Summary
Background
Gonorrhoea is a common sexually transmitted infection for which ceftriaxone is the current first-line treatment, but antimicrobial resistance is emerging. The objective of this study was to assess the effectiveness of gentamicin as an alternative to ceftriaxone (both combined with azithromycin) for treatment of gonorrhoea.
Methods
G-ToG was a multicentre, parallel-group, pragmatic, randomised, non-inferiority trial comparing treatment with gentamicin to treatment with ceftriaxone for patients with gonorrhoea. The patients, treating physician, and assessing physician were masked to treatment but the treating nurse was not. The trial took place at 14 sexual health clinics in England. Adults aged 16–70 years were eligible for participation if they had a diagnosis of uncomplicated genital, pharyngeal, or rectal gonorrhoea. Participants were randomly assigned to receive a single intramuscular dose of either gentamicin 240 mg (gentamicin group) or ceftriaxone 500 mg (ceftriaxone group). All participants also received a single 1 g dose of oral azithromycin. Randomisation (1:1) was stratified by clinic and performed using a secure web-based system. The primary outcome was clearance of Neisseria gonorrhoeae at all initially infected sites, defined as a negative nucleic acid amplification test 2 weeks post treatment. Primary outcome analyses included only participants who had follow-up data, irrespective of the baseline visit N gonorrhoeae test result. The margin used to establish non-inferiority was a lower confidence limit of 5% for the risk difference. This trial is registered with ISRCTN, number ISRCTN51783227.
Findings
Of 1762 patients assessed, we enrolled 720 participants between Oct 7, 2014, and Nov 14, 2016, and randomly assigned 358 to gentamicin and 362 to ceftriaxone. Primary outcome data were available for 306 (85%) of 362 participants allocated to ceftriaxone and 292 (82%) of 358 participants allocated to gentamicin. At 2 weeks after treatment, infection had cleared for 299 (98%) of 306 participants in the ceftriaxone group compared with 267 (91%) of 292 participants in the gentamicin group (adjusted risk difference −6·4%, 95% CI −10·4% to −2·4%). Of the 328 participants who had a genital infection, 151 (98%) of 154 in the ceftriaxone group and 163 (94%) of 174 in the gentamicin group had clearance at follow-up (adjusted risk difference −4·4%, −8·7 to 0). For participants with a pharyngeal infection, a greater proportion receiving ceftriaxone had clearance at follow-up (108 [96%] in the ceftriaxone group compared with 82 [80%] in the gentamicin group; adjusted risk difference −15·3%, −24·0 to −6·5). Similarly, a greater proportion of participants with rectal infection in the ceftriaxone group had clearance (134 [98%] in the ceftriaxone group compared with 107 [90%] in the gentamicin group; adjusted risk difference −7·8%, −13·6 to −2·0). Thus, we did not find that a single dose of gentamicin 240 mg was non-inferior to a single dose of ceftriaxone 500 mg for the treatment of gonorrhoea, when both drugs were combined with a 1 g dose of oral azithromycin. The side-effect profiles were similar between groups, although severity of pain at the injection site was higher for gentamicin (mean visual analogue pain score 36 of 100 in the gentamicin group vs 21 of 100 in the ceftriaxone group).
Interpretation
Gentamicin is not appropriate as first-line treatment for gonorrhoea but remains potentially useful for patients with isolated genital infection, or for patients who are allergic or intolerant to ceftriaxone, or harbour a ceftriaxone-resistant isolate. Further research is required to identify and test new alternatives to ceftriaxone for the treatment of gonorrhoea.
Funding
UK National Institute for Health Research.
Introduction
Each year gonorrhoea accounts for over 40 000 infections in the UK and around 78 million infections globally,1 with a disproportionate burden in young adults, men who have sex with men, and specific ethnic groups. Infection leads to local inflammation causing genital pain and discomfort, and localised immune activation that facilitates the acquisition and transmission of HIV. For women, infection can spread to the fallopian tubes and ovaries causing pelvic inflammatory disease with resultant tubal scarring, infertility, chronic pelvic pain, and an increased risk of ectopic pregnancy. For men, infection can spread to the testicles leading to epididymo-orchitis, and men who have sex with men are at an increased risk of proctitis, which can lead to abscess and fistula formation.
Research in context.
Evidence before this study
Two systematic reviews evaluated the efficacy of gentamicin for the treatment of gonorrhoea. They included randomised trials, quasi-randomised trials, and prospective studies with concurrent controls published between Jan 1, 1950, and June 2, 2014. We also searched MEDLINE and Embase for studies published between Jan 1, 2013, and Dec 12, 2017, using the terms “gonorrhoea/gonorrhea/Neisseria gonorrhoeae” and “gentamicin”. In total, six studies assessed single-dose gentamicin treatment, of which three were randomised trials, one was quasi-randomised, and two were non-randomised. Cure rates of 62% to 100% were reported with gentamicin treatment. Methodology was poorly described and there was a high risk of bias within most studies. The largest and best quality study was a non-comparative evaluation of 157 patients, which reported that gentamicin cured 100% of infections. This study used a relatively less sensitive culture technique to diagnose and assess cure, and included few extra-genital gonorrhoea infections (ten pharyngeal, one rectal). Gentamicin was administered with a 2 g dose of azithromycin. The combined regimen was poorly tolerated, causing nausea in 26% of patients and vomiting in 10%.
Added value of this study
Because of antibiotic resistance, treatment options for gonorrhoea are diminishing. G-ToG is the first randomised trial to compare gentamicin with the current first-line treatment, ceftriaxone, for gonorrhoea. We were unable to conclude that gentamicin was non-inferior to ceftriaxone, and treatment failure with gentamicin was higher than with ceftriaxone for patients with extra-genital infections. Cure rates for genital infections were similar between groups, so for these patients gentamicin might be a candidate for second-line therapy. Single-dose gentamicin was safe and well tolerated.
Implications of all the available evidence
Ceftriaxone should remain the first-line treatment for gonorrhoea, with gentamicin as an alternative particularly for patients with genital infections, and those who are allergic or intolerant to ceftriaxone, or harbour ceftriaxone-resistant gonococci. Further research is required to identify and test new alternatives to ceftriaxone for the treatment of gonorrhoea.
The causative organism, Neisseria gonorrhoeae, readily develops resistance to antibiotics. High-level resistance to penicillins, sulphonamides, tetracyclines, and quinolones has led to these no longer being recommended as treatment. Current guidance is to treat with intramuscular ceftriaxone, either as monotherapy or as dual therapy combined with azithromycin.2, 3, 4 Surveillance data in the UK show a reduction in susceptibility to ceftriaxone over time, with an upward drift in the minimum inhibitory concentration (MIC).1 A similar reduction in susceptibility to other antimicrobials used for gonorrhoea was followed by widespread treatment failure, and sporadic clinical failure of cephalosporins has been reported.5, 6 If ceftriaxone becomes ineffective, options for treatment are limited. With the exception of gentamicin, alternative drugs have either not been assessed in patients (eg, ertapenem, piperacillin-tazobactam), are still in development before licensing (eg, zoliflodacin, gepotidicin), are reserved for other infections (eg, rifampicin for tuberculosis), or have the potential for resistance to develop rapidly (eg, azithromycin, spectinomycin). Untreatable, multidrug-resistant gonorrhoea is a real possibility, and new clinical trial data are needed to inform treatment guidelines.7 WHO and the European Centre for Disease Prevention and Control have called for urgent research into the efficacy of new regimens to treat gonorrhoea, including combination regimens and the assessment of antimicrobial efficacy at extra-genital sites.8, 9 Effective, safe, and low-cost treatment in low-income and middle-income countries is particularly needed; many of these countries have a high burden of gonorrhoea infection.
Gentamicin is an aminoglycoside antibiotic that inhibits protein synthesis by irreversibly binding to 30S ribosomal subunits. Studies in the 1970s and 1980s assessed gentamicin for treatment of gonorrhoea, but all studies were small and had a high risk of bias.10, 11 The dose used in these studies was usually 240 mg (ranging from 160 mg to 5mg/kg), with no apparent dose response effect across studies and no reported adverse events associated with the drug. In-vitro susceptibility testing suggests that N gonorrhoeae remains susceptible to gentamicin12 although the in-vivo response and associated susceptibility breakpoints have been poorly characterised. Gentamicin can cause ototoxicity and nephrotoxicity,13 but the frequency and severity of these adverse events following a single dose is not known.
Recent systematic reviews of gentamicin10, 11 for the treatment of (mostly urogenital) gonorrhoea report its clinical and microbiological cure rate to be around 62–98%. Data on its efficacy when treating pharyngeal or rectal gonorrhoea are scarce, although antibiotics for gonorrhoea are sometimes less effective at these sites.14 A large randomised non-comparative trial reported a 100% cure rate when gentamicin was combined with 2 g oral azithromycin, but a high incidence of gastrointestinal adverse effects reduced the tolerability of this regimen.15
The aim of our study was to assess whether single-dose gentamicin therapy is an acceptable alternative to ceftriaxone for the treatment of gonorrhoea, when both antibiotics are combined with azithromycin.
Methods
Study design and participants
G-ToG was a multicentre, parallel-group, pragmatic, randomised, non-inferiority trial comparing treatment with gentamicin to treatment with ceftriaxone for patients with gonorrhoea. The trial took place at 14 sexual health clinics in England. Ethics approval was obtained from the Health Research Authority South Central–Oxford C Research Ethics Committee (14/SC/1030). The study protocol is available online.16
Adults aged 16–70 years were eligible for participation if they had a diagnosis of untreated genital, pharyngeal, or rectal gonorrhoea (ie, they had not received any antibiotic in the previous 28 days that could have treated gonorrhoea, either partially or completely). To reflect normal practice, all patients who had an initial positive test for gonorrhoea and presented for treatment were eligible for inclusion. Diagnosis was based on detection of intracellular Gram-negative diplococci by microscopy (urethral, cervical, vaginal, or rectal specimens), or by nucleic acid amplification test (NAAT) from first void urine, urethral, endocervical, vulvovaginal, pharyngeal or rectal swabs. Any licensed NAAT test platform result was accepted for assessing eligibility for inclusion into the trial. Exclusion criteria were known concurrent bacterial sexually transmitted infections apart from chlamydia; known bacterial vaginosis or Trichomonas vaginalis infection; known contraindications or allergy to gentamicin, ceftriaxone, azithromycin, or lidocaine; complicated gonorrhoea infection, for example pelvic inflammatory disease or epididymo-orchitis; and patient weight being less than 40 kg. Women who were pregnant or breastfeeding were also excluded. Patients were only eligible to participate in the trial once. They provided written informed consent at their initial consultation.
Randomisation and masking
Participants were randomly assigned (1:1) to receive a single intramuscular dose of either gentamicin 240 mg (gentamicin group) or ceftriaxone 500 mg (ceftriaxone group). All participants also received a single 1 g dose of oral azithromycin. Randomisation was stratified by clinic and performed with a secure web-based system. We used a computer-generated pseudo-random code with permuted blocks of randomly varying size created by the Nottingham Clinical Trials Unit in accordance with their standard operating procedure. The allocated treatment was administered from routine clinic stock. To maintain blinding the system confirmed that randomisation had been successful when a member of the research team randomised a participant, but did not reveal the allocated treatment. A nurse who was trained only in the trial's treatment administration procedure and not involved with any other trial procedures then logged onto the randomisation system to determine which treatment had been allocated, and administered the injection and oral azithromycin. The nurse who gave the injection did not reveal the treatment allocation to participants, research staff or investigators, who all remained masked to treatment. The allocation sequence remained concealed until the database was locked at the end of the trial.
Procedures
Ceftriaxone 500 mg in powder formulation was dissolved in 1% lidocaine and administered as a single 2 mL intramuscular injection. Gentamicin 240 mg (3 × 80 mg in 2 mL vials) was administered as a single 6 mL intramuscular injection. All participants also received a single oral dose of 1 g azithromycin. All participants were asked to avoid sexual contact until review after 2 weeks.
Participants provided samples for N gonorrhoeae testing before treatment. These samples varied by gender and sexual orientation: for heterosexual men, NAAT and culture testing were done from urethra samples (a first pass urine sample could be taken as an alternative to the urethra for NAAT); for men who have sex with men, NAAT and culture testing were done from urethra, pharynx, and rectum samples (a urine sample could be taken as an alternative to the urethra for NAAT); for women, NAAT and culture testing were done from cervix, pharynx, and rectum samples (a vaginal sample could be taken as an alternative to the cervix for NAAT). Follow-up was 2 weeks after treatment, when NAAT and culture testing for N gonorrhoeae was repeated for sites that had been positive at baseline. All baseline and post-treatment samples were required to be tested with NAAT (Aptima Combo 2, Hologic, MA, USA). If the local laboratory did not use Aptima Combo 2 NAAT, additional samples were tested at Public Health England (London, UK). Culture specimens were processed according to local laboratory procedures, and pure viable cultures confirmed to be N gonorrhoeae were frozen to −70°C or below and shipped to Public Health England for antimicrobial sensitivity testing. Blood samples for creatinine measurement (allowing calculation of the estimated glomerular filtration rate) were taken at baseline and at follow-up 2 weeks after treatment.
Outcomes
The primary outcome was clearance of N gonorrhoeae at all initially infected sites, defined as a negative NAAT 2 weeks after treatment.17 Secondary outcomes were clinical resolution of symptoms, change in renal function (estimated glomerular filtration rate) and comparative cost-effectiveness at 2 weeks. The relationship between clearance of N gonorrhoeae and in-vitro measurement of antibiotic minimum inhibitory concentration (MIC) was also investigated as a secondary outcome, using Etests (BioMérieux, Marcy-l'Étoile, France) on GC base agar (Becton Dickinson, NJ, USA) with 1% Vitox (Oxoid, Thermo Fisher Scientific, Basingstoke, UK). Safety outcomes were the frequency of known side-effects (nausea, vomiting, hearing loss, dizziness, rash), frequency of any other adverse events reported by participants, and tolerability of the treatment injection measured on a visual analogue scale, where 0 represented no pain and 100 the worst imaginable pain. The results of the cost-effectiveness analyses will not be presented in this paper.
Statistical analysis
Based on 96% clearance for the ceftriaxone regimen, a total sample size of 646 participants (323 in each group) was required to detect non-inferiority with a lower confidence limit of 5% for the risk difference, with 90% power and 0·025 one-sided significance. To allow for loss to follow-up of 10%, the trial had a target recruitment of 720 participants.
The primary approach to between-group comparisons was to analyse participants according to randomised allocation without imputation of missing outcome data. Planned analysis of the primary outcome was modified, before the database was locked and treatment codes revealed. The initial analysis plan in the protocol was to compare gentamicin with ceftriaxone with a general linear model for binary outcome adjusted by clinic site, with the primary efficacy parameter being the risk difference in the proportion of participants clear of infection at follow-up, along with the 95% confidence interval. However, additional clinics joined the trial, some of which recruited small numbers of participants. This meant that there was the chance that some clinics would have no participants whose infection had not cleared, making the inclusion of clinic as a fixed effect inappropriate. Therefore, we modified the between-group comparative analyses to use generalised estimating equations for binary outcomes adjusted by recruiting clinic as a random effect with robust standard errors. The generalised estimating equation model used an identity link function to enable estimation of adjusted risk difference. Gentamicin was to be regarded as non-inferior if the lower 95% confidence limit for the risk difference (gentamicin group vs ceftriaxone group) in confirmed clearance was −5 percentage points or greater (ie, closer to zero). Analysis of the primary outcome included only participants who had follow-up data, irrespective of the baseline visit N gonorrhoeae test result (since this was a pragmatic trial). Sensitivity analyses were done to assess the robustness of the primary outcome analysis, and included multiple imputation using chained equations, assuming all missing data were cleared and not cleared, excluding participants who did not have any positive baseline samples, excluding those who had not received their allocated treatment, and excluding those who did not have a full set of baseline samples.
Secondary outcomes were similarly analysed using appropriate regression models dependent on data type, adjusted for clinic site and baseline value of the outcome variable if collected. All participants who had follow-up data were included in the analyses of secondary outcomes. Clearance at each site was investigated separately for each infection site. MIC data were summarised per participant. The relationship between clinical effectiveness and MIC was examined by plotting the distribution of the highest MIC detected per participant categorised by clearance at all sites at 2 weeks.
Safety and tolerability analyses were descriptive; all participants who received treatment were included in the safety analyses. Frequency counts and percentages of the pre-specified main categories of side-effects were presented by treatment group. Adverse events were coded using MedDRA (version 17.1) and summarised by system organ class.
All analyses were done with Stata/SE 13.1. Full details of the analysis are documented in the statistical analysis plan, which was finalised before database lock and release of treatment allocation codes for analysis. An independent data monitoring committee oversaw the trial and had access to unblinded data by treatment group, prepared by a statistician who was independent to the trial team. This trial is registered with ISRCTN, number ISRCTN51783227.
Role of the funding source
The study was funded by the UK National Institute for Health Research (NIHR) Health Technology Assessment programme. NIHR had input into trial design through peer review of the funding proposal. The funders had no role in data collection, data analysis, data interpretation, or writing of the report but had sight of the paper prior to publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
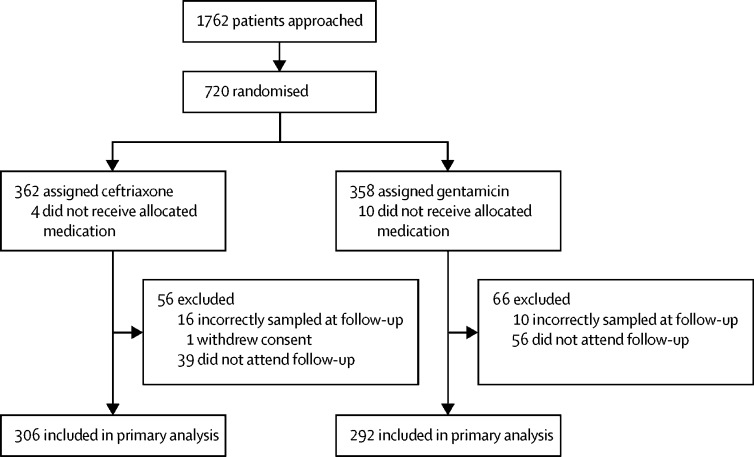
Of 1762 patients approached at 14 sexual health clinics in England, 720 were enrolled between Oct 7, 2014, and Nov 14, 2016 (362 were assigned to receive ceftriaxone and 358 to receive gentamicin). The main reasons for exclusion were participants not being interested, the trial taking too much time, a belief that the standard treatment would be successful and the trial taking too many extra or intrusive samples. 14 participants did not receive their allocated medication; four in the ceftriaxone group and ten in the gentamicin group (figure 1). 96 participants did not return for their follow-up visit. 26 participants who returned for their follow-up visit did not have primary outcome data because of incorrect sampling. Primary outcome data were therefore available for 306 (85%) of 362 participants allocated to ceftriaxone and 292 (82%) of 358 participants allocated to gentamicin.
Figure 1.
Trial profile
Baseline characteristics were well balanced across treatment groups (table 1). Treatment groups appeared to be balanced with respect to participants' history of sexually transmitted infections: 294 (41%) of 720 participants had at least one previous diagnosis of gonorrhoea, 248 (34%) of chlamydia, and 101 (14%) of syphilis; 4 (3%) of 135 women had a previous diagnosis of pelvic inflammatory disease.
Table 1.
Baseline characteristics of participants
| Ceftriaxone group (n=362) | Gentamicin group (n=358) | ||
|---|---|---|---|
| Mean age, years (SD) | 30·2 (10·1) | 30·4 (9·9) | |
| Gender | |||
| Female | 69 (19%) | 65 (18%) | |
| Male | 293 (81%) | 292 (82%) | |
| Other | 0 | 1 (<1%) | |
| Ethnicity | |||
| White | 241 (67%) | 255 (71%) | |
| Black | 53 (15%) | 48 (13%) | |
| Asian | 26 (7%) | 18 (5%) | |
| Mixed race | 27 (7%) | 26 (7%) | |
| Other | 15 (4%) | 11 (3%) | |
| Country of birth | |||
| UK | 258 (71%) | 253 (71%) | |
| Europe (excluding UK) | 51 (14%) | 56 (16%) | |
| North America | 8 (2%) | 5 (1%) | |
| Asia Pacific | 18 (5%) | 14 (4%) | |
| Latin America | 7 (2%) | 11 (3%) | |
| Middle East | 2 (1%) | 5 (1%) | |
| Africa | 18 (5%) | 14 (4%) | |
| Creatinine (μmol/L) | |||
| Mean (SD) | 78·6 (15·4) | 78·3 (15·8) | |
| Range | 42–137 | 26–154 | |
| n | 343 | 332 | |
| Estimated glomerular filtration rate | |||
| Mean (SD) | 110·6 (18·2) | 111·5 (17·7) | |
| Range | 56·3–179 | 52·4–157·7 | |
| n | 341 | 328 | |
| Medical history* | |||
| Diabetes | 3 (1%) | 1 (<1%) | |
| Otitis media | 9 (2%) | 7 (2%) | |
| Renal disease | 3 (1%) | 4 (1%) | |
| Liver disease | 8 (2%) | 5 (1%) | |
| Gonorrhoea | 152 (42%) | 142 (40%) | |
| Chlamydia | 121 (33%) | 127 (35%) | |
| Syphilis | 48 (13%) | 53 (15%) | |
| Pelvic inflammatory disease (women) | 2/69 (3%) | 2/65 (3%) | |
| HIV status (participant self-report) | |||
| Positive | 53 (15%) | 43 (12%) | |
| Unknown | 10 (3%) | 8 (2%) | |
| Sites of infection | |||
| Genital | 190 (52%) | 219 (61%) | |
| Pharyngeal | 128 (35%) | 128 (36%) | |
| Rectal | 159 (44%) | 147 (41%) | |
| Number of sites infected | |||
| One | 189 (52%) | 180 (50%) | |
| Two | 96 (27%) | 94 (26%) | |
| Three | 32 (9%) | 42 (12%) | |
| Positive diagnosis of gonorrhoea at baseline visit | 317 (87%) | 316 (88%) | |
| Positive diagnosis of gonorrhoea by Gram stain at baseline visit | 139/224 (38%) | 166/239 (46%) | |
| Positive diagnosis of gonorrhoea by nucleic acid amplification test at baseline visit† | 308/358 (86%) | 309/353 (88%) | |
Data are n (%) unless otherwise specified.
Medical history was based on the participant ever having had that condition.
Data not available for four participants in the ceftriaxone group and five in the gentamicin group.
Protocol deviations were reported in 121 (33%) of 362 of participants receiving ceftriaxone and in 124 (35%) of 358 participants receiving gentamicin, but the majority of these deviations were considered minor. Two major protocol deviations were identified: not receiving treatment according to randomisation (14 participants, four allocated to ceftriaxone, and ten allocated to gentamicin) and not fulfilling eligibility criteria (18 participants, five allocated to ceftriaxone and 13 allocated to gentamicin; appendix). The imbalance in the proportion of major protocol deviations was considered unlikely to be caused by selection bias or knowledge of treatment allocation, so these violations were not believed to affect the validity of the trial. Overall, 322 (89%) of 362 participants allocated to ceftriaxone and 302 (84%) of 358 participants allocated gentamicin attended their follow-up visit. The median time from randomisation to follow-up was 16 days (IQR 14–20) in the ceftriaxone group and 15 days (IQR 14–20) in the gentamicin group. 267 (83%) of 322 participants in the ceftriaxone group and 248 (82%) of 302 participants in the gentamicin group returned within 21 days.
At 2 weeks after treatment, infection had cleared (as defined by a negative NAAT) for 299 (98%) of 306 participants allocated to ceftriaxone compared with 267 (91%) of 292 participants allocated to gentamicin (adjusted risk difference −6·4%, 95% CI −10·4% to −2·4%; table 2). Sensitivity analyses were consistent with the primary analysis (figure 2).
Table 2.
Clearance of Neisseria gonorrhoeae at infected sites at 2 weeks
| Ceftriaxone group (n=362) | Gentamicin group (n=358) | Adjusted risk difference for clearance*(95% CI) | |
|---|---|---|---|
| Participants cleared at all sites | 299/306 (98%, 95–99) | 267/292 (91%, 88–94) | −6·4% (−10·4 to −2·4%) |
| Participants with genital gonorrhoea cleared | 151/154 (98%, 96–100) | 163/174 (94%, 90–97) | −4·4% (−8·7 to 0) |
| Participants with pharyngeal gonorrhoea cleared | 108/113 (96%, 92–99) | 82/102 (80%, 72–88) | −15·3% (−24·0 to −6·5) |
| Participants with rectal gonorrhoea cleared | 134/137 (98%, 95–100) | 107/119 (90%, 84–95) | −7·8% (−13·6 to −2·0) |
Data are n/N (%, 95% CI) unless otherwise specified.
Adjusted by recruiting site (for gentamicin group vs ceftriaxone group)
Figure 2.
Sensitivity analyses of Neisseria gonorrhoeae clearance at all sites
The red line indicates the −5% non-inferiority margin. BD=Becton Dickinson. AC=Aptima Combo. NAAT=nucleic acid amplification test. *Age, gender, ethnicity, country of birth, and past history of gonorrhoea were included in the multiple imputation with chained equations.
Of the 328 participants who had a genital infection, 151 (98%) of 154 in the ceftriaxone group and 163 (94%) of 174 in the gentamicin group had clearance at follow-up (table 2). For participants with a pharyngeal infection, a greater proportion receiving ceftriaxone had clearance at follow-up (108 [96%] in the ceftriaxone group compared with 82 [80%] in the gentamicin group). Similarly, a greater proportion of participants with rectal infection in the ceftriaxone group had clearance (134 [98%] in the ceftriaxone group compared with 107 [90%] in the gentamicin group). There was no difference between treatment groups in resolution of symptoms (table 3).
Table 3.
Resolution of symptoms present at baseline
| n (overall) | n (gentamicin group) | n (ceftriaxone group) | Adjusted risk difference*(95% CI) | |
|---|---|---|---|---|
| Genital discharge | 276 | 147 | 129 | −0·1% (−5·5 to 5·2) |
| Dysuria | 234 | 128 | 106 | −7·7% (−13·6 to 1·9) |
| Sore throat | 92 | 45 | 47 | 4·0% (−7·4 to 15·4) |
| Anorectal pain | 20 | 7 | 13 | −24·4% (−62·5 to 13·7) |
| Rectal bleeding | 15 | 7 | 8 | 12·5% (−10·4 to 35·4) |
| Rectal discharge | 20 | 8 | 12 | −9·9% (−43·7 to 23·9) |
| Tenesmus | 10 | 3 | 7 | 12·5% (−10·4 to 35·4) |
| Constipation | 15 | 4 | 11 | −12·6% (−57·8 to 32·6) |
| Intermenstrual bleeding (women only) | 14 | 5 | 9 | −9·4% (−9·4 to 31·6) |
Adjusted by clinic (for gentamicin group vs ceftriaxone group). Risk difference is unadjusted for rectal bleeding, tenesmus, and intermenstrual bleeding. No between-group difference could be measured for post-coital bleeding because of insufficient observations.
Changes in estimated glomerular filtration rate between baseline and follow-up were similar in both groups (median difference −1·3 mL/min [IQR −6·7 to 4.3] in the ceftriaxone group vs −1·4mL/min [IQR −6·9 to 3·7] in the gentamicin group). No between-group differences were calculated. A similar proportion of participants had nausea in the ceftriaxone and gentamicin groups. Vomiting, reduction in hearing, dizziness, unsteadiness, and skin rash were rare and proportions were similar across the two treatment groups (table 4). The majority of participants reported injection site pain, 98% of participants in the ceftriaxone group and 99% of participants in the gentamicin group, with the mean pain score higher in the gentamicin group (mean pain score 36 of 100 in the gentamicin group vs 21 of 100 in the ceftriaxone group). The median time to resolution of injection pain was 1 h (IQR 0–12) for ceftriaxone and 1·5 h (IQR 0–24) for gentamicin. At least one adverse event was reported by 15% of participants allocated to ceftriaxone and 13% of participants allocated to gentamicin, the majority of these were mild (83% of adverse events for ceftriaxone and 81% adverse events for gentamicin, table 4). Three adverse events were considered severe: grade 4 dizziness (ceftriaxone), diarrhoea (gentamicin), and sickness (gentamicin). One serious adverse event (grade 4 dizziness) was reported and was not considered to be related to the trial medication. In addition to the side effects participants were specifically asked about, 86 (14%) of 618 participants who received treatment (48 receiving ceftriaxone and 38 receiving gentamicin) reported at least one other adverse event, most commonly gastrointestinal disorders (14 of 54 events in the ceftriaxone group and 22 of 43 events in the gentamicin group; table 4).
Table 4.
Side-effects and adverse events
| Ceftriaxone group (n=320) | Gentamicin group (n=298) | ||
|---|---|---|---|
| Nausea | 38 (12%) | 41 (14%) | |
| Vomiting | 3 (1%) | 12 (4%) | |
| Reduction in hearing | 5 (2%) | 3 (1%) | |
| Dizziness or unsteadiness | 24 (7%) | 21 (7%) | |
| Skin rash | 5 (2%) | 12 (4%) | |
| Injection pain | 315 (98%) | 294 (99%) | |
| Participants with at least one adverse event | 48 (15%) | 38 (13%) | |
| Total number of adverse events | 54 | 43 | |
| Adverse event severity | |||
| Mild | 45/54 | 35/43 | |
| Moderate | 8/54 | 6/43 | |
| Severe | 1/54 | 2/43 | |
| Participants with at least one adverse event thought to be related to trial medication | 15 (5%) | 17 (6%) | |
| Total number of adverse events thought to be related to trial medication | 16 | 19 | |
| Serious adverse events | 1 (<1%) | 0 | |
| Most frequently reported adverse events (>5%) | |||
| Gastrointestinal disorders | 14/54 | 22/43 | |
| Nervous system disorders | 10/54 | 3/43 | |
| General disorders and administration site conditions | 6/54 | 3/43 | |
| Infections and infestations | 6/54 | 5/43 | |
Data are n (%) for the number of participants, or n/N for the number of adverse events. All side-effects and adverse events were self-reported by the participant. Adverse event categories are from MedDRA coding.
There were no differences between treatment groups with respect to additional medications (including antibiotics) taken during the trial, reported sexual behaviour, or condom use during the trial. We did not find a clear association between in-vitro gentamicin, ceftriaxone, or azithromycin MICs and the response to treatment, with the majority of treatment failures occurring in isolates expected to be susceptible according to EUCAST resistance breakpoints18 (figure 3).
Figure 3.
Pre-treatment MICs of gentamicin, ceftriaxone, and azithromycin
(A) Distribution of gentamicin MICs by treatment response in 132 participants who received gentamicin. (B) Distribution of ceftriaxone MICs by treatment response in 145 participants who received ceftriaxone. Azithromycin MICs for the four participants who did not clear were 0·125 mg/L (cervix), 0·125 mg/L (rectum), 0·125 mg/L (pharynx), and 0·25 mg/L (urethra). (C) Distribution of azithromycin MICs by treatment response in 276 participants who received azithromycin. MIC=minimum inhibitory concentration.
Discussion
Our study did not find that a single dose of gentamicin 240 mg was non-inferior to a single dose of ceftriaxone 500 mg for the treatment of gonorrhoea, when both drugs were combined with a 1 g dose of oral azithromycin. The trial was not designed to assess superiority, but the 6·4% greater clearance of infection in the ceftriaxone group and the consistency of the findings on sensitivity analyses suggest that ceftriaxone is than gentamicin for the microbiological cure of gonorrhoea. Clearance of infection with gentamicin was markedly lower for pharyngeal and rectal gonorrhoea, although gentamicin performed better for genital gonorrhoea, achieving microbiological cure in 94% of infections compared with 98% of infections for ceftriaxone.
Two systematic reviews10, 11 have reported wide variation in the efficacy of gentamicin for the treatment of gonorrhoea and noted a substantial risk of bias in previous studies. A more recent study15 evaluating intramuscular gentamicin 240 mg combined with oral azithromycin 2 g reported a 100% cure rate (95% CI 97·6–100). This study differed from G-ToG by including few women and only a small number of participants with pharyngeal and rectal infections, and by using cultures to diagnose gonorrhoea and a 2 g dose of azithromycin. The large number of extra-genital sites of infection analysed in G-ToG, with their associated lower cure rates, provides a partial explanation for the different treatment efficacies reported in previous studies.
Dual therapy with azithromycin 1 g did not prevent treatment failure in a substantial proportion of participants receiving gentamicin. Azithromycin monotherapy as a single dose of either 1 g or 2 g has been previously shown as an effective treatment for gonorrhoea,19 when culture was used to diagnose infection and assess cure. However, a reduced in vitro sensitivity to azithromycin has been reported in many geographical locations20, 21 and occurs in 5% of gonorrhoea infections in England and Wales;1 an outbreak of high-level resistance was recently reported in England.22
Most gonococcal isolates from participants in G-ToG (262 [96%] of 274) had azithromycin MICs of 0·5 mg/L or lower. Two (17%) of the 12 azithromycin-resistant isolates with MICs greater than 0·5 mg/L were from patients who had treatment failure, but the majority of treatment failures (11 [69%] of 16) occurred in participants who had isolates with a MIC of 0·25 mg/L or lower, with the remaining three [19%] harbouring azithromycin MICs of 0·5 mg/L (intermediate susceptibility). Thus, we found in vitro azithromycin resistance did not reliably predict treatment failure with the 1 g azithromycin dose if we assume gentamicin had failed to treat the infection. A poor association between pre-treatment azithromycin MIC and cure has been reported by others, with emergence of in vivo resistance.23, 24 A higher dose of azithromycin than the 1 g dose used in G-ToG (eg, 2 g)15 might be more effective, but without a direct comparative study this is speculative, and a 2 g dose is also poorly tolerated leading to nausea in 26% and vomiting in 10% of patients in a recent study.15 An extended-release formulation of azithromycin with lower peak drug concentrations might reduce the incidence of side effects and improve tolerability compared with the immediate-release formulation, but there are limited data comparing these formulations.
Current treatment guidelines from the US Centers for Disease Control and Prevention3 and WHO4 recommend dual therapies which incorporate azithromycin 1 g, to reduce the development of resistance in N gonorrhoeae by providing additional microbiological cover.25, 3, 4 In GToG we found substantial microbiological failure when 1 g of azithromycin was used as part of the dual therapy, suggesting that azithromycin component might not be achieving this microbiological cover, particularly in patients with extra-genital infections.
Both ceftriaxone and gentamicin were well tolerated when combined with azithromycin. Nausea was the most common side effect, occurring in 12% of participants receiving ceftriaxone plus azithromycin and in 14% of participants receiving gentamicin plus azithromycin. Nausea and vomiting are uncommon side-effects of ceftriaxone (incidence ≥1/1000 to <1/100 exposures) and have been reported in association with gentamicin, but are common following use of oral azithromycin (≥1/100 to <1/10 exposures). The gastrointestinal side-effects reported in G-ToG were likely principally caused by azithromycin, although the higher reported frequency of vomiting in those receiving gentamicin suggests that gentamicin might also have been a contributing factor. Gentamicin was associated with more injection site pain than ceftriaxone (mean pain score was 36 with gentamicin compared with 21 with ceftriaxone) and it took longer to resolve (median 1·5 h with gentamicin compared with 1 h with ceftriaxone); probably related to the larger volume of injection administered (6 mL for gentamicin vs 2 mL for ceftriaxone) and the local anaesthetic effect of lidocaine as the dissolving agent for ceftriaxone.
Gentamicin is potentially vestibulotoxic and can cause dizziness, ataxia, and nystagmus. Most previous gentamicin studies have evaluated a prolonged course of treatment and the safety of a single dose is less well characterised, but a recent systematic review26 of single dose therapy found vestibulotoxicity to be rare, which is consistent with our findings. Gentamicin can also cause renal impairment following reuptake of the drug in the proximal renal tubule where it is concentrated. A transient rise in creatinine is common when single dose gentamicin is used as antibiotic prophylaxis in elderly, surgical patients,26 but this rise is less likely to occur in younger, healthier individuals and the estimated glomerular filtration rate did not significantly change in G-ToG participants (mean difference −1·4 mL/min).
The mechanisms for development of gentamicin resistance are not fully understood but might include decreased cell membrane permeability and modification of the drug by cellular enzymes.27 In vitro measurement of the MIC provides a phenotypic assessment of antimicrobial susceptibility, but the breakpoint MIC value (below which clinical cure occurs and above which gentamicin is ineffective) has not been established. The extent to which gentamicin penetrates into rectal and pharyngeal tissue is not known but has been reported to be suboptimal in the pharynx for spectinomycin, which belongs to a similar antibiotic class.25 It has been tentatively suggested that an isolate with a MIC lower than 8 mg/L is susceptible, with a MIC of 8 to 16 mg/L has intermediate susceptibility, and a MIC greater than 16 mg/L is resistant.28 The European Network for Sexually Transmitted Infection Surveillance found that 95% of gonococcal isolates had gentamicin MICs in the range of 4 to 8 mg/L,12 similar to GToG participants after accounting for differences in testing methodology. The MIC in G-ToG participants was not, however, predictive of treatment failure; only three isolates had a MIC greater than 4 mg/L and all three participants were cleared of infection. Of those isolates with a MIC of 4 mg/L treated with gentamicin, 12 (13%) failed therapy compared with 81 (87%) which were cleared. It is possible that a higher dose of gentamicin would be more effective, although the limited association between gentamicin MIC and clinical response does not directly support this.
An antagonistic interaction between gentamicin and azithromycin could potentially reduce the efficacy of this drug combination; in vitro testing does not suggest either antagonism or synergy,29 and the potential for a high cure rate with this regimen for genital infections diagnosed by culture has been shown.15 A clinically important interaction between both drugs is therefore unlikely.
The robust design of the G-ToG trial resulted in well-balanced treatment groups and a low risk of bias. The trial was appropriately powered, pragmatic in design, and likely to be relevant to clinical practice in the UK and other countries with similar health-care systems. It included symptomatic and asymptomatic patients, a wide age range, HIV-positive and HIV-negative individuals, men and women, heterosexual men and men who have sex with men, cases of genital and extra-genital infections, and a wide variety of ethnic groups. The distribution of age, gender, ethnicity, and sites of infection for participants in G-ToG were comparable to those in the UK Gonoccocal Resistance to Antimicrobials Surveillance Programme suggesting that our results are widely applicable.
Unexpectedly, a number of participants who were recruited to the trial were found to have a negative NAAT at their baseline visit (50 [14%] of 358 participants in the ceftriaxone group and 44 [12%] of 353 in the gentamicin group) despite having been tested previously and found to be positive, and being recalled to the clinic to be given antibiotic treatment. In routine clinical practice a NAAT would not be repeated before treatment. The apparent spontaneous reversion from positive to negative NAAT observed in these trial participants could have resulted from an initial false positive NAAT before trial entry, a false negative NAAT at the baseline trial visit, or natural clearance of gonorrhoea without antibiotic therapy. A previous large study30 has reported spontaneous clearance of pharyngeal gonorrhoea in 139 (6%) of 2204 of patients, which would be consistent with our findings. However, although NAATs for N gonorrhoeae have high sensitivities and specificities we cannot exclude the possibility of some false positive or false negative results, especially when testing a low-prevalence population. The occurrence of negative tests in some patients at their baseline visit does not bias our results since they were equally distributed between the treatment groups, and a secondary sensitivity analyses excluding these participants (adjusted risk difference −7·1%, 95% CI −11·4% to −2·8%) was consistent with the primary intention-to-treat analysis. The NAAT test can remain positive for several days following effective treatment of gonorrhoea, but the test of cure was taken at least 14 days after receiving antibiotics in accordance with UK national guidance to minimise this possibility. Additionally, because of the randomised trial design, a false positive test-of-cure would not bias our results.
In conclusion, we found that gentamicin plus azithromycin cannot be considered non-inferior to ceftriaxone plus azithromycin, with a relatively higher frequency of treatment failure occurring in patients with extra-genital gonorrhoea who were treated with gentamicin. Gentamicin cannot therefore be recommended to replace ceftriaxone as first-line therapy for gonorrhoea. However, gentamicin combined with 1 g azithromycin achieved a cure rate of 94% for genital gonorrhoea and its use might be appropriate in patients who are allergic, intolerant, or harbour a ceftriaxone-resistant infection. A 1 g dose of azithromycin as a component of dual therapy for gonorrhoea had limited efficacy in treating gentamicin-resistant infections and this suggests that its widespread use to prevent development of resistance requires review.
This online publication has been corrected. The corrected version first appeared at thelancet.com on June 20, 2019
Acknowledgments
Acknowledgments
This project was funded by the NIHR Health Technology Assessment Programme (project number 12/127/10). The views and opinions expressed in this Article are those of the authors and do not necessarily reflect those of the Health Technology Assessment Programme, NIHR, NHS or the UK Department of Health. The trial was sponsored by the University of Nottingham, coordinated from the Nottingham Clinical Trials Unit, and supported by the NIHR Clinical Research Network. We wish to thank the study investigators, Nottingham Clinical Trials Unit staff, site staff, and study participants. We would particularly like to thank our co-applicants and acknowledge the contributions of the members of the trial steering committee: Prof Judith Stevenson (Chair) Professor of Reproductive and Sexual Health, University College London; Prof John McLeod Professor in Clinical Epidemiology and Primary Care, University of Bristol; Dr Andy Winter, Consultant in Sexual Health and HIV, NHS Greater Glasgow and Clyde; David Roberts-Jones, Patient Representative. And the data monitoring committee Prof Chris Butler, (Chair) Professor of Primary Care, University of Oxford; Dr Mike Bradburn, Senior Statistician, University of Sheffield; Prof Charles Lacey, University of York. We would also like to thank all study sites: Barts Health NHS Trust, Burrell Street Clinic, Chelsea and Westminster Hospital NHS Foundation Trust, Coventry and Warwickshire Partnership Trust, John Hunter Clinic, Chelsea and Westminster Hospital NHS Trust, Manchester Royal Infirmary, Central Manchester University Hospitals NHS Foundation Trust, Royal Berkshire NHS Foundation Trust, Royal Free London NHS Foundation Trust, Sheffield Teaching Hospitals NHS Foundation Trust, St James University Hospital, Leeds Teaching Hospitals NHS Trust, St Mary's Hospital, Imperial College Healthcare NHS Trust, University Hospitals Birmingham NHS Foundation Trust, Whittall Street Clinic, Western Community Hospital, Solent NHS Trust, West Middlesex Clinic, West Middlesex University Hospital NHS Trust, Chelsea and Westminster Hospital NHS Trust.
Contributors
JDCR was the chief investigator and conceived the study. JDCR, AAM, LD, TH, LJ, and TER contributed to the design of the study. JDCR, CD, JWh, and JW were principal investigators at recruiting sites. MK was responsible for patient and public input to delivery of the trial. CB, JH, TL, KS, and ST were responsible for managing the trial. WT, TH, and AAM were responsible for the statistical analysis plan and carried out the statistical analyses. LJ and TER were responsible for the health economic component. MC was responsible for the microbiological component of the trial at Public Health England. JDCR, WT, KS, TH, LJ, and MC drafted the manuscript. All authors assisted with interpretation of the data and reviewed and approved the final manuscript.
Declaration of interests
JDCR reports personal fees from GlaxoSmithKline, Hologic Diagnostics, Tallis, and Janssen Pharmaceutica outside the submitted work, as well as ownership of shares in GlaxoSmithKline and AstraZeneca. He is an author of the United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease and European Guideline for the Management of Pelvic Inflammatory Disease. He is also a member of the editorial board for the European sexually transmitted infection guidelines and of the National Institute for Health Research (NIHR) Health Technology Assessment commissioning board; was previously a member of the NIHR Health Technology Assessment primary care, community and preventative interventions panel (2013–2016); and is a NIHR Journals Editor. AAM is a member of the NIHR Health Technology Assessment clinical evaluation and trials board. JW reports non-financial support from Gen-Probe (Hologic) and personal fees from Becton Dickinson outside the submitted work. JWh reports personal fees from Hologic, GlaxoSmithKline, and Becton Dickinson outside the submitted work, as well as personal fees from SAGE Publications. He is also Editor-in-Chief of the International Journal of STD & AIDS. TH reports ownership of shares in AstraZeneca. During the trial LD was the Director of the Nottingham Clinical Trials Unit, a unit with NIHR Clinical Trials Unit Support Funding. All other authors declare no competing interests.
Supplementary Material
References
- 1.Public Health England Surveillance of antimicrobial resistance in Neisseria gonorrhoeae in England and Wales: key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) October, 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/651636/GRASP_Report_2017.pdf; Public Health England. Surveillance of antimicrobial resistance in Neisseria gonorrhoeae in England and Wales: key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP). October, 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/651636/GRASP_Report_2017.pdf (accessed April 27, 2018).
- 2.Fifer H, Saunders J, Soni S, Sadiq T, FitzGerald M. National guideline for the management of infection with Neisseria gonorrhoeae in adults (2019). British Association for Sexual Health and HIV. https://www.bashhguidelines.org/media/1208/gc-2019.pdf [DOI] [PubMed]; Fifer H, Saunders J, Soni S, Sadiq T, FitzGerald M. National guideline for the management of infection with Neisseria gonorrhoeae in adults (2019). British Association for Sexual Health and HIV. https://www.bashhguidelines.org/media/1208/gc-2019.pdf (accessed March 20, 2019).
- 3.Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. http://www.cdc.gov/std/tg2015/default.htm; Centers for Disease Control and Prevention. 2015 sexually transmitted diseases treatment guidelines. http://www.cdc.gov/std/tg2015/default.htm (accessed Feb 14, 2018).
- 4.WHO . World Health Organization; Geneva: 2016. WHO guidelines for the treatment of Neisseria gonorrhoeae. [PubMed] [Google Scholar]; WHO. WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva: World Health Organization, 2016. [PubMed]
- 5.Fifer H, Natarajan U, Jones L. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med. 2016;374:2504–2506. doi: 10.1056/NEJMc1512757. [DOI] [PubMed] [Google Scholar]; Fifer H, Natarajan U, Jones L, et al. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 2016; 374: 2504–06. [DOI] [PubMed]
- 6.Golparian D, Ohlsson A, Janson H. Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.30.20862. pii:20862. [DOI] [PubMed] [Google Scholar]; Golparian D, Ohlsson A, Janson H, et al. Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014. Euro Surveill 2014; 19: pii:20862. [DOI] [PubMed]
- 7.Alirol E, Wi TE, Bala M. Multidrug-resistant gonorrhea: a research and development roadmap to discover new medicines. PLoS Med. 2017;14:e1002366. doi: 10.1371/journal.pmed.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alirol E, Wi TE, Bala M, et al. Multidrug-resistant gonorrhea: a research and development roadmap to discover new medicines. PLoS Med 2017; 14: e1002366. [DOI] [PMC free article] [PubMed]
- 8.European Centre for Disease Prevention and Control Response plan to control and manage the threat of multidrug-resistant gonorrhoea in Europe. June, 2012. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/1206-ECDC-MDR-gonorrhoea-response-plan.pdf; European Centre for Disease Prevention and Control. Response plan to control and manage the threat of multidrug-resistant gonorrhoea in Europe. June, 2012. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/1206-ECDC-MDR-gonorrhoea-response-plan.pdf (accessed Feb 13, 2018).
- 9.WHO . World Health Organization; Geneva: 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. [Google Scholar]; WHO. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Geneva: World Health Organization, 2012.
- 10.Dowell D, Kirkcaldy RD. Effectiveness of gentamicin for gonorrhoea treatment: systematic review and meta-analysis. Sex Transm Infect. 2012;88:589–594. doi: 10.1136/sextrans-2012-050604. [DOI] [PubMed] [Google Scholar]; Dowell D, Kirkcaldy RD. Effectiveness of gentamicin for gonorrhoea treatment: systematic review and meta-analysis. Sex Transm Infect 2012; 88: 589–94. [DOI] [PubMed]
- 11.Hathorn E, Dhasmana D, Duley L, Ross JD. The effectiveness of gentamicin in the treatment of Neisseria gonorrhoeae: a systematic review. Syst Rev. 2014;3:104. doi: 10.1186/2046-4053-3-104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hathorn E, Dhasmana D, Duley L, Ross JD. The effectiveness of gentamicin in the treatment of Neisseria gonorrhoeae: a systematic review. Syst Rev 2014; 3: 104. [DOI] [PMC free article] [PubMed]
- 12.Chisholm SA, Quaye N, Cole MJ. An evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Europe. J Antimicrob Chemother. 2011;66:592–595. doi: 10.1093/jac/dkq476. [DOI] [PubMed] [Google Scholar]; Chisholm SA, Quaye N, Cole MJ, et al. An evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Europe. J Antimicrob Chemother 2011; 66: 592–95. [DOI] [PubMed]
- 13.Kahlmeter G, Dahlager JI. Aminoglycoside toxicity—a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1984;13(suppl A):9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]; Kahlmeter G, Dahlager JI. Aminoglycoside toxicity—a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother 1984; 13 (suppl A): 9–22. [DOI] [PubMed]
- 14.Judson FN, Ehret JM, Handsfield HH. Comparative study of ceftriaxone and spectinomycin for treatment of pharyngeal and anorectal gonorrhea. JAMA. 1985;253:1417–1419. [PubMed] [Google Scholar]; Judson FN, Ehret JM, Handsfield HH. Comparative study of ceftriaxone and spectinomycin for treatment of pharyngeal and anorectal gonorrhea. JAMA 1985; 253: 1417–19. [PubMed]
- 15.Kirkcaldy RD, Weinstock HS, Moore PC. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083–1091. doi: 10.1093/cid/ciu521. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 2014; 59: 1083–91. [DOI] [PMC free article] [PubMed]
- 16.Brittain C, Childs M, Duley L. Gentamicin versus ceftriaxone for the treatment of gonorrhoea (G-TOG trial): study protocol for a randomised trial. Trials. 2016;17:558. doi: 10.1186/s13063-016-1683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brittain C, Childs M, Duley L, et al. Gentamicin versus ceftriaxone for the treatment of gonorrhoea (G-TOG trial): study protocol for a randomised trial. Trials 2016; 17: 558. [DOI] [PMC free article] [PubMed]
- 17.British Association for Sexual Health and HIV Clinical Effectiveness Group guidelines. https://www.bashh.org/guidelines; British Association for Sexual Health and HIV. Clinical Effectiveness Group guidelines. https://www.bashh.org/guidelines (accessed June 20, 2017).
- 18.European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters: version 7.1. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf; European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters: version 7.1. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf (accessed June 5, 2017).
- 19.Bignell C, Garley J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex Transm Infect. 2010;86:422–426. doi: 10.1136/sti.2010.044586. [DOI] [PubMed] [Google Scholar]; Bignell C, Garley J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex Transm Infect 2010; 86: 422–26. [DOI] [PubMed]
- 20.Barbee LA, Soge OO, Dombrowski JC, Katz DA, Holmes KK, Golden MR. Azithromycin-resistant Neisseria gonorrhoeae in men who have sex with men (MSM) in Seattle, Washington: 2014–2015. Sex Transm Infect. 2015;91:A25. [Google Scholar]; Barbee LA, Soge OO, Dombrowski JC, Katz DA, Holmes KK, Golden MR. Azithromycin-resistant Neisseria gonorrhoeae in men who have sex with men (MSM) in Seattle, Washington: 2014–2015. Sex Transm Infect 2015; 91: A25.
- 21.Ni C, Xue J, Zhang C, Zhou H, van der Veen S. High prevalence of Neisseria gonorrhoeae with high-level resistance to azithromycin in Hangzhou, China. J Antimicrob Chemother. 2016;71:2355–2357. doi: 10.1093/jac/dkw131. [DOI] [PubMed] [Google Scholar]; Ni C, Xue J, Zhang C, Zhou H, van der Veen S. High prevalence of Neisseria gonorrhoeae with high-level resistance to azithromycin in Hangzhou, China. J Antimicrob Chemother 2016; 71: 2355–57. [DOI] [PubMed]
- 22.Fifer H, Cole M, Hughes G. Sustained transmission of high-level azithromycin-resistant Neisseria gonorrhoeae in England: an observational study. Lancet Infect Dis. 2018;18:573–581. doi: 10.1016/S1473-3099(18)30122-1. [DOI] [PubMed] [Google Scholar]; Fifer H, Cole M, Hughes G, et al. Sustained transmission of high-level azithromycin-resistant Neisseria gonorrhoeae in England: an observational study. Lancet Infect Dis 2018; 18: 573–81. [DOI] [PubMed]
- 23.Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill. 2011;16 pii:19833. [PubMed] [Google Scholar]; Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill 2011; 16: pii:19833. [PubMed]
- 24.Soge OO, Harger D, Schafer S. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011) Sex Transm Dis. 2012;39:877–879. doi: 10.1097/OLQ.0b013e3182685d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]; Soge OO, Harger D, Schafer S, et al. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011). Sex Transm Dis 2012; 39: 877–79. [DOI] [PMC free article] [PubMed]
- 25.Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis. 1995;20(suppl 1):S47–S65. doi: 10.1093/clinids/20.supplement_1.s47. [DOI] [PubMed] [Google Scholar]; Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis 1995; 20 (suppl 1): S47–65. [DOI] [PubMed]
- 26.Hayward RS, Harding J, Molloy R. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br J Clin Pharmacol. 2018;84:223–238. doi: 10.1111/bcp.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hayward RS, Harding J, Molloy R, et al. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br J Clin Pharmacol 2018; 84: 223–38. [DOI] [PMC free article] [PubMed]
- 27.Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother. 1999;43:727–737. doi: 10.1128/aac.43.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother 1999; 43: 727–37. [DOI] [PMC free article] [PubMed]
- 28.Brown LB, Krysiak R, Kamanga G. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex Transm Dis. 2010;37:169–172. doi: 10.1097/OLQ.0b013e3181bf575c. [DOI] [PubMed] [Google Scholar]; Brown LB, Krysiak R, Kamanga G, et al. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex Transm Dis 2010; 37: 169–72. [DOI] [PubMed]
- 29.Wind CM, de Vries HJC, van Dam AP. Determination of in vitro synergy for dual antimicrobial therapy against resistant Neisseria gonorrhoeae using Etest and agar dilution. Int J Antimicrob Agents. 2015;45:305–308. doi: 10.1016/j.ijantimicag.2014.10.020. [DOI] [PubMed] [Google Scholar]; Wind CM, de Vries HJC, van Dam AP. Determination of in vitro synergy for dual antimicrobial therapy against resistant Neisseria gonorrhoeae using Etest and agar dilution. Int J Antimicrob Agents 2015; 45: 305–08. [DOI] [PubMed]
- 30.Hananta IPY, De Vries HJC, van Dam AP, van Rooijen MS, Soebono H, Schim van der Loeff MF. Persistence after treatment of pharyngeal gonococcal infections in patients of the STI clinic, Amsterdam, the Netherlands, 2012–2015: a retrospective cohort study. Sex Transm Infect. 2017;93:467–471. doi: 10.1136/sextrans-2017-053147. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hananta IPY, De Vries HJC, van Dam AP, van Rooijen MS, Soebono H, Schim van der Loeff MF. Persistence after treatment of pharyngeal gonococcal infections in patients of the STI clinic, Amsterdam, the Netherlands, 2012–2015: a retrospective cohort study. Sex Transm Infect 2017; 93: 467–71. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.