Abstract
Inflammation is involved in many prostate pathologies including infection, benign prostatic hyperplasia, and prostate cancer. Preclinical models are critical to our understanding of disease mechanisms, yet few models are genetically tractable. Here, we present a comparative quantitative proteomic analysis of urine from mice with and without prostate-specific inflammation induced by conditional prostate epithelial IL-1β expression. Relative quantification and sample multiplexing was achieved using custom 4-plex N,N-dimethyl leucine (DiLeu) isobaric tags and nanoflow ultrahigh-performance liquid chromatography coupled to high-resolution tandem mass spectrometry. Each set of 4-plex DiLeu reagents allows four urine samples to be analyzed simultaneously, providing high-throughput and accurate quantification of urinary proteins. Proteins involved in the acute phase response, including haptoglobin, inter-α-trypsin inhibitor, and α1-antitrypsin 1-1, were differentially represented in the urine of mice with prostate inflammation. Mass spectrometry-based quantitative urinary proteomics represents a promising bioanalytical strategy for biomarker discovery and the elucidation of molecular mechanisms in urological research.
Keywords: benign prostatic hyperplasia; inflammation; interleukin-1β; lower urinary tract symptoms, mass spectrometry; urine proteomics
INTRODUCTION
Prostatic inflammation commonly cooccurs with prostate infection, benign prostatic hyperplasia (BPH), and prostate cancer (CaP) and contributes to lower urinary tract symptoms (LUTS) (21, 24, 31, 32). To better understand the molecular signature of prostate inflammation and to determine if this inflammation could be monitored in a noninvasive manner, we undertook a global urinary proteomics analysis of mice with prostate epithelium-targeted IL-1β. Urine proteins can offer valuable insight into the status of urological and systemic diseases (42). Additionally, the noninvasive nature of its sample collection makes urine a valuable sample material for clinical and basic scientists alike (42).
The proinflammatory cytokine IL-1β mediates inflammation for the innate immune response through the IL-1 receptor type I (IL-1RI), which signals in the nucleus after heterodimerization with IL-1R accessory protein (IL-1RAcP) and phosphorylation by IL-1R-associated kinases (6). Normal IL-1β signaling is tightly controlled at several levels, with transcripts generated in response to injury and infection and IL-1β cytokine maturation and secretion regulated by the inflammasome (38). Inflammasome assembly can be induced by pathogen- or damage-associated molecular patterns, and its activity is regulated by signals from cytokines and reactive oxygen species (39). Inactivation of IL-1β signaling is a promising therapeutic avenue for numerous autoinflammatory diseases including rheumatoid arthritis, gout, and osteoarthritis (6). Prostate abnormalities like prostatitis, BPH, and CaP may be among these IL-1β-related diseases. For instance, IL-1β in the seminal plasma of patients with chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome was significantly higher than in healthy controls (14). In BPH, elevated IL-1β has been reported relative to the normal prostate via immunohistochemistry (36). In CaP, expression of key inflammasome components are enriched in malignant prostate tissue as compared with benign adjacent tissue (21). Single-nucleotide polymorphisms in the IL1B gene are associated with increased CaP risk in African-American and Caucasian men (47). Additionally, elevated tissue IL-1β has significantly associated with CaP progression (as biochemical recurrence) (7).
To test the hypothesis that prostate inflammation produces objective, quantifiable changes to the urinary proteome, a noninvasive urinary proteomics analysis was performed in a novel model of prostate-specific IL-1β induction, known as the induced mouse prostate inflammation-IL-1-driven (IMPI-1) model (4). The IMPI-1 prostate inflammation model offers strict control over this important signaling pathway. When used in a paired experimental design, the model also offers minimized biological variability and is conducive to paired statistical comparison while reducing animal use. We aimed to shed light on the molecular processes involved in signaling prostatic inflammation, particularly those that can be detectable in a urine sample. This information could one day help diagnose or stratify treatment for patients with chronic prostatitis/chronic pelvic pain syndrome, a syndrome that can include many different symptoms and can be challenging to diagnose and treat (33). These data could also inform future efforts in drug development or other mechanistic studies related to inflammatory diseases of the prostate.
Urine contains protein information from both urological and systemic sources and can be collected in a relatively noninvasive manner, making it a valuable sample material for biomarker discovery (42). The noninvasive nature of urine collection can allow for the monitoring of disease onset across time, as in a repeated-measures study of small research animals. Urine collection also offers minimal burden imposed on the subject and is enriched for proteins derived from the urogenital tract (5). These qualities are appealing in the context of clinical analysis and basic urological research alike. Excluding prostatic secretory proteins, ~26% of the proteins found in urine are of systemic origin (17). The remaining fraction is contributed directly from the kidneys, ureter, bladder, urethra, and prostate. Consequently, the urine proteome is a good source of prostatic proteins. Indeed, urinary proteomics has become a valuable strategy for the discovery of biomarkers in patients with CaP (2, 41), LUTS, and BPH (13). Expressed prostatic secretions in urine can also contain valuable proteins (23). Urinary proteomics has been successfully applied to murine models of inflammation-related diseases like kidney disease (46, 48), bladder cancer (9), and atherosclerosis (43). Murine models can provide valuable insights into disease mechanisms and offer minimized interindividual variability for the potential discovery of biomarkers.
Mass spectrometry (MS) has emerged as the dominant analytical tool for the identification and quantification of biological molecules. Modern high-resolution, accurate-mass instruments are capable of measurements with low single digit part-per-million mass accuracy, leading to confident peptide and small-molecule identifications, especially when combined with a plethora of gas-phase fragmentation techniques. When coupled to ultrahigh-performance liquid chromatography, MS allows the analysis of complex analyte mixtures, as found in most biological matrixes (e.g., serum and urine). A major challenge in biomarker discovery in these complex matrixes is the wide dynamic range of analyte concentrations, which have been estimated at around 12 orders of magnitude in plasma, with hemoglobin and albumin at high abundance and ILs at very low (3). Typical MS analyses rely on data-dependent acquisition, which is inherently biased against low-abundance proteins. Orthogonal chromatographic separations, fractionation, and enrichment strategies (e.g., combinatorial peptide ligand library beads) can provide deeper proteome profiling but are resource intensive. Efforts to deepen the observable human urine proteome have resulted in remarkable protein counts at the expense of increased sample preparation and analysis time (37, 49). MS-based stable isotope labeling is a key technique for accurate and multiplexed peptide quantification. Our laboratory has developed a set of custom isobaric labeling reagents, called N,N-dimethyl leucine (DiLeu) (10, 13, 15, 16, 45). Here, we used a streamlined, multiplexed MS workflow using DiLeu isobaric peptide labeling for relative quantification of urinary proteins in a genetically induced prostatic inflammation mouse model. Modest sample fractionation after pooling can improve proteome coverage while retaining the multiplexing efficiency offered by the DiLeu labeling strategy. This method would translate well to high-throughput biomarker discovery or validation experiments seeking reliable relative protein quantification.
MATERIALS AND METHODS
Materials and reagents.
DTT and sequencing grade trypsin were purchased from Promega (Madison, WI). Iodoacetamide, trifluoroacetic acid, 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride, and triethylammonium bicarbonate were purchased from Sigma (St. Louis, MO). N-methylmorpholine was purchased from TCI (Tokyo, Japan). Hydroxylamine was purchased from Alfa Aesar (Ward Hill, MA). Ammonium formate was purchased from Fluka Honeywell (Muskegon, MI). Urea, Tris buffer, high-purity LC-MS grade Optima water, acetonitrile, and formic acid solvents were purchased from Fisher Scientific (Fair Lawn, NJ). Anhydrous dimethylformamide was supplied via dry solvent dispenser (University of Wisconsin-Madison School of Pharmacy). The BCA protein concentration assay was purchased from Thermo Scientific (no. 23225, Pierce, Rockford, IL). Centrifugal filter units (3-kDa Amicon Ultra 0.5 ml) were purchased from Millipore (Billerica, MA). Solid phase extraction cartridges (SepPak C18) were purchased from Waters (Milford, MA). Solid-phase extraction pipette tips (OMIX ZipTip C18) were purchased from Agilent Technologies (Santa Clara, CA). The strong cation exchange column for offline fractionation (2.1 mm × 200 mm, 5 µm, 300 Å) was purchased from PolyLC (Columbia, MD). Reversed-phase ethylene-bridged hybrid beads for the self-made online column (75.1 µm × 150 mm, 1.7 µm, 100 Å) were purchased from Waters.
Genetically engineered mouse model of prostatic inflammation.
The IMPI-1 mice used in this study are transgenic for Hoxb13-rtTA (29, 35) and TetO-IL-1B (IMPI-1+/+) on the FVB/NJ background, as previously described in detail elsewhere (4). Doxycycline administration drives expression of the human proinflammatory cytokine IL-1β in Hoxb13-expressing prostate luminal epithelial cells.
Mouse treatment and sample collection.
Mouse handling and sample collection procedures were approved by the University of Maryland-Baltimore County Animal Care and Use Committee. To induce prostatic IL-1β, 8-wk-old IMPI-1 mice (n = 6) were administered doxycycline in drinking water (ad libitum, 2 mg/ml) for 6 wk. This treatment generates chronic-active inflammation (4). Urine was collected via abdominal massage at baseline and again after 6 wk of IL-1β induction. Urine samples were collected on sterile weigh boats, transferred to microcentrifuge tubes, and placed on dry ice before storage at −80°C. When mice produced inadequate urine volume, collections across 2 h were pooled. Mice were euthanized after the 6-wk urine collection time point. Ventral prostates were collected, preserved in formalin, embedded in paraffin, sectioned (5 µm), and stained with hematoxylin and eosin before histological examination.
Mouse urine sample preparation.
Each mouse urine sample was thawed on ice, vortexed to redissolve protein, and centrifuged at 10,000 g for 10 min to remove cells, cell debris, and other particulates. The urinary protein fraction was obtained using 3-kDa molecular weight cutoff centrifugal filters following the manufacturer’s protocol. Total protein concentration of each sample was measured via the BCA assay (see Supplemental Table S1 in the Supplemental Data; Supplemental Data are available online at https://doi.org/10.6084/m9.figshare.7840760.v4). Two hundred micrograms of each sample were freeze dried in a SpeedVac and redissolved in 8 M urea/50 mM Tris buffer (pH 8). Protein disulfide bonds were reduced with 5 mM DTT for 1 h, and free thiol groups were alkylated with 15 mM iodoacetamide in the dark for 30 min followed by 5 mM DTT to quench the reaction. Each protein sample was diluted with 50 mM Tris buffer to a urea concentration of <1 M and digested with trypsin enzyme at a 50:1 protein-to-enzyme ratio at 37°C for 16 h. Protein digestion was quenched by reducing the pH to <3 with 10% trifluoroacetic acid, and the digested sample was purified via SepPak C18 solid-phase extraction cartridge.
Four-plex isobaric DiLeu labeling.
The 4-plex isobaric DiLeu labeling reagents were synthesized following a modified procedure as previously described (45). DiLeu reagents are activated to the triazine ester form to react with amine groups. One milligram of each DiLeu reagent was mixed with 1.86 mg 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride and 0.74 µl N-methylmorpholine in 50 µl anhydrous dimethylformamide. The reaction vial was vortexed at room temperature for 1 h. After activation, peptide samples were labeled immediately with DiLeu triazine ester to obtain the optimal labeling efficiency. To accomplish this, lyophilized peptides were reconstituted in 0.5 M triethylammonium bicarbonate solution and labeled with a 5× (wt/wt) excess of activated DiLeu. Anhydrous dimethylformamide was added to reach a 70% (vol/vol) organic-to-aqueous ratio, and the reaction vials were vortexed for 2 h at room temperature. The labeling reaction was quenched with 0.25% (vol/vol) hydroxylamine, and 4-plex labeled samples were dried separately.
Sample cleanup with strong cation exchange.
Offline SCX chromatography fractionation was used to remove unreacted DiLeu reagent and reaction byproducts from the labeled peptides and separate each multiplexed sample into three fractions for subsequent nanoLC-MS/MS analysis. SCX fractionation was performed using an SCX column on a Waters Alliance e2695 HPLC instrument. Buffer A was 10 mM NH4HCO2 and 25% acetonitrile (ACN; pH 3), and buffer B was 500 mM NH4HCO2 and 25% ACN (pH 6−8. Individual labeled peptide samples were redissolved in buffer A, and each of four DiLeu channels were pooled in equal parts to generate multiplexed samples for SCX fractionation. The binary gradient at a flow rate of 0.2 ml/min was performed as follows: 0–20 min, 0% B, 20–90 min, 0–50% B, 90–100 min, 50–100% B, 100–110 min, 100% B. Fractions were collected every 2 min, combined into three vials based on UV-Vis at 280 nm, and dried down. Solid-phase extraction was performed via C18 OMIX ZipTips for subsequent peptide desalting, with 0.1% trifluoroacetic acid in H2O as the reconstitution and washing solution and 0.1% FA in 50% ACN and 0.1% formic acid (FA) in 70% ACN as elution solutions. The eluate was dried and stored at −20°C.
NanoLC-ESI-MS/MS.
Multiplexed urinary peptides were analyzed using a Waters nanoLC system coupled to a Thermo Q Exactive mass spectrometer. Mobile phase A was 0.1% FA in H2O, and mobile phase B was 0.1% FA in ACN. The flow rate was 0.3 µl/min. A C18 column was fabricated in house with an integrated electrospray ionization emitter (75.1 µm × 150 mm, 1.7 µm, 100 Å). Top 20 data-dependent acquisitions were conducted, and the MS scanned from m/z 400–1,500 at a resolving power of 120 K (at m/z 200) and an S-lens radio frequency of 30. Parent masses were isolated in the quadrupole with an isolation window of 1.0 m/z and fragmented with higher-energy collisional dissociation with a normalized collision energy of 30%. MS/MS scans were detected in the linear ion trap using the rapid scan rate and a dynamic exclusion time of 60 s. Automatic gain control targets were 1 × 106 for MS and 2 × 105 for MS/MS acquisitions. Maximum injection times were 100 ms for MS and 50 ms for MS/MS.
Data analysis.
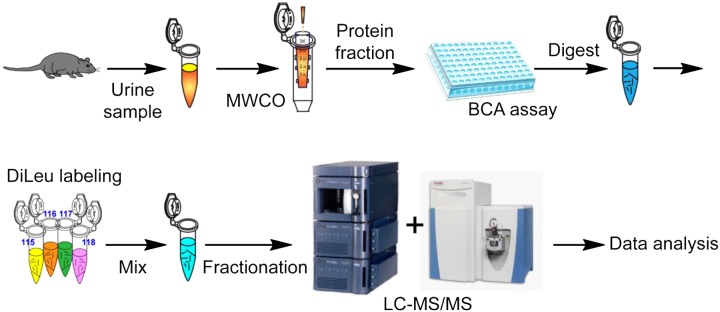
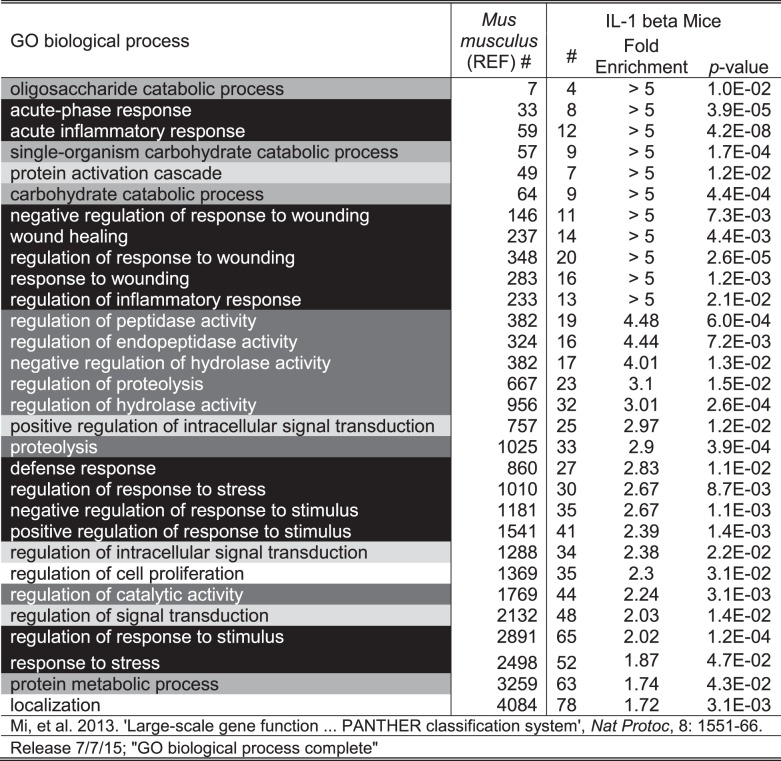
Data analysis was carried out using the Proteome Discoverer 1.4 software for peptide and protein identification and quantification. Protein identification was achieved by searching against a Mus musculus reference database (UniProtKB release-2014_10). Methionine oxidation was chosen as variable modification. Cysteine carbamidomethylation, NH2-terminus DiLeu labeling, and lysine residue DiLeu labeling were chosen as static modifications. Statistical analysis was conducted using a paired Student's t-test (α = 0.05) with Benjamini-Hochberg correction for multiple hypothesis testing (false discovery rate = 0.1) (28). A support vector machine (SVM) supervised machine learning strategy was used to identify important classifying features. The SVM constructs a hyperplane that optimally separates two groups and individual data points (proteins) can be ranked by separation efficacy (40). Significantly overrepresented biological processes were identified via a binomial test with Bonferroni correction (P < 0.05, PANTHER online) (30). The overall analytical workflow was illustrated, including urine sample collection, protein extraction, normalization, digestion, DiLeu labeling, offline SCX fractionation, nanoLC-MS/MS analysis, and data analysis (Fig. 1).
Fig. 1.
Overall workflow of quantitative urinary proteomics analysis using 4-plex N,N-dimethyl leucine (DiLeu) labeling. Data analysis was conducted using Proteome Discoverer software followed by offline analysis. MWCO, molecular weight cutoff; LC-MS/MS, liquid chromotography tandem mass spectroscopy.
RESULTS AND DISCUSSION
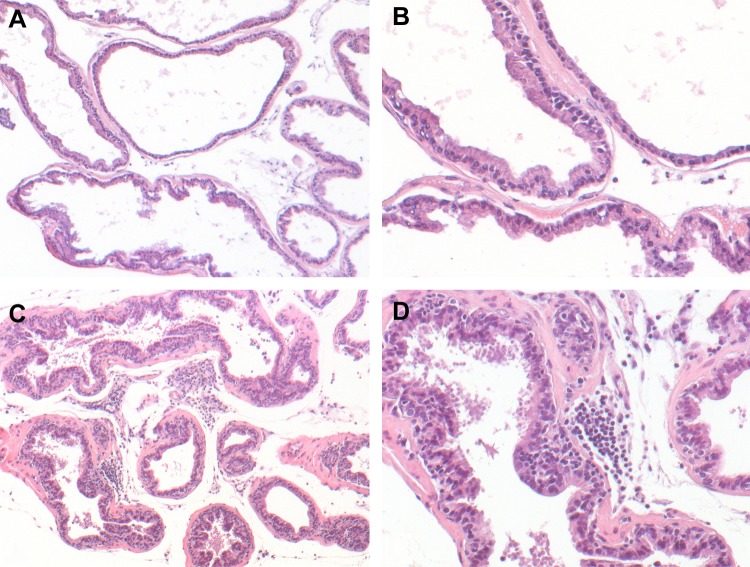
Histological examination showed increased inflammatory infiltrates in the prostate after 6 wk of IL-1β induction compared with a noninduced mouse of the same genotype (Fig. 2). A total of 261 urine proteins were identified and quantified (Fig. 3 and Supplemental Table S2). Thirty proteins were significantly different after IL-1β induction in the prostate epithelium (paired Student’s t-test α = 0.05). Six proteins remained significant after false discovery rate correction for multiple comparisons, and many of these were also useful in separating treatment from control via the SVM machine learning strategy (Table 1). An example tandem MS spectrum is shown of DiLeu-labeled FHLIVNEECTEMTAIGEQTEK, a peptide representative of major urinary protein 3, in Fig. 4. A rich ladder of b- and y-product ions were generated from DiLeu labeled peptides for confident identification. Partial least-squares discriminant analysis was conducted for all quantified proteins, and a score plot showing the complete separation of baseline samples from induced samples was generated (Fig. 5). Cross-validation accuracy of the established partial least-squares discriminant analysis model was over 0.9, suggesting negligible model overfitting. Thirty significantly overrepresented processes were found via PANTHER gene ontology (Fig. 6). The largest category represents inflammation-related processes like wound healing and regulation of the inflammatory response, as would be expected in this model of genetically induced inflammation (Fig. 7). The acute phase protein response is often signaled by cytokines like IL-1β and is known to modulate serum levels of coagulation, transport, and protease-inhibiting proteins (11).
Fig. 2.
Histological analysis of IL-1β-mediated inflammation in induced mouse prostate inflammation-IL-1-driven (IMPI-1) prostate lobes at 6.5 wk after doxycycline administration. A and B: ventral IMPI-1 prostate from an animal maintained on normal drinking water. C and D: ventral IMPI-1 prostate from an animal maintained continuously on doxycycline-supplemented drinking water (2 mg/ml). Note the presence of pockets of inflammatory cells in the stroma and epithelium. Original magnifications: ×10 for A and C and ×25 for B and D.
Fig. 3.
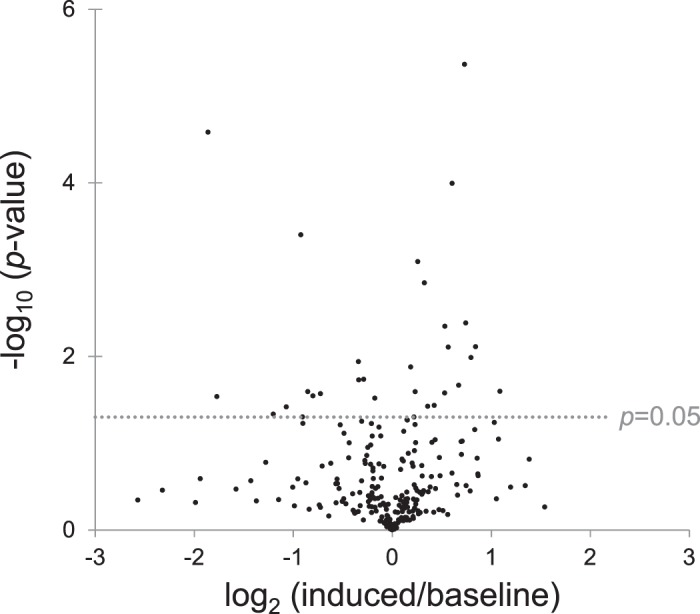
Volcano plot of mouse urine proteins; -log10 P value (paired Student's t-test) versus log2 of the prostatic IL-1β induced-to-baseline ratio.
Table 1.
Top 20 mouse prostate inflammation urinary proteins
| Accession Number | Name | Gene | Induced-to-Baseline | P Value | Support Vector Machine Rank |
|---|---|---|---|---|---|
| Q61646 | Haptoglobin | Hp | 0.28 | 2.6 e−5* | 1 |
| P01837 | Igκ chain C region | Not applicable | 1.66 | 4.3 e−6* | 2 |
| O70570 | Polymeric Ig receptor | Pigr | 1.52 | 1.0 e−4* | 3 |
| Q07456 | Protein AMBP | Ambp | 1.25 | 1.4 e−3* | 4 |
| Q9EQ21 | Hepcidin | Hamp | 0.53 | 4.0 e−4* | 5 |
| P01868 | Igγ1 chain C region secreted form | Ighg1 | 2.12 | 2.5 e−2 | 7 |
| P13634 | Carbonic anhydrase 1 | Ca1 | 1.73 | 1.0 e−2 | 8 |
| P07758 | α1-Antitrypsin 1-1 | Serpina1a | 1.79 | 7.7 e−3 | 9 |
| Q9EP95 | Resistin-like alpha | Retnla | 1.48 | 7.8 e−3 | 10 |
| Q91X17 | Uromodulin | Umod | 1.19 | 8.1 e−4* | 13 |
| P23953 | Carboxylesterase 1C | Ces1c | 1.44 | 4.5 e−3 | 14 |
| Q99P86 | Resistin like β | Retnlb | 1.67 | 4.1 e−3 | 15 |
| F6XQ00 | Complement factor B | Cfb | 0.79 | 1.1 e−2 | 16 |
| D3YY79 | Glutathione hydrolase 1 proenzyme | Ggt1 | 1.17 | 2.5 e−2 | 17 |
| P04939 | Major urinary protein 3 | Mup3 | 1.14 | 1.3 e−2 | 19 |
| P07724 | Serum albumin | Alb | 1.59 | 2.1 e−2 | 20 |
| Q80YX8 | Major urinary protein 26 | Mup26 | 1.44 | 2.6 e−2 | 23 |
| A9C497 | Protein Mup19 | Mup19 | 0.79 | 1.9 e−2 | 24 |
| Q9DAU7 | WAP four-disulfide core domain protein 2 | Wfdc2 | 0.82 | 1.8 e−2 | 25 |
| O09159 | Lysosomal α-mannosidase | Man2b1 | 0.55 | 2.5 e−2 | 27 |
P values are from a paired Student's t-test.
Proteins that remained significant after Benjamini-Hochberg correction (false discovery rate = 0.1). Proteins were ranked by importance as classifiers in separating induced from baseline via Support Vector Machine.
Fig. 4.
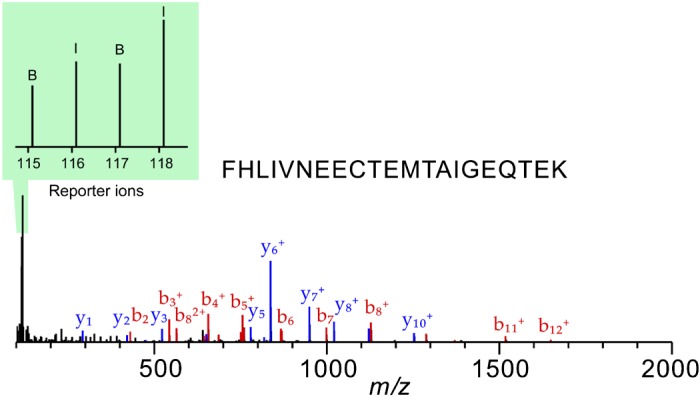
Example MS/MS fragmentation of N,N-dimethyl leucine (DiLeu) labeled urinary peptide representing major urinary protein 3. The b- and y-product ions represent the backbone fragmentation of the peptide for identification. The intensities of reporter ions are used for relative quantification.
Fig. 5.
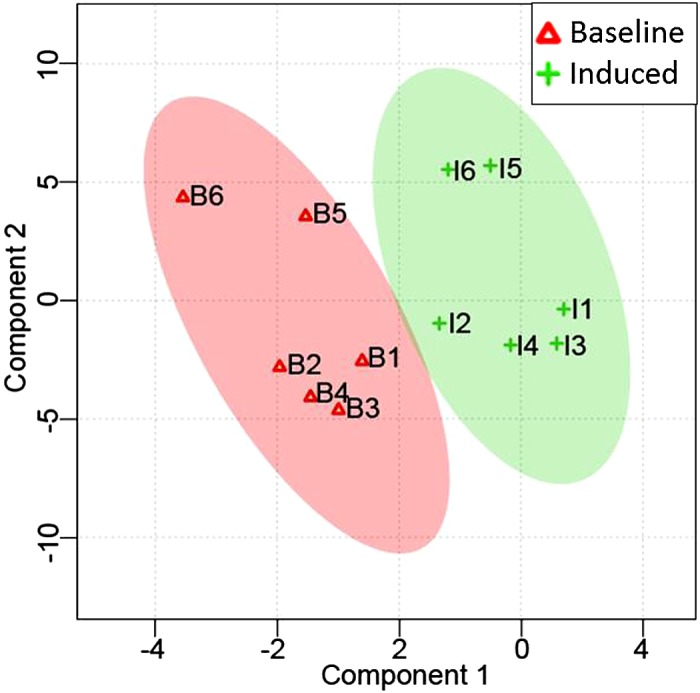
Partial least-squares discriminant analysis score plot of all identified and quantified mouse prostate inflammation urinary proteins. Each data point represents a biological replicate at baseline or induced, averaged across technical replicates.
Fig. 6.
PANTHER overrepresentation test: 261 total mouse prostate inflammation urinary proteins; binomial test with Bonferroni correction, α = 0.05 for induced to baseline versus the program-generated reference mouse proteome. Gray shading corresponds to Fig. 7. GO, Gene Ontology.
Fig. 7.
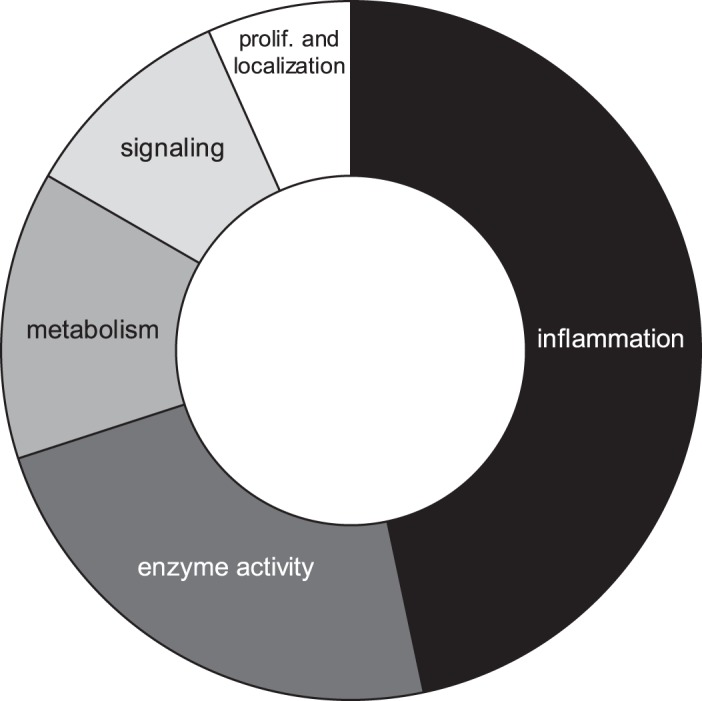
PANTHER overrepresentation test: 261 total mouse prostate inflammation urinary proteins; binomial test with Bonferroni correction, α = 0.05 for induced to baseline versus the program-generated reference mouse proteome. Categories were manually assigned from Fig. 6.
Haptoglobin, an acute phase protein whose synthesis by the liver is upregulated in response to inflammation (25), showed the lowest P value in our analysis and was the strongest classifier by our SVM machine learning strategy (Table 1). Interestingly, while haptoglobin has been viewed as positively associating with inflammatory diseases (11, 34), our data show its decrease (~3.6-fold) with IL-1β induction in this model of inflammation. It is possible that haptoglobin is repressed by IL-1β, as haptoglobin is a type II acute phase protein, relying on IL-6 alone for induction (12). Alternatively, at 6 wk this model reflects a chronic-active stage of inflammation, which could lead to effects like these that may be inconsistent with a predominantly acute phase response.
Other acute phase proteins, related to protease inhibition, were increased with IL-1β induction, consistent with the expected acute phase protein response (11). The first, inter-α-trypsin inhibitor (Ambp), was upregulated with treatment and remained significant after false discovery rate correction (Table 1). The second, α1-antitrypsin 1-1 (Serpina1), was increased 1.79-fold and was the ninth most effective protein classifier in separating treatment from control via SVM (Table 1). Plasminogen was increased slightly at 1.16-fold, consistent with the acute phase response (11), and was significant by paired t-test but did not remain significant after false discovery rate correction. Serum albumin is generally expected to decrease in the acute phase response (11) but was increased 1.59-fold in our analysis. As above, effects like these, which appear inconsistent with the acute phase response, may be related to the 6-wk length of treatment, which reflects a state of chronic-active inflammation.
Immune response processes are also expected in this model. Igκ chain C region (Igkc gene) and polymeric immunoglobulin receptor (Pigr gene) proteins were both upregulated with IL-1β induction, had the second and third lowest P values respectively, and proved second and third best at separating treatment from control via SVM (Table 1). Of relevance here, Pigr is expressed in many tissues, including the genitourinary tract, and is inducible by cytokines like IL-1β (20). As such, Pigr protein is a valuable putative biomarker for IL-1β signaling and potentially inflammation in this model. This receptor would normally be induced by cytokines signaling viral or bacterial infection through the NF-κB pathway (20). Pigr is expressed at the surface of epithelial secretory cells and, upon liganding by polymeric IgA, is transported through the cytoplasm to the apical surface (20). There Pigr is cleaved, releasing the Pigr secretory component that signals in the innate and adaptive immune responses (20). Less is known about Igkc, but it has been studied in the context of CaP, where the Igκ chain was detected in human CaP but not in normal prostate specimens (26). Additionally, the Igkc gene was elevated in prostate cancers of African-American men versus their European-American counterparts (44).
Prostatic inflammation is involved in abnormalities of the prostate and lower urinary tract. As such, the urinary proteins and associated processes found herein may be important in human diseases such as prostatitis, BPH, LUTS, and CaP. Interestingly, a previous study identified 50 significantly modulated proteins in the urine of patients with LUTS (n = 25) compared with urology patients without significant LUTS (n = 15) (13). Relevant process-level similarities were found between mice with inflamed prostates and men with LUTS, but no coinciding proteins were identified here. Common significantly overrepresented processes include an acute inflammatory response, response to wounding, and regulation of the inflammatory response. Among the proteins involved in these processes, a coagulation factor was identified in both mice and men. In men, coagulation factor XII was upregulated in patients with LUTS ~1.36-fold and was significant by t-test (P = 0.01) (13). Here, coagulation factor X was upregulated 1.34-fold in IL-1β-induced mice and was significant by paired t-test (P = 0.037) but not after false discovery rate correction. Inflammation and coagulation show strong cooccurrence. Inflammation can initiate coagulation and anticoagulants can also be anti-inflammatory (8). The acute protein response includes many coagulation-related proteins (11). IL-1β is known to promote coagulation and inhibit antithrombotic processes (19). Reciprocally, inhibition of coagulation factor Xa decreased proinflammatory cytokines, including IL-1β (18). Coagulation factor XII can promote inflammation and coagulation and has been highlighted as a promising drug target for the treatment of thrombosis and inflammation (22). Coagulation factors have been discussed in the context of BPH and CaP, where coagulation factor X was found significantly higher in BPH versus malignant tumors (1) and higher in BPH than in histologically normal prostate tissue (27). Coagulation signaling and wound healing are promising biological processes in the search for useful markers of prostatic inflammation.
The proteome coverage achieved here, while not entirely comprehensive, is satisfactory given current methodologies and the inherent dynamic range limitations of urinary proteins. Isobaric labeling strategies generally favor high-throughput efficiency over sheer protein identification count, and one should expect this when undertaking such an analysis. It is likely that proteome coverage by isobaric labeling techniques will improve as the performance of mass spectrometers and chromatographic systems continues to improve.
In conclusion, mass spectrometry-based urinary proteomics is a viable and powerful bioanalytical platform for comparative analysis of global protein expression in mouse models of prostate diseases. Custom isobaric DiLeu labeling provided an accurate and high-throughput strategy for urinary proteomic analysis. This model of prostate-specific IL-1β induction offers strict control over inflammation and, when coupled with in-host controls, offers minimized biological variability and paired statistical comparison. We have shed light on the downstream impact of IL-1β-induced inflammation in the mouse prostate and have highlighted proteins that could serve as putative biomarkers or targets of future mechanistic studies. The overrepresented processes found were consistent with the expected effects of this treatment: wound healing and inflammation. Proteins of the acute phase response, including haptoglobin, inter-α-trypsin inhibitor, and α1-antitrypsin 1-1, typify these processes. Future studies will assess IL-1β-induced prostatic inflammation and its role in prostate diseases.
GRANTS
S. Thomas is supported by National Institutes of Health (NIH) Training Program Predoctoral Fellowship Grant T32-ES-007015. This work was also supported by NIH Grants U54-DK-104310, R01-ES-01332 (to C. M. Vezina and W. A. Ricke), R01-DK-071801, P20-DK-090921 (to C. Bajpai), and P41-GM-108538 (to L. Li). The Orbitrap instruments were purchased through the support of NIH Shared Instrument Grant S10-RR-029531.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.H., T.G., S.B., A.A., A.M.D.M., and C.B. performed experiments; L.H., S.T., and T.G. analyzed data; L.H. and S.T. prepared figures; L.H., S.T., C.M.V., L.L., and W.A.R. edited and revised manuscript; L.H., S.T., T.G., C.M.V., S.B., A.A., A.M.D.M., C.B., L.L., and W.A.R. approved final version of manuscript; S.T. interpreted results of experiments; S.T. drafted manuscript; W.A.R. conceived and designed research.
ACKNOWLEDGMENTS
We appreciate Dr. Dustin Frost and Dr. Amanda Buchberger in the Li Research Group for assisting with the synthesis of 4-plex DiLeu reagents and Michael Rubenstein for assistance in figure preparation.
REFERENCES
- 1.Adamson AS, Francis JL, Witherow RO, Snell ME. Procoagulant properties of benign and malignant prostatic tissue. Br J Urol 74: 204–209, 1994. doi: 10.1111/j.1464-410X.1994.tb16587.x. [DOI] [PubMed] [Google Scholar]
- 2.Adeola HA, Soares NC, Paccez JD, Kaestner L, Blackburn JM, Zerbini LF. Discovery of novel candidate urinary protein biomarkers for prostate cancer in a multiethnic cohort of South African patients via label-free mass spectrometry. Proteomics Clin Appl 9: 597–609, 2015. doi: 10.1002/prca.201400197. [DOI] [PubMed] [Google Scholar]
- 3.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 1: 845–867, 2002. doi: 10.1074/mcp.R200007-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Ashok A, Keener R, Rubenstein M, Stookey S, Bajpai S, Hicks J, Alme AK, Drake CG, Zheng Q, Trabzonlu L, Yegnasubramanian S, De Marzo AM, Bieberich CJ. Consequences of interleukin 1β-triggered chronic inflammation in the mouse prostate gland: altered architecture associated with prolonged CD4+ infiltration mimics human proliferative inflammatory atrophy. Prostate 79: 732–745, 2019. doi: 10.1002/pros.23784. [DOI] [PubMed] [Google Scholar]
- 5.Decramer S, Gonzalez de Peredo A, Breuil B, Mischak H, Monsarrat B, Bascands JL, Schanstra JP. Urine in clinical proteomics. Mol Cell Proteomics 7: 1850–1862, 2008. doi: 10.1074/mcp.R800001-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720–3732, 2011. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eiró N, Bermudez-Fernandez S, Fernandez-Garcia B, Atienza S, Beridze N, Escaf S, Vizoso FJ. Analysis of the expression of interleukins, interferon β, and nuclear factor-κ B in prostate cancer and their relationship with biochemical recurrence. J Immunother 37: 366–373, 2014. doi: 10.1097/CJI.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 8.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol 131: 417–430, 2005. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira R, Oliveira P, Martins T, Magalhães S, Trindade F, Pires MJ, Colaço B, Barros A, Santos L, Amado F, Vitorino R. Comparative proteomic analyses of urine from rat urothelial carcinoma chemically induced by exposure to N-butyl-N-(4-hydroxybutyl)-nitrosamine. Mol Biosyst 11: 1594–1602, 2015. doi: 10.1039/C4MB00606B. [DOI] [PubMed] [Google Scholar]
- 10.Frost DC, Greer T, Li L. High-resolution enabled 12-plex DiLeu isobaric tags for quantitative proteomics. Anal Chem 87: 1646–1654, 2015. doi: 10.1021/ac503276z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340: 448–454, 1999. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 12.Gianazza E, Miller I, Palazzolo L, Parravicini C, Eberini I. With or without you - Proteomics with or without major plasma/serum proteins. J Proteomics 140: 62–80, 2016. doi: 10.1016/j.jprot.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Greer T, Hao L, Nechyporenko A, Lee S, Vezina CM, Ricke WA, Marker PC, Bjorling DE, Bushman W, Li L. Custom 4-plex DiLeu isobaric labels enable relative quantification of urinary proteins in men with lower urinary tract symptoms (LUTS). PLoS One 10: e0135415, 2015. doi: 10.1371/journal.pone.0135415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H, Xu Y-M, Ye Z-Q, Yu J-H, Fu Q, Sa Y-L, Hu X-Y, Song L-J. Heat-shock protein 70 expression in the seminal plasma of patients with chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome. Prostate Cancer Prostatic Dis 13: 338–342, 2010. doi: 10.1038/pcan.2010.22. [DOI] [PubMed] [Google Scholar]
- 15.Hao L, Greer T, Page D, Shi Y, Vezina CM, Macoska JA, Marker PC, Bjorling DE, Bushman W, Ricke WA, Li L. In-depth characterization and validation of human urine metabolomes reveal novel metabolic signatures of lower urinary tract symptoms. Sci Rep 6: 30869, 2016. doi: 10.1038/srep30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao L, Zhong X, Greer T, Ye H, Li L. Relative quantification of amine-containing metabolites using isobaric N,N-dimethyl leucine (DiLeu) reagents via LC-ESI-MS/MS and CE-ESI-MS/MS. Analyst (Lond) 140: 467–475, 2015. doi: 10.1039/C4AN01582G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia L, Zhang L, Shao C, Song E, Sun W, Li M, Gao Y. An attempt to understand kidney’s protein handling function by comparing plasma and urine proteomes. PLoS One 4: e5146, 2009. doi: 10.1371/journal.pone.0005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joo SS, Won TJ, Kim JS, Yoo YM, Tak ES, Park SY, Park HY, Hwang KW, Park SC, Lee DI. Inhibition of coagulation activation and inflammation by a novel factor Xa inhibitor synthesized from the earthworm Eisenia andrei. Biol Pharm Bull 32: 253–258, 2009. doi: 10.1248/bpb.32.253. [DOI] [PubMed] [Google Scholar]
- 19.Joseph L, Fink LM, Hauer-Jensen M. Cytokines in coagulation and thrombosis: a preclinical and clinical review. Blood Coagul Fibrinolysis 13: 105–116, 2002. doi: 10.1097/00001721-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev 206: 83–99, 2005. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 21.Karan D, Tawfik O, Dubey S. Expression analysis of inflammasome sensors and implication of NLRP12 inflammasome in prostate cancer. Sci Rep 7: 4378, 2017. doi: 10.1038/s41598-017-04286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenne E, Renné T. Factor XII: a drug target for safe interference with thrombosis and inflammation. Drug Discov Today 19: 1459–1464, 2014. doi: 10.1016/j.drudis.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Jeon J, Mejia S, Yao CQ, Ignatchenko V, Nyalwidhe JO, Gramolini AO, Lance RS, Troyer DA, Drake RR, Boutros PC, Semmes OJ, Kislinger T. Targeted proteomics identifies liquid-biopsy signatures for extracapsular prostate cancer. Nat Commun 7: 11906, 2016. doi: 10.1038/ncomms11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol 51: 1202–1216, 2007. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, Lache O, Nakhoul FM, Asaf R, Farbstein D, Pollak M, Soloveichik YZ, Strauss M, Alshiek J, Livshits A, Schwartz A, Awad H, Jad K, Goldenstein H. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal 12: 293–304, 2010. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Chen Z, Niu N, Chang Q, Deng R, Korteweg C, Gu J. IgG gene expression and its possible significance in prostate cancers. Prostate 72: 690–701, 2012. doi: 10.1002/pros.21476. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Dunn T, Ewing C, Sauvageot J, Chen Y, Trent J, Isaacs W. Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate 51: 189–200, 2002. doi: 10.1002/pros.10087. [DOI] [PubMed] [Google Scholar]
- 28.McDonald JH. Handbook of Biological Statistics. Baltimore, MD: Sparky House Publishing, 2014. [Google Scholar]
- 29.McMullin RP, Mutton LN, Bieberich CJ. Hoxb13 regulatory elements mediate transgene expression during prostate organogenesis and carcinogenesis. Dev Dyn 238: 664–672, 2009. doi: 10.1002/dvdy.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc 8: 1551–1566, 2013. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickel JC, Freedland SJ, Castro-Santamaria R, Moreira DM. Chronic prostate inflammation predicts symptom progression in patients with chronic prostatitis/chronic pelvic pain. J Urol 198: 122–128, 2017. doi: 10.1016/j.juro.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 32.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol 54: 1379–1384, 2008. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis 19: 132–138, 2016. doi: 10.1038/pcan.2016.8. [DOI] [PubMed] [Google Scholar]
- 34.Quaye IK. Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg 102: 735–742, 2008. doi: 10.1016/j.trstmh.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Rao V, Heard JC, Ghaffari H, Wali A, Mutton LN, Bieberich CJ. A Hoxb13-driven reverse tetracycline transactivator system for conditional gene expression in the prostate. Prostate 72: 1045–1051, 2012. doi: 10.1002/pros.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Berriguete G, Fraile B, Paniagua R, Royuela M.. TNF alpha/IL-1 transduction pathway in benign prostate hyperplasia. In: Horizons in Cancer Research, edited by Morrison E. Hauppauge, NY: Nova Science, 2010, p. 235–247. [Google Scholar]
- 37.Santucci L, Candiano G, Petretto A, Bruschi M, Lavarello C, Inglese E, Righetti PG, Ghiggeri GM. From hundreds to thousands: widening the normal human urinome (1). J Proteomics 112: 53–62, 2015. doi: 10.1016/j.jprot.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Schroder K, Tschopp J. The inflammasomes. Cell 140: 821–832, 2010. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 481: 278–286, 2012. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 40.Suykens J, Vandewalle J. Least squares support vector machine classifiers. Neural Process Lett 9: 293–300, 1999. doi: 10.1023/A:1018628609742. [DOI] [Google Scholar]
- 41.Theodorescu D, Schiffer E, Bauer HW, Douwes F, Eichhorn F, Polley R, Schmidt T, Schöfer W, Zürbig P, Good DM, Coon JJ, Mischak H. Discovery and validation of urinary biomarkers for prostate cancer. Proteomics Clin Appl 2: 556–570, 2008. doi: 10.1002/prca.200780082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas S, Hao L, Ricke WA, Li L. Biomarker discovery in mass spectrometry-based urinary proteomics. Proteomics Clin Appl 10: 358–370, 2016. doi: 10.1002/prca.201500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von zur Muhlen C, Schiffer E, Sackmann C, Zürbig P, Neudorfer I, Zirlik A, Htun N, Iphöfer A, Jänsch L, Mischak H, Bode C, Chen YC, Peter K. Urine proteome analysis reflects atherosclerotic disease in an ApoE−/− mouse model and allows the discovery of new candidate biomarkers in mouse and human atherosclerosis. Mol Cell Proteomics 11: 013847, 2012. doi: 10.1074/mcp.M111.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, Stephens RM, Caporaso NE, Loffredo CA, Ambs S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res 68: 927–936, 2008. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 45.Xiang F, Ye H, Chen R, Fu Q, Li L. N,N-dimethyl leucines as novel isobaric tandem mass tags for quantitative proteomics and peptidomics. Anal Chem 82: 2817–2825, 2010. doi: 10.1021/ac902778d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan Y, Zhang F, Wu J, Shao C, Gao Y. Urinary candidate biomarker discovery in a rat unilateral ureteral obstruction model. Sci Rep 5: 9314, 2015. doi: 10.1038/srep09314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zabaleta J, Lin HY, Sierra RA, Hall MC, Clark PE, Sartor OA, Hu JJ, Ochoa AC. Interactions of cytokine gene polymorphisms in prostate cancer risk. Carcinogenesis 29: 573–578, 2008. doi: 10.1093/carcin/bgm277. [DOI] [PubMed] [Google Scholar]
- 48.Zhao M, Li M, Li X, Shao C, Yin J, Gao Y. Dynamic changes of urinary proteins in a focal segmental glomerulosclerosis rat model. Proteome Sci 12: 42, 2014. doi: 10.1186/1477-5956-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao M, Li M, Yang Y, Guo Z, Sun Y, Shao C, Li M, Sun W, Gao Y. A comprehensive analysis and annotation of human normal urinary proteome. Sci Rep 7: 3024, 2017. doi: 10.1038/s41598-017-03226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]