Abstract
The gut microbiome is composed of a diverse population of bacteria that have beneficial and adverse effects on human health. The microbiome has recently gained attention and is increasingly noted to play a significant role in health and a number of disease states. Increasing urea concentration during chronic kidney disease (CKD) leads to alterations in the intestinal flora that can increase production of gut-derived toxins and alter the intestinal epithelial barrier. These changes can lead to an acceleration of the process of kidney injury. A number of strategies have been proposed to interrupt this pathway of injury in CKD. The purpose of this review is to summarize the role of the gut microbiome in CKD, tools used to study this microbial population, and attempts to alter its composition for therapeutic purposes.
Keywords: chronic kidney disease, disease progression, gastrointestinal tract, indoxyl sulfate, metaproteomics, microbiome
COMPOSITION OF THE GUT MICROBIOME
The human gastrointestinal (GI) tract contains >100 trillion bacteria (32). Although >200 microbial strains exist in an individual, the two bacterial phyla Firmicutes and Bacteroidetes dominate and, in the healthy gut, contribute >90% of the bacterial species (8). Each section of the GI tract contains a characteristic microbial population. In general, the concentration of microbial cells increases through the length of the GI tract (32). Given a constant diet, the composition of the gut microbiome is stable over time. In healthy volunteers, it was found that 70% of the bacterial strains on initial sampling were present after 1 yr and that few changes in composition occurred after that point (12). Despite this stability, changes in diet can quickly alter the composition of the gut microbiome. In another study of healthy human subjects, 5 days of altered diet significantly changed the composition of the gut microbiome (6).
FUNCTION OF THE GUT MICROBIOME
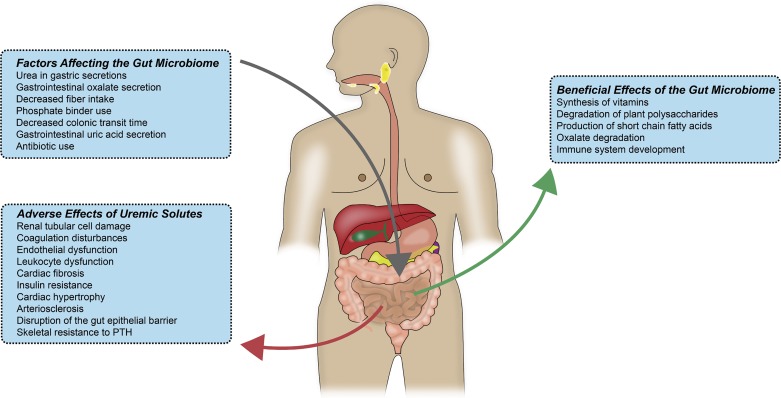
The gut microbiome participates in a variety of activities during normal function of the human body (Fig. 1). Synthesis of vitamins and degradation of indigestible plant polysaccharides and oxalates are functions of the gut microbiome. In contrast to monosaccharides and disaccharides, animals have a limited ability to use ingested polysaccharides. The symbiosis of human and gut bacteria has resulted in high colonization of the distal gut with species of the genus Bacteroides, which are able to metabolize a wide variety of plant polysaccharides, making available to the human host nutrition that would otherwise be unavailable (13). Another interesting mechanism has developed to ensure that the products of microbial metabolism of polysaccharides can be used. As the distal GI tract is an anaerobic environment, polysaccharide fermentation produces short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate. Colonic epithelial cells have the ability to use SCFAs as nutrition (3, 15). Thus, the metabolism of colonic bacteria contributes to the health of colonic epithelial cells. Normal gut flora helps restore tight junctions in the intestinal epithelium (11). In addition, gut colonization of germ-free mice has been shown to upregulate the expression of the Na+-glucose cotransporter as well as genes used in lipid absorption (13).
Fig. 1.
The gut microbiome has a variety of beneficial effects during normal human metabolism. Factors that affect the environment of the gut microbiome alter its composition. In a dysbiotic intestinal microbiota, uremic solutes affect many different tissue types. Some of these effects, such as renal tubular damage and progression of renal failure, result in further gut microbiome dysbiosis, causing a positive-feedback loop. PTH, parathyroid hormone.
The development of the immune system is assisted through the ability of Bacteroides fragilis polysaccharide A to cultivate beneficial T helper (Th) cell ratios (Th1/Th2) (23). Dendritic cells and gut-associated lymphoid tissues in the GI tract sample components of B. fragilis, migrate to lymphoid organs, and encourage Th1 lineage differentiation (23).
ALTERATION OF THE GUT MICROBIOME IN CHRONIC KIDNEY DISEASE
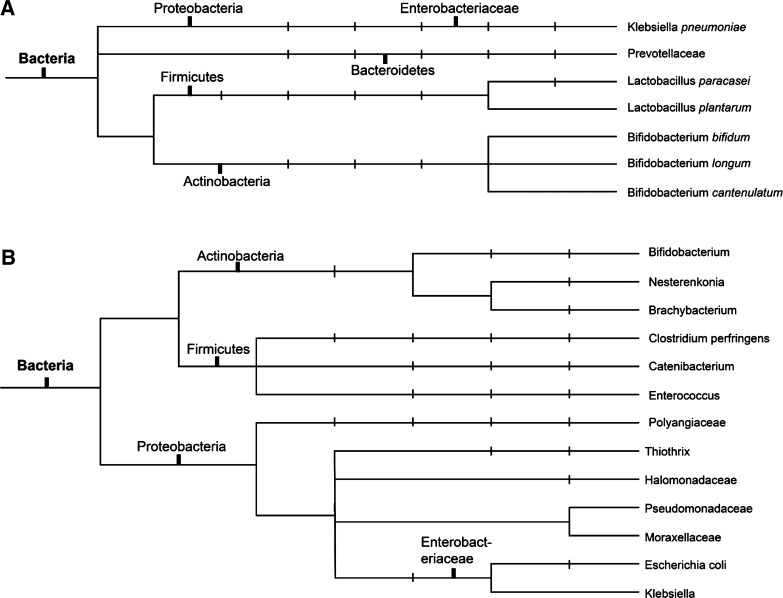
Progressive renal failure results in higher concentrations of urea in blood. Exposure of intestinal bacteria to urea through GI secretions results in the conversion of urea to ammonia via bacterial urease. This high concentration of urea causes overgrowth of bacterial families containing urease. Expansion of bacterial families producing uricase and indole- and p-cresyl-forming enzymes occurs in patients with end-stage renal disease (ESRD) compared with healthy controls (54). It is interesting to speculate as to how changes in appetite that occur with renal failure may affect the intake of foods that alter the microbiome. Decreases in resistant starch content associated with changes in appetite could lead to increases in rates of progression of chronic kidney disease (CKD). In severe renal failure, the colon becomes the main route of uric acid as well as oxalate secretion (48). This can account for the expansion of bacterial species that produce uricase. The microbial flora of rats with 5/6 nephrectomy, as well as patients with ESRD on hemodialysis, is different from that of healthy controls (48). The bacterial families of Actinobacteria, Firmicutes, and Proteobacteria were shown in one study to have the largest increases in patients with ESRD compared with healthy controls (48). This differing microbial population has also been reported in patients on peritoneal dialysis (Fig. 2) compared with healthy controls (51). Patients on peritoneal dialysis were less likely to have Bifidobacterium catenulatum, Bifidobacterium longum, Bifidobacterium bifidum, Lactobacillus plantarum, Lactobacillus paracasei, and Klebsiella pneumoniae (51). Potential additional mechanisms for the change in microbiome composition between patients with uremia and healthy controls include decreased fiber intake in patients with CKD and ESRD and decreased colonic transit time in patients with uremia as well as phosphate binders and comorbidities such as diabetes. In support of this concept, the rate of constipation is high: 63% in patients on hemodialysis and 29% in patients on peritoneal dialysis (56).
Fig. 2.
A: bacteria that are decreased in abundance in patients with end-stage renal disease. B: bacteria that are increased in abundance in patients with end-stage renal disease. Taxonomic terms commonly used in gut microbiome literature are noted on the phylogenetic trees.
Interestingly, a high-salt diet has also been shown to affect the gut microbiome in ways that could interact with the progression of CKD. Decreases in several species of bacteria, including those of the genus Lactobacillus, have been reported in mice fed a high-salt diet (53). Moreover, a high-salt diet altered the frequency of Th17 lymphocytes, and this could be ameliorated by the reintroduction of Lactobacillus species. This raises the interesting prospect that the effects of resistant starch in the diet could interact with the effects of salt on the microbiome to affect salt-sensitive hypertension as well as the progression of kidney disease through other mechanisms.
EFFECTS OF COLON-DERIVED UREMIC TOXINS
p-Cresyl sulfate and indoxyl sulfate, the most extensively studied gut-derived uremic toxins, have been shown to originate from the colon and increase in concentration with decline of renal function (1, 32). Indoxyl sulfate is ultimately derived from dietary protein. Intestinal flora produces indole during metabolism of tryptophan, which is then metabolized by the liver to produce indoxyl sulfate (4). p-Cresol is a metabolite of tyrosine and phenylalanine (4). In healthy individuals, the protein binding of these solutes is close to 100%. However, in patients with chronic renal failure, ~90% of p-cresol and 85% of indoxyl sulfate are protein bound (7). p-Cresol is excreted by the kidney mainly through proximal tubular secretion (19). In patients with chronic renal failure, serum levels of p-cresol and indoxyl sulfate are increased ~10- and 50-fold, respectively (7).
These two toxins have been noted to have a variety of deleterious effects on body tissues, including renal tubular cell damage, coagulation disturbances, endothelial dysfunction, leukocyte activation, cardiac fibrosis and hypertrophy, and insulin resistance (46). p-Cresol induces insulin resistance in vivo in mice (19). p-Cresyl sulfate, administered to mice for 4 wk, induced insulin resistance, reduction of fat mass, and redistribution of lipid mass.
Subtotal nephrectomy also led to insulin resistance, and dietary supplementation with the prebiotic arabino-xylo-oligosaccharide reduced p-cresyl sulfate levels and reversed insulin resistance. CKD is a known risk factor for cardiovascular disease (36). Pathogenic mechanisms that contribute to a higher level of coronary artery disease in patients with CKD include protein carbamylation and oxidative stress (44). Gut-derived uremic toxins, such as p-cresyl sulfate and indoxyl sulfate, may also be related to cardiovascular mortality (46), although a recent publication from the Hemodialysis (HEMO) study did not confirm this (40). p-Cresyl sulfate has been shown to mediate a proinflammatory effect on unstimulated leukocytes, highlighting one mechanism for increased cardiovascular disease in patients with CKD (37). Indoxyl sulfate and p-cresyl sulfate are not well cleared by dialysis, largely because of high protein binding, leading to higher levels of these solutes (39). In addition, serum levels of p-cresyl sulfate are associated with the severity of atherosclerosis in patients with CKD, as seen on coronary angiography (51). Indoxyl sulfate is also associated with cardiovascular mortality and vascular disease, as measured by aortic calcification and pulse wave velocity, in patients with CKD (2). It has been shown to induce inflammatory reactions, reduce cholesterol efflux in macrophages, and increase leukocyte-endothelial interactions (14). In vitro studies have shown that uremic toxins affect cross talk between leukocytes and blood vessels. Exposure of leukocytes to p-cresyl sulfate enhances leukocyte rolling. A prospective cohort study found that serum concentrations of indoxyl sulfate were associated with the first heart failure event in patients on hemodialysis, independent of other risk factors (28). Elevated levels of gut microbiome-derived trimethylamine N-oxide are associated with cardiovascular disease and long-term mortality and are an independent predictor of coronary atherosclerosis (4, 17).
Oxidative phosphorylation and ATP synthesis are improved in germ-free mice in ischemia-reperfusion injury models by providing acetate, propionate, and butyrate (3). In uremic rats, indoxyl sulfate promotes progression of renal failure and glomerular sclerosis (7). p-Cresyl sulfate has also been shown to damage renal tissue and cause renal toxicity through increased NADPH oxidase activity and production of reactive oxygen species (52).
THE GUT EPITHELIAL BARRIER
There is significant evidence that products of bacterial metabolism are associated with the integrity of the gut epithelial barrier. SCFAs, derived from resistant starches, are the focus of much of this research. Resistant starches are obtained from vegetables, fruits, wheat, corn, and nuts. However, since potassium is often restricted in patients with CKD, they are told to avoid beans, lentils, bananas, bran, granola, and nuts. These foods are high in potassium but are also rich sources of resistant starch. Resistant starches are degraded, primarily in the colon, to produce metabolites, the majority of which are SCFAs. Butyrate is a four-carbon SCFA that comprises 15% of the SCFAs produced. More than 95% of the SCFAs produced are absorbed in the colon. Butyrate is a preferred energy source for colonocytes (34). In addition, SCFAs can produce beneficial effects through receptors. SCFA receptors are associated with epithelial barrier integrity. Expression of the G protein-coupled receptor GPR109A, a SCFA receptor found on epithelial cells in the colon, is associated with bacterial presence in the colon, inasmuch as its expression is reduced in germ-free mice (15). Activation of GPR109A leads to suppression of the proinflammatory mediators inducible nitric oxide synthase, cyclooxygenase 2, and TNF-α (34). Some of the beneficial effects of SCFAs may be mediated through changes in the ability of tight junctions to mediate the integrity of the gut epithelial barrier. Claudin-1, occludin, and zonula occludens-1 proteins are significantly depleted in the colonic mucosa of rats with CKD compared with healthy controls (32). This is the underlying mechanism for the increased gut permeability in CKD. Colonocyte exposure to urea partially affects tight junctions, and this allows translocation of endotoxin and microbial fragments, causing inflammation, which increases epithelial barrier disruption (48). It is not clear how alterations in the integrity of the gut epithelial barrier may participate in infections, which are more common in patients with ESRD. Alterations of the inflammatory milieu also play a role. Colonic T regulatory (Treg) cells play a role in maintaining integrity of the colon. SCFAs regulate population growth of colonic Treg cells and help prevent inflammation of the colon (42). Bowel wall edema, as well as intermittent hypotension, during hemodialysis exacerbates impairment of the colonic epithelial barrier (49). Urea-derived ammonia and ammonium hydroxide play a central role in breakdown of the gut’s epithelial barrier. In patients with CKD, this breakdown of the gut epithelial barrier allows absorption of microbial toxins, resulting in systemic inflammation, which can lead to cardiovascular disease, anemia, and protein wasting (54).
METAPROTEOMICS IN THE ASSESSMENT OF THE GUT MICROBIOME
Metagenomics aims to characterize the full genetic content of a community (20, 31, 45). Similarly, metaproteomics aims at characterizing the full proteome content, irrespective of the species present. Metaproteomics complements metagenomics, as it analyzes the end result of gene expression. Characterization of taxonomies by 16S RNA has limited resolution at the species level, whereas metaproteomics can reveal many details about the species of bacteria present. Metaproteomics has the potential to elucidate species diversity and to obtain protein abundances, providing comprehensive insights into phenotype. From the clinical perspective, proteins can serve as prognostic and diagnostic biomarkers as well as potential drug targets.
Human GI microbiota in stool is arguably the best-studied host-associated microbiome, but metaproteomic approaches have also been developed for studying the microbial mucosa-lumen interface of different intestinal sites (21) and have been applied in a cohort study of inflammatory bowel disease (30) that demonstrated strong dissimilarities between inflamed and healthy mucosa linked to the resident microbiome.
The first metaproteomic study of the human fecal microbiome identified >1,340 nonredundant proteins/sample (50). This study also established that fecal metaproteomics is impacted extensively by host proteins, with 30% of the measured spectra being matched to a human protein database. Depletion of host cells before measurements can significantly increase the depth of coverage of the microbial proteome (55). However, these human proteome measurements can also be very informative. A collation of metaproteomes of fecal samples with Crohn's disease and healthy samples mirrored the previous findings from metagenomic studies, including a reduced functional richness in the fecal microbiota of individuals with Crohn's disease, while differential abundances of proteins relating to carbohydrate degradation and human recognition of bacteria were also detected (9, 22). In CKD, better understanding of the host response to either the stress of CKD or microbiome-related treatment for CKD can help elucidate the mechanisms and aid in the selection of drug targets.
A comparison of metagenomic and metaproteomic data identified similar abundances from the same organismal groups, with a few notable exceptions (such as highly active but low-abundance Bifidobacteria) (18). This study also described temporal stability of the core metaproteome, similar to previous metagenomic observations. However, disturbance of such a stable state was observed in a multiomic time series study of the effect of antibiotic treatment (27). A metaproteomic study of the microbiome in rats with CKD revealed species-level resolution and demonstrated significantly higher levels of the butyrate producer Ruminococcus bromii in stool from resistant starch-fed rats with CKD than controls (57). These studies showed the ability of metaproteomics to resolve clinically relevant activities of gut-associated microbiota, which may be exploited as biomarkers. In addition, metaproteomics has the potential to estimate species diversity along with bacterial protein abundances. For analysis of microbiome-related metabolic pathways, the protein abundances may be more relevant than simple reliance on metagenomics diversity estimates. Data integration from multiple -omics approaches is being developed (25).
WHAT THERAPIES HAVE BEEN ATTEMPTED TO ALTER THE GUT MICROBIOME OR PRODUCTS OF ITS METABOLISM?
AST-120, an oral adsorbent made of porous carbon microspheres, adsorbs gut-derived uremic toxins and has been shown to restore epithelial tight junction proteins and reduce endotoxin levels and markers of oxidative stress and inflammation in rats with reduced renal function (32). Two large trials evaluating prevention of progression in CKD, EPPIC-1 and EPPIC-2, evaluated the effect of AST-120 on patients. The primary end point was a composite of dialysis initiation, kidney transplantation, or serum creatinine doubling. These trial results were limited by a more gradual decrease in kidney function than expected in the trial populations and did not show a difference in CKD progression end points (38). A recent trial of AST-120 in patients with stage 3–4 CKD showed no benefit relative to change in renal disease progression, proteinuria, mortality, and health-related quality of life (5). Coadministration of pre- and probiotics during the Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY) trial reduced serum levels of p-cresyl sulfate and favorably altered the stool microbiome as determined by 16S RNA sequencing (35). Oral administration of B. longum has been shown to reduce serum levels of indoxyl sulfate (43). Sevelamer hydrochloride is a nonmetal-based phosphate binder, which, in addition to binding phosphate, binds uremic retention solutes in vitro, including indole and p-cresol. However, it does not lower concentrations of indoxyl sulfate or p-cresol in mouse models of CKD or in patients on hemodialysis (29).
Increased resistant starch intake could slow progression of CKD in several ways. Increased intake of resistant starches may increase the proportion of bacteria that produce SCFAs, promoting colonocyte and Treg cell nutrition. In an adenine-induced CKD rat model, a diet high in resistant starch reduces loss of renal function, interstitial fibrosis, renal tubular damage, and activation of proinflammatory molecules (47).
Increases in dietary fiber reduce plasma levels of indoxyl sulfate in patients on hemodialysis (41). High-amylose maize-resistant starch type 2 was shown in a rat model to increase the Bacteroides-to-Firmicutes ratio and reduce concentrations of indoxyl sulfate and p-cresyl sulfate in serum and urine (16). Along this same line, the α-glucosidase inhibitor acarbose has been examined as a way to increase the mass of polysaccharides that reaches the colonic lumen. The action of acarbose is to inhibit α-glucosidase, an enzyme present in the brush border of the small intestine. In doing so, the amount of oligo- and polysaccharides that reaches the distal lumen of the GI tract is increased. In one study (10), serum concentrations of p-cresyl sulfate and indoxyl sulfate were decreased after treatment with acarbose, and excretion of these two uremic toxins increased. This is a potential mechanism to increase fermentation of polysaccharides to produce SCFAs (22). Probiotics have been examined for potential restoration of the gut microbiome in CKD. The results of these studies have not been encouraging, possibly because the dysbiotic gut microbiome in patients with renal failure is due to an unfavorable uremic milieu in the GI tract (24, 26, 33, 46). Introduction of organisms without restoration of this environment will result in an inability to alter its composition. Together, the results of these studies support further research for amelioration of human CKD and its consequences.
GRANTS
This work was supported by the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R01 DK-101034 as well as National Institute of General Medical Sciences Institutional Development Award Centers of Biomedical Research Excellence Grant 1-P20-GM-121293.
DISCLAIMERS
The contents do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.P.H., O.K., B.L.Z., and J.M.A. conceived and designed research; G.P.H. prepared figures; G.P.H. drafted manuscript; G.P.H., O.K., G.F.D., M.S., B.L.Z., and J.M.A. edited and revised manuscript; G.P.H., O.K., G.F.D., M.S., B.L.Z., and J.M.A. approved final version of manuscript.
REFERENCES
- 1.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW. Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011. doi: 10.1681/ASN.2010121220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin Work Group (EUTox) . Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrows IR, Ramezani A, Raj DS. Gut feeling in AKI: the long arm of short-chain fatty acids. J Am Soc Nephrol 26: 1755–1757, 2015. doi: 10.1681/ASN.2014111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao XS, Chen J, Zou JZ, Zhong YH, Teng J, Ji J, Chen ZW, Liu ZH, Shen B, Nie YX, Lv WL, Xiang FF, Tan X, Ding XQ. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol 10: 111–119, 2015. doi: 10.2215/CJN.04730514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha RH, Kang SW, Park CW, Cha DR, Na KY, Kim SG, Yoon SA, Han SY, Chang JH, Park SK, Lim CS, Kim YS. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin J Am Soc Nephrol 11: 559–567, 2016. doi: 10.2215/CJN.12011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65: 442–451, 2004. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 8.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 308: 1635–1638, 2005. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson AR, Cantarel BL, Lamendella R, Darzi Y, Mongodin EF, Pan C, Shah M, Halfvarson J, Tysk C, Henrissat B, Raes J, Verberkmoes NC, Fraser CM, Hettich RL, Jansson JK. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn’s disease. PLoS One 7: e49138, 2012. doi: 10.1371/journal.pone.0049138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y. Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: a pilot study. Kidney Int 70: 192–198, 2006. doi: 10.1038/sj.ki.5001523. [DOI] [PubMed] [Google Scholar]
- 11.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295: G1025–G1034, 2008. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 12.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science 341: 1237439, 2013. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 22: 283–307, 2002. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 14.Ito S, Osaka M, Higuchi Y, Nishijima F, Ishii H, Yoshida M. Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin. J Biol Chem 285: 38869–38875, 2010. doi: 10.1074/jbc.M110.166686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7: 2839–2849, 2015. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, Nazertehrani S, Moore ME, Marco ML, Martin RJ, Adams SH. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol 310: F857–F871, 2016. doi: 10.1152/ajprenal.00513.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitai T, Kirsop J, Tang WH. Exploring the microbiome in heart failure. Curr Heart Fail Rep 13: 103–109, 2016. doi: 10.1007/s11897-016-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolmeder CA, de Been M, Nikkilä J, Ritamo I, Mättö J, Valmu L, Salojärvi J, Palva A, Salonen A, de Vos WM. Comparative metaproteomics and diversity analysis of human intestinal microbiota testifies for its temporal stability and expression of core functions. PLoS One 7: e29913, 2012. doi: 10.1371/journal.pone.0029913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y, Soula HA, Fouque D, Soulage CO. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol 24: 88–99, 2013. doi: 10.1681/ASN.2012050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, Takami H, Morita H, Sharma VK, Srivastava TP, Taylor TD, Noguchi H, Mori H, Ogura Y, Ehrlich DS, Itoh K, Takagi T, Sakaki Y, Hayashi T, Hattori M. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res 14: 169–181, 2007. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, LeBlanc J, Truong A, Vuthoori R, Chen SS, Lustgarten JL, Roth B, Allard J, Ippoliti A, Presley LL, Borneman J, Bigbee WL, Gopalakrishnan V, Graeber TG, Elashoff D, Braun J, Goodglick L. A metaproteomic approach to study human-microbial ecosystems at the mucosal luminal interface. PLoS One 6: e26542, 2011. doi: 10.1371/journal.pone.0026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 55: 205–211, 2006. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122: 107–118, 2005. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Miranda Alatriste PV, Urbina Arronte R, Gómez Espinosa CO, Espinosa Cuevas ML. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp 29: 582–590, 2014. doi: 10.3305/nh.2014.29.3.7179. [DOI] [PubMed] [Google Scholar]
- 25.Mondot S, Lepage P. The human gut microbiome and its dysfunctions through the meta-omics prism. Ann NY Acad Sci 1372: 9–19, 2016. doi: 10.1111/nyas.13033. [DOI] [PubMed] [Google Scholar]
- 26.Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, Mallappallil MC, Norin AJ, Friedman EA, Saggi SJ. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. BioMed Res Int 2014: 1–9, 2014. doi: 10.1155/2014/568571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M, Neulinger SC, Däumer C, Heinsen FA, Latorre A, Barbas C, Seifert J, dos Santos VM, Ott SJ, Ferrer M, Moya A. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 62: 1591–1601, 2013. doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pletinck A, Glorieux G, Schepers E, Cohen G, Gondouin B, Van Landschoot M, Eloot S, Rops A, Van de Voorde J, De Vriese A, van der Vlag J, Brunet P, Van Biesen W, Vanholder R. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 24: 1981–1994, 2013. doi: 10.1681/ASN.2012030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poesen R, Meijers B, Evenepoel P. The colon: an overlooked site for therapeutics in dialysis patients. Semin Dial 26: 323–332, 2013. doi: 10.1111/sdi.12082. [DOI] [PubMed] [Google Scholar]
- 30.Presley LL, Ye J, Li X, Leblanc J, Zhang Z, Ruegger PM, Allard J, McGovern D, Ippoliti A, Roth B, Cui X, Jeske DR, Elashoff D, Goodglick L, Braun J, Borneman J. Host-microbe relationships in inflammatory bowel disease detected by bacterial and metaproteomic analysis of the mucosal-luminal interface. Inflamm Bowel Dis 18: 409–417, 2012. doi: 10.1002/ibd.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J; MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65, 2010. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657–670, 2014. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, Tam P, Rao AV, Anteyi E, Musso CG. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther 27: 634–647, 2010. doi: 10.1007/s12325-010-0059-9. [DOI] [PubMed] [Google Scholar]
- 34.Richards JL, Yap YA, McLeod KH, Mackay CR, Mariño E. Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clin Transl Immunology 5: e82, 2016. doi: 10.1038/cti.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto CC, McWhinney BC, Ungerer JP, Campbell KL. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol 11: 223–231, 2016. doi: 10.2215/CJN.05240515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065, 2003. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 37.Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R. p-Cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 22: 592–596, 2007. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 38.Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M. Randomized placebo-controlled EPPIC Trials of AST-120 in CKD. J Am Soc Nephrol 26: 1732–1746, 2015. doi: 10.1681/ASN.2014010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafi T, Meyer TW, Hostetter TH, Melamed ML, Parekh RS, Hwang S, Banerjee T, Coresh J, Powe NR. Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: results from the Retained Organic Solutes and Clinical Outcomes (ROSCO) Investigators. PLoS One 10: e0126048, 2015. doi: 10.1371/journal.pone.0126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafi T, Sirich TL, Meyer TW, Hostetter TH, Plummer NS, Hwang S, Melamed ML, Banerjee T, Coresh J, Powe NR. Results of the HEMO Study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int 92: 1484–1492, 2017. doi: 10.1016/j.kint.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 9: 1603–1610, 2014. doi: 10.2215/CJN.00490114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis 41, Suppl 1: S142–S145, 2003. doi: 10.1053/ajkd.2003.50104. [DOI] [PubMed] [Google Scholar]
- 44.Tonelli M, Karumanchi SA, Thadhani R. Epidemiology and mechanisms of uremia-related cardiovascular disease. Circulation 133: 518–536, 2016. doi: 10.1161/CIRCULATIONAHA.115.018713. [DOI] [PubMed] [Google Scholar]
- 45.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 457: 480–484, 2009. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 25: 1897–1907, 2014. doi: 10.1681/ASN.2013101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, Kieffer DA, Adams SH, Martin RJ. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 9: e114881, 2014. doi: 10.1371/journal.pone.0114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 49.Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant 31: 737–746, 2016. doi: 10.1093/ndt/gfv095. [DOI] [PubMed] [Google Scholar]
- 50.Verberkmoes NC, Russell AL, Shah M, Godzik A, Rosenquist M, Halfvarson J, Lefsrud MG, Apajalahti J, Tysk C, Hettich RL, Jansson JK. Shotgun metaproteomics of the human distal gut microbiota. ISME J 3: 179–189, 2009. doi: 10.1038/ismej.2008.108. [DOI] [PubMed] [Google Scholar]
- 51.Wang IK, Lai HC, Yu CJ, Liang CC, Chang CT, Kuo HL, Yang YF, Lin CC, Lin HH, Liu YL, Chang YC, Wu YY, Chen CH, Li CY, Chuang FR, Huang CC, Lin CH, Lin HC. Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl Environ Microbiol 78: 1107–1112, 2012. doi: 10.1128/AEM.05605-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, Noguchi T, Kadowaki D, Ishima Y, Kotani S, Nakajima M, Kataoka K, Kim-Mitsuyama S, Tanaka M, Fukagawa M, Otagiri M, Maruyama T. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int 83: 582–592, 2013. doi: 10.1038/ki.2012.448. [DOI] [PubMed] [Google Scholar]
- 53.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Frätzer C, Krannich A, Gollasch M, Grohme DA, Côrte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Müller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551: 585–589, 2017. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39: 230–237, 2014. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong W, Giannone RJ, Morowitz MJ, Banfield JF, Hettich RL. Development of an enhanced metaproteomic approach for deepening the microbiome characterization of the human infant gut. J Proteome Res 14: 133–141, 2015. doi: 10.1021/pr500936p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, Tochikubo O. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis 39: 1292–1299, 2002. doi: 10.1053/ajkd.2002.33407. [DOI] [PubMed] [Google Scholar]
- 57.Zybailov BL, Glazko GV, Rahmatallah Y, Andreyev DS, McElroy T, Karaduta O, Byrum SD, Orr L, Tackett AJ, Mackintosh SG, Edmondson RD, Kieffer DA, Martin RJ, Adams SH, Vaziri ND, Arthur JM. Metaproteomics reveals potential mechanisms by which dietary resistant starch supplementation attenuates chronic kidney disease progression in rats. PLoS One 14: e0199274, 2019. doi: 10.1371/journal.pone.0199274. [DOI] [PMC free article] [PubMed] [Google Scholar]