Abstract
Renal Na+-glucose cotransporter SGLT1 mediates glucose reabsorption in the late proximal tubule, a hypoxia-sensitive tubular segment that enters the outer medulla. Gene deletion in mice (Sglt1−/−) was used to determine the role of the cotransporter in acute kidney injury induced by ischemia-reperfusion (IR), including the initial injury and subsequent recovery phase. On days 1 and 16 after IR, absolute and fractional urinary glucose excretion remained greater in Sglt1−/− mice versus wild-type (WT) littermates, consistent with a sustained contribution of SGLT1 to tubular glucose reabsorption in WT mice. Absence of SGLT1 did not affect the initial kidney impairment versus WT mice, as indicated by similar increases on day 1 in plasma concentrations of creatinine and urinary excretion of the tubular injury marker kidney injury molecule-1 as well as a similar rise in plasma osmolality and fall in urine osmolality as indicators of impaired urine concentration. Recovery of kidney function on days 14/16, however, was improved in Sglt1−/− versus WT mice, as indicated by lower plasma creatinine, higher glomerula filtration rate (by FITC-sinistrin in awake mice), and more completely restored urine and plasma osmolality. This was associated with a reduced tubular injury score in the cortex and outer medulla, better preserved renal mRNA expression of tubular transporters (Sglt2 and Na+-K+-2Cl– cotransporter Nkcc2), and a lesser rise in renal mRNA expression of markers of injury, inflammation, and fibrosis [kidney injury molecule-1, chemokine (C-C motif) ligand 2, fibronectin 1, and collagen type I-α1] in Sglt1−/− versus WT mice. These results suggest that SGLT1 activity in the late proximal tubule may have deleterious effects during recovery of IR-induced acute kidney injury and identify SGLT1 as a potential therapeutic target.
Keywords: acute kidney injury, glomerular filtration rate, sodium-glucose cotransport, sodium-glucose cotransporter 2, tubular function
INTRODUCTION
Acute kidney injury (AKI) is associated with high morbidity and mortality (1). It is defined by an abrupt decline in renal excretory function (within hours to days) accompanied by plasma retention of uremic toxins and metabolic waste products and dysregulation of fluid and electrolyte homeostasis (5). Renal ischemia-reperfusion (IR) injury is an important cause of AKI. It is characterized by a sudden decrease or interruption of renal blood perfusion followed by restoration of blood flow and organ reoxygenation, and it can occur in shock, episodes of congestive heart failure decompensation, cardiac bypass surgery, or kidney transplantation (6, 18). IR can induce ischemic injury of the tubular epithelium, associated with oxidative stress, mitochondrial dysfunction, and endothelial dysfunction, resulting in microcirculatory impairment, induction of tubulointerstitial inflammation, and acute tubular necrosis (6, 18, 30). Moreover, recurring episodes of AKI contribute to the development of chronic kidney disease and end-stage renal disease (9). Current therapies consist mainly of supportive care rather than effective prevention or curative treatments, indicating the need for a better understanding of the pathophysiology and the development of new therapeutic strategies (7).
The outer medulla (OM) of the kidney is particularly vulnerable to hypoxia. Renal blood flow is unequally distributed between the renal cortex and medulla, with the latter receiving only ~10% of renal blood flow, supplied by the descending vasa recta, which branch from the efferent arterioles of juxtamedullary glomeruli. However, the OM is a site of active solute reabsorption, including the S3 segment of the proximal tubule (PT) and medullary thick ascending limb of the loop of Henle. The combination of a high energy and oxygen demand and a relatively low blood supply results in a lower Po2 in the OM compared with cortical values (~10–20 vs. ~50 mmHg, respectively) (11, 23). As a consequence, a further decrease in oxygen supply, as in IR, can particularly affect and damage the OM (4, 12). Moreover, increases in transport activity in the OM may contribute to the pathogenesis of renal IR injury or worsen the subsequent recovery (24, 26).
In a healthy normoglycemic individual, large amounts of glucose are filtered by the glomeruli (~180 g/day). Almost all filtered glucose is reabsorbed by the tubular system, predominantly by the PT. In the early PT (or S1/S2 segments), apical low-affinity, high-capacity Na+-glucose cotransporter SGLT2 is responsible for the majority of glucose uptake from the lumen to the cytoplasm (~97% of filtered glucose) (28). Facilitative glucose transporter GLUT2 allows glucose to diffuse across the basolateral membrane and return to the systemic circulation or the reabsorbed glucose is taken up and used by more distal tubular cells. The filtered glucose that is not reabsorbed by SGLT2 reaches the downstream late PT (or S2/S3 segment), which enters and ends in the OM and reabsorbs glucose through apical higher-affinity, lower-capacity SGLT1 and basolateral GLUT2 and GLUT1 (22, 29). SGLT-mediated glucose transport is coupled to Na+ reabsorption and relies on the Na+ concentration gradient that is established by basolateral Na+-K+-ATPase. Thus, SGLT1 is a secondary active transporter that requires energy and oxygen to reabsorb glucose and Na+ in the vulnerable S3 segment. While SGLT2 transports Na+ and glucose in a 1:1 ratio, SGLT1 transports 2 Na+ per glucose (29). This enhances the concentrative power of glucose reabsorption via SGLT1 but also enhances the amount of oxygen required to reabsorb glucose. Moreover, the AKI-associated damage and tubular malfunction in the S1/S2 segments can enhance the glucose and Na+ delivery to the S2/S3 segment. Thus, an enhanced SGLT1-dependent oxygen consumption could induce deleterious effects in the early phase of AKI but also during the subsequent recovery of tubular function, which may be a prerequisite for the recovery of glomerular filtration rate (GFR) (26). On the other hand, the glucose uptake associated with SGLT1 may provide a much needed energy substrate as glycolysis has been proposed to increase in response to AKI in the proximal tubule (15).
Therefore, we aimed to determine whether genetic deletion of SGLT1 is beneficial or detrimental in a murine model of AKI induced by bilateral renal artery clamping, including the initial phase of kidney injury and the early recovery phase over the subsequent 16 days.
MATERIALS AND METHODS
Animals.
All animal experiments were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and were approved by the local Institutional Animal Care and Use Committee. Experiments were performed on 26- to 28-wk-old male Sglt1-deficient (Sglt1−/−) mice and wild-type (WT) littermates (on C57BL/6J background), the generation of which has been previously described (10). All animals were housed in the same room with a 12:12-h light-dark cycle and free access to water and food. All mice were fed a low-glucose (1.5% carbohydrate by weight), high-protein (52.4% by weight) diet to prevent diarrhea in Sglt1−/− mice, due to the role of SGLT1 in intestinal glucose reabsorption (10).
Induction of renal IR injury.
Nonfasted mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg body wt)-xylazine (8 mg/kg body wt) and placed on a temperature-controlled surgical table with a rectal probe to maintain body temperature between 36 and 37°C. The kidneys were exposed with bilateral flank incisions. The surrounding fat tissue was dissected to expose renal pedicles. Both renal arteries were carefully dissected and clamped for 20 min using nontraumatic microvessel clamps. Renal veins remained unoccluded. Cessation of blood flow was documented by visual inspection. After the clamps were released, wounds were sutured using 9-mm wound clips. Sterile saline (0.9% NaCl) was injected subcutaneously (30 μl/g body wt) to replenish for fluid loss. Mice recovering from anesthesia were then returned to a warm cage and placed on a heating pad until fully ambulatory (~2 h). Sham-operated (sham) mice underwent the same procedures without artery clamping. The analgesic buprenorphine (50 μg/kg) was injected subcutaneously immediately after completion of surgery and twice a day for the first 3 days postsurgery. The following four groups of mice were studied: WT sham (n = 7), WT IR (n = 7), Sglt1−/− sham (n = 8) and Sglt1−/− IR (n = 8).
Urine and blood collection.
Urine collection was paired with body weight and blood glucose measurements in awake mice 2 days before surgery to obtain basal values as well as 1, 3, 7, and 16 days after surgery. First, blood glucose was measured by tail snip (Contour glucometer, Bayer, Mishawaka, IN) to minimize stress-induced blood glucose elevation. Urine was then obtained by grabbing the mice and provoking spontaneous urination. Samples were stored at −80°C for later analyses. Blood samples were collected 2 days before surgery to obtain basal values as well as on days 1 and 16 after surgery. Briefly, under short isoflurane anesthesia (3 min, 3% isoflurane, 2.5 l/min O2), the retroorbital plexus was punctured using a 10-μl Na-heparin microcapillary (Hirschmann Laborgeräte, Eberstadt, Germany), and blood was collected into a 70-μl heparinized microhematocrit capillary tube (Fisherbrand, Hampton, NH). Capillaries were sealed (Châ-Seal tube sealing compound, Kimble Chase, Vineland, NJ) and centrifuged for 3 min at 12,000 rpm. Plasma was stored at −80°C for later analyses.
Determination of GFR in conscious mice.
Fourteen days after surgery, GFR was determined using plasma elimination kinetics of FITC-sinistrin as previously described (20, 21, 27). Briefly, 2 μl/g body wt of FITC-sinistrin (Fresenius-Kabi, Linz, Austria, 2% in 0.85% NaCl, which also served to establish the standard curve) was injected into the retroorbital plexus during brief isoflurane anesthesia (3 min, 3% isoflurane, 2.5 l/min O2). Blood was then collected into Na+-heparinized 10-μl microcapillaries from a small tail nick at 3, 5, 7, 10, 15, 35, 56, and 75 min after injection. After centrifugation (3 min at 12,000 rpm), 2 μl of plasma were diluted 1:10 in 0.5 mol/l HEPES (pH 7.4), and the fluorescence signal was measured at 470 nm in 2-μl samples using a NanoDrop ND-3300 Fluorospectrometer (Nanodrop Technologies, Wilmington, DE). GFR was calculated using a two-compartment model of two-phase exponential decay (GraphPad Prism, San Diego, CA).
Kidney harvest.
Sixteen days after surgery and under terminal isoflurane anesthesia, blood was collected by retroorbital puncture, kidneys were harvested, and the renal capsule was gently removed. Kidneys were weighed, cut in half, frozen in liquid nitrogen, and stored at −80°C for later quantitative RT-PCR analyses. One kidney half was kept in 3.5 ml of 10% formalin fixation solution for 48 h and at 4°C. Fixed samples were transferred in tissue cassettes, immersed into 70% ethanol, and prepared for histological analyses (see below).
Plasma and urine analyses.
Urine glucose concentration was determined by the hexokinase/glucose-6-phosphate dehydrogenase method (Infinity, Thermo Scientific, Louisville, CO) and plasma creatinine concentration by the isotope dilution liquid chromatography tandem-mass spectrometry method (LC-MS/MS) at the O’Brien Center for Acute Kidney Injury Research at the University of Alabama at Birmingham (Birmingham, AL). Urine creatinine was measured by the kinetics of the alkaline picrate Jaffe reaction (Infinity, Thermo Scientific). The fractional excretion of glucose (FE-glucose) was calculated as the urine-to-plasma concentration ratio of glucose divided by the urine-to-plasma concentration ratio of creatinine. Plasma and urine electrolytes (Na+, K+, and Cl−) were measured using an EasyElectrolytes electrolyte analyzer (Medica, Bedford, MA). Plasma and urine urea concentrations were determined by the urease-glutamate dehydrogenase method (Infinity, Thermo Scientific). Plasma and urine osmolality were measured using a Vapro vapor pressure osmometer 5520 (Wescor, Logan, UT). Twenty-four hours postsurgery, urinary kidney injury molecule (KIM)-1 concentrations were determined in samples diluted 1:10,000 using the mouse TIM-1/KIM-1/HAVCR Quantikine ELISA kit (Bio-Techne, Minneapolis, MN) following the manufacturer’s instructions.
Reverse transcription and real-time PCR.
Total kidney RNA was isolated using the RNeasy Plus Mini Kit (Qiagen, Germantown, MD), and cDNA was prepared using the SuperScript IV First-Strand Synthesis System (Thermo Scientific, Louisville, CO). For quantification, TaqMan Universal PCR Master Mix (Applied Biosystems) and specific primers were used in a 7500 Real-Time PCR System (2 min at 50°C, 10 min at 95°C with 40 cycles of 15 s at 95°C and 1 min at 60°C). Each experiment was performed in duplicate to determine the relative expression of the following genes: KIM-1 [hepatitis A virus cellular receptor 1 (Havcr1)], fibronectin (Fn1), collagen type I-α1 (Col1a1), monocyte chemoattractant protein 1 [chemokine (C-C motif) ligand 2 (Ccl2)], peroxisome proliferator-activated receptor-γ coactivator-1α (Ppargc1a), hexokinase-2 (Hk2), erythropoietin (Epo), aquaporin 2 (Aqp2), solute carrier family 5 member 1 (Slc5a1, SGLT1), solute carrier family 5 member 2 (Slc5a2, SGLT2), solute carrier family 12 member 1 [Slc12a1, Na+-K+-2Cl– cotransporter (NKCC2)] and renin. See primer details in Table 1.
Table 1.
Real-time PCR primers used
| Target Gene | Assay ID |
|---|---|
| Aqp2 | Mm00437575_m1 |
| MCP1/Ccl2 | Mm00441242_m1 |
| Col1a1 | Mm00801666_g1 |
| Epo | Mm01202755_m1 |
| Fn1 | Mm01256744_m1 |
| KIM-1/Havcr1 | Mm00506686_m1 |
| Hk2 | Mm00443385_m1 |
| Hprt | Mm00446968_m1 |
| Ppargc1a | Mm01208835_m1 |
| Ren | Mm02342889_g1 |
| SGLT1/Slc5a1 | Mm00451203_m1 |
| SGLT2/Slc5a2 | Mm00453831_m1 |
| NKCC2/Slc12a1 | Mm01275821_m1 |
Aqp2, aquaporin-2; MCP1/Ccl2, monocyte chemoattractant protein-1/chemokine (C-C motif) ligand 2; Col1a1, collagen type I-α1; Epo, erythropoietin; Fn1, fibronectin 1; KIM-1/Havcr1, kidney injury molecule-1/hepatitis A virus cellular receptor 1, Hk2, hexokinase-2; Hprt, hypoxanthine-guanine phosphoribosyltransferase; Ppargc1a, peroxisome proliferator-activated receptor-γ coactivator-1α; Ren, renin; SGLT, Na+-glucose cotransporter; NKCC2, Na+-K+-2Cl– cotransporter.
Histological analyses.
Formalin-fixed kidney samples were embedded in paraffin blocks, and 5-µm sections were prepared for histological analyses by the University of California-San Diego Tissue Technology Shared Resource (supported by National Cancer Institute Cancer Center Support Grant P30-CA-23100). For tubular injury semiquantification, periodic acid-Schiff staining was performed, and slides were analyzed by a pathologist who was blinded to experimental groups. For each animal, analysis consisted in estimating the total amount of tubular and tubulointerstitial damages in the cortical and OM regions of the entire kidney section. Injuries included loss of normal tubular architecture and brush border, inflammation, intratubular casts, and necrosis. The scoring scale was defined as follows: 0 = normal tubular architecture, 1 = 0–25% of tubules and the tubulointerstitium are injured, 2 = 25–50% of tubules and the tubulointerstitium are injured, 3 = 50–75% of tubules and the tubulointerstitium are injured, and 4 ≥75% of tubules and the tubulointerstitium are injured (14).
Immunolocalization of KIM-1.
Paraffin-embedded kidney tissue sections were rehydrated, and heat-induced antigen retrieval was performed in 10 mM citrate buffer (pH 6.0). Tissue sections were blocked using 2.5% normal donkey serum in Tris-buffered saline and Tween 20 and incubated with goat anti-KIM-1 antibodies (1:200, AF1817, R&D Systems) overnight at 4°C. After being washed, tissue sections were incubated with donkey anti-goat IgG antibodies conjugated with Alexa Fluor 555 (1:500, A21432, Invitrogen) for 1 h at room temperature followed by counterstaining using DAPI. Autofluorescence background signals were quenched by a TrueBlack Lipofuscin Autofluorescence Quencher reagent (no. 23007, Biotium) according to the manufacturer’s instruction. Slides were scanned via a Axio Scan.Z1 (Zeiss) slide scanner.
Statistical analyses.
Data are presented as means ± SE. Data were analyzed by two-way ANOVA to test for an effect of IR or SGLT1 knockout or a significant interaction. This was followed by pairwise multiple comparison procedures using the Holm-Sidak method. An unpaired t-test was used when only two groups were compared for basal data. An overall significance level of P < 0.05 was considered as statistically significant.
RESULTS
Absence of SGLT1 caused mild glucosuria under basal conditions.
Quantitative RT-PCR analyses confirmed the effective elimination of renal Sglt1 mRNA expression in Sglt1−/− sham and Sglt1−/− IR mice (Fig. 1A). Consistent with a previous study (10), Sglt2 mRNA was not significantly altered in Sglt1−/− sham versus WT sham mice, suggesting that SGLT2 may not compensate for the absence of SGLT1. Moreover, a previous study (10) reported unchanged renal mRNA and total membrane protein expression of GLUT1 and GLUT2 in Sglt1−/− versus WT mice.
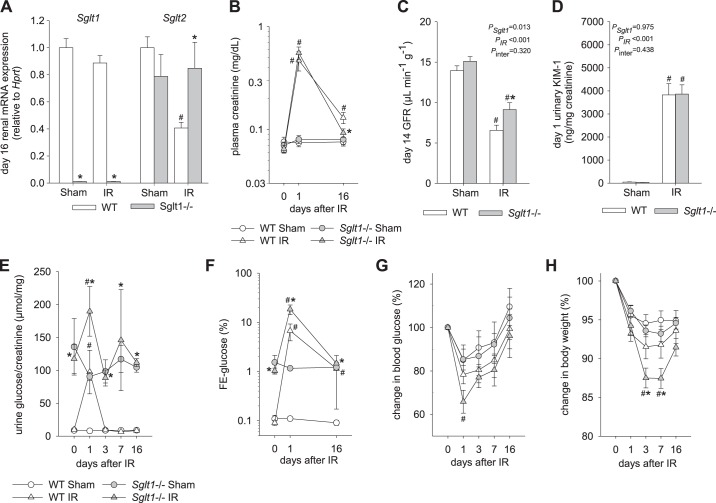
Fig. 1.
Absence of Na+-glucose cotransporter SGLT1 did not affect the early glomerular and tubular impairment in response to ischemia-reperfusion (IR) but did improve recovery. A: renal mRNA expression of Slc5a1 (Sglt1) and Slc5a2 (Sglt2) were measured on day 16 after IR or sham surgery [normalized to the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (Hprt)]. B: plasma creatinine rose to similar levels in Sglt1−/− and wild-type (WT) mice on day 1 after IR, indicating a similar initial glomerular filtration rate (GFR) reduction. On day 16 after IR, plasma creatinine was more fully recovered and lower in Sglt1−/− versus WT mice. C: direct measurement on day 14 after IR confirmed higher GFR values in Sglt1−/− versus WT mice. D: urinary levels of kidney injury molecule (KIM)-1 were similarly increased in Sglt1−/− and WT mice on day 1 after IR, suggesting similar initial proximal tubule injury. E and F: transient increases in urinary glucose-to-creatinine ratios (E) and in the fractional excretion of glucose (FE-glucose; F) in both genotypes on day 1 after IR, with higher levels observed in Sglt1−/− versus WT mice, indicating a robust contribution of SGLT1 to glucose reabsorption after IR. G: blood glucose was significantly reduced in Sglt1−/− mice on day 1 after IR versus sham surgery. H: a transient decrease in body weight was observed in Sglt1−/− mice after IR. Results are means ± SE; n = 7–8 mice/group in A, n = 6–8 mice per group and per time point in B; n = 4 sham mice/group and 6–7 IR mice/group in C; n = 6–8 mice/group in D, and n = 5–8 mice per group and per time point in E−H. #P < 0.05 vs. sham and *P < 0.05 vs. WT mice using two-way ANOVA and Holm-Sidak post hoc analysis for multiple comparisons.
At baseline, plasma creatinine concentrations were not different between genotypes, indicating similar GFR (Table 2). This was confirmed by additional plasma creatinine measurements on days 1 and 16 in sham groups (Fig. 1B) and by actual GFR measurements on day 14 (Fig. 1C). Urinary KIM-1-to-creatinine ratios, a marker of proximal tubular injury, were not different between Sglt1−/− and WT sham groups (Fig. 1D). Moreover, blood glucose levels were similar between genotypes (Table 2) and urinary glucose-to-creatinine ratios and FE-glucose were higher in Sglt1−/− compared with WT sham mice (Fig. 1, E and F), consistent with the previously described minor contribution of SGLT1 to basal renal glucose reabsorption in the normal kidney under euglycemia (10, 22). Absence of SGLT1 did not significantly alter plasma or urine osmolality in sham groups (Table 2).
Table 2.
Baseline values in WT and Sglt1−/− mice
| Genotype | Blood Glucose, mg/dl | Urinary Glucose, µmol/mg creatinine | Renal Fractional Excretion of Glucose, % | Body Weight, g | Hematocrit, % | Plasma Creatinine, mg/dl | Urine Osmolality, mosm/kg | Plasma Osmolality, mosm/kg |
|---|---|---|---|---|---|---|---|---|
| WT | 121 ± 6 | 9.4 ± 0.6 | 0.10 ± 0.01 | 28.3 ± 0.7 | 46.7 ± 0.3 | 0.065 ± 0.003 | 1951 ± 106 | 306 ± 2 |
| Sglt1−/− | 127 ± 7 | 126 ± 22* | 1.26 ± 0.28* | 27.6 ± 0.6 | 47.1 ± 1.0 | 0.065 ± 0.003 | 1776 ± 101 | 304 ± 3 |
Results are means ± SE; n = 13–16 mice/group. Sglt1−/−, Na+-glucose cotransporter 1 deficient. Baseline data were not significantly different between sham and ischemia-reperfused groups for a given genotype and thus were pooled.
P < 0.05 vs. WT mice using an unpaired t-test.
Absence of SGLT1 did not affect the early glomerular and tubular impairment in response to IR.
On day 1 after IR, plasma creatinine rose to similar levels in Sglt1−/− and WT mice (Fig. 1B), potentially indicating a similar initial GFR decrease. This was associated with a similar increase in urinary KIM-1-to-creatinine ratios in both genotypes, suggesting a similar degree of initial PT injury (Fig. 1D). Moreover, transient increases in urinary glucose-to-creatinine ratios (Fig. 1E) and in FE-glucose (Fig. 1F) were observed on day 1 after IR in both genotypes, with greater values for FE-glucose found in Sglt1−/− compared with WT mice (~19% vs. ~7%, P = 0.003). The greater absolute glucosuria on day 1 after IR in Sglt1−/− mice may have contributed to the greater drop in blood glucose (Fig. 1G) and moderate body weight loss in this group on days 3 and 7 after IR (Fig. 1H), possibly due to a greater caloric loss or due to the osmotic effect of enhanced glucose excretion.
IR also increased plasma osmolality and reduced urine osmolality to a similar extent in both genotypes on day 1 after IR (Fig. 2, A and B), indicating a similar initial impairment in urine concentration.
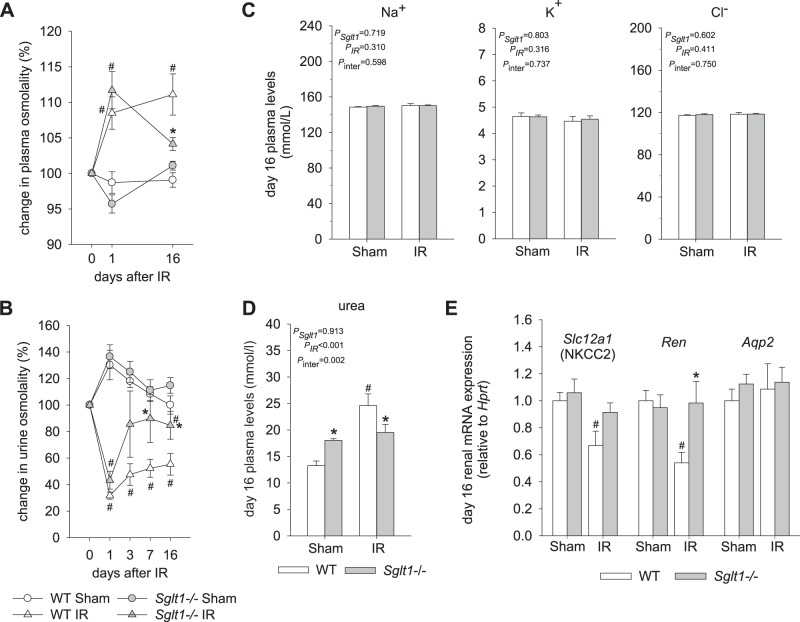
Fig. 2.
Absence of Na+-glucose cotransporter SGLT1 improved the recovery of plasma and urine osmolality after ischemia-reperfusion (IR). A: on day 1 after IR, plasma osmolality was increased to a similar extent in Sglt1−/− and wild-type (WT) mice. The subsequent recovery was nearly complete in Sglt1−/− mice, but plasma osmolality remained elevated in WT IR mice on day 16. B: on day 1 after IR, urine osmolality was reduced to a similar extent in Sglt1−/− and WT mice. The subsequent recovery was improved in Sglt1−/− mice. C: plasma concentrations of Na+, K+, and Cl− were not different between groups. D: on day 16 after IR, plasma urea concentration had recovered in Sglt1−/− mice but remained elevated versus WT sham mice. E: on day 16 after IR, renal mRNA expression of Na+-K+-2Cl– cotransporter (Nkcc2) and renin (Ren) was unchanged in Sglt1−/− mice versus Sglt1−/−sham mice but was reduced in WT mice. Results are means ± SE; n = 5–8 mice per group and per time point in A and B, n = 6–8 mice/group in C, n = 7–8 mice/group in D, and n = 7–8 mice/group in E. #P < 0.05 vs. sham and *P < 0.05 vs. WT mice using two-way ANOVA and Holm-Sidak post hoc analysis for multiple comparisons.
Absence of SGLT1 improved the recovery of GFR and markers of tubular function and kidney injury after IR.
On day 14 after IR, GFR was greater in Sglt1−/− IR versus WT IR groups (Fig. 1C). In accordance, on day 16 after IR, plasma creatinine was close to normal in the Sglt1−/− IR group, whereas the levels remained significantly elevated in the WT IR group (Fig. 1B).
On day 16 after IR, FE-glucose remained slightly elevated in WT mice (Fig. 1F). This was associated with a ~60% downregulation in Sglt2 mRNA in WT IR versus WT sham mice, whereas renal Sglt1 mRNA levels were similar in these two groups (Fig. 1A), indicating a better preservation or quicker recovery of Sglt1 versus Sglt2 mRNA expression at day 16 after IR in WT mice. In comparison, FE-glucose had returned to baseline on day 16 after IR in Sglt1−/−, associated with unchanged Sglt2 mRNA expression versus Sglt1−/− sham mice.
Lack of SGLT1 was associated with improved recovery of plasma osmolality and urine osmolality on day 16 after IR (Fig. 2, A and B). Plasma concentrations of Na+, K+, and Cl− were not affected by genotype or IR (Fig. 2C). The enhanced plasma osmolality in WT IR versus Sglt1−/− IR mice on day 16 was in part due to an increase in plasma urea concentration (Fig. 2D), potentially reflecting impaired urea filtration. Plasma urea concentration was greater in Sglt1−/− sham versus WT sham mice, but the levels were not significantly affected by IR versus sham in Sglt1−/− mice on day 16 after IR, potentially due to better recovery of GFR and thus renal urea filtration.
Quantitative RT-PCR analyses on day 16 after IR revealed that renal mRNA expression of Nkcc2 and renin was reduced compared with sham in WT mice but not in Sglt1−/− mice (Fig. 2E). This may reflect a sustained impairment of NaCl reabsorption in the thick ascending limb in WT mice, which is expected to impair urine concentration and, through effects on NaCl delivery to the juxtaglomerular apparatus, lower renin expression and GFR. In comparison, mRNA expression of Aqp2, a marker of the connecting tubule and collecting duct, was similar in IR versus sham mice in both genotypes (Fig. 2E).
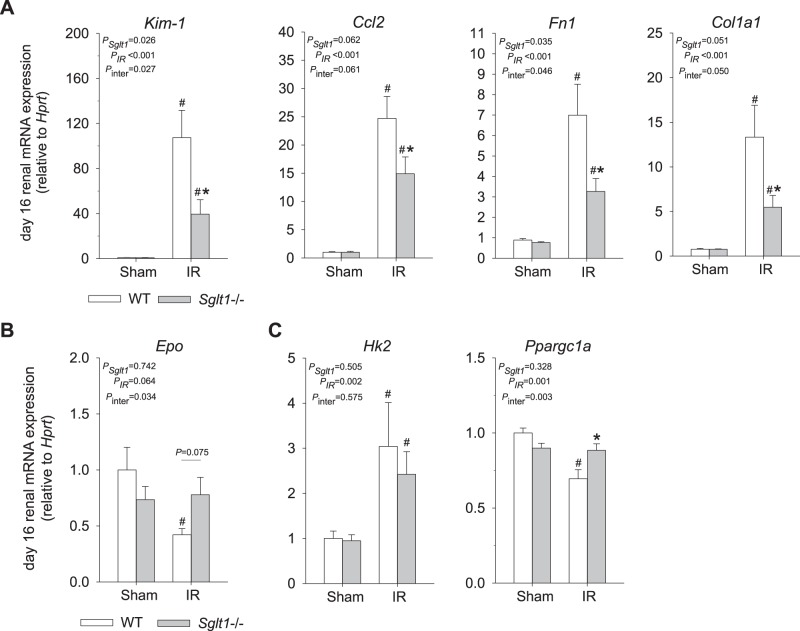
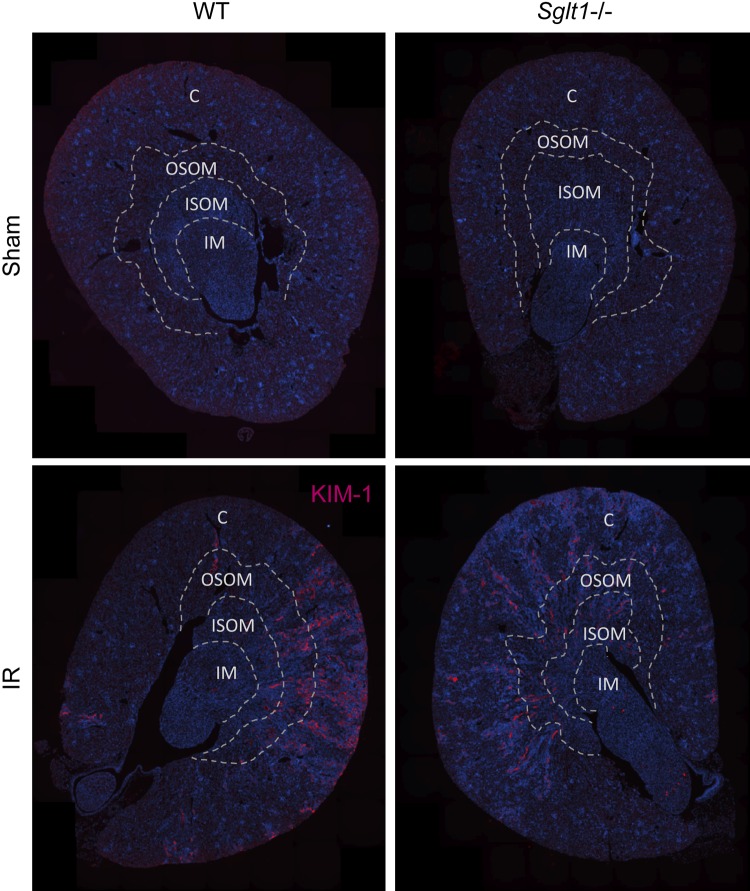
Tubular injury was quantified in kidneys harvested on day 16 after IR by a kidney pathologist blinded to study groups. In sham animals, no significant differences were observed between genotypes. IR induced cortical and medullary tubular damage and cast formation, brush border loss, tubular simplification, and immune cell infiltration in both genotypes. Absence of SGLT1, however, significantly reduced both cortical and medullary tubular and interstitial injury scores (Fig. 3, A and B). This was associated with a lesser upregulation of renal mRNA expression of Kim-1 as well as renal inflammation and fibrosis markers Ccl2, Fn1, and Col1a1 in Sglt1−/− versus WT mice (Fig. 4A). Immunostaining indicated that IR enhanced renal KIM-1 expression in the OM and cortex in both genotypes. Absence of SGLT1 appeared to attenuate this increase in KIM-1 immunostaining in response to IR in both regions (Fig. 5). Furthermore, renal mRNA expression of Epo, a gene primarily expressed in interstitial fibroblast-like cells in the OM and inner cortex, was significantly decreased in WT but not Sglt1−/− mice (Fig. 4B). mRNA expression of Hk2, a key glycolytic gene, was similarly upregulated in both genotypes in response to IR, whereas the Ppargc1a mRNA, a master regulator of mitochondrial biogenesis, was reduced in WT but not Sglt1−/− mice on day 16 after IR (Fig. 4C).
Fig. 3.
Absence of Na+-glucose cotransporter SGLT1 reduced cortical and outer medullary tubular injury during recovery from ischemia-reperfusion (IR). On day 16 after IR, kidneys were harvested for histological analysis and tubular injury quantification by a renal pathologist blinded with regard to study groups. A: representative pictures of cortical and outer medullary tubular injury [periodic acid-Schiff (PAS) staining]. Arrows indicate damaged tubules, some with loss of tubular brush borders and some with dilatation; arrowheads indicate interstitial inflammation. *Tubular casts. B: semiquantitative analysis of cortical and outer medullary tubular injury. Sglt1−/− sham animals had normal histology features similar to those of WT sham animals. IR injury in WT mice was characterized by interstitial inflammation and patchy tubular injury with some tubular segments containing sloughed cells and/or cell cytoplasm and proteinaceous cast material. Other portions of the tubular parenchyma showed attenuation or loss of proximal tubular brush borders. Sglt1−/− IR kidneys displayed less severe tubular and tubulointerstitial damage in the cortex and outer medulla compared with WT IR kidneys. The number of medullary tubular casts appeared to be similar in both genotypes after IR. Results are means ± SE; n = 4–6 sham mice/group and 6 IR mice/group. #P < 0.05 vs. sham and *P < 0.05 vs. WT mice using two- way ANOVA and Holm-Sidak post hoc analysis for multiple comparisons.
Fig. 4.
Absence of Na+-glucose cotransporter SGLT1 reduced renal mRNA expression of markers of tubular injury, inflammation, and fibrosis during recovery from ischemia-reperfusion (IR). Kidneys were harvested on day 16 after IR for quantitative RT-PCR analyses of mRNA expression. mRNA expression was normalized to the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (Hprt). Absence of SGLT1 attenuated the increase in renal expression of markers of proximal tubular injury [kidney injury molecule-1 (Kim-1)], inflammation [chemokine (C-C motif) ligand 2 (Ccl2)], and fibrosis [fibronectin 1 (Fn1) and collagen type I-α1 (Col1a1)] (A) and prevented the decrease in erythropoietin (Epo) expression (B), a marker of interstitial cells in the outer medulla and inner cortex. Absence of SGLT1 did not prevent the upregulation of hexokinase-2 (Hk2), a key glycolytic enzyme, but prevented the downregulation of peroxisome proliferator-activated receptor-γ coactivator-1α (Ppargc1a), a master regulator of mitochondrial biogenesis and function (C). Results are means ± SE; n = 6–8 mice/group. #P < 0.05 vs. sham and *P < 0.05 vs. WT mice using two-way ANOVA and Holm-Sidak post hoc analysis for multiple comparisons.
Fig. 5.
Enhanced kidney injury molecule (KIM)-1 immunostaining in the renal outer medulla and cortex during recovery from ischemia-reperfusion (IR). On day 16 after IR, kidneys were harvested to localize KIM-1 protein expression by immunostaining. IR enhanced renal KIM-1 immunostaining in the outer medulla and cortex in both genotypes. Absence of Na+-glucose cotransporter SGLT1 appeared to attenuate the increase in KIM-1 immunostaining in both regions. Representative images are shown from n = 6 mice/group. C, cortex; OSOM, outer stripe of the outer medulla; ISOM, inner stripe of the outer medulla; IM, inner medulla; WT, wild type.
DISCUSSION
The present study demonstrates that genetic deletion of SGLT1 did not affect the acute impairment in glomerular and tubular function in a mouse model of IR-induced AKI. Absence of SGLT1, however, improved the subsequent recovery of glomerular and tubular functions, alleviated the upregulation of renal markers of tubular injury, inflammation, and fibrosis, and reduced the severity of cortical and medullary tubular damage. Thus, the present study provides the first evidence for a potential deleterious role of SGLT1 in the recovery phase of renal IR injury (Fig. 6).
Fig. 6.
Proposed deleterious role for Na+-glucose cotransporter SGLT1-mediated reabsorption during recovery from ischemia-reperfusion (IR)-induced acute kidney injury. IR initially suppresses SGLT2 and SGLT1-mediated reabsorption in the early and later proximal tubule, respectively, which is associated with glucosuria. Early recovery of SGLT1-mediated Na+ reabsorption in late proximal tubule/outer medulla sustains IR-induced hypoxia. This enhances cell injury in the outer medulla and further inhibits Na+-K+-2Cl– cotransporter (NKCC2)-mediated NaCl reabsorption in the thick ascending limb, which impairs urine concentration and enhances NaClK delivery to the macula densa ([Na/Cl/K]MD). The latter reduces renin expression and lowers glomerular filtration rate (GFR) via tubuloglomerular feedback. The reduction in GFR enhances plasma creatinine and urea, with the latter contributing to enhanced plasma osmolality. The increased hypoxia and cell injury further enhances mitochondrial dysfunction, inflammation, and fibrosis, which can spread to the cortex and further suppress tubular function. A sustained suppression of SGLT2 maintains a high glucose load to SGLT1, thereby sustaining the detrimental influence of SGLT1. Kim-1, kidney injury molecule-1; Epo, erythropoietin; Col1a1, collagen type I-α1; Fn1, fibronectin 1; Ccl2, chemokine (C-C motif) ligand 2; Ppargc1a, peroxisome proliferator-activated receptor-γ coactivator-1α.
The PT is the primary site of renal ischemic injury, particularly the S3 segment, which enters the vulnerable OM (4, 6, 24). A typical hallmark of IR injury is the rapid and reversible loss of the PT brush border accompanied with a substantial decrease in fluid and solute reabsorption, both resulting from ischemic ATP depletion. Previous studies in rats have demonstrated that renal ischemia is associated with a transient decrease in SGLT-mediated glucose transport activity in PT brush border vesicles and decreased SGLT2 expression at the apical membrane (13, 19). Consistent with impaired tubular glucose reabsorption, the present study found that IR in WT mice induced a transient increase in urinary glucose excretion accompanied with an increase in FE-glucose on day 1. The absolute contribution of SGLT1 to glucose reabsorption appeared to be maintained on day 1 of reperfusion, as indicated by a maintained absolute difference in urinary glucose to creatinine ratios between Sglt1−/− and WT mice despite a strong increase in plasma creatinine as an indicator of a reduction in GFR and thus filtered glucose in both groups. This would be consistent with an IR-induced decrease in SGLT2-mediated glucose transport in the early PT, shifting more glucose reabsorption to the downstream S2/S3 segments where SGLT1 compensates in part. Little is known about the responses in SGLT1 expression and transport activity when the luminal glucose delivery to the late PT is increased. Genetic deletion of Sglt2 in mice upregulated SGLT1-mediated glucose reabsorption, but this was associated with a 40% suppression of renal SGLT1 mRNA and protein levels, potentially as a protective mechanism to prevent excessive reabsorption of glucose in the hypoxia-sensitive OM (28). Preliminary RNA sequencing data indicated that renal Sglt1 and Sglt2 mRNA expression was downregulated on day 1 of reperfusion after 15 or 25 min of bilateral renal artery clamping in C57BL/6J mice (8). The present study found in WT mice at day 16 after IR that mRNA expression of Sglt1 was similar to sham mice, whereas Sglt2 mRNA was downregulated, suggesting that SGLT1 may have recovered more quickly than SGLT2. In other words, early downregulation of SGLT1 after IR may have limited its functional contribution on day 1 of reperfusion. This may contribute to the finding of the present study that absence of SGLT1 did not measurably affect initial proximal tubular injury or glomerular and tubular function on that day, as indicated by similar increases in plasma concentrations of creatinine and urinary excretion of the tubular injury marker KIM-1 as well as a similar fall in urine osmolality and rise in plasma osmolality as indicators of impaired urine concentration. In comparison, renal Sglt1 mRNA expression appeared normal on day 16 after IR in WT mice, potentially indicating a significant contribution of SGLT1 to glucose reabsorption during IR recovery. The finding that absence of SGLT1 improved the recovery of glomerular and tubular function, associated with improved renal histology and lower mRNA expression of kidney markers of injury, inflammation, and fibrosis, indicated that the overall influence of SGLT1 on the kidney during IR recovery was deleterious.
Beneficial effects of SGLT1 deletion may have originated in the corticomedullary region, the primary site of SGLT1 expression. Indeed, renal mRNA expression of Epo, which derives from interstitial fibroblast-like cells in the deep cortex and OM (25), was significantly reduced on day 16 of reperfusion in WT mice, whereas levels were similar to sham mice lacking SGLT1. In accordance, mRNA expression of Nkcc2, mostly expressed in the thick ascending limb of the OM and deeper cortex, also was better preserved in the absence of SGLT1. Ultimately, facilitated restoration of tubular transport activity via NKCC2 in the absence of SGLT1 could explain the observed better recovery of urine concentration as well as the lesser suppression of renin mRNA and improved GFR recovery via the physiology of the tubuloglomerular feedback mechanism. SGLT1 deletion potentially promoted tubular recovery also in cortical regions, as indicated by histological analysis, KIM-1 immunostaining, and a better preservation of renal SGLT2 mRNA expression.
Transcript analyses of several AKI-relevant genes may provide the first clues on how SGLT1 impairs or retards kidney recovery after IR. Kidney recovery depends on the ability to restore and maintain tubular cell energetics to drive tubular reabsorption, which is ultimately required to concentrate the urine and allows GFR to increase without risking a negative fluid and electrolyte balance due to urinary loss. Previous studies have indicated that ischemic AKI leads to mitochondrial dysfunction and a metabolic shift of the recovering PTs toward anaerobic glycolysis (2, 15). This shift is characterized by increased activity of hexokinase in the kidney cortex and the outer stripe of the OM at 14 days after IR injury (15). In the present study, whole kidney mRNA expression of Hk2, a key glycolytic enzyme, was similarly upregulated on day 16 after IR in both genotypes, suggesting that the transition to anaerobic glycolysis may not have been affected by the absence of SGLT1. However, renal mRNA expression of the transcription factor Ppargc1a, a master regulator of mitochondrial biogenesis and function (17), was improved during recovery in mice lacking SGLT1. Further studies are needed to determine whether SGLT1 affects mitochondria number, architecture, and function during IR recovery and whether this is restricted to the OM S3 segment, where SGLT1 is primarily expressed, or whether other nephron segments or cell types are also affected.
Sham mice lacking SGLT1 had increased plasma urea levels compared with WT mice. This finding is consistent with the hypothesis that SGLT1 mediates tubular secretion of urea in the proximal straight tubule (3). The use of a high-protein diet in the present study may have unraveled such a contribution of SGLT1; however, additional studies are needed to further support this hypothesis.
In summary, genetic deletion of SGLT1 did not affect the early tubular injury and impairment of kidney function after IR but improved the subsequent glomerular and tubular recovery. The findings thus indicate a deleterious role of SGLT1 during recovery from renal IR, which is reminiscent of a recently proposed deleterious role of cardiac SGLT1 in heart IR injury (16).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK-106102 and R01-DK-112042, University of Alabama at Birmingham/University of California-San Diego O’Brien Center of Acute Kidney Injury NIDDK Grant P30-DK-079337, and the Department of Veterans Affairs.
DISCLOSURES
Over the past 36 mo, V. Vallon has served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceutical, and Merck and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen Pharmaceutical.
AUTHOR CONTRIBUTIONS
J.N., H.K., C.E.A., and V.V. conceived and designed research; J.N., R.P., K.L.H., W.H., B.F., and Y.K. performed experiments; J.N., Y.K., and V.V. analyzed data; J.N., K.L.H., Y.K., C.E.A., and V.V. interpreted results of experiments; J.N., K.L.H., Y.K., and V.V. prepared figures; J.N. and V.V. drafted manuscript; J.N., R.P., K.L.H., W.H., B.F., Y.K., H.K., C.E.A., and V.V. edited and revised manuscript; J.N., R.P., K.L.H., W.H., B.F., Y.K., H.K., C.E.A., and V.V. approved final version of manuscript.
References
- 1.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18: 1292–1298, 2007. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 2.Ash SR, Cuppage FE. Shift toward anaerobic glycolysis in the regenerating rat kidney. Am J Pathol 60: 385–402, 1970. [PMC free article] [PubMed] [Google Scholar]
- 3.Bankir L, Yang B. New insights into urea and glucose handling by the kidney, and the urine concentrating mechanism. Kidney Int 81: 1179–1198, 2012. doi: 10.1038/ki.2012.67. [DOI] [PubMed] [Google Scholar]
- 4.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Busse LW. Novel therapies for acute kidney injury. Kidney Int Rep 2: 785–799, 2017. doi: 10.1016/j.ekir.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattah H, Shigeoka A, Huang W, Patel R, Kasimsetty S, Singh P, McKay DB, Vallon V. Diverse gene expression patterns of renal transporters in AKI (Abstract). J Am Soc Nephrol 29: 1045, 2018. [Google Scholar]
- 9.Fiorentino M, Grandaliano G, Gesualdo L, Castellano G. Acute kidney injury to chronic kidney disease transition. Contrib Nephrol 193: 45–54, 2018. doi: 10.1159/000484962. [DOI] [PubMed] [Google Scholar]
- 10.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61: 187–196, 2012. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol 40: 123–137, 2013. doi: 10.1111/1440-1681.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyman SN, Rosen S, Brezis M. The renal medulla: life at the edge of anoxia. Blood Purif 15: 232–242, 1997. doi: 10.1159/000170341. [DOI] [PubMed] [Google Scholar]
- 13.Johnston PA, Rennke H, Levinsky NG. Recovery of proximal tubular function from ischemic injury. Am J Physiol 246: F159–F166, 1984. doi: 10.1152/ajprenal.1984.246.2.F159. [DOI] [PubMed] [Google Scholar]
- 14.Khalid U, Pino-Chavez G, Nesargikar P, Jenkins RH, Bowen T, Fraser DJ, Chavez R. Kidney ischaemia reperfusion injury in the rat: the EGTI scoring system as a valid and reliable tool for histological assessment. J Histol Histopathol 3: article 1, 2016. doi: 10.7243/2055-091X-3-1. [DOI] [Google Scholar]
- 15.Lan R, Geng H, Singha PK, Saikumar P, Bottinger EP, Weinberg JM, Venkatachalam MA. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol 27: 3356–3367, 2016. doi: 10.1681/ASN.2015020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Agrawal V, Ramratnam M, Sharma RK, D’Auria S, Sincoular A, Jakubiak M, Music ML, Kutschke WJ, Huang XN, Gifford L, Ahmad F. Cardiac sodium-glucose co-transporter 1 (SGLT1) is a novel mediator of ischemia/reperfusion injury. Cardiovasc Res, 2019. doi: 10.1093/cvr/cvz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch MR, Tran MT, Parikh SM. PGC1α in the kidney. Am J Physiol Renal Physiol 314: F1–F8, 2018. doi: 10.1152/ajprenal.00263.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev 4: 20–27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molitoris BA, Kinne R. Ischemia induces surface membrane dysfunction. Mechanism of altered Na+-dependent glucose transport. J Clin Invest 80: 647–654, 1987. doi: 10.1172/JCI113117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pill J, Kraenzlin B, Jander J, Sattelkau T, Sadick M, Kloetzer HM, Deus C, Kraemer U, Gretz N. Fluorescein-labeled sinistrin as marker of glomerular filtration rate. Eur J Med Chem 40: 1056–1061, 2005. doi: 10.1016/j.ejmech.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 22.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt KA, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188−F193, 2014. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen S, Epstein FH, Brezis M. Determinants of intrarenal oxygenation: factors in acute renal failure. Ren Fail 14: 321–325, 1992. doi: 10.3109/08860229209106636. [DOI] [PubMed] [Google Scholar]
- 24.Shanley PF, Brezis M, Spokes K, Silva P, Epstein FH, Rosen S. Transport-dependent cell injury in the S3 segment of the proximal tubule. Kidney Int 29: 1033–1037, 1986. doi: 10.1038/ki.1986.103. [DOI] [PubMed] [Google Scholar]
- 25.Shih HM, Wu CJ, Lin SL. Physiology and pathophysiology of renal erythropoietin-producing cells. J Formos Med Assoc 117: 955–963, 2018. doi: 10.1016/j.jfma.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Vallon V. Tubular transport in acute kidney injury: relevance for diagnosis, prognosis and intervention. Nephron 134: 160–166, 2016. doi: 10.1159/000446448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 30.Zuk A, Bonventre JV. Acute Kidney Injury. Annu Rev Med 67: 293–307, 2016. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]