Abstract
Thiamin (vitamin B1) is essential for normal cellular metabolism and function. Pancreatic acinar cells (PACs) obtain thiamin from the circulation via a specific carrier-mediated process that involves the plasma membrane thiamin transporters 1 and 2 (THTR-1 and THTR-2; products of SLC19A2 and SLC19A3 genes, respectively). There is nothing known about the effect of bacterial products/toxins on thiamin uptake by PACs. We addressed this issue in the present investigation by examining the effect of bacterial flagellin on physiological and molecular parameters of thiamin uptake by PACs. We used human primary PACs, mice in vivo, and cultured mouse-derived pancreatic acinar 266-6 cells in our investigation. The results showed that exposure of human primary PACs to flagellin led to a significant inhibition in thiamin uptake; this inhibition was associated with a significant decrease in expression of THTR-1 and -2 at the protein and mRNA levels. These findings were confirmed in mice in vivo as well as in cultured 266-6 cells. Subsequent studies showed that flagellin exposure markedly suppressed the activity of the SLC19A2 and SLC19A3 promoters and that this effect involved the Sp1 regulatory factor. Finally, knocking down Toll-like receptor 5 by use of gene-specific siRNA was found to lead to abrogation in the inhibitory effect of flagellin on PAC thiamin uptake. These results show, for the first time, that exposure of PACs to flagellin negatively impacts the physiological and molecular parameters of thiamin uptake and that this effect is mediated at the level of transcription of the SLC19A2 and SLC19A3 genes.
NEW & NOTEWORTHY The present study demonstrates, for the first time, that prolonged exposure of pancreatic acinar cells to flagellin inhibits uptake of vitamin B1, a micronutrient that is essential for energy metabolism and ATP production. This effect is mediated at the level of transcription of the SLC19A2 and SLC19A3 genes and involves the Sp1 transcription factor.
Keywords: flagellin, pancreatic acinar cells, thiamin uptake, THTR-1, THTR-2
INTRODUCTION
Vitamin B1 (thiamin; also referred to as the “energy vitamin”) is indispensable for normal oxidative energy metabolism and ATP production (2, 28, 41) due to its role as a cofactor (mainly in the form of thiamin pyrophosphate) for multiple enzymes (transketolase, pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and branched-chain keto acid dehydrogenase). The vitamin also plays an important role in reducing cellular oxidative stress (5, 7, 19, 28). Deficiency of thiamin at the cellular level “weakens the cell” via reduction in its oxidative energy metabolism/ATP level, impairment in function/structure of its mitochondria, and induction in oxidative stress (3, 5, 19, 28, 41). At the systemic level, thiamin deficiency leads to a variety of clinical abnormalities that include neurological and cardiovascular disorders (41, 42). Optimizing body/cellular thiamin level, on the other hand, brings about positive health/metabolic benefits under certain conditions (e. g., thiamin use in sepsis and septic shock; 11, 16, 18, 29, 30).
Pancreatic acinar cells (PACs) perform important exocrine functions and are among the most metabolically active cells in the body; these cells require ample amounts of energy for their high rate of protein synthesis and secretion. Like all other cells, PACs cannot synthesize thiamin and thus must obtain the vitamin from the circulation, and that deficiency of this micronutrient impairs both its exocrine and endocrine functions (20, 22, 23, 32). Using a variety of physiological, cellular, and molecular approaches (including appropriate knockout and knockdown approaches), we have previously shown that thiamin uptake by mouse and human pancreatic acinar cells is via a specific carrier-mediated process and involves thiamin transporters 1 and 2 (THTR-1 and THTR-2) (38, 39). Other studies from our laboratory have examined the effect of chronic exposure of PACS to alcohol and to specific components of cigarette smoke on thiamin uptake and have delineated the cellular/molecular mechanisms involved in the identified effects (34, 35, 36, 39).
To date, however, there has been nothing known about the effect of bacterial products/toxins that the pancreas encounters under certain conditions on the ability of its cells to acquire thiamin across their plasma membrane. An example of such toxins is flagellin, a primary structural protein of flagella, which is produced by many gram-negative bacteria (37); levels of this toxin increase in the circulation under a variety of conditions (8, 9, 15). This bacterial product exerts profound effects on cell physiology (21, 26, 43) and in addition has significant systemic effects (4, 6, 13). The cellular effects of flagellin are mediated mainly via Toll-like receptor 5 (TLR-5) (10), a receptor that is expressed in both human and mouse PACs (level of expression in PACs appears to be comparable to that in the gut) (12). In this study, we examined the effect of prolonged (24-h) exposure of human primary PACs as well as mouse PACs in vivo and in cultured mouse-derived pancreatic acinar 266-6 cells to flagellin and their ability to transport thiamin. Our results show that such exposure leads to a significant inhibition in thiamin uptake and that this effect appears to be mediated at the level of transcription of the SLC19A2 and SLC19A3 genes and is exerted via the TLR-5 receptor.
MATERIALS AND METHODS
Materials
[3H]thiamin (specific activity 20 Ci/mmol; radiochemical purity of >98%) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Nitrocellulose filters (0.45-μm pore size) were obtained from Millipore (Fisher Scientific). Flagellin (cat. no. tlrl-stfla) was purchased from InvivoGen, (San Diego, CA). Oligonucleotide primers were synthesized by Sigma Genosys (Sigma, Woodland, TX). TLR-5 Silencer Select siRNA was obtained from Ambion. All other chemicals, including unlabeled thiamin and molecular biology reagents, were of analytical grade and were obtained from commercial vendors.
Flagellin Exposure of Human PACs and Uptake Studies
Human primary pancreatic acinar cells (hPAC) were isolated from cadaveric pancreatic tissues of organ donors at Islet Cell Laboratory at the University of Louisville (Louisville, KY), and at City of Hope (Duarte, CA). Briefly, after informed consent had been obtained, pancreata of subjects (25- to 65-yr-old males) were removed from the donors and digested to separate islets from ductal-acinar trees and purified as described previously (36). The resulting acini were transported to the University of California, Irvine (<48 h after isolation) and cultured in Ham’s F-12K growth medium supplemented with the following: 10% FCS, 5% BSA, 10 ng/ml epidermal growth factor, and 0.1 mg/ml soybean trypsin inhibitor, as described by us and others before (31, 36). The study protocols were approved by the institutional review boards of the City of Hope, Duarte, CA; the University of Louisville, Louisville, KY; and the University of California, Irvine, CA (Institutional Review Board: 2017-1593). The hPACs formed attached monolayers after 24 h in culture under these incubation conditions and were used in transport investigations after an additional 48 h in culture (total time between removal of the pancreas and performance of the uptake investigations was ~96 h). Their viability at the time of use was >80% (as estimated by the trypan blue exclusion method), which is similar to what we and others have seen previously (31, 36). Twenty-four hours before their use in transport investigations, cells were treated with flagellin (100 ng/ml), as described before (4). For thiamin uptake studies, the flagellin-exposed and control hPACs were suspended in Krebs-Ringer (KR) buffer. Labeled and unlabeled thiamin were added to the incubation buffer at the onset of incubation, and the reaction was terminated after 7 min [initial rate of uptake (39)] by adding 1 ml of ice-cold KR buffer followed by rapid filtration, as described previously (39). The level of incorporated 3H was determined in a liquid scintillation counter. Protein concentrations of cell digests were determined using a Bio-Rad (Hercules, CA) Dc protein assay kit.
Cell Culture and Thiamin Uptake Studies
The mouse-derived pancreatic acinar 266-6 cells were obtained from the American Type Tissue Collection (ATCC, Rockville, MD). Cells were cultured in complete DMEM growth medium at 37°C in a 5% CO2 environment. Confluent 266-6 cells were used to investigate the effect of flagellin on pancreatic thiamin uptake and determine the protein and mRNA expression of thiamin transporters and specificity protein-1 (Sp1), as well as SLC19A2 and SLC19A3 promoter activity. To measure thiamin uptake in vitro, confluent 266-6 cells were treated with 100 ng/ml flagellin for 24 h, and thiamin uptake was performed using KR buffer containing either [3H]-labeled or unlabeled thiamin (39). The reaction was terminated by the addition of 2 ml of ice-cold KR buffer. After the cells were rinsed two times with ice-cold KR buffer, they were digested with 1 ml of 1 N NaOH and then neutralized with 10 N HCl. Counting for radioactivity was assessed in a liquid scintillation counter. The protein content of the cell digests was measured using the Dc protein kit. To study the effect of flagellin on thiamin uptake in vivo, C57BL/6 mice (8 wk old) were injected intraperitoneally with flagellin (5 µg/mouse), and control mice were injected with PBS (6, 26). After 48 h, the mice were euthanized, and PACs were isolated by collagenase type-IV (Sigma, St. Louis, MO) digestion method as described previously (33, 35). Freshly isolated PACs were suspended in KR buffer and either [3H]-labeled or unlabeled thiamin was added to initiate uptake. The uptake reaction was terminated by adding 1 ml of ice-cold KR buffer, and the cell suspension was placed on nitrocellulose filters under negative pressure by use of a vacuum manifold (Hoefer Scientific Instruments, Holliston, MA), washed with 5 ml of ice-cold KR buffer and dissolved in scintillation fluid. The radioactivity was measured using a scintillation counter (LS6500; Beckman Coulter, Brea, CA), and digested samples were used for determining protein concentration (Dc protein assay kit). The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee, University of California, Irvine, CA.
Quantitative Real-Time PCR Analysis
Total RNA isolated from 266-6 cells, mouse (m)PACs and hPACs treated with flagellin, were subjected to reverse transcription (RT) using the iScript cDNA synthesis kit (Bio-Rad). The human and mouse THTR-1 and THTR-2 as well as mouse Sp1 were amplified using human and mouse gene-specific primers (Table 1), and the PCR conditions were as previously described (34–36). Data were normalized to β-actin and quantified using a relative relationship method (14).
Table 1.
Primers used for amplifying coding regions of respective genes by real-time PCR
| Gene Name | Forward and Reverse Primers (5′–3′) |
|---|---|
| m-THTR-1 | GTTCCTCACGCCCTACCTTC; GCATGAACCACGTCACAATC |
| m-THTR-2 | TCATGCAAACAGCTGAGTTCT; CTCCGACAGTAGCTGCTCA |
| m-Sp1 | TATGTTGTGGCTGCTACC; TGTGGGATTACTTGATACTGAA |
| m-TLR5 | GCAGGATCATGGCATGTCAAC; ATCTGGGTGAGGTTACAGCCT |
| m-β-Actin | ATCCTCTTCCTCCCTGGA; TTCATGGATGCCACAGGA |
| h-THTR-1 | GCCAGACCGTCTCCTTGTA; TAGAGAGGGCCCACCACAC |
| h-THTR-2 | TTCCTGGATTTACCCCACTG; GTATGTCCAAACGGGGAAGA |
| h-β-Actin | CATCCTGCGTCTGGACCT; TAATGTCACGCACGATTTCC |
THTR-1/2, thiamin transporter receptor 1/2; m-, murine; h-, human; Sp-1, specificity protein-1.
siRNA Transfection
TLR-5 (50 pmol)-specific small interfering RNA (siRNA; Thermo Scientific) was transfected into 266-6 cells at 60–80% confluence using Lipofectamine RNAiMAX (Life Technologies) according to the manufacturer’s protocol. Twenty-four hours posttransfection, cells were used for flagellin treatment for 24 h followed by thiamin uptake.
Transfection and Firefly Luciferase Assay
The SLC19A2 and SLC19A3 (wild-type, WT) full-length, minimal, and mutated minimal promoter-luciferase reporter constructs utilized in this study were generated previously (17, 24). SLC19A2 and SLC19A3 WT and mutant promoter constructs (3 µg/ml), along with 100 ng of Renilla luciferase-thymidine kinase (pRL-TK) plasmid (Promega, Madison, WI), were transiently transfected into 266-6 cells with Lipofectamine 2000 reagent (Life Technologies) for 24 h, and then, cells were subsequently treated with flagellin. After 24 h, cells were lysed with passive lysis buffer, and Renilla-normalized firefly luciferase activity was measured using a dual-luciferase assay system (Promega).
Isolation of Protein and Western Blot Analysis
Flagellin-treated and untreated 266-6 cells or m- or hPACs were lysed in radioimmunoprecipitation assay buffer (Sigma) containing complete protease inhibitor cocktail (Roche, Branchburg, NJ). The soluble protein fraction was isolated, followed by centrifugation (14,000 rpm, 20 min). Protein concentrations were measured using the Dc protein assay kit. To determine the level of hTHTR and mTHTR protein expression, an equal amount of protein (40 µg) was loaded onto 4–12% Bis-Tris gradient minigels (Invitrogen) and transferred onto Immobilon polyvinylidene difluoride membrane (Fisher Scientific). Subsequently, the blot was probed with hTHTR-1 (1:500), hTHTR-2 (1:200), and hβ-actin (1:3,000), mTHTR-1 (1:200), mTHTR-2 (1:200), mSp1 (1:200) and mβ-actin (1:3,000) antibodies. The anti-THTR-1 and anti-THTR-2 polyclonal antibodies were raised against respective synthetic peptides in rabbits by a commercial vendor (Thermo Fisher Scientific, Huntsville, AL, and Sigma-Genosys, The Woodlands, TX, respectively). Specificity of the individual transporter band was determined previously in our laboratory on the basis of studies utilizing antigenic peptides, knockout animal models, and overexpression of His-tagged protein (1, 25, 40). The anti-Sp1 antibody (no. 07-645; Millipore, Billerica, MA) produces a single band, and it has been characterized and validated in several publications. The secondary antibodies used were anti-rabbit IRDye-800 and anti-mouse IRDye-680 (both at 1:30,000 dilution). The specific immunoreactive bands were detected using the Odyssey infrared imaging system (LI-COR Bioscience, Lincoln, NE), and their densities were quantified using the LI-COR software.
Statistical Analysis
Uptake data presented in this study represent means ± SE of at least three independent experiments with multiple determinants and are expressed as percentages relative to simultaneously performed controls. The RT-qPCR, Western blotting, and firefly luciferase assays were determined from at least three independent sample preparations. Student’s t-test, using Microsoft Excel, was used for statistical analysis, and P < 0.05 was considered statistically significant.
RESULTS
Effect of Flagellin on Thiamin Uptake by PACs
Effect of flagellin exposure on thiamin uptake by hPACs.
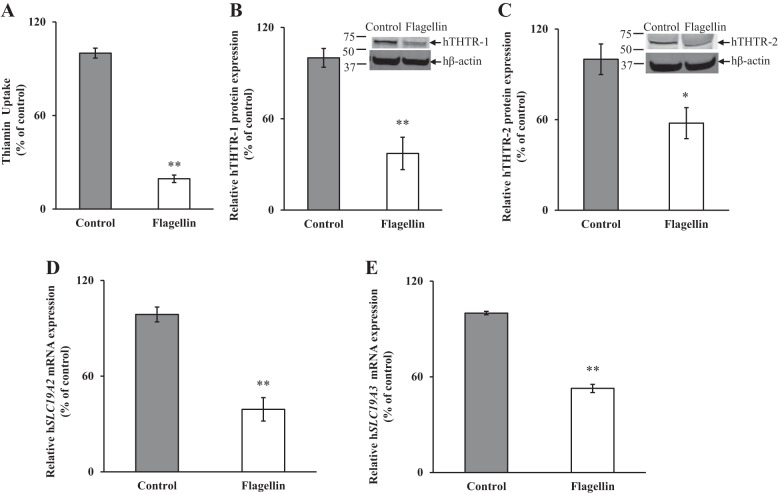
In this study, we examined the effect of exposure of freshly isolated hPACs to flagellin [100 ng/ml, 24 h (4)] on carrier-mediated thiamin uptake. The results showed a significant (P < 0.01) inhibition of the vitamin uptake by cells exposed to flagellin compared with simultaneously processed untreated controls (Fig. 1A). In another study, we examined the effect of exposure of hPACs to flagellin on the levels of expression of hTHTR-1 and hTHTR-2 proteins. This was done by Western blot analysis using specific polyclonal antibodies against these transporters. The results showed a significant reduction in the levels of expression of hTHTR-1 (P < 0.01) and hTHTR-2 (P < 0.05) in flagellin-treated cells compared with controls (Fig. 1, B and C). We also examined (by qPCR) the effect of exposure of hPACs to flagellin on the levels of expression of SLC19A2 and SLC19A3 mRNA. The results showed a significant (P < 0.01 for both) reduction in the levels of expression of both mRNAs in hPACs treated with flagellin compared with the untreated-controls (Fig. 1, D and E).
Fig. 1.
Effect of flagellin exposure of human primary pancreatic acinar cells (hPACs) on carrier-mediated [3H]thiamin uptake (A) and on levels of expression of human thiamin transporters 1 and 2 (hTHTR-1 and hTHTR-2) proteins (B and C) and mRNAs (D and E). hPACs were exposed to 100 ng/ml flagellin for 24 h followed by determination of carrier-mediated [3H]thiamin uptake. Levels of protein expression were determined by Western blotting, and mRNA was determined by qPCR. Data are means ± SE of 3 independent experiments. **P < 0.01, *P < 0.05.
Effect of in vivo exposure of mice to flagellin on thiamin uptake by PACs.
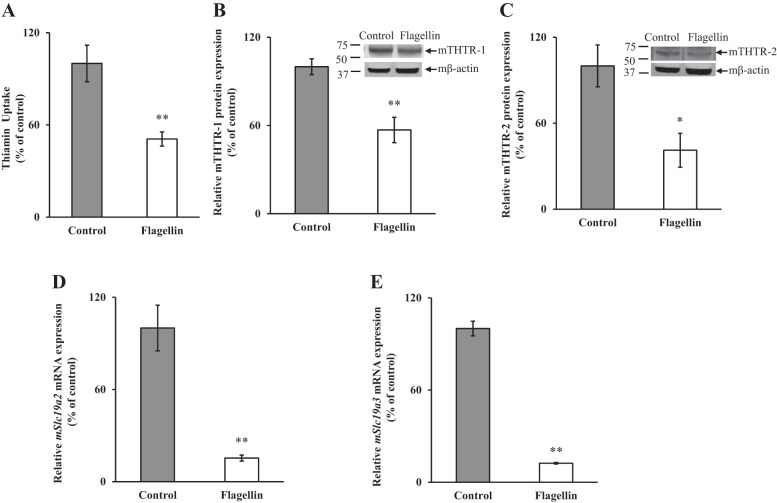
To validate our in vitro findings, we examined the effect of in vivo exposure of mice to flagellin [injection of 5 µg ip per mouse (6, 26)]followed (48 h later) by determination of carrier-mediated thiamin uptake by freshly isolated primary mPACs. The results showed that flagellin exposure led to a significant (P < 0.01) inhibition in thiamin uptake by PACs from mice injected with flagellin compared with those from untreated controls (Fig. 2A). We also examined by Western blot analysis the levels of expression of mTHTR-1 and mTHTR-2 proteins treated with flagellin compared with their controls. The results showed a significant reduction in the levels of expression of mTHTR-1 (P < 0.01) and mTHTR-2 (P < 0.05) proteins in mice treated with flagellin compared with the controls (Fig. 2, B and C). In another study, we determined the levels of expression of Slc19a2 and Slc19a3 mRNA by qPCR. The results showed a significant decrease (P < 0.01 for both) in the expression of both Slc19a2 and Slc19a3 mRNA in mice treated with flagellin compared with its controls (Fig. 2, D and E).
Fig. 2.
Effect of exposure of mice to flagellin on carrier-mediated [3H]thiamin uptake (A) and on levels of expression of mouse thiamin transporters 1 and 2 (mTHTR-1 and mTHTR-2) proteins (B and C) and mRNAs (D and E). Primary pancreatic acinar cells (PACs) were isolated from mice injected with flagellin (5 µg/mouse) for 48 h, and carrier-mediated thiamin uptake was determined as described in materials and methods. Western blot analysis was done using PAC whole cell proteins (40 µg). Levels of protein expression were determined by Western blotting; that of mRNA was determined by qPCR. Data are means ± SE of 3 pairs of mice. **P < 0.01, *P < 0.05.
In vitro studies using mouse-derived PAC 266-6 cells.
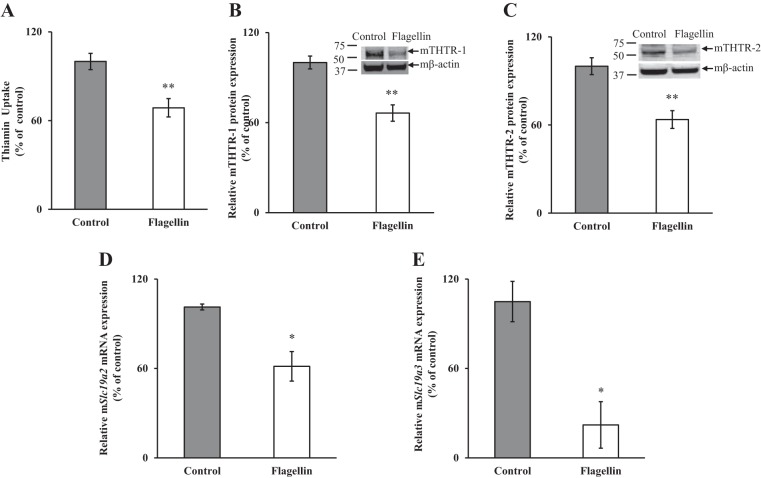
To further extend the findings on the effect of flagellin on PACs and to understand the molecular mechanism involved, we used mouse-derived PAC 266-6 cells. We first confirmed the effect of flagellin on thiamin uptake in 266-6 cells by exposing them to flagellin (100 ng/ml; 24 h); such treatment did not significantly affect cell viability, as estimated by trypan blue exclusion method; data not shown) and observed a significant (P < 0.01) inhibition in thiamin uptake compared with untreated control (Fig. 3A). Interestingly, such treatment also led to a significant (P < 0.05) reduction in the cellular level of ATP (100 ± 5.2 and 72.8 ± 12.8% for control and flagellin-treated PAC 266-6 cells, respectively; the cellular ATP level was determined as described previously; Ref. 27). In other studies, we examined (by Western blotting) the effect of flagellin exposure of 266-6 cells on levels of expression of mTHTR-1 and mTHTR-2 proteins. The results showed a significant (P < 0.01 for both) reduction in the levels of expression of mTHTR-1 and -2 proteins in 266-6 cells exposed to flagellin compared with controls (Fig. 3, B and C). We also examined (by qPCR) the expression of Slc19a2 and Slc19a3 mRNA in flagellin-treated cells and observed a significant (P < 0.05 for both) reduction in levels of both mRNAs in PAC 266-6 cells treated with flagellin compared with untreated controls (Fig. 3, D and E).
Fig. 3.
Effect of flagellin exposure of mouse-derived pancreatic acinar 266-6 cells on carrier-mediated [3H]thiamin uptake (A) and on levels of expression of mouse thiamin transporter 1 and 2 (mTHTR-1 and mTHTR-2) proteins (B and C) and mRNAs (D and E). Acinar 266-6 cells were treated with 100 ng/ml flagellin for 24 h followed by determination of carrier-mediated thiamin uptake. Levels of protein expression were determined by Western blotting; that of mRNA was determined by qPCR. Data are means ± SE of 3 independent experiments. **P < 0.01, *P < 0.05.
Collectively, the findings above show that flagellin negatively impacts thiamin uptake by PACs. The similarities in the effect of flagellin on thiamin uptake physiology and molecular biology in 266-6 cells and in human primary PACs and mice in vivo demonstrate the suitability of the former cells as a model for detailed analysis of the molecular mechanism(s) involved in flagellin effect on thiamin uptake by PACs.
Involvement of Transcriptional Mechanism(s) in Mediating Effect of Flagellin on Thiamin Uptake by PACs
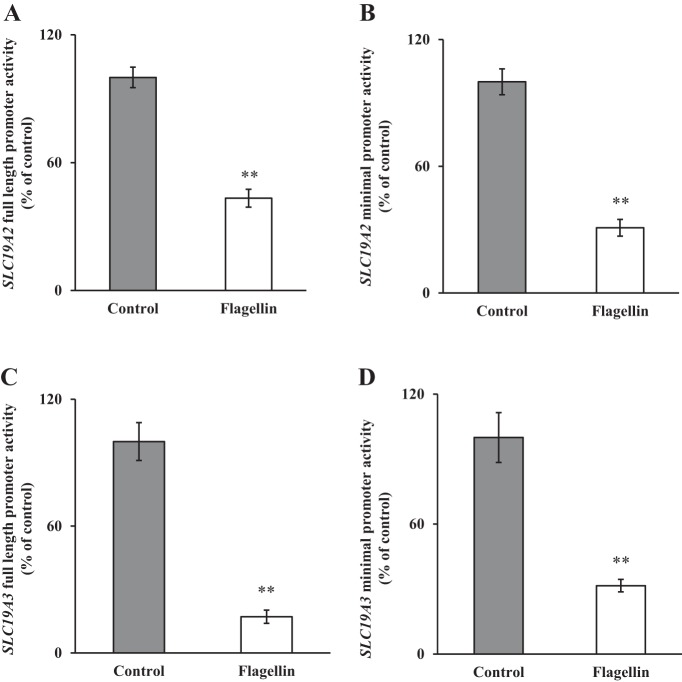
Changes in the level of mRNA expression of a gene by external factors could be achieved via different mechanisms. One such prominent mechanism is alteration in the transcription rate of the gene involved. To determine whether such a mechanism is involved in mediating the effect of flagellin on thiamin uptake by PACs, we tested the effect of exposure of 266-6 cells to flagellin on the activity of the human SLC19A2 and SLC19A3 full-length (and minimal) promoter-luciferase constructs transfected into these cells. The results showed a significant (P < 0.01 for both) inhibition in the activities of the SLC19A2 and SLC19A3 full-length (as well as the minimal) promoters of these genes in cells exposed to flagellin compared with controls (Fig. 4, A–D). These findings suggest the involvement of transcriptional mechanisms in mediating the effect of flagellin on thiamin uptake by PACs; the data also suggest that the flagellin-responsive region is located in the minimal promoters of these genes.
Fig. 4.
Effect of exposure of pancreatic acinar 266-6 cells to flagellin on the activities of SLC19A2 and SLC19A3 promoters. Full-length and minimal SLC19A2 (A and B) and SLC19A3 (C and D) promoters in pGL3-Basic were transfected into 266-6 cells and exposed to 100 ng/ml flagellin for 24 h followed by determination of luciferase activity. Data were normalized relative to Renilla luciferase activity and presented as means ± SE of 3 independent experiments. **P < 0.01.
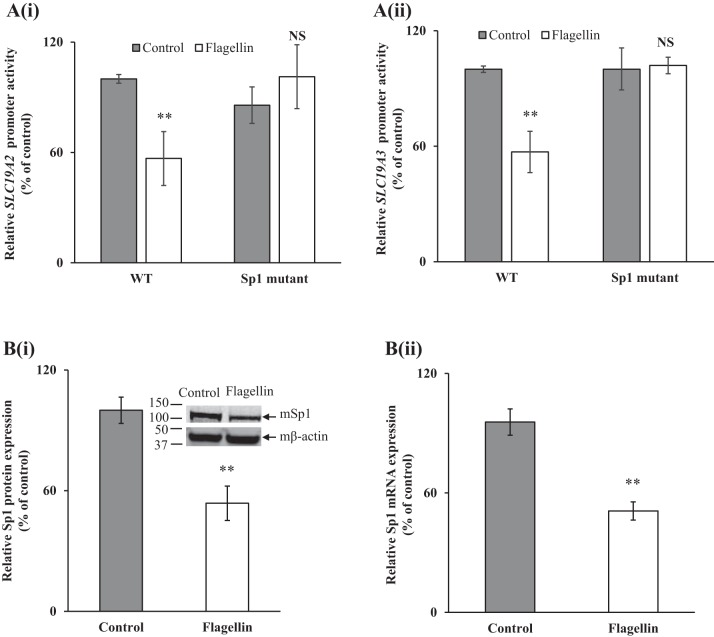
Previous studies from our laboratory have shown that the minimal region of the SLC19A2 and SLC19A3 promoters contain Sp1 cis-regulatory elements (−228/−230 for SLC19A2 and −48/−45 for SLC19A3) that are important for their promoter activity (17, 24). Thus, we examined the effect of mutating the specific Sp1 sites on the inhibitory effect of flagellin on activity of these promoters. In this study, we used the previously generated and characterized SLC19A2/A3 promoter-luciferase constructs with mutations at the Sp1 cis-element (17, 24). The WT (SLC19A2/A3 promoter without mutations) and mutant Sp1 constructs of both SLC19A2 and SLC19A3 minimal promoters fused to luciferase were transiently transfected into 266-6 cells followed by exposure of the cells to flagellin (24 h). The results showed that mutating the Sp1 binding site abrogated the inhibitory effect of flagellin on activity of both of these promoters (Fig. 5, Ai and Aii).
Fig. 5.
A: role of specificity protein-1 (Sp1) sites in SLC19A2 and SLC19A3 promoters in mediating the effect of flagellin on promoter activity. Wild-type (WT) and mutant constructs of SLC19A2 (i) and SLC19A3 (ii) were transfected into 266-6 cells and exposed to 100 ng/ml flagellin for 24 h followed by determination of luciferase activity. B: effect of exposure of pancreatic acinar 266-6 cells to flagellin on levels of expression of mSp1 protein (i) and mRNA (ii). Protein and mRNA levels were determined by Western blot and RT-qPCR, respectively. Data are means ± SE of 3 independent experiments. **P < 0.01; NS, not significant.
In another study, we examined whether flagellin treatment of 266-6 cells also affects the level of expression of the transcription factor Sp1 [which is necessary for the basal activity of SLC19A2 and SLC19A3 promoters (17, 24)]. The results showed that flagellin treatment of 266-6 cells led to a significant (P < 0.01 for both) reduction in the expression of Sp1 at both protein and mRNA levels (Fig. 5, Bi and Bii).
Effect of Flagellin on Thiamin Uptake by PACs is Mediated via TLR-5 Receptor
Flagellin transduces its effects on cells via the TLR-5 receptor, which is located at the cell membrane (10). To determine whether the inhibitory effect of flagellin on thiamin uptake by PACs is mediated by TLR-5, we knocked down the TLR-5 receptor by using gene-specific TLR-5 siRNA and examined the effect of flagellin on thiamin uptake by PACs. The results showed that knocking down TLR-5 led to abrogation of the flagellin inhibition in carrier-mediated thiamin uptake by PACs (Fig. 6), suggesting that the bacterial toxin exerts its effect(s) on the vitamin transport via the TLR-5 receptor.
Fig. 6.
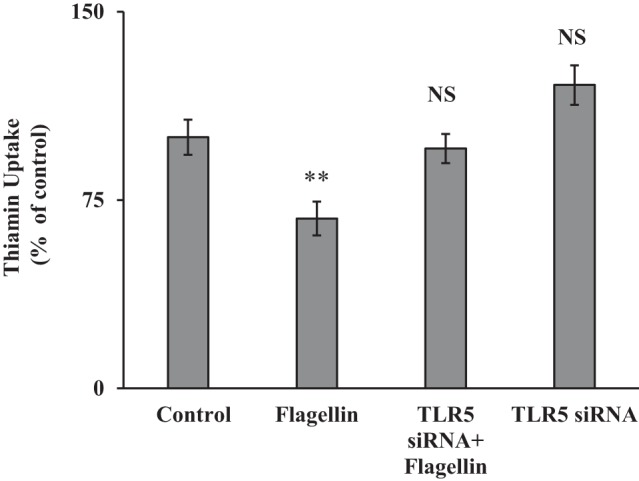
Toll-like receptor 5 (TLR-5) mediates the inhibitory effect of flagellin on thiamin uptake by pancreatic acinar cells. Acinar 266-6 cells were transiently transfected with TLR-5 small interfering (si)RNA followed by treatment with flagellin (100 ng/ml for 24 h); thiamin uptake was then determined (see materials and methods). Data are means ± SE of 3 independent experiments. **P < 0.01; NS, not significant.
DISCUSSION
The aim of this study was to examine the effect of flagellin on PAC thiamin uptake and to delineate the molecular mechanism(s) involved. Flagellin, a primary structural protein of flagella (37), is generated by gram-negative bacteria, and the level of this bacterial product in circulation increases in a variety of pathological conditions including sepsis and intestinal diseases (9, 13, 15). Flagellin exerts profound effects on the physiology of cells (21, 26, 43); it also has significant systemic effects (4, 6, 13). There is nothing known about the effect(s) of this bacterial product on uptake of water-soluble vitamins (including thiamin) by PACs. We addressed this issue in the present study, using primary human PACs, mice (in vivo), and cultured mouse-derived pancreatic acinar 266-6 cells as models.
Our results showed that prolonged (24-h) exposure of primary hPACs to flagellin led to a significant inhibition in carrier-mediated thiamin uptake. This inhibition was associated with a significant decrease in expression of THTR-1 and -2 at the protein and mRNA levels. These findings were confirmed in vivo in mice treated with flagellin; again, a significant inhibition in carrier-mediated thiamin uptake by PACs was observed, which was associated with a reduction in the levels of expression of THTR-1 and -2 protein and mRNA.
To further delineate the cellular/molecular mechanisms involved in mediating the effect of flagellin on thiamin uptake by PACs, we sought an in vitro PAC model that would similarly respond to flagellin. For that, we used the mouse-derived pancreatic acinar 266-6 cells and found that exposure of these cells to flagellin also led to a significant inhibition in carrier-mediated thiamin uptake that was associated with a reduction in expression of THTR-1 and −2 proteins and mRNAs. Using these cells, we examined whether the effect of flagellin on thiamin uptake and on the expression of THTR-1 and -2 was mediated at the level of transcription of these two thiamin transporters; i.e., we determined whether the effect was mediated at the level of transcription of the SLC19A2 and SLC19A3 genes. For this we examined the effect of exposure to flagellin on activity of the SLC19A2 and SLC19A3 full-length promoters transfected into 266-6 cells. A significant decrease in the activities of both promoters was observed, suggesting the involvement of transcriptional mechanisms in mediating the effect of flagellin on levels of expression of THTR-1 and -2. Similar effects were observed when minimal promoters of the SLC19A2 and SLC19A3 genes were used, suggesting that the flagellin-responsive region is located in the minimal promoter regions of these genes.
Previous studies from our laboratory have shown that the nuclear factor Sp1 plays an important role in driving promoter activity of the SLC19A2 and SLC19A3 genes (17, 24). To investigate the role of Sp1 in mediating the flagellin effects on activity of the SLC19A2 and SLC19A3 promoters, we mutated the Sp1 binding sites in these promoters and then examined the effect of flagellin on promoter activity. The results showed a significant reversal in the inhibitory effect of flagellin on activity of the promoters of both genes. We also examined the effect of exposure of PAC 266-6 cells to flagellin on the level of expression of the Sp1 nuclear factor and observed a significant reduction in its level in cells treated with flagellin compared with untreated control cells. Together, these findings point to a role for Sp1 in mediating the effect of flagellin on activity of the SLC19A2 and SLC19A3 promoters in PACs.
The cellular effects of flagellin are known to be mediated mainly via the TLR-5 receptor located at the cell membrane (10). To investigate the involvement of TLR-5 in mediating the effect of flagellin on thiamin uptake by PACs, we knocked down the TLR-5 receptor using gene-specific siRNA and then examined the effect of such manipulation on carrier-mediated thiamin uptake. The results showed that knocking down the TLR-5 receptor led to a significant reversal in the inhibitory effect of flagellin on thiamin uptake by PACs. This clearly suggests that the effect of flagellin is mediated via the cell membrane TLR-5 receptor.
In summary, results of these investigations show for the first time that exposure of PACs to flagellin negatively impacts the physiology and molecular biology of thiamin uptake and that these effects are mediated at the level of transcription of the SLC19A2 and SLC19A3 genes. Increased flagellin levels have been seen in a variety of pathological conditions, and there are reports of pancreatic damage in such conditions. Exposure to flagellin could lead to deficient/suboptimal levels of thiamin in pancreatic acinar cells, a situation that could impair oxidative energy metabolism, increase oxidative stress, and compromise the mitochondrial structure and function in these cells. This compromised status of pancreatic acinar cells would weaken the cells and predispose them to development of disease.
GRANTS
This study was supported by grants from the National Institutes of Health (AA-018071, DK-56061, and DK-58057) and the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.S., K.Y.A., and H.M.S. conceived and designed research; P.S., K.Y.A., V.R., and E.T.G. performed experiments; P.S., K.Y.A., V.R., and E.T.G. analyzed data; P.S., K.Y.A., V.R., and E.T.G. interpreted results of experiments; P.S. and K.Y.A. prepared figures; P.S., K.Y.A., and H.M.S. drafted manuscript; P.S., K.Y.A., and H.M.S. edited and revised manuscript; P.S., K.Y.A., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Balamurugan K, Said HM. Functional role of specific amino acid residues in human thiamine transporter SLC19A2: mutational analysis. Am J Physiol Gastrointest Liver Physiol 283: G37–G43, 2002. doi: 10.1152/ajpgi.00547.2001. [DOI] [PubMed] [Google Scholar]
- 2.Berdanier CD. Advanced Nutrition: Micronutrients. New York: CRC, 1998. [Google Scholar]
- 3.Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem 64: 2013–2021, 1995. doi: 10.1046/j.1471-4159.1995.64052013.x. [DOI] [PubMed] [Google Scholar]
- 4.Caballero I, Boyd J, Almiñana C, Sánchez-López JA, Basatvat S, Montazeri M, Maslehat Lay N, Elliott S, Spiller DG, White MR, Fazeli A. Understanding the dynamics of toll-like receptor 5 response to flagellin and its regulation by estradiol. Sci Rep 7: 40981, 2017. doi: 10.1038/srep40981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calingasan NY, Gandy SE, Baker H, Sheu KF, Smith JD, Lamb BT, Gearhart JD, Buxbaum JD, Harper C, Selkoe DJ, Price DL, Sisodia SS, Gibson GE. Novel neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine deficiency. Am J Pathol 149: 1063–1071, 1996. [PMC free article] [PubMed] [Google Scholar]
- 6.Eaves-Pyles T, Murthy K, Liaudet L, Virág L, Ross G, Soriano FG, Szabó C, Salzman AL. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IκBα degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol 166: 1248–1260, 2001. doi: 10.4049/jimmunol.166.2.1248. [DOI] [PubMed] [Google Scholar]
- 7.Frederikse PH, Farnsworth P, Zigler JS Jr. Thiamine deficiency in vivo produces fiber cell degeneration in mouse lenses. Biochem Biophys Res Commun 258: 703–707, 1999. doi: 10.1006/bbrc.1999.0560. [DOI] [PubMed] [Google Scholar]
- 8.Gewirtz AT, Simon PO Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O’Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest 107: 99–109, 2001. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gewirtz AT. TLRs in the gut. III. Immune responses to flagellin in Crohn’s disease: good, bad, or irrelevant? Am J Physiol Gastrointest Liver Physiol 292: G706–G710, 2007. doi: 10.1152/ajpgi.00347.2006. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103, 2001. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 11.Holmberg MJ, Moskowitz A, Patel PV, Grossestreuer AV, Uber A, Stankovic N, Andersen LW, Donnino MW. Thiamine in septic shock patients with alcohol use disorders: An observational pilot study. J Crit Care 43: 61–64, 2018. doi: 10.1016/j.jcrc.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huhta H, Helminen O, Kauppila JH, Salo T, Porvari K, Saarnio J, Lehenkari PP, Karttunen TJ. The expression of toll-like receptors in normal human and murine gastrointestinal organs and the effect of microbiome and cancer. J Histochem Cytochem 64: 470–482, 2016. doi: 10.1369/0022155416656154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liaudet L, Szabó C, Evgenov OV, Murthy KG, Pacher P, Virág L, Mabley JG, Marton A, Soriano FG, Kirov MY, Bjertnaes LJ, Salzman AL. Flagellin from gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock 19: 131–137, 2003. doi: 10.1097/00024382-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest 113: 1296–1306, 2004. doi: 10.1172/JCI200420295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskowitz A, Andersen LW, Cocchi MN, Karlsson M, Patel PV, Donnino MW. Thiamine as a renal protective agent in septic shock: a secondary analysis of a randomized, double-blind, placebo-controlled trial. Ann Am Thorac Soc 14: 737–741, 2017. doi: 10.1513/AnnalsATS.201608-656BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am J Physiol Gastrointest Liver Physiol 287: G822–G829, 2004. doi: 10.1152/ajpgi.00234.2004. [DOI] [PubMed] [Google Scholar]
- 18.Nichols HK, Basu TK. Thiamin status of the elderly: dietary intake and thiamin pyrophosphate response. J Am Coll Nutr 13: 57–61, 1994. doi: 10.1080/07315724.1994.10718372. [DOI] [PubMed] [Google Scholar]
- 19.Portari GV, Marchini JS, Vannucchi H, Jordao AA. Antioxidant effect of thiamine on acutely alcoholized rats and lack of efficacy using thiamine or glucose to reduce blood alcohol content. Basic Clin Pharmacol Toxicol 103: 482–486, 2008. doi: 10.1111/j.1742-7843.2008.00311.x. [DOI] [PubMed] [Google Scholar]
- 20.Prasannan KG, Sundaresan R, Venkatesan D. Thiamine deficiency and protein secretion by pancreatic slices in vitro. Experientia 33: 169–170, 1977. doi: 10.1007/BF02124046. [DOI] [PubMed] [Google Scholar]
- 21.Ramos HC, Rumbo M, Sirard JC. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol 12: 509–517, 2004. doi: 10.1016/j.tim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Rathanaswami P, Pourany A, Sundaresan R. Effects of thiamine deficiency on the secretion of insulin and the metabolism of glucose in isolated rat pancreatic islets. Biochem Int 25: 577–583, 1991. [PubMed] [Google Scholar]
- 23.Rathanaswami P, Sundaresan R. Effects of thiamine deficiency on the biosynthesis of insulin in rats. Biochem Int 24: 1057–1062, 1991. [PubMed] [Google Scholar]
- 24.Reidling JC, Subramanian VS, Dudeja PK, Said HM. Expression and promoter analysis of SLC19A2 in the human intestine. Biochim Biophys Acta 1561: 180–187, 2002. doi: 10.1016/S0005-2736(02)00341-3. [DOI] [PubMed] [Google Scholar]
- 25.Reidling JC, Lambrecht N, Kassir M, Said HM. Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology 138: 1802–1809, 2010. doi: 10.1053/j.gastro.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolli J, Loukili N, Levrand S, Rosenblatt-Velin N, Rignault-Clerc S, Waeber B, Feihl F, Pacher P, Liaudet L. Bacterial flagellin elicits widespread innate immune defense mechanisms, apoptotic signaling, and a sepsis-like systemic inflammatory response in mice. Crit Care 14: R160, 2010. doi: 10.1186/cc9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabui S, Subramanian VS, Kapadia R, Said HM. Adaptive regulation of pancreatic acinar mitochondrial thiamin pyrophosphate uptake process: possible involvement of epigenetic mechanism(s). Am J Physiol Gastrointest Liver Physiol 313: G448–G455, 2017. doi: 10.1152/ajpgi.00192.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Said HM. Thiamin. In: Encyclopedia of Dietary Supplements, edited by Coats PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD. New York: Informa Health Care USA, 2010, p. 252–257. [Google Scholar]
- 29.Seligmann H, Halkin H, Rauchfleisch S, Kaufmann N, Motro M, Vered Z, Ezra D. Thiamine deficiency in patients with congestive heart failure receiving long-term furosemide therapy: a pilot study. Am J Med 91: 151–155, 1991. doi: 10.1016/0002-9343(91)90007-K. [DOI] [PubMed] [Google Scholar]
- 30.Shimon I, Almog S, Vered Z, Seligmann H, Shefi M, Peleg E, Rosenthal T, Motro M, Halkin H, Ezra D. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med 98: 485–490, 1995. doi: 10.1016/S0002-9343(99)80349-0. [DOI] [PubMed] [Google Scholar]
- 31.Singh L, Bakshi DK, Vasishta RK, Arora SK, Majumdar S, Wig JD. Primary culture of pancreatic (human) acinar cells. Dig Dis Sci 53: 2569–2575, 2008. doi: 10.1007/s10620-007-0162-1. [DOI] [PubMed] [Google Scholar]
- 32.Singh M. Effect of thiamin deficiency on pancreatic acinar cell function. Am J Clin Nutr 36: 500–504, 1982. doi: 10.1093/ajcn/36.3.500. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan P, Kapadia R, Biswas A, Said HM. Chronic alcohol exposure inhibits biotin uptake by pancreatic acinar cells: possible involvement of epigenetic mechanisms. Am J Physiol Gastrointest Liver Physiol 307: G941–G949, 2014. doi: 10.1152/ajpgi.00278.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivasan P, Subramanian VS, Said HM. Effect of the cigarette smoke component, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), on physiological and molecular parameters of thiamin uptake by pancreatic acinar cells. PLoS One 8: e78853, 2013. doi: 10.1371/journal.pone.0078853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan P, Subramanian VS, Said HM. Mechanisms involved in the inhibitory effect of chronic alcohol exposure on pancreatic acinar thiamin uptake. Am J Physiol Gastrointest Liver Physiol 306: G631–G639, 2014. doi: 10.1152/ajpgi.00420.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan P, Thrower EC, Loganathan G, Balamurugan AN, Subramanian VS, Gorelick FS, Said HM. Chronic nicotine exposure in vivo and in vitro inhibits vitamin b1 (thiamin) uptake by pancreatic acinar cells. PLoS One 10: e0143575, 2015. doi: 10.1371/journal.pone.0143575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steiner TS. How flagellin and toll-like receptor 5 contribute to enteric infection. Infect Immun 75: 545–552, 2007. doi: 10.1128/IAI.01506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian VS, Subramanya SB, Said HM. Relative contribution of THTR-1 and THTR-2 in thiamin uptake by pancreatic acinar cells: studies utilizing Slc19a2 and Slc19a3 knockout mouse models. Am J Physiol Gastrointest Liver Physiol 302: G572–G578, 2012. doi: 10.1152/ajpgi.00484.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanya SB, Subramanian VS, Sekar VT, Said HM. Thiamin uptake by pancreatic acinar cells: effect of chronic alcohol feeding/exposure. Am J Physiol Gastrointest Liver Physiol 301: G896–G904, 2011. doi: 10.1152/ajpgi.00308.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol 299: G23–G31, 2010. doi: 10.1152/ajpgi.00132.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanphaichitr V. Modern Nutrition in Health and Disease, edited by Shils ME, Olsen JA, Shike M. New York: Lea and Febiger, 1994. [Google Scholar]
- 42.Victor MG, Adams RD, Collins A. The Wernicke-Korsakoff Syndrome and Related Neurological Disorders due to Alcoholism and Malnutrition (2nd ed.). Philadelphia, PA: F.A. Davis, 1989. [Google Scholar]
- 43.Xiao Y, Liu F, Yang J, Zhong M, Zhang E, Li Y, Zhou D, Cao Y, Li W, Yu J, Yang Y, Yan H. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cell Mol Immunol 12: 729–742, 2015. doi: 10.1038/cmi.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]