Selfridge JE, Artigues A, Swerdlow RH, Slawson C. Altering O-linked β-N-acetylglucosamine cycling disrupts mitochondrial function. J Biol Chem 289: 14719–14730, 2014. doi: 10.1074/jbc.M113.525790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas RE, Andrews LA, Burman JL, Lin WY, Pallanck LJ. PINK1-Parkin pathway activity is regulated by degradation of PINK1 in the mitochondrial matrix. PLoS Genet 10: e1004279, 2014. doi: 10.1371/journal.pgen.1004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259: 3308–3317, 1984. [PubMed] [Google Scholar]
- 49.Van Laar VS, Roy N, Liu A, Rajprohat S, Arnold B, Dukes AA, Holbein CD, Berman SB. Glutamate excitotoxicity in neurons triggers mitochondrial and endoplasmic reticulum accumulation of Parkin, and, in the presence of N-acetyl cysteine, mitophagy. Neurobiol Dis 74: 180–193, 2015. doi: 10.1016/j.nbd.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venkatesh S, Li M, Saito T, Tong M, Rashed E, Mareedu S, Zhai P, Bárcena C, López-Otín C, Yehia G, Sadoshima J, Suzuki CK. Mitochondrial LonP1 protects cardiomyocytes from ischemia/reperfusion injury in vivo. J Mol Cell Cardiol 128: 38–50, 2019. doi: 10.1016/j.yjmcc.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci USA 110: 6400–6405, 2013. doi: 10.1073/pnas.1221132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walgren JL, Vincent TS, Schey KL, Buse MG. High glucose and insulin promote O-GlcNAc modification of proteins, including α-tubulin. Am J Physiol Endocrinol Metab 284: E424–E434, 2003. doi: 10.1152/ajpendo.00382.2002. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Feng Z, Wang X, Yang L, Han S, Cao K, Xu J, Zhao L, Zhang Y, Liu J. O-GlcNAcase deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis. Diabetologia 59: 1287–1296, 2016. doi: 10.1007/s00125-016-3919-2. [DOI] [PubMed] [Google Scholar]
- 54.Wani WY, Boyer-Guittaut M, Dodson M, Chatham J, Darley-Usmar V, Zhang J. Regulation of autophagy by protein post-translational modification. Lab Invest 95: 14–25, 2015. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wani WY, Chatham JC, Darley-Usmar V, McMahon LL, Zhang J. O-GlcNAcylation and neurodegeneration. Brain Res Bull 133: 80–87, 2017. doi: 10.1016/j.brainresbull.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wani WY, Ouyang X, Benavides GA, Redmann M, Cofield SS, Shacka JJ, Chatham JC, Darley-Usmar V, Zhang J. O-GlcNAc regulation of autophagy and α-synuclein homeostasis; implications for Parkinson’s disease. Mol Brain 10: 32, 2017. doi: 10.1186/s13041-017-0311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J, Chen W, Zhang B, Tian F, Zhou Z, Liao X, Li C, Zhang Y, Han Y, Wang Y, Li Y, Wang GQ, Shen XL. Lon in maintaining mitochondrial and endoplasmic reticulum homeostasis. Arch Toxicol 92: 1913–1923, 2018. doi: 10.1007/s00204-018-2210-3. [DOI] [PubMed] [Google Scholar]
- 58.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 4: 483–490, 2008. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol 1: 19–23, 2013. doi: 10.1016/j.redox.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z, Tan EP, VandenHull NJ, Peterson KR, Slawson C. O-GlcNAcase expression is sensitive to changes in O-GlcNAc homeostasis. Front Endocrinol (Lausanne) 5: 206, 2014. doi: 10.3389/fendo.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu-Mauldin X, Marsh SA, Zou L, Marchase RB, Chatham JC. Modification of STIM1 by O-linked N-acetylglucosamine (O-GlcNAc) attenuates store-operated calcium entry in neonatal cardiomyocytes. J Biol Chem 287: 39094–39106, 2012. doi: 10.1074/jbc.M112.383778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou L, Yang S, Champattanachai V, Hu S, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-κB signaling. Am J Physiol Heart Circ Physiol 296: H515–H523, 2009. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zurita Rendón O, Shoubridge EA. LONP1 is required for maturation of a subset of mitochondrial proteins, and its loss elicits an integrated stress response. Mol Cell Biol 38: e00412-17, 2018. doi: 10.1128/MCB.00412-17. [DOI] [PMC free article] [PubMed] [Google Scholar]