INTRODUCTION
Benign prostatic hyperplasia (BPH) is an aging disease associated with histological changes within the transition zone of the prostate; postmortem studies have indicated these changes in 50% of men over 50 yr old and 90% of men over 80 yr old (9). Lower urinary tract symptoms (LUTS), with symptoms including urinary urgency, frequency, and nocturia, affects 44% of all men over 20 yr old but affects 30–50% of aging men with BPH (9). Although BPH/LUTS is a nonmalignant disease, there is a significant impact on quality of life, and the burden on healthcare exceeds $4 billion annually (9).
BPH/LUTS is most commonly associated with epithelial and stromal proliferation, leading to prostate nodules, and an increase in prostate volume that impinges on the urethra, causing bladder outlet obstruction. 5α-Reductase inhibitors (5ARI) target the conversion of testosterone to dihydrotestosterone, blocking androgen receptor-mediated proliferation, decreasing prostate size, and resolving LUTS (6). Prostate size alone does not predict LUTS severity, suggesting other mechanisms of disease progression in addition to proliferation (9). Changes in smooth muscle contractility, another contributing factor in BPH/LUTS, occurs via an increase in contractility around the bladder neck or urethra, leading to decreased urinary flow. α-Blockers target α1-adrenergic receptors found within the prostate and lower urinary tract and prevent smooth muscle contraction (6). Although α-blockers and 5ARI, singly or in combination, are widely used to treat BPH/LUTS, the risk of disease progression remains, increasing the need for surgical intervention. This suggests additional mechanisms of BPH/LUTS development and progression.
In several studies of BPH/LUTS, increases in collagen deposition and fibrosis have been identified as a contributing factor to urinary dysfunction (2). Extracellular remodeling leads to increased rigidity in the prostate and around the urethra, potentially causing a “fibrotic bottleneck” and decreased urinary flow. One cause of fibrosis is an increase in inflammation, and a variety of proinflammatory processes have been associated with BPH/LUTS (3, 8). About 8% of men experience prostatitis, which could be acutely infectious, chronically infectious, chronically inflammatory, or chronically noninfectious, noninflammatory, with noninfectious categories accounting for at least 95% of prostatitis (3). Men diagnosed with prostatitis were significantly more likely to be diagnosed and treated for BPH/LUTS later in life (3, 8). Additionally, inflammatory infiltrates associated with both acute and chronic inflammation could be detected in a large percentage of prostate tissue from patients with BPH/LUTS, leading to higher prostate volume. Taken together, inflammation-driven fibrosis in BPH/LUTS could be targeted for treatment.
INFLAMMATION AND FIBROSIS
To study the contribution of inflammation on fibrosis, rodent models that recapitulate human BPH/LUTS are necessary but challenging. Several prostate inflammation models exist in mice and rats, including an inducible IL-1β model that mediates prostatic inflammation through cytokine expression (1). While these models can be used to examine the effect of inflammation on the prostate, the effect on urinary function is less well characterized. Although infectious prostatitis accounts for a mere 2% of cases, Bell-Cohn et al. (2a) elaborated on an Escherichia coli mouse prostatitis model resulting in prostatic fibrosis. Unlike other infectious inflammation models, E. coli CP1 can cause chronic prostatic inflammation after resolution of the infection (day 35 postinfection) (10). This model produces urodynamic and symptomatic changes along the lines of those observed in human BPH/LUTS. In addition to physiological changes, CP1 infection increases the percentage of collagen within the prostate, predominantly in the dorsolateral prostate with an increase in expression of collagen type I mRNA; C57BL6 mice showed more collagen deposition than NOD mice. This collagen remodeling in the prostate occurs before stable collagen remodeling in the bladder, suggesting the contribution of bladder fibrosis to BPH/LUTS as a secondary effect. Corresponding to the increase in collagen, there is an elevated expression of smooth muscle α-actin. Previous studies have implicated the conversion of fibroblasts to myofibroblasts with subsequent expression of α-smooth muscle actin and collagen type I as a wound healing mechanism; the specific myofibroblast precursor cell within the prostate remains unknown but is an active and exciting area of study (8). As acknowledged by Bell-Cohn et al. (2a), prostatic fibrosis may vary by location and collagen type. This underscores the need for better collagen detection methods as well as the identification of the cell types responsible for the extracellular matrix remodeling and collagen production. MRI techniques have been used to quantify collagen type I in various cancer models using diffusion MRI or a collagen-targeted contrast agent (4). Multiplex immunohistochemical techniques can identify various cell types within one tissue sample; this can be paired with in situ hybridization for collagen type I gene expression for the identification of collagen-producing cells (7).
Cytokines and chemokines that mediate inflammation and lead to fibrosis in BPH/LUTS need to be better characterized. Bell-Cohn et al. (2a) also identified the type 2 cytokine pathway as a mediator of inflammation-driven fibrosis leading to urinary dysfunction. Examination of type 2 cytokine gene expression showed a more significant increase in cytokine production in C57BL6 mice corresponding to the higher percentage of collagen production. Disruption of type 2 cytokine signaling through STAT6 ablation showed a significant decrease in collagen deposition in all prostate tissue. Serum and urine proteomics/metabolomics have been used to screen biomarkers for a variety of diseases, including prostate cancer and those mediated by chronic inflammation. With the goal of stratifying patients with BPH/LUTS that would not benefit from current therapies, urine proteomics has been used to identify candidate biomarkers between normal individuals and those with BPH/LUTS, and, similarly, urine metabolomics are being used to identify differences in cytokines and chemokines (5).
FUTURE DIRECTIONS
As more studies have implicated fibrosis in BPH/LUTS, future translational studies must focus on fibrosis as a causative factor. Fibrosis is clinically intriguing because current treatments (i.e., α-blockers and 5ARI) are ineffective in treating fibrosis, so new drugs and strategies for stratification and treatment are necessary (Fig. 1). Animal models that can address the various causes of BPH remain elusive, but several different models that recapitulate BPH/LUTS exist and can be helpful in assessing the efficacy of antifibrotics singly or in combination with current treatment standards in preventing or alleviating urinary dysfunction. By quantifying the amount of inflammation within the prostate through urine metabolomics/metabolomics and the degree of collagen remodeling through MRI, these minimally invasive techniques could assess the degree of prostatic fibrosis in a patient compared with other mechanisms of disease progression (e.g., proliferation) and direct future treatment modalities.
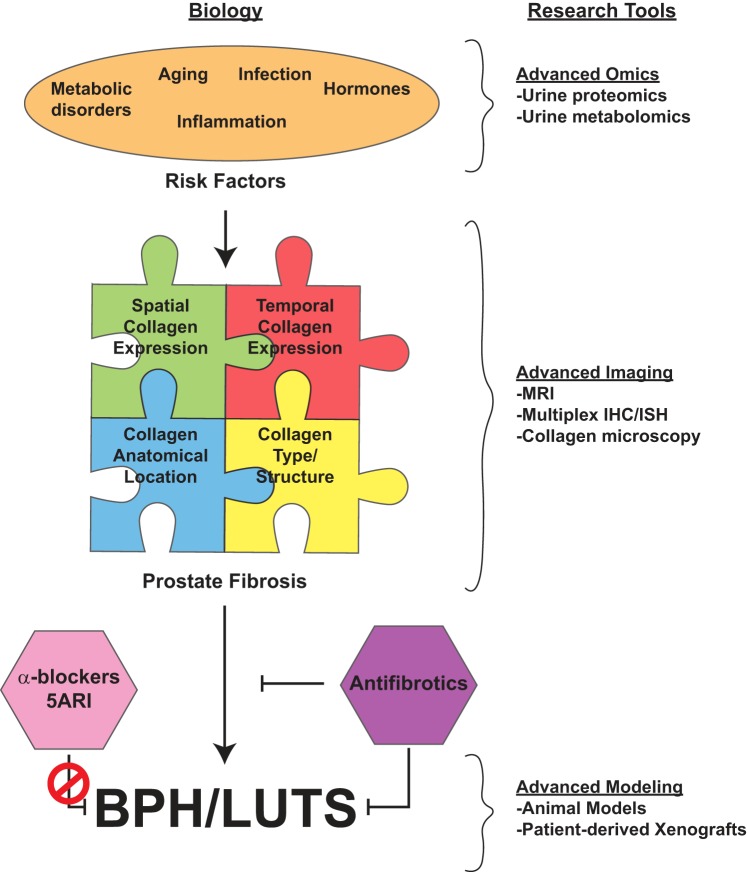
Fig. 1.
Schematic of prostate fibrosis and benign prostatic hyperplasia (BPH)/lower urinary tract symptoms (LUTS). Some factors contributing to the development of fibrosis include infection (2a), metabolic disorders (e.g., type 2 diabetes and obesity), hormones, and aging. These can be interrogated for biomarkers with proteomic and metabolomics tools. The impact of fibrosis on BPH/LUTS must include an understanding of spatial (i.e., cell types) and temporal (progressive remodeling) collagen expression, anatomic location (periurethral vs. diffuse throughout prostate) of collagen expression, and type of collagen (e.g., collagen-1, -2,…-29) and structure (i.e., collagen fiber metrics) within the prostate. The research tools necessary to examine fibrosis include a variety of advanced imaging and staining modalities, including picrosirius red, Masson’s trichrome, PolScope, and multiphoton microscopy. Current treatments for BPH/LUTS [i.e., 5α-reductase inhibitors (5ARIs) and α-blockers] are ineffective at treating fibrosis-mediated disease. Instead, antifibrotic treatments need to be developed using preclinical animal and patient-derived models to prevent or treat fibrosis-mediated BPH/LUTS. IHC, immunohistochemistry; ISH, in situ hybridization.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.A.R. and T.T.L. drafted manuscript; W.A.R. and T.T.L. edited and revised manuscript; W.A.R., R.C.B., and T.T.L. approved final version of manuscript; T.T.L. prepared figures.
REFERENCES
- 1.Ashok A, Keener R, Rubenstein M, Stookey S, Bajpai S, Hicks J, Alme AK, Drake CG, Zheng Q, Trabzonlu L, Yegnasubramanian S, De Marzo AM, Bieberich CJ. Consequences of interleukin 1β-triggered chronic inflammation in the mouse prostate gland: altered architecture associated with prolonged CD4+ infiltration mimics human proliferative inflammatory atrophy. Prostate. In press. doi: 10.1002/pros.23784. [DOI] [PubMed] [Google Scholar]
- 2.Bauman TM, Nicholson TM, Abler LL, Eliceiri KW, Huang W, Vezina CM, Ricke WA. Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS One 9: e109102, 2014. doi: 10.1371/journal.pone.0109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Bell-Cohn A, Mazur DJ, Hall C, Schaeffer AJ, Thumbikat P. Uropathogenic Escherichia coli-induced fibrosis, leading to lower urinary tract symptoms, is associated with type 2 cytokine signaling. Am J Physiol Renal Physiol 316: F682–F692, 2019. doi: 10.1152/ajprenal.00222.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushman WA, Jerde TJ. The role of prostate inflammation and fibrosis in lower urinary tract symptoms. Am J Physiol Renal Physiol 311: F817–F821, 2016. doi: 10.1152/ajprenal.00602.2015. [DOI] [PubMed] [Google Scholar]
- 4.Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun WC, Zhang Z. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem Int Ed Engl 46: 8171–8173, 2007. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 5.Hao L, Greer T, Page D, Shi Y, Vezina CM, Macoska JA, Marker PC, Bjorling DE, Bushman W, Ricke WA, Li L. In-depth characterization and validation of human urine metabolomes reveal novel metabolic signatures of lower urinary tract symptoms. Sci Rep 6: 30869, 2016. doi: 10.1038/srep30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM Jr, Clarke HS, Crawford ED, Diokno A, Foley JP, Foster HE, Jacobs SC, Kaplan SA, Kreder KJ, Lieber MM, Lucia MS, Miller GJ, Menon M, Milam DF, Ramsdell JW, Schenkman NS, Slawin KM, Smith JA; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group . The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 349: 2387–2398, 2003. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson TM, Sehgal PD, Drew SA, Huang W, Ricke WA. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation 85: 140–149, 2013. doi: 10.1016/j.diff.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol 10: 546–550, 2013. doi: 10.1038/nrurol.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roehrborn CG. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin North Am 95: 87–100, 2011. doi: 10.1016/j.mcna.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Rudick CN, Berry RE, Johnson JR, Johnston B, Klumpp DJ, Schaeffer AJ, Thumbikat P. Uropathogenic Escherichia coli induces chronic pelvic pain. Infect Immun 79: 628–635, 2011. doi: 10.1128/IAI.00910-10. [DOI] [PMC free article] [PubMed] [Google Scholar]