Abstract
Acid retention associated with reduced glomerular filtration rate (GFR) exacerbates nephropathy progression in partial nephrectomy models of chronic kidney disease (CKD) and might be reflected in patients with CKD with reduced estimated GFR (eGFR) by increased anion gap (AG). We explored the presence of AG and its association with CKD in 14,924 adults aged ≥20 yr with eGFR ≥ 15 ml·min−1·1.73 m−2 enrolled in the National Health and Nutrition Examination Survey III, 1988–1994, using multivariable regression analysis. The model was adjusted for sociodemographic characteristics, diabetes, and hypertension. We further examined the association between AG and incident end-stage renal disease (ESRD) using frailty models, adjusting for demographics, clinical factors, body mass index, serum albumin, bicarbonate, eGFR, and urinary albumin-to-creatinine ratio by following 558 adults with moderate CKD for 12 yr via the United States Renal Data System. Laboratory measures determined AG using the traditional, albumin-corrected, and full AG definitions. Individuals with moderate CKD (eGFR: 30–59 ml·min−1·1.73 m−2) had a greater AG than those with eGFR ≥ 60 ml·min−1·1.73 m−2 in multivariable regression analysis with adjustment for covariates. We found a graded relationship between the adjusted mean for all three definitions of AG and eGFR categories (P trend < 0.0001). During followup, 9.2% of adults with moderate CKD developed ESRD. Those with AG in the highest tertile had a higher risk of ESRD after adjusting for covariates in a frailty model [relative hazard (95% confidence interval) for traditional AG: 1.76 (1.16–2.32)] compared with those in the middle tertile. The data suggest that high AG, even after adjusting for serum bicarbonate, is a contributing acid-base mechanism to CKD progression in adults with moderate chronic kidney disease.
Keywords: acid-base, anion gap, chronic kidney disease, progression
INTRODUCTION
Chronic kidney disease (CKD) is a major public health challenge, affecting nearly 30 million people in the United States (5) with now >650,000 patients with stage 5 CKD enrolled in the Medicare End-Stage Renal Disease (ESRD) Program. Although major risk factors for CKD progression to ESRD, such as proteinuria, poor control of hypertension, and diabetes, have been previously described (30, 42), identification of additional risk factors could aid in the goal of reducing ESRD.
We previously showed a direct association between high dietary acid load (DAL) and the risk for progression of prevalent CKD to ESRD (3), suggesting that DAL may be a modifiable risk factor for CKD progression. This finding also supports the theory that factors related to high dietary acid contribute to CKD progression. Increased dietary acid is associated with the intake of suboptimal amounts of potassium from fruits and vegetables, which translates into suboptimal amounts of bicarbonate precursors, high sulfur-containing protein intake, and high phosphorus intake. High acid diets cause acid retention in partial nephrectomized animals with reduced glomerular filtration rate (GFR), despite no metabolic acidosis characterized by serum acid-base parameters (38, 39). This acid retention, even in the absence of metabolic acidosis, exacerbates GFR decline (36, 38). Urine acid excretion in individuals because of increments in dietary acid is less than the dietary increment (20), and individuals with reduced estimated GFR (eGFR) and no metabolic acidosis appear to have acid retention when eating a high acid diet typical of Western societies (40). Reduced GFR makes experimental animals (37) and humans (12) more likely to develop acid retention in response to high dietary acid.
Lower acid diets have higher proportions of fruits and vegetables, which promote gut flora that metabolize some ingested food to yield substances such as p-cresol sulfate and indoxyl sulfate (9), which can enter the bloodstream from the gastrointestinal tract, thereby increasing unmeasured serum anions. Acid accumulation that is insufficient to cause metabolic acidosis yet causes positive acid balance (production of more acid than is excreted) might be present in humans eating such diets and correlates in degree with the amount of endogenous acid produced by the metabolism of foods in American diets abundant in acid precursors (19). The existence of this acid-base imbalance is characterized by higher tissue acid loads with lower buffering capacity, which eventually may lead to tissue and organ damage (38). Also, the net renal acid excretion does not fully account for endogenous acid production (19, 21). Consequently, high DAL because of high intake of animal-source protein might also be reflected by increased serum anion gap (AG) but not by changes in serum acid-base parameters, characteristic of metabolic acidosis. In addition, impaired acid excretion in CKD occurs even in the setting of clinically normal serum bicarbonate concentration (31). Intake of dietary alkali can reduce acid retention and slow eGFR decline, despite the absence of metabolic acidosis, in experimental animal models of CKD (38, 39).
Previous studies of AG in persons with CKD included mostly individuals with advanced kidney disease (13, 33, 41) with few studies examining the accumulation of anions earlier in CKD (1, 9a). However, to the best of our knowledge, whether elevated anions play a role in the progression to ESRD in moderate CKD has not been elucidated. In the present study, we tested the following hypotheses: 1) AG is increased in adults with CKD with moderate reductions of eGFR (between 30 and 59 ml·min−1·1.73 m−2), 2) DAL is directly associated with AG in individuals with moderate CKD, 3) AG is directly associated with an increased risk of ESRD, and 4) serum bicarbonate is not associated with an increased risk of ESRD in the setting of elevated serum AG in moderate CKD.
MATERIALS AND METHODS
Study population and baseline data.
The present study used cross-sectional data of adults from the United States with CKD participating in the third National Health and Nutrition Evaluation Survey (NHANES III) to examine the relation of eGFR and CKD progression risk group [defined by Kidney Disease Improving Global Outcome (KDIGO) (16) guidelines] to serum AG in the general population. Elevated serum AG is a known characteristic of advanced CKD. To examine whether elevated serum AG is present earlier in patients with moderate CKD (eGFR: 30–59 ml·min−1·1.73 m−2), we longitudinally examined the relation of serum AG with risk of progression to ESRD in moderate CKD. NHANES III was a national probability sample of noninstitutionalized civilians from the United States conducted between 1988 and 1994 by the Centers for Disease Control and Prevention’s National Center for Health Statistics. Our eligibility requirements were participants of ≥20 yr of age, who did not have missing data on serum measures and urine albumin and creatinine (n = 16,110), had an eGFR of ≥15 ml·min−1·1.73 m−2, and were not pregnant at the time of the survey (n = 14,924). eGFR was calculated through determination of serum creatinine (sCr) using the Chronic Kidney Disease Epidemiology Collaboration (22) equation.
Sociodemographic and clinical measurements.
Medical history and demographic data were collected through a standardized questionnaire conducted at the participant’s home followed by a medical examination and laboratory testing in a mobile examination center (2).
Racial/ethnic categories were self-reported by participants and recorded as non-Hispanic white, non-Hispanic black, and Mexican-American. Education was categorized as a high school diploma or lower, some college, and bachelor’s degree or higher. Self-reported income was represented using the poverty income ratio, which is a ratio of household income to the United States household poverty level (2).
Diabetes was defined by self-report of a health care provider’s diagnosis or measured hemoglobin A1c of ≥6.5% (14). Hypertension was defined by self-report of being told by a health care provider of having the condition, an average of three measures of systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, or reported use of antihypertensive medications (6).
Serum chemistry measures.
Serum for the biochemistry profile was frozen at less than or equal to −20°C, transported on dry ice to the central laboratory, and stored at −20°C until analysis. Serum sodium, potassium, chloride, bicarbonate, albumin, total calcium, and phosphorus were measured using a Hitachi 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Serum bicarbonate was measured using the phosphoenolpyruvate method, with the normal range using this assay being 23.0–29.0 mM. An ion-selective electrode was used for measurements of sodium and chloride in serum. sCr was measured using the kinetic rate Jaffe method and recalibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, OH) as standardized creatinine = −0.184 + 0.960 × NHANES III-measured sCr (7).
Serum AG.
Serum AG was calculated as the traditional AG as well as in two other ways using ionic contributions of electrolytes and albumin based on the published literature (18): 1) traditional AG = serum sodium (meq/l) − [serum chloride (meq/l) + serum bicarbonate (meq/l)]; 2) albumin-corrected AG (10) = [(4.4 − serum albumin (g/dl)] × 2.5 + traditional AG, and 3) full AG = albumin-corrected AG + serum potassium (meq/l) + ionized calcium (meq/l) − serum phosphate (meq/l), in which ionized calcium (meq/l) = 0.5 × [total calcium (mg/dl) + 0.8 × (4 − serum albumin (g/dl)]/2 and serum phosphate (meq/l) = [0.323 × serum phosphate (mg/dl)] × 1.8 (1, 4).
Dietary acid load.
The 24-h dietary recall data collected were used to estimate the types and amounts of foods and beverages consumed during the 24-h period before the interview (midnight to midnight) and to estimate intake of energy, nutrients, and other food components from those foods and beverages. The potential renal acid load (PRAL) of foods consumed by the participants was calculated using the Remer and Manz (29) model: PRAL (meq/day) = 0.493 protein (g) + 0.0373 phosphorus (mg) − 0.0213 potassium (mg) − 0.0263 magnesium (mg) − 0.0125 calcium (mg). DAL was estimated as PRAL.
KDIGO risk groups.
Using recent CKD nomenclature by KDIGO (16), we defined CKD according to prognostic groups for progression by eGFR and albuminuria categories, in which the risk groups ranged from low, moderately increased, and high to very high. Moderate CKD was defined as an eGFR of 30–59 ml·min−1·1.73 m−2 measured through determination of sCr using the Chronic Kidney Disease Epidemiology Collaboration (22) equation.
Outcomes.
To address our first two hypotheses, serum AG was the outcome variable. We used NHANES III data linked to the United States Renal Data System Registry (USRDS) and National Death Index records to address our last two hypotheses. To test the association of AG and serum bicarbonate with ESRD, ESRD was defined as entry into the USRDS from the time of the survey through December 31, 2006 (11). We had 105 ESRD events in our cohort. All-cause mortality data was based on the results from a probabilistic match between NHANES III participants and National Death Index records linked through December 31, 2006 (26). Since the proportionality assumption under the Cox proportional hazards model was not met for serum AG and bicarbonate, we estimated separate hazard ratios for early and late effects of serum AG and bicarbonate with time-dependent covariates. Based on the log likelihood criterion, the early effects were estimated for a cut point at 12 yr. Moreover, assuming a simple random sample, to obtain a confidence level of 95% and a power of 80%, 101 events were required to study the association between serum AG and ESRD. A followup of 12 yr provided us with the required number of events to carry out the analysis.
Statistical analysis.
We followed the analytical guidelines for NHANES data proposed by the Centers for Disease Control and Prevention (25). Baseline characteristics of the study participants across the categories of AG or eGFR were compared using ANOVA for continuous variables and χ2-tests for dichotomous variables. A Kruskal-Wallis test was used for continuous variables if the normality assumption of the residuals was not met.
For hypothesis 1, we used multivariable linear regression analysis to examine whether the eGFR category or CKD progression risk group was independently associated with the magnitude of AG after adjusting for demographics (age, sex, and race/ethnicity), education history, poverty income ratio, diabetes, and hypertension status.
For hypothesis 2, we used a multivariable linear regression model to examine the association between DAL and serum AG in adults with moderate CKD after adjusting for diabetes, hypertension, eGFR, and urinary albumin-to-creatinine ratio (UACR).
In our cohort with moderate CKD, some individuals will be more susceptible or prone to progressing to ESRD than other individuals. To account for this heterogeneity in our cohort, we used the Weibull frailty model parametrized as the proportional hazards model to examine whether serum AG or serum bicarbonate tertile was associated with the subsequent development of ESRD, as proposed in hypotheses 3 and 4. The random effect (frailty) in the model was specified to follow a normal distribution (1a, 24, 32). The middle tertile of traditional AG, albumin-corrected AG, full AG, and serum bicarbonate category served as the reference groups in the respective analysis. The rationale for selecting the middle tertile was based on previous work in which serum bicarbonate values between 26 and 30 mM were associated with the lowest risk of CKD progression and death in individuals with CKD (17, 28). We adjusted frailty models for demographics, diabetes, hypertension, body mass index, serum albumin, total serum proteins, serum bicarbonate, eGFR, and UACR. Our fully adjusted models are adjusted for serum protein and bicarbonate because changes in the concentration of the protein (other than serum albumin) may alter serum AG and we wanted to examine whether serum AG still shows an association with risk of ESRD on adjustment for serum bicarbonate.
We also used Cox proportional hazard models to examine the association of AG and bicarbonate with the development of ESRD. Multivariable Cox regression was done in a stepwise manner with an inclusion criterion of P < 0.05 and exclusion criterion of P > 0.10.
Secondary analyses using the Weibull frailty model explored whether serum AG and bicarbonate were associated with an increased risk of all-cause mortality in adults with moderate CKD. Time of development of ESRD was censored for this analysis.
All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Of the 14,924 adults meeting the eligibility criteria, the mean baseline of traditional AG was 9.5 meq/l. Baseline characteristics by the CKD stages are shown in Table 1. Adults with lower eGFR were older, poorer, less educated, and more likely to have diabetes and hypertension (for all P < 0.05). Baseline characteristics by KDIGO CKD risk groups are provided in Supplemental Table S1 (available online at https://doi.org/10.6084/m9.figshare.7807829.v2). On comparing the distributions of traditional AG across sex, race/ethnicity, and CKD stages, we did not find statistical significance across the tertiles of AG (P > 0.05; see appendix).
Table 1.
Baseline characteristics of United States adults according to stages of CKD
| CKD Stages |
||||||
|---|---|---|---|---|---|---|
| Characteristic | No CKD (n = 12,424) | Stage 1 (n = 826) | Stage 2 (n = 471) | Stage 3 (n = 1,145) | Stage 4 (n = 58) | P Value |
| Age [means (SE)] | 46.8 (0.2) | 45.7 (0.5) | 69.0 (0.6) | 72.1 (0.4) | 73.1 (1.7) | <0.0001 |
| 20–49 yr | 63.5 (7,889) | 60.4 (499) | 17.2 (81) | 4.3 (49) | * | |
| 50–69 yr | 24.1 (2,994) | 27.6 (228) | 38.3 (180) | 29.5 (338) | 30.6 (18) | |
| ≥70 yr | 12.4 (1,541) | 12.0 (99) | 44.6 (210) | 66.2 (758) | 67.3 (39) | |
| Men/women | 47.8/53.2 (5,939/6,485) | 37.4/63.6 (309/517) | 48.3/52.7 (227/244) | 43.8/57.2 (502/643) | 42.9/58.1 (25/33) | 0.0003 |
| Race/ethnicity | <0.0001 | |||||
| Other | 4.1 (509) | 3.6 (30) | 4.5 (21) | 3.3 (38) | * | |
| Mexican-American | 28.6 (3,553) | 34.3 (283) | 24.6 (116) | 10.3 (118) | 16.3 (9) | |
| Non-Hispanic black | 27.3 (3,392) | 38.1 (315) | 24.0 (113) | 16.7 (191) | 28.6 (17) | |
| Non-Hispanic white | 40.0 (4,970) | 24.0 (198) | 46.9 (221) | 69.7 (798) | 53.1 (31) | |
| Poverty income ratio (≤2) | 49.6 (6,162) | 62.4 (515) | 58.0 (273) | 53.2 (609) | 70.0 (41) | <0.0001 |
| Education level | <0.0001 | |||||
| <High school | 37.9 (4,709) | 48.1 (397) | 54.9 (259) | 54.1 (619) | 70.8 (41) | |
| High school/some college | 49.1 (6,100) | 41.8 (345) | 36.4 (171) | 34.7 (397) | 25.0 (15) | |
| >College | 13.1 (1,628) | 10.1 (83) | 8.7 (41) | 11.2 (128) | * | |
| Diabetes | 5.6 (696) | 23.3 (192) | 26.5 (125) | 20.3 (232) | 34.7 (20) | <0.0001 |
| Hypertension | 33.2 (4,125) | 52.0 (430) | 74.9 (353) | 79.1 (906) | 93.9 (54) | <0.0001 |
Values are percentages with numbers of patients (n) in parentheses unless otherwise indicated. All analyses included the total third National Health and Nutrition Evaluation Survey (NHANES III) mobile examination center-examined sample final weight to account for the complex sample design following the analytical guidelines for NHANES III data (32). For variance estimates, we used Fay’s balanced repeated replication procedure, an approach for estimation of standard errors for multistage samples that consists of many sampling units. The poverty income ratio is a ratio of family income to poverty threshold. Hypertension was defined as self-reported, average blood pressure > 140/90 mmHg, or use of medications. Diabetes was defined as self-reported or hemoglobin A1c ≥ 6.5%. No chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) of ≥60 ml·min−1·1.73 m−2 and urinary albumin-to-creatinine ratio (UACR) of ≤200 mg/g. Stage 1 was defined as an eGFR of ≥90 ml·min−1·1.73 m−2 and UACR of ≥30 mg/g. Stage 2 was defined as an eGFR of 60–89 ml·min−1·1.73 m−2 and UACR of ≥30 mg/g. Stage 3 was defined as an eGFR of 30–59 ml·min−1·1.73 m−2. Stage 4 was defined as an eGFR of 15–29 ml·min−1·1.73 m−2. *Missing data represent estimates that were suppressed because of a relative standard error of 30% or more.
AG increased in adults with moderate CKD.
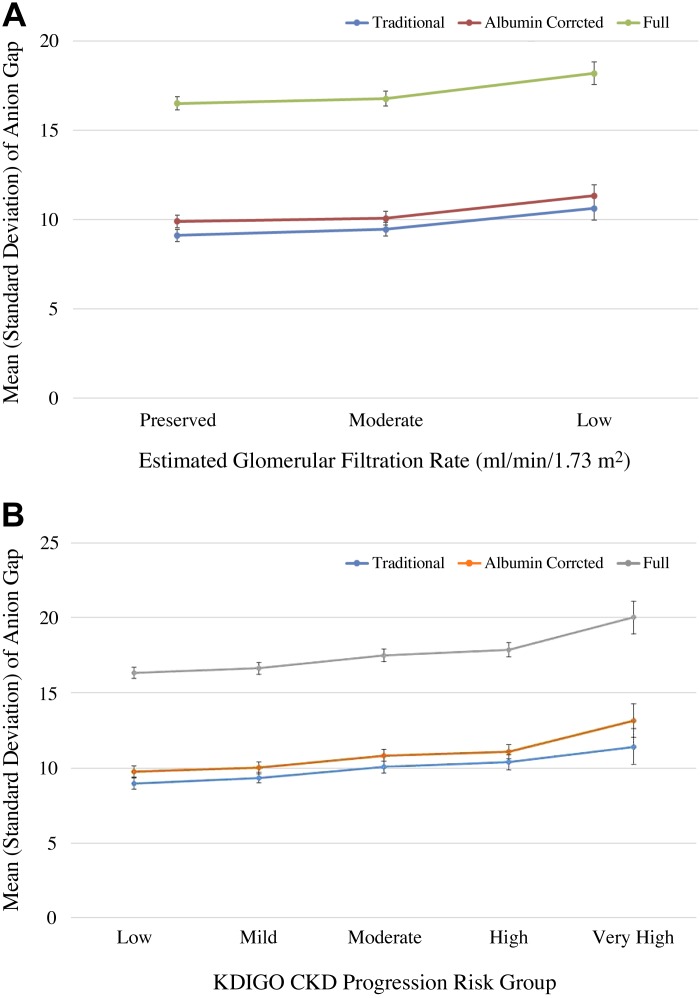
Of all adults included, 7.7% had an eGFR between 30 and 59 ml·min−1·1.73 m−2. The adjusted mean of traditional AG was progressively higher across eGFR categories (P trend = 0.007), with a mean of 9.1 meq/l in eGFR ≥ 60 ml·min−1·1.73 m−2, 9.5 meq/l in eGFR 30–59 ml·min−1·1.73 m−2, and 10.6 mEq/l in eGFR 15–29 ml·min−1·1.73 m−2 (Fig. 1A). There was a graded relation of the full AG as well, with increasing eGFR categories in the adjusted models (P trend = 0.02; Table 2 and Fig. 1A). The results were similar when using CKD progression risk groups as the independent variable [Fig. 1B and Supplemental Table S2 (available online at https://doi.org/10.6084/m9.figshare.7807829.v2)]. The adjusted mean for albumin-corrected and full AG showed similar trends across CKD risk groups as well (P trend = 0.001 and 0.0003, respectively).
Fig. 1.
Adjusted means with error bars for traditional, albumin-corrected, and full anion gap across the estimated glomerular filtration rate (eGFR) groups (A) and Kidney Disease Improving Global Outcome (KDIGO) risk groups (B). The adjusted mean for traditional, albumin-corrected, and full anion gap was progressively higher across eGFR categories and chronic kidney disease (CKD) risk groups.
Table 2.
Relation of eGFR to traditional, albumin-corrected, and full anion gap in United States adults
| Traditional Anion Gap |
Albumin-Corrected Anion Gap |
Full Anion Gap |
||||
|---|---|---|---|---|---|---|
| Model | β (95% CI)* | P value | β (95% CI)* | P value | β (95% CI)* | P value |
| Unadjusted | ||||||
| Preserved (eGFR ≥ 60 ml·min−1·1.73 m−2) | Reference† | Reference† | Reference† | |||
| Moderate (eGFR: 30–59 ml·min−1·1.73 m−2) | 0.30 (0.008, 0.59) | 0.04 | 0.70 (0.39, 0.99) | <0.0001 | 0.79 (0.47, 1.11) | <0.0001 |
| Low (eGFR: 15–29 ml·min−1·1.73 m−2) | 1.64 (0.39, 2.89) | 0.01 | 2.42 (1.26, 3.56) | 0.0004 | 2.75 (1.61, 3.89) | <0.0001 |
| Trend | 0.01 | <0.0001 | <0.0001 | |||
| Adjusted | ||||||
| Preserved (eGFR: ≥60 ml·min−1·1.73 m−2) | Reference‡ | Reference‡ | Reference‡ | |||
| Moderate (eGFR: 30–59 ml·min−1·1.73 m−2) | 0.35 (0.08, 0.62) | 0.01 | 0.18 (−0.09, 0.45) | 0.19 | 0.26 (−0.02, 0.54) | 0.07 |
| Low (eGFR: 15–29 ml·min−1·1.73 m−2) | 1.54 (0.30, 2.78) | 0.01 | 1.47 (0.30, 2.64) | 0.01 | 1.73 (0.57, 2.89) | 0.004 |
| Trend | 0.007 | 0.07 | 0.02 | |||
All analyses included the total third National Health and Nutrition Evaluation Survey (NHANES III) mobile examination center-examined sample final weight to account for the complex sample design following the analytical guidelines for NHANES III data (32). For variance estimates, we used Fay’s balanced repeated replication procedure, an approach for estimation of standard errors for multistage samples that consists of many sampling units adjusted for demographics (age, sex, and race/ethnicity), education history, poverty income ratio, diabetes, and hypertension. Hypertension was defined as self-reported, average blood pressure > 140/90 mmHg, or use of medications. Diabetes was defined as self-reported or hemoglobin A1c ≥ 6.5%. eGFR, estimated glomerular filtration rate; CI, confidence interval. *From multivariable linear regression analysis. †See Fig. 1A.‡See Fig. 1B.
DAL is directly associated with AG in individuals with moderate CKD.
A multivariable linear regression of DAL and serum AG was used to estimate the associated differences in serum AG. Each 25 meq/day higher DAL was associated with significantly higher traditional serum AG of 0.61 meq/l (0.35–1.10). Adjustments made for clinical risk factors of diabetes, hypertension, baseline eGFR, and UACR attenuated but did not eliminate this association [0.58 meq/l (0.30–1.09)]. We noted a significant association between DAL and albumin-corrected and full AG as well after adjustment for potential confounders [0.51 meq/l (0.20–0.97) for albumin-corrected AG and 0.54 meq/l (0.24–1.10) for full AG per 25 meq/day DAL; Supplemental Table S3, available online at https://doi.org/10.6084/m9.figshare.7807829.v2)].
AG is directly associated with increased risk of ESRD.
Adults with moderate CKD linked to USRDS over a period of 12 yr (n = 1,145) yielded 105 (9.2%) ESRD events. There was a greater percentage of people with eGFR of 45–60 ml·min−1·1.73 m−2 and with elevated UACR with higher traditional AG tertile (P < 0.05) at baseline. No significant association was observed between the tertiles of traditional AG and other baseline characteristics (P > 0.05; Table 3).
Table 3.
Baseline characteristics of United States adults with moderate CKD (n = 1,145) by tertile of traditional AG
| Characteristic | Lowest AG (minimal−8.1 meq/l) (n = 381) | Middle AG (8.1–11.8 meq/l) (n = 385) | Highest AG (11.8−maximal meq/l) (n = 379) | P Value |
|---|---|---|---|---|
| Age | 0.24 | |||
| 20–49 yr | 5.1 (19) | 8.4 (32) | 12.0 (45) | |
| 50–69 yr | 30.0 (114) | 33.4 (129) | 50.5 (191) | |
| ≥70 yr | 64.9 (247) | 58.3 (224) | 37.5 (142) | |
| Men/women | 42.5/58.5 (162) | 33.3/67.7 (128) | 49.2/51.8 (186) | 0.23 |
| Race/ethnicity | 0.10 | |||
| Mexican-American | * | 1.5 (6) | 1.9 (7) | |
| Non-Hispanic black | 12.0 (46) | 6.8 (26) | 17.0 (64) | |
| Non-Hispanic white | 85.6 (326) | 85.9 (331) | 76.3 (289) | |
| Diabetes | 19.2 (73) | 30.3 (117) | 25.6 (97) | 0.51 |
| Hypertension | 85.0 (324) | 81.0 (312) | 87.7 (332) | 0.29 |
| eGFR | 0.02 | |||
| 30–44 ml·min−1·1.73 m−2 | 30.6 (117) | 30.9 (119) | 36.7 (139) | |
| 45–59 ml·min−1·1.73 m−2 | 69.4 (264) | 69.1 (266) | 63.3 (240) | |
| Urinary albumin-to creatinine ratio (≥30 mg/g) | 29.2 (111) | 42.7 (164) | 50.9 (193) | 0.04 |
| KDIGO CKD progression risk group | 0.09 | |||
| Mild | 21.0 (80) | 27.7 (107) | 43.6 (165) | |
| Moderate | 15.4 (59) | 15.4 (59) | 13.2 (50) | |
| High | 63.6 (242) | 56.8 (219) | 43.2 (164) |
Values are percentages with numbers of patients (n) in parentheses unless otherwise indicated. The mild progression group was defined as estimated glomerular filtration rate (eGFR) ≥ 90 ml·min−1·1.73 m−2 or eGFR = 60–89 ml·min−1·1.73 m−2 and urinary albumin-to-creatinine ratio (UACR) = 30–300 mg/g and eGFR = 45–59 ml·min−1·1.73 m−2 and UACR < 30 mg/g. The moderate progression group was defined as eGFR ≥ 90 ml·min−1·1.73 m−2 or eGFR = 60–89 ml·min−1·1.73 m−2 and UACR > 300 mg/g, eGFR = 45–59 ml·min−1·1.73 m−2 and UACR = 30–300 mg/g, and eGFR = 30–44 ml·min−1·1.73 m−2 and UACR < 30 mg/g. The high progression group was defined as eGFR = 45–59 ml·min−1·1.73 m−2 or eGFR = 30–44 ml·min−1·1.73 m−2 and UACR > 300 mg/g, eGFR = 30–44 ml·min−1·1.73 m−2 or eGFR = 15–29 ml·min−1·1.73 m−2 and UACR = 30–300 mg/g, and eGFR = 15–29 ml·min−1·1.73 m−2 and UACR < 30 mg/g. Moderate chronic kidney disease (CKD) was defined as eGFR = 30–59 ml·min−1·1.73 m2. These linkage data were limited to linkage-eligible participants. All analyses included the total third National Health and Nutrition Evaluation Survey (NHANES III) mobile examination center-examined sample final weight to account for the complex sample design following the analytical guidelines for NHANES III data (32). For variance estimates, we used Fay’s balanced repeated replication procedure, an approach for estimation of standard errors for multistage samples that consists of many sampling units. AG, anion gap; KDIGO, Kidney Disease Improving Global Outcome. *Missing data represent estimates that were suppressed as the cell frequency was <5.
Adults with moderate CKD in the highest tertile of traditional AG after adjustment for demographics, body mass index, serum albumin, total protein, and serum bicarbonate had a higher risk of developing ESRD compared with adults in the middle tertile using a frailty model [relative hazard (95% confidence interval): 1.76 (1.16–2.32); Table 4]. The relation of albumin-corrected AG with risk of ESRD was higher in the highest tertile [1.94 (1.06–2.81)] compared with the middle tertile. The relation of the full AG with risk of ESRD was similar for the lowest and highest tertile compared with the middle tertile; however, the risk in the lowest tertile was not statistically significant. When examining the relation of serum bicarbonate with risk of ESRD after adjustment for serum AG, we observed a high risk associated with the highest tertile [2.98 (2.05–3.91)] compared with the reference category, whereas the risk of ESRD within the lowest tertile was not statistically significantly higher (Table 4).
Table 4.
Association of traditional anion gap, albumin-corrected anion gap, full anion gap, and serum bicarbonate with risk of ESRD in United States adults with moderate chronic kidney disease (n = 1,145) with a followup of 12 yr using a frailty model
| Relative Hazard for ESRD (95% Confidence Interval)a |
|||
|---|---|---|---|
| Model | Lowest tertile | Middle tertile | Highest tertile |
| Traditional anion gap | (minimal−8.1 meq/l) | (8.1–11.8 meq/l) | (11.8−maximal meq/l) |
| Unadjusted | 2.92 (2.40–5.44) | 1.0 (reference) | 3.16 (2.07–4.24) |
| Adjustedb | 2.07 (1.12–3.01) | 1.0 (reference) | 2.09 (1.29–3.48) |
| Fully adjustedc | 1.71 (0.99–2.42) | 1.0 (reference) | 1.76 (1.16–2.32) |
| Albumin-corrected anion gap | (minimal−9.2 meq/l) | (9.2–12.7 meq/l) | (12.7–maximal meq/l) |
| Unadjusted | 1.19 (1.09–1.28) | 1.0 (reference) | 1.43 (1.21–1.66) |
| Adjustedb | 1.35 (1.19–1.50) | 1.0 (reference) | 2.59 (1.80–3.99) |
| Fully adjustedd | 0.82 (0.58–1.08) | 1.0 (reference) | 1.94 (1.06–2.81) |
| Full anion gap | (minimal−15.93 meq/l) | (15.93–19.54 meq/l) | (19.54−maximal meq/l) |
| Unadjusted | 1.24 (1.08–1.43) | 1.0 (reference) | 1.48 (1.27–1.71) |
| Adjustedb | 1.81 (1.44–2.31) | 1.0 (reference) | 2.13 (1.50–3.07) |
| Fully adjustedd | 1.29 (0.92–1.70) | 1.0 (reference) | 1.35 (1.05–1.83) |
| Serum bicarbonate | (minimal−26 mmol/l) | (26–29 mmol/l) | (29−maximal mmol/l) |
| Unadjusted | 1.12 (0.78–2.02) | 1.0 (reference) | 1.31 (0.88–2.20) |
| Adjustedb | 2.77 (1.90–3.64) | 1.0 (reference) | 2.97 (1.79–4.17) |
| Fully adjustede | 1.30 (0.88–1.84) | 1.0 (reference) | 2.98 (2.05–3.91) |
Moderate chronic kidney disease was defined as an estimated glomerular filtration rate of 30–59 ml·min−1·1.73 m−2. These linkage data were limited to linkage-eligible participants. All analyses included the total third National Health and Nutrition Evaluation Survey (NHANES III) mobile examination center-examined sample final weight to account for the complex sample design following the analytical guidelines for NHANES III data (32). For variance estimates, we used Fay’s balanced repeated replication procedure, an approach for estimation of standard errors for multistage samples that consists of many sampling units. ESRD, end-stage renal disease. aFrom the frailty model; badjusted for demographic factors (age, sex, and race), diabetes, hypertension, estimated glomerular filtration rate, and urinary albumin-to-creatinine ratio; cadjusted for demographic factors (age, sex, and race), diabetes, hypertension, estimated glomerular filtration rate, urinary albumin-to-creatinine ratio, body mass index, serum albumin, total protein, and serum bicarbonate; dadjusted for demographic factors (age, sex, and race), diabetes, hypertension, estimated glomerular filtration rate, urinary albumin-to-creatinine ratio, body mass index, serum total protein, and serum bicarbonate; eadjusted for demographic factors (age, sex, and race), diabetes, hypertension, estimated glomerular filtration rate, urinary albumin-to-creatinine ratio, body mass index, and serum anion gap.
The proportional hazards model yielded a similar risk of ESRD associated with traditional and albumin-corrected AG to that in the frailty model. For full AG, neither the highest nor lowest tertiles showed a significant risk of ESRD in adults compared with the middle tertile. A significant risk of ESRD was noted for adults in the highest tertile [2.06 (1.08–2.92)] of serum bicarbonate (Supplemental Table S4, available online at https://doi.org/10.6084/m9.figshare.7807829.v2).
AG, serum bicarbonate, and all-cause mortality.
The risk of all-cause mortality was greater in the highest levels of AG in fully adjusted multivariable models compared with the middle tertile in full AG [relative hazard (95% confidence interval): 1.20 (1.01–1.39)]. No statistically significant association was noted between the highest levels of AG in traditional and albumin-corrected AG and all-cause mortality. In addition, there was no statistically significant association between the lowest tertiles of AG and mortality compared with the middle tertile (Table 5). After adjusting for potential confounders, the risks of all-cause mortality for individuals in the lowest and highest tertiles of serum bicarbonate were not significantly different from those in the middle tertile.
Table 5.
Association of traditional anion gap, albumin-corrected anion gap, full anion gap, and serum bicarbonate with all-cause mortality in United States adults with moderate chronic kidney disease (n = 1,145) with a followup of 12 yr using a frailty model
| Relative Hazard for Mortality (95% Confidence Interval)a |
|||
|---|---|---|---|
| Model | Lowest tertile | Middle tertile | Highest tertile |
| Traditional anion gap | (minimal−8.1 meq/l) | (8.1–11.8 meq/l) | (11.8−maximal meq/l) |
| Unadjusted | 1.05 (0.92–1.18) | 1.0 (reference) | 1.28 (1.14–1.43) |
| Adjustedb | 0.97 (0.84–1.09) | 1.0 (reference) | 1.16 (0.99–1.32) |
| Fully adjustedc | 0.91 (0.78–1.06) | 1.0 (reference) | 1.18 (0.98–1.40) |
| Albumin-corrected anion gap | (minimal−9.2 meq/l) | (9.2–12.7 meq/l) | (12.7−maximal meq/l) |
| Unadjusted | 1.10 (1.00–1.21) | 1.0 (reference) | 1.25 (1.08–1.42) |
| Adjustedb | 1.01 (0.87–1.15) | 1.0 (reference) | 1.14 (0.97–1.30) |
| Fully adjustedd | 0.98 (0.81–1.10) | 1.0 (reference) | 1.13 (0.95–1.31) |
| Full anion gap | (minimal−15.93 meq/l) | (15.93–19.54 meq/l) | (19.54−maximal meq/l) |
| Unadjusted | 1.11 (0.96–1.25) | 1.0 (reference) | 1.23 (1.06–1.39) |
| Adjustedb | 1.04 (0.90–1.19) | 1.0 (reference) | 1.15 (0.99–1.32) |
| Fully adjusted d | 1.01 (0.85–1.16) | 1.0 (reference) | 1.20 (1.01–1.39) |
| Serum bicarbonate | (minimal−26 mmol/l) | (26–29 mmol/l) | (29−maximal mmol/l) |
| Unadjusted | 0.89 (0.89–1.00) | 1.0 (reference) | 0.97 (0.83–1.11) |
| Adjustedb | 0.95 (0.83–1.07) | 1.0 (reference) | 0.99 (0.84–1.13) |
| Fully adjusted e | 0.98 (0.80–1.15) | 1.0 (reference) | 1.01 (0.70–1.31) |
Moderate chronic kidney disease was defined as an estimated glomerular filtration rate of 30–59 ml·min−1·1.73 m−2. These linkage data were limited to linkage-eligible participants. All analyses included the total third National Health and Nutrition Evaluation Survey (NHANES III) MEC-examined sample final weight to account for the complex sample design following the analytical guidelines for NHANES III data (32). For variance estimates, we used Fay’s balanced repeated replication procedure, an approach for estimation of standard errors for multistage samples that consists of many sampling units. aFrom the frailty model; badjusted for demographic factors (age, sex, and race), diabetes, hypertension, estimated glomerular filtration rate, and urinary albumin-to-creatinine ratio. cadjusted for demographic factors (age, sex, and race), diabetes, hypertension, estimated glomerular filtration rate, urinary albumin-to-creatinine ratio, body mass index, serum albumin, total protein, and serum bicarbonate; dadjusted for demographic factors (age, sex, and race), diabetes, hypertension, estimated glomerular filtration rate, urinary albumin-to-creatinine ratio, body mass index, serum total protein, and serum bicarbonate; eadjusted for demographic factors (age, sex, and race), diabetes, hypertension, estimated glomerular filtration rate, urinary albumin-to-creatinine ratio, body mass index, and serum anion gap.
DISCUSSION
We found in this former nationally representative cohort that adults with moderate CKD had higher serum AG compared with their counterparts with earlier stages of CKD, a finding that was consistent across serum AG definitions. AG, which has previously received little attention as a possible risk factor in population-based studies, was identified as a contributing acid-base mechanism (i.e., underlying acid retention) for CKD progression to ESRD in adults with moderate CKD (Fig. 2). The excess risk associated with higher levels of AG compared with lower levels of AG in this cohort translates to almost 3,200 people in the United States population (calculated using 4.5% prevalence of moderate CKD in NHANES III) with CKD having a greater risk of progression.
Fig. 2.
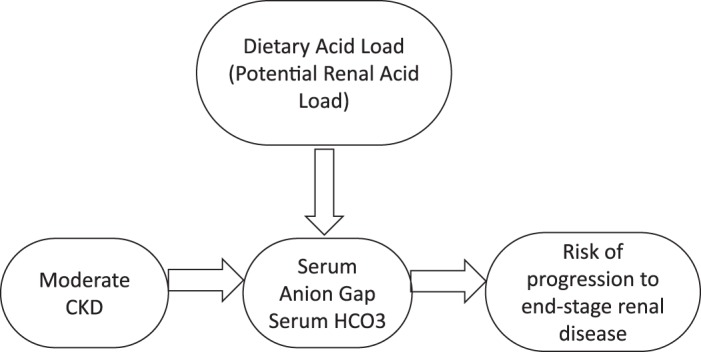
Conceptual framework of our hypotheses. CKD, chronic kidney disease.
The acid-base imbalance caused by an increase in the unmeasured anions (a drop in serum bicarbonate levels) may be prominent earlier in the course of CKD than thought earlier. During the early stages, the degree of hyperchloremia encountered is sufficient in magnitude to offset the decrement in bicarbonate concentration, and thereby the concentration of unmeasured anions remaining within the normal range. Thus, individuals might have had an increment in AG even though the absolute values did not appear high. Moreover, as shown in some animal and patient studies by Wesson et al. (34–36, 38–40), acid retention in patients with CKD with reduced eGFR might be present without a measurable decrease in serum bicarbonate. Another study by Vallet et al. (31) showed that as eGFR decreased over time, urine net acid excretion (the renal contribution to maintain the acid-base balance with the excretion of and titratable acids) decreased slightly, whereas net endogenous acid production (production of more acid than is excreted because of consumption of a diet high in acid precursors) remained unchanged, yielding an increase in net acid balance. Authors found that the increase in net acid balance was not associated with an accompanying decrease in serum bicarbonate, which supports the presence of acid retention despite no decrease in serum bicarbonate. In our study, we noted a statistically significant association between DAL and serum AG after accounting for differences in comorbid conditions, eGFR, and UACR. Our findings further strengthen the argument that increased serum AG is, at least in part, due to increase in the acid balance, even in the absence of decreased serum bicarbonate. Also, high net acid balance may influence the progression of eGFR decline, as shown by Wesson et al. (34–36, 38–40). An increase in acid balance might exacerbate CKD progression through mechanisms associated with adaptive responses meant to enhance acid excretion in the face of progressive loss of kidney function (24a, 39). When the kidney is challenged with acid, it increases levels of hormones (i.e., angiotensin II, aldosterone, and endothelin) that promote excretion of acid in the short term (15, 35), but high levels of these hormones may worsen kidney function in the long term (36, 39).
We observed that lower levels of serum bicarbonate were not associated with a higher risk of progression in adults with moderate CKD when adjusted for serum AG. This finding suggests that the increase in the acid balance marked by serum AG blunts the association between low serum bicarbonate concentration and risk of ESRD. Our results are in contrast with the findings of some observational studies (8, 23, 28) that have reported lower serum bicarbonate concentrations as a risk factor for CKD progression. In the Chronic Renal Insufficiency Cohort for participants with eGFR > 45 ml·min−1·1.73 m−2, low serum bicarbonate was an independent risk factor for kidney disease progression (8). In the African-American Study of Kidney Disease, each 1-meq/l increase in serum bicarbonate level was associated with a 7% reduction in eGFR events (defined as a GFR reduction by 50% or by 25 ml·min−1·1.73 m−2 from the mean of two GFR measurements at baseline) (28). In the Modification of Diet in Renal Disease study, patients in the lowest quartile of bicarbonate levels had higher risk of kidney failure (hazard ratio = 2.22) compared with patients in the highest quartile. However, this association was rendered nonsignificant with adjustment for eGFR (23). None of these studies adjusted for serum AG, which eliminated the association between lower serum bicarbonate levels and risk of ESRD in our analysis.
This study provides further evidence in patients with CKD that states of “acid stress” that fall short of metabolic acidosis reflected by relevant changes in serum acid-base parameters (including reduced serum bicarbonate) not outside their clinically adjudged “normal” ranges nevertheless subject patients with CKD to an increased risk for progression to ESRD. As noted earlier, in patients with CKD with reduced GFR and with no metabolic acidosis eating acid-producing diets, acid retention when corrected by oral NaHCO3 (and not KHCO3) slows or stops GFR decline and further reduces urine excretion of endothelin and aldosterone, whose high levels are associated with progressive nephropathy (34, 39). There exists an association between the slowed eGFR decline and reduced urine excretion of endothelin. In addition, as eGFR decreases in patients with CKD, acid-base imbalance worsens (12). We further demonstrated a significant association between DAL and increased risk of ESRD in adults with CKD (3). Together, these data support that increase in acid balance in adults with CKD can be caused by a combination of high DAL and reduced eGFR. We propose that the level of AG reflects the algebraic sum of DAL and reduced eGFR and is a simple, clinically available way by which to determine if an adult with CKD has underlying acid retention not associated with metabolic acidosis. AG can be increased by a very high DAL with a modestly reduced eGFR or conversely by a modest DAL with a very low eGFR. The data reported in this study support that AG serves this purpose better than serum bicarbonate. Future studies should attempt to replicate these results in other cohorts and evaluate the underlying mechanism as well as validate clinically meaningful threshold values.
The major strength of this analysis is the large sample representative of the United States population and the longitudinal design with moderately long followup for ESRD. Although metabolic acidosis is concomitant of advanced CKD, AG has not been identified as a risk factor for progression in moderate CKD until now. Large prospective followup cohorts are needed to validate our conclusions. We attempted to address potential confounders between AG and progression of CKD such as reduced eGFR, UACR, serum albumin, total protein, and serum bicarbonate.
Despite the strengths, there are several limitations. First, the observational design certainly precludes conclusions about causation. Second, we did not have laboratory followup data beyond baseline measurements. Thus, there is a possibility of misclassification from measurements at a single time point. Third, our results may have been influenced by unmeasured confounders. Fourth, we did not have information on conditions that might lead to the accumulation of lactate and keto acids and therefore could not account for these factors that affect endogenous acid production. Fifth, this survey was conducted in the early 1990s with a followup that ended in 2006. However, we used this cohort because it is the most recent nationally representative sample that is linked to the ESRD registry with a long followup and data for AG measure and serum bicarbonate.
In conclusion, our findings suggest that a greater AG is present even among individuals with moderate CKD and that high serum AG, independent of low serum bicarbonate, gives insight to an acid-base mechanism contributing to CKD progression in adults with moderate CKD.
GRANTS
The Chronic Kidney Disease Surveillance System project is supported under a cooperative agreement from the Centers for Disease Control and Prevention through Grant NU58DP003836-05-01.
DISCLAIMERS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.B. and N.R.P. conceived and designed research; T.B. performed experiments; T.B. analyzed data; T.B., D.E.W., C.E.M., and N.R.P. interpreted results of experiments; T.B. prepared figures; T.B. drafted manuscript; T.B., D.C.C., D.E.W., C.E.M., K.L.J., S.S., N.R.B., R.S., B.G., J.B.-G., and N.R.P. edited and revised manuscript; T.B., D.C.C., D.E.W., C.E.M., K.L.J., S.S., N.R.B., R.S., B.G., J.B.-G., and N.R.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants and staff of the National Health and Nutrition Examination Survey.
The Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team consists of a group members led by the University of California-San Francisco [Neil Powe (Principal Investigator), Tanushree Banerjee, Delphine Tuot, Chi-yuan Hsu, Charles McCulloch, Deidra Crews, Raymond Hsu, Vanessa Grubbs, Kirsten Bibbins-Domingo, Michael Shlipak, Carmen Peralta, Anna Rubinsky, Josef Coresh, and Joanne Rodrigue], the University of Michigan [Rajiv Saran (Principal Investigator), Vahakn Shahinian, Brenda Gillespie, Hal Morgenstern, Michael Heung, William Herman, William McClellan, Jennifer Bragg-Gresham, Diane Steffick, Anca Tilea, Maggie Yin, Ian Robinson, Kara Zivin, Vivian Kurtz, and April Wyncott], and the Centers for Disease Control and Prevention [Nilka Rios Burrows (Technical Advisor), Mark Eberhardt, Linda Geiss, Juanita Mondesire, Bernice Moore, Priti Patel, Meda Pavkov, Deborah Rolka, Sharon Saydah, Sundar Shrestha, and Larry Waller].
APPENDIX
Figures A1, A2, and A3 show distributions of traditional AG by sex, race/ethnicity, and CKD stage, respectively. We did not find statistical significance by the tertiles of AG.
Fig. A1.
Distribution of traditional anion gap by sex.
Fig. A2.
Distribution of traditional anion gap by race/ethnicity.
Fig. A3.
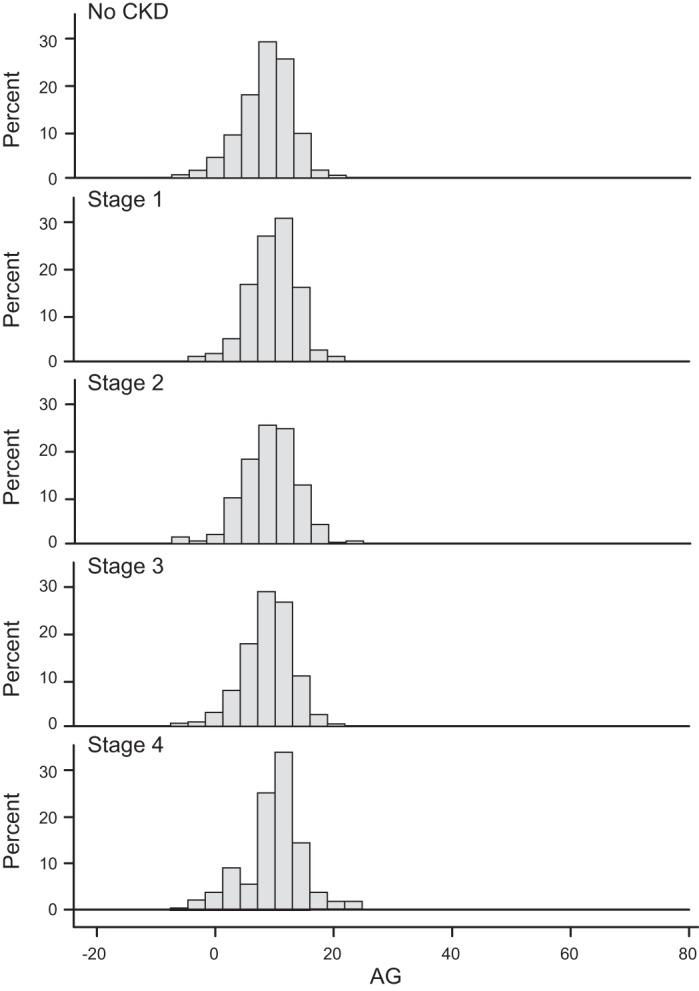
Distribution of traditional anion gap by chronic kidney disease (CKD) stage.
REFERENCES
- 1.Abramowitz MK, Hostetter TH, Melamed M. The serum anion gap is altered in early kidney disease and associates with mortality. Kidney Int 82: 701–709, 2012. doi: 10.1038/ki.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Allison PD. Survival Analysis Using SAS: a Practical Guide (2nd ed.). Cary, NC: SAS Institute, 2010. [Google Scholar]
- 2.Anonymous Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1 32: 1–407, 1994. [PubMed] [Google Scholar]
- 3.Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Ríos-Burrows N, Williams DE, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol 26: 1693–1700, 2015. doi: 10.1681/ASN.2014040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal VK. Serum inorganic phosphorus. In: Clinical Methods: the History, Physical, and Laboratory Examinations (3rd ed.), edited by Walker HK, Hall WD, Hurst JW. Boston: Butterworths, 1990, p. 895–899. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention National Chronic Kidney Disease Fact Sheet, 2017. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2017. [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 8.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, Ojo A, Scialla J, Steigerwalt S, Teal V, Wolf M, Rahman M; CRIC Investigators . Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 62: 670–678, 2013. doi: 10.1053/j.ajkd.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 76: S12–S19, 2009. doi: 10.1038/ki.2009.402. [DOI] [PubMed] [Google Scholar]
- 9a.Farwell WR, Taylor EN. Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey. CMAJ 182: 137–141, 2010. doi: 10.1503/cmaj.090329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med 26: 1807–1810, 1998. doi: 10.1097/00003246-19981100000019. [DOI] [PubMed] [Google Scholar]
- 11.Golden C, Driscoll AK, Simon AE, Judson DH, Miller EA, Parker JD. Linkage of NCHS population health surveys to administrative records from Social Security Administration and Centers for Medicare Medicaid Services. Vital Health Stat 1: 1–53, 2015. [PubMed] [Google Scholar]
- 12.Goraya N, Simoni J, Sager LN, Pruszynski J, Wesson DE. Acid retention in chronic kidney disease is inversely related to GFR. Am J Physiol Renal Physiol 314: F985–F991, 2018. doi: 10.1152/ajprenal.00463.2017. [DOI] [PubMed] [Google Scholar]
- 13.Hakim RM, Lazarus JM. Biochemical parameters in chronic renal failure. Am J Kidney Dis 11: 238–247, 1988. doi: 10.1016/S0272-6386(88)80156-2. [DOI] [PubMed] [Google Scholar]
- 14.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32: 1327–1334, 2009. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna A, Simoni J, Wesson DE. Endothelin-induced increased aldosterone activity mediates augmented distal nephron acidification as a result of dietary protein. J Am Soc Nephrol 16: 1929–1935, 2005. doi: 10.1681/ASN.2004121054. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease Improving Global Outcomes KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 5–14, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24: 1232–1237, 2009. doi: 10.1093/ndt/gfn633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2: 162–174, 2007. doi: 10.2215/CJN.03020906. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int 24: 670–680, 1983. doi: 10.1038/ki.1983.210. [DOI] [PubMed] [Google Scholar]
- 20.Lemann J Jr, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol Renal Physiol 285: F811–F832, 2003. doi: 10.1152/ajprenal.00115.2003. [DOI] [PubMed] [Google Scholar]
- 21.Lennon EJ, Lemann J Jr, Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest 45: 1601–1607, 1966. doi: 10.1172/JCI105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon V, Tighiouart H, Vaughn NS, Beck GJ, Kusek JW, Collins AJ, Greene T, Sarnak MJ. Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis 56: 907–914, 2010. doi: 10.1053/j.ajkd.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Molenberghs G, Verbeke G, Efendi A, Braekers R, Demétrio CGB. A combined gamma frailty and normal random-effects model for repeated, overdispersed time-to-event data. Stat Methods Med Res 24: 434–452, 2015. doi: 10.1177/0962280214520730. [DOI] [PubMed] [Google Scholar]
- 24a.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics Analytical and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, 1988–1994. Hyattsville, MD: National Center for Health Statistics, 1996. [Google Scholar]
- 26.National Center for Health Statistics The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File: Matching Methodology. Hyattsville, MD: National Center for Health Statistics, 2005. [Google Scholar]
- 28.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011. doi: 10.1038/ki.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95: 791–797, 1995. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 30.Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care 35: 329–344, 2008. doi: 10.1016/j.pop.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallet M, Metzger M, Haymann JP, Flamant M, Gauci C, Thervet E, Boffa JJ, Vrtovsnik F, Froissart M, Stengel B, Houillier P; NephroTest Cohort Study group . Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int 88: 137–145, 2015. doi: 10.1038/ki.2015.52. [DOI] [PubMed] [Google Scholar]
- 32.Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16: 439–454, 1979. doi: 10.2307/2061224. [DOI] [PubMed] [Google Scholar]
- 33.Wallia R, Greenberg A, Piraino B, Mitro R, Puschett JB. Serum electrolyte patterns in end-stage renal disease. Am J Kidney Dis 8: 98–104, 1986. doi: 10.1016/S0272-6386(86)80119-6. [DOI] [PubMed] [Google Scholar]
- 34.Wesson DE. Assessing acid retention in humans. Am J Physiol Renal Physiol 301: F1140–F1142, 2011. doi: 10.1152/ajprenal.00346.2011. [DOI] [PubMed] [Google Scholar]
- 35.Wesson DE, Jo CH, Simoni J. Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int 82: 1184–1194, 2012. doi: 10.1038/ki.2012.267. [DOI] [PubMed] [Google Scholar]
- 36.Wesson DE, Jo CH, Simoni J. Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol Dial Transplant 30: 762–770, 2015. doi: 10.1093/ndt/gfu388. [DOI] [PubMed] [Google Scholar]
- 37.Wesson DE, Pruszynski J, Cai W, Simoni J. Acid retention with reduced glomerular filtration rate increases urine biomarkers of kidney and bone injury. Kidney Int 91: 914–927, 2017. doi: 10.1016/j.kint.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Wesson DE, Simoni J. Increased tissue acid mediates a progressive decline in the glomerular filtration rate of animals with reduced nephron mass. Kidney Int 75: 929–935, 2009. doi: 10.1038/ki.2009.6. [DOI] [PubMed] [Google Scholar]
- 39.Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int 78: 1128–1135, 2010. doi: 10.1038/ki.2010.348. [DOI] [PubMed] [Google Scholar]
- 40.Wesson DE, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300: F830–F837, 2011. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 41.Widmer B, Gerhardt RE, Harrington JT, Cohen JJ. Serum electrolyte and acid base composition. The influence of graded degrees of chronic renal failure. Arch Intern Med 139: 1099–1102, 1979. doi: 10.1001/archinte.1979.03630470021010. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, Ojo A, Rahman M, Nessel L, Kusek JW, Feldman HI; CRIC Study Investigators . Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 63: 236–243, 2014. doi: 10.1053/j.ajkd.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]