Abstract
Background
Variability in standard-of-care classifications precludes accurate predictions of early tumor recurrence for individual patients with meningioma, limiting the appropriate selection of patients who would benefit from adjuvant radiotherapy to delay recurrence. We aimed to develop an individualized prediction model of early recurrence risk combining clinical and molecular factors in meningioma.
Methods
DNA methylation profiles of clinically annotated tumor samples across multiple institutions were used to develop a methylome model of 5-year recurrence-free survival (RFS). Subsequently, a 5-year meningioma recurrence score was generated using a nomogram that integrated the methylome model with established prognostic clinical factors. Performance of both models was evaluated and compared with standard-of-care models using multiple independent cohorts.
Results
The methylome-based predictor of 5-year RFS performed favorably compared with a grade-based predictor when tested using the 3 validation cohorts (ΔAUC = 0.10, 95% CI: 0.03–0.018) and was independently associated with RFS after adjusting for histopathologic grade, extent of resection, and burden of copy number alterations (hazard ratio 3.6, 95% CI: 1.8–7.2, P < 0.001). A nomogram combining the methylome predictor with clinical factors demonstrated greater discrimination than a nomogram using clinical factors alone in 2 independent validation cohorts (ΔAUC = 0.25, 95% CI: 0.22–0.27) and resulted in 2 groups with distinct recurrence patterns (hazard ratio 7.7, 95% CI: 5.3–11.1, P < 0.001) with clinical implications.
Conclusions
The models developed and validated in this study provide important prognostic information not captured by previously established clinical and molecular factors which could be used to individualize decisions regarding postoperative therapeutic interventions, in particular whether to treat patients with adjuvant radiotherapy versus observation alone.
Keywords: methylation, meningioma, nomogram, predictor, recurrence
Key Points.
Combining DNA methylation with clinical factors results in reliable individualized estimations of recurrence risk.
Individualized recurrence risk can be used to guide decisions for postoperative therapeutic interventions.
Importance of the Study.
Our work is the first to demonstrate the transformative utility of integrating clinical and molecular factors for use beyond simple classification into the realm of individualized prognostication for any brain tumor. Using our developed and validated tools that are publicly available, clinicians will be able combine clinical and molecular factors to determine an individualized probability of recurrence for patients with meningiomas. This represents a major advance in the field of personalized medicine for neuro-oncology, and the use of this tool can help clinicians overcome one of the most challenging limitations we face when treating patients with meningiomas.
Meningiomas are the most common primary intracranial tumor. They account for 37% of all central nervous system tumors and are continuing to increase in incidence with the aging population.1 They result in significant neurological morbidity and loss of quality of life by exerting mass effect on critical adjacent brain regions.1 The current standard of care for nearly all patients with symptomatic meningiomas includes gross total tumor resection with removal of involved dura and bone when possible.2 However, despite radical surgical resection, approximately 20% of meningiomas display aggressive behavior, with early tumor recurrence resulting in a clinical course of repetitive disease- and treatment-related morbidity. Radiation therapy can be used to provide disease control as an adjunct to surgery for a subset of tumors.3,4 However, radiotherapy can often result in adverse radiation effects that lead to considerable morbidity and neurological dysfunction long term, precluding universal use in all patients.5 One of the greatest clinical challenges faced by clinicians is the inability to predict early tumor recurrence at an individual patient level, which limits the appropriate selection of patients who would benefit from adjuvant radiation therapy.
To date, the most reliable clinical factors associated with recurrence in meningiomas have been the World Health Organization (WHO) grade of the tumor and extent of tumor resection at surgery.2,6 Although both are crudely associated with recurrence rates on a population level, they are challenged with interrater variability of grading and considerable within-grade variation of recurrence risk for individual patients.2,6 In the past decade, several studies have focused on molecular profiling of meningiomas to refine biological subgroups.7–13 With the exception of mutations in BAP1 (breast cancer associated protein 1) and telomerase reverse transcriptase (TERT) promoter, each of which occurs rarely in these tumors, the mutations identified in meningioma have not been shown to be tightly correlated to patient outcome with current standard of care.7–10 We and others have independently shown that global DNA methylation profiling reveals robust methylome-based meningioma subtypes; however, the clinical translation of this to predict recurrence risk for individual patients has not been demonstrated to date.
To examine whether methylation profiles can be defined and validated for clinical utility, we aimed to develop and validate a methylome-based predictor of early meningioma recurrence that could be combined with established prognostic clinical factors to individualize decisions regarding the need for postoperative therapeutic interventions—in particular, whether to treat patients with adjuvant radiation therapy versus observation alone. Our work is the first in neuro-oncology to demonstrate the transformative utility of integrating clinical and molecular factors for use beyond simple classification into the realm of individualized prognostication.
Materials and Methods
Study Design and Data Sources
This multicenter retrospective study was carried out in accordance with individual institutional ethics and review board guidelines and comprised a total of 486 patients with clinically annotated and available meningioma samples. Institutional waivers of informed consent were obtained due to minimal patient risk associated with this study. Two-hundred and eighty-two (N = 282) fresh frozen or formalin fixed paraffin embedded (FFPE) meningioma tumor samples from multiple institutions (N = 76 from Princess Margaret Cancer Research Centre; N = 206 from European centers, including University of Heidelberg, Goethe-University, University of Tubingen, University Hospital Zurich, and Medical University of Vienna) composed the discovery cohort, which was split into a training cohort (81%, N = 228 samples) and a first validation cohort (19%, N = 54 samples), each balanced for tumor grade, tissue type, recurrence status, and time to recurrence. One hundred and forty (N = 140) FFPE meningioma tumor samples from a separate institution (The MD Anderson Cancer Center) were used as a second validation cohort, and N = 64 fresh frozen meningioma tumor samples from 2 other institutions were used as a third validation cohort (N = 46 from Princess Margaret Cancer Research Centre; N = 18 from The Chinese University of Hong Kong). The sample sets from Europe and MD Anderson composed the subset of previously published samples for which clinical data (recurrence-free survival [RFS], WHO grade) were available.11,12 Moreover, TERT promoter mutation status was available on a subset of previously published European samples.10,11 Gene expression analysis was performed on publicly available microarray data on 98 patients with meningiomas of all grades (GSE1658114 and GSE943815). The outline for the overall study design is demonstrated in Supplementary Fig. 1
Definitions
Hematoxylin and eosin (H&E) slides for each patient were reviewed for meningioma diagnosis, and WHO grading was performed according to the current WHO 2016 criteria at local institutions by experienced neuropathologists. Tumor recurrence and time to recurrence were the primary outcomes of interest and were collected locally for each sample as previously described.11,12 Briefly, recurrence was defined as tumor growth following gross total resection or tumor progression following subtotal resection. Time to recurrence was determined by reviewing postoperative imaging and calculating the duration from the date of surgery to first postoperative imaging documenting tumor recurrence in concordance with documentation in the medical charts. The extent of resection (Simpson grade2) was determined based on the surgeon’s operative report in correlation with postoperative cranial imaging.
Generation of an Individualized Methylome Predictor of 5-Year RFS
For DNA methylation and copy number analysis of the samples, DNA was extracted from each tumor and DNA methylation profiling was performed using Illumina 450k HumanMethylation BeadChip or 850k EPIC arrays as per manufacturer instructions at each institution. Raw data files (*.idat) were imported, processed, and normalized to integrate data from multiple generations of Infinium methylation arrays. Copy number aberrations were inferred from methylation array data,16 and burden of copy number alterations was computed per sample as previously described.17 Probes that were common in both 450k HumanMethylation Beadchip and 850k EPIC arrays were selected as possible features for development of our predictor such that our predictor would be applicable to the landscape of available technologies. We used a multi-step strategy to select the probes to be used in the generation of our predictor (see Supplementary Methods and Supplementary Fig. 2).
To develop the methylome-based predictor of early meningioma recurrence, we performed generalized boosted regression modeling using the final selected probes in samples from the training cohort to predict 5-year RFS. Boosted regression modeling using WHO grade as the sole feature in the training cohort was also performed and tested in each validation cohort to compare methylation-based predictor performance with a standard-of-care model. Performance of both models was assessed by generating time-dependent receiver operating characteristic (ROC) curves and computing average areas under the ROC curves (AUCs) for each validation cohort independently, along with their 95% confidence intervals using the bootstrap resampling method with 10 000 resamples.18
Methylation probe annotation was performed using the University of California Santa Cruz Genome Browse (GRCh38/hg38 assembly). We used the Functional Annotation Clustering algorithm19 of DAVID (Database for Annotation, Visualization, and Integrated Discovery)19 Bioinformatics Resource 6.8 to identify redundant functional clusters represented by genes annotated with a minimum of 5 probes (see Supplementary Fig. 3). Two publicly available microarray datasets (GSE1658114 and GSE943815) reporting on 22 486 genes for 98 patients with meningiomas were pooled to correlate methylation data with gene expression data as an exploratory analysis.
Further details regarding the steps for generation, validation, and characterization of the methylome predictor of 5-year RFS can be found in the Supplementary Methods.
Generation of a Meningioma Recurrence Score
To create a contemporary meningioma recurrence score that could be utilized by clinicians to predict early risk of recurrence for individual patients, we generated a nomogram based on a Cox model that incorporated the methylome-based predictor, WHO grade, and extent of resection using samples from the training cohort and second validation cohort to predict 5-year RFS. Both the training cohort and second validation cohort were used to train this nomogram in order to increase the number of samples available to capture the heterogeneity in the spectrum of data available. This is important to note, as none of the samples to train this model were used to assess model performance on external validation. Global performance of the meningioma recurrence was assessed by generating time-dependent ROC curves and computing average AUC for the first and third validation cohort independently, along with their 95% CIs using the bootstrap resampling method with 10 000 resamples.18,20 For comparison, a nomogram that incorporated only WHO grade and extent of resection as the sole features was also developed in similar fashion. Internal validation using bootstrap resampling of 10 000 resamples was also performed. Model calibration was assessed visually by plotting observed event rates against nomogram predicted probabilities for 2 risk groups. Detailed descriptions on nomogram calculations and use are described in the Supplementary Methods and Supplementary Fig. 4.
Statistical Analysis
Summary statistics are reported as counts (and proportions) for categorical variables and median (and range) for continuous variables, unless otherwise indicated. Cohort size was determined by availability of samples. Statistical analyses were performed in consultation with 2 expert biostatisticians (L.P. and O.S.).
To investigate the clinical relevance of the methylome-based predictor and meningioma recurrence score, distribution of survival times was performed using Kaplan–Meier methods and compared across groups using log-rank testing. The frequency of genome-wide copy number alterations across groups was computed and plotted using a custom algorithm. The performance of a methylome-based predictor was compared with a grade-based predictor by computing an average ΔAUC (AUCmethylome − AUCgrade) and 95% CI from all bootstraps, and the performance of the meningioma recurrence score incorporating the methylome predictor was compared with a nomogram excluding the methylome predictor by computing average ΔAUC (AUCcombined nomogram –AUCclinical only nomogram) and 95% CI in similar fashion.
Hazard ratios (HRs), including 95% CIs, were calculated based on univariable and multivariable Cox regression modeling for the methylome-based predictor and other covariates, including WHO grade, extent of resection, and burden of copy number alterations. Proportional hazards assumption was tested by computing Schoenfeld residuals for each covariate, and testing was performed according to Grambsch and Therneau21 (Supplementary Fig. 5 and Supplementary Table 1). Martingale residuals were plotted against methylation predictor probabilities to determine appropriateness of linear modeling (Supplementary Fig. 6). Sensitivity analyses evaluating the possible confounding effects of TERT promoter mutations, receipt of adjuvant radiation therapy, and center effects were performed using multivariable Cox regression in samples with available information. Comparison of proportions across groups was completed using the χ2 test and Fisher’s exact test, where appropriate.
Two-sided P-values are reported, and the threshold for statistical significance was set a priori at α = 0.05. We used R v3.3.1 for all statistical analyses, model generation, and model validation.
Results
A set of 9529 probes were selected from an initial training cohort, and generalized boosted regression modeling was performed to develop a DNA methylation-based predictor of 5-year recurrence risk in meningioma (Supplementary Fig. 1). Three validation cohorts (Supplementary Table 2) were used to test the performance of the methylation-based predictor compared with a grade-based predictor.
Validation of a Methylome Predictor of Early Meningioma Recurrence
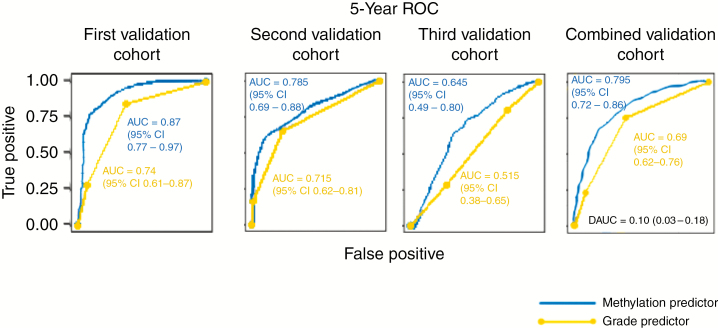
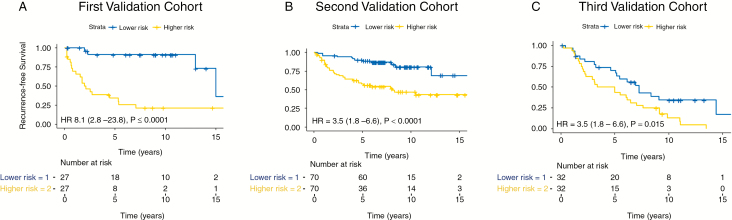
The methylome-based predictor performed favorably compared with the grade-based predictor at 5 years in each of the 3 validation cohorts (Fig. 1), with substantial statistical improvements in performance when tested in all combined validation cohorts (ΔAUC = 0.10, 95% CI: 0.03–0.18). When stratified by median, the 5-year methylome predictor distinguished risk groups (lower and higher risk) in all 3 validation cohorts (Fig. 2). Patients in the higher risk group had a median RFS of 2.1 years, 8.1 years, and 4.2 years in the first, second, and third validation cohorts, respectively, compared with patients in the lower risk groups, which had median RFS of “unreached” in the first and second validation cohorts and median RFS of 7.2 years in the third validation cohort (HR 8.1, 95% CI: 2.8–23.8; HR 3.5, 95% CI: 1.8–6.6, and HR 2.0, 95% CI: 1.2–3.7, respectively).
Fig. 1.
Comparison of grade-based and methylome-based RFS predictor performance. Data presented are time-dependent ROC curves and average AUC as well as ΔAUC (DAUC) with 95% CI using 10 000 bootstrap resampling validation approach for methylome-based and grade-based predictors in the (A) first validation cohort, (B) second validation cohort, (C) third validation cohort, and (D) combined validation cohorts.
Fig. 2.
RFS analysis of the first validation cohort (A), second validation cohort (B), and (C) third validation cohort using the 5-year methylome-based RFS predictor, based on separation into distinct risk groups by median.
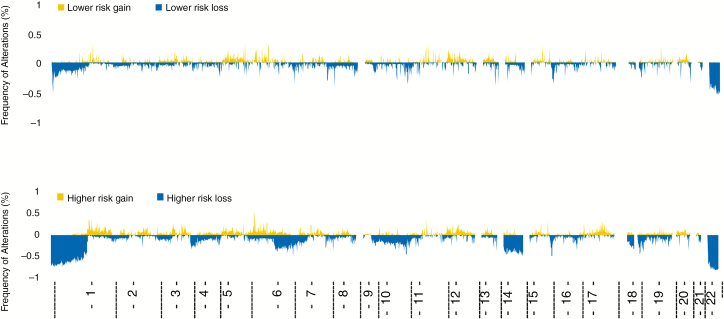
DNA copy number analysis demonstrated increased frequency of copy number aberrations in the higher risk groups by a high proportion of chromosomal deletions in 1p, 4p, 6q, 10q, 14q, and 18q (Fig. 3). The total burden of copy number alterations was also correlated with risk groups, with greatest proportion of burden of copy number alterations found in the higher risk groups in all 3 validation cohorts (Supplementary Table 3). It is noteworthy that of all patients with a high burden of copy number aberrations from all 3 validation cohorts, only 12 patients (4.6%) were in the lower risk group.
Fig. 3.
Frequency of copy number alterations across the genome stratified in risk groups according to the methylome-based predictor. Groups correspond to same groups seen in Fig. 2.
Multivariable Cox regression analysis demonstrated that the 5-year methylome-based predictor was independently associated with RFS in samples from all validation cohorts (HR 3.6, 95% CI: 1.8–7.2, P < 0.001) after controlling for tumor grade, extent of resection, and burden of copy number alterations (Supplementary Table 4). Sensitivity analyses including receipt of adjuvant therapy, TERT promoter mutations, and center of treatment as covariates did not alter this relationship and these covariates were not independently associated with RFS (Supplementary Tables 5–7).
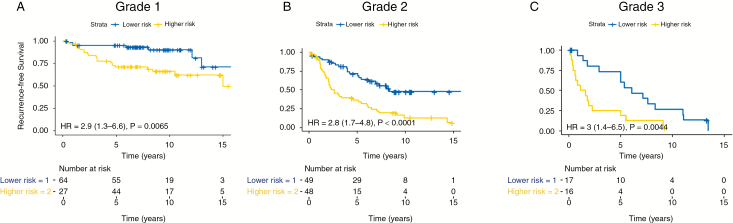
Current clinical practice relies on histopathologic grade to inform the decision of postsurgical management of meningioma. Patients with grade I tumors are most commonly monitored with serial imaging after surgery, while patients with grade II and III tumors are considered for adjuvant therapy, such as radiation, to prevent recurrence. There is, however, unexplained clinical variability in RFS within all grades of meningioma, and we examined the use of the 5-year methylome predictor to address this issue. Among patients with WHO grade II tumors in all validation cohorts, the median RFS for the higher risk group was 2.6 years compared with a median RFS of 8.4 years in the lower risk group (HR 2.8, 95% CI: 1.7–4.8, P < 0.001; Fig. 4B). Patients with WHO grade I tumors in all validation cohorts also had increased risk for recurrence in the higher risk group (HR 2.9, 95% CI: 1.3–6.6, P = 0.006; Fig. 4A), with only 3 patients in the lower risk group recurring within the first 5 years compared with 18 patients in the higher risk group. Lastly, patients with WHO grade III tumors from all validation cohorts had poor median RFS in the higher risk group (1.3 y), compared with a median RFS of 6.0 years in the lower risk group (HR 3.0, 95% CI: 1.4–6.5, P = 0.004; Fig. 4C).
Fig. 4.
Survival analysis of all WHO grade I (A), WHO grade II (B) and WHO grade III (C) tumors in all 3 validation cohorts stratified by methylation-based predictor risk groups.
Characterization of Predictor Cytosine-Phosphate-Guanine Sites
The selected 9529 probes used in our model make up only 1% of all probes included on the 850k Illumina Array. These probes were enriched to be found in the promoter regions (N = 3057, 32.1%) and located on cytosine-phosphate-guanine (CpG) islands (N = 4633, 48.6%) compared with all probes found on the 850k Illumina Array (29.6%, P < 0.001 and 18.0%, P < 0.001, respectively; Supplementary Table 8). Of the 9529 probes, only 1261 (13.2%) were “favorable,” associated with lower risk of recurrence when methylated (HR ranging from 0.002 to 0.38 on univariable Cox regression with associated P < 0.001). The remaining 8237 (86.8%) probes were “unfavorable,” associated with higher risk of recurrence when methylated (HR ranging from 2.45 to 517.82 on univariable Cox regression with associated P < 0.001).
There were 2332 probes annotated to 294 genes with at least 5 probes represented per gene. Of these (Supplementary Table 9), only 68 of 2332 probes (2.9%) were found to be “favorable” (associated with lower recurrence risk when methylated, HR ranging from 0.008 to 0.252 on univariable Cox regression with associated P < 0.001). The remaining 2265 probes (97.1%) were found to be “unfavorable,” associated with greater recurrence risk (associated with higher recurrence risk when methylated, HR ranging from 2.95 to 517.82 on univariable Cox regression with associated P < 0.001). Functional annotation clustering of these 2265 “unfavorable” probes revealed that homeobox (enrichment score = 54.33) and T-box (enrichment score = 4.12) were highly significant redundant functional clusters (Supplementary Fig. 3). Gene expression analysis of the homeobox family of genes and T-box genes for which methylation data were also available demonstrated that although these genes were relatively hypermethylated in recurrence-prone tumors, they were either upregulated or non-differentially expressed (Supplementary Table 10).
Validation and Clinical Utility of a Meningioma Recurrence Score
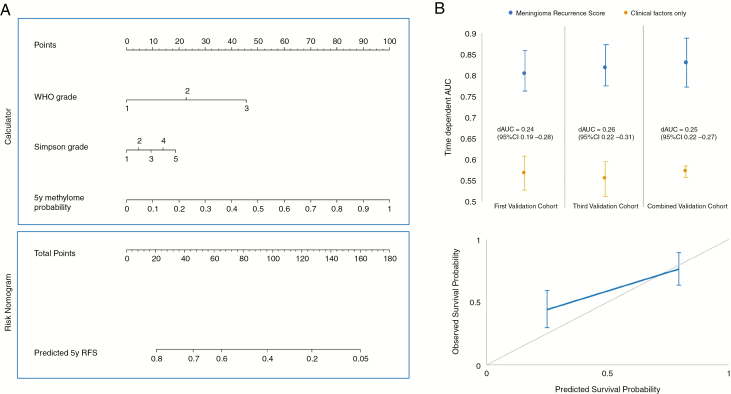
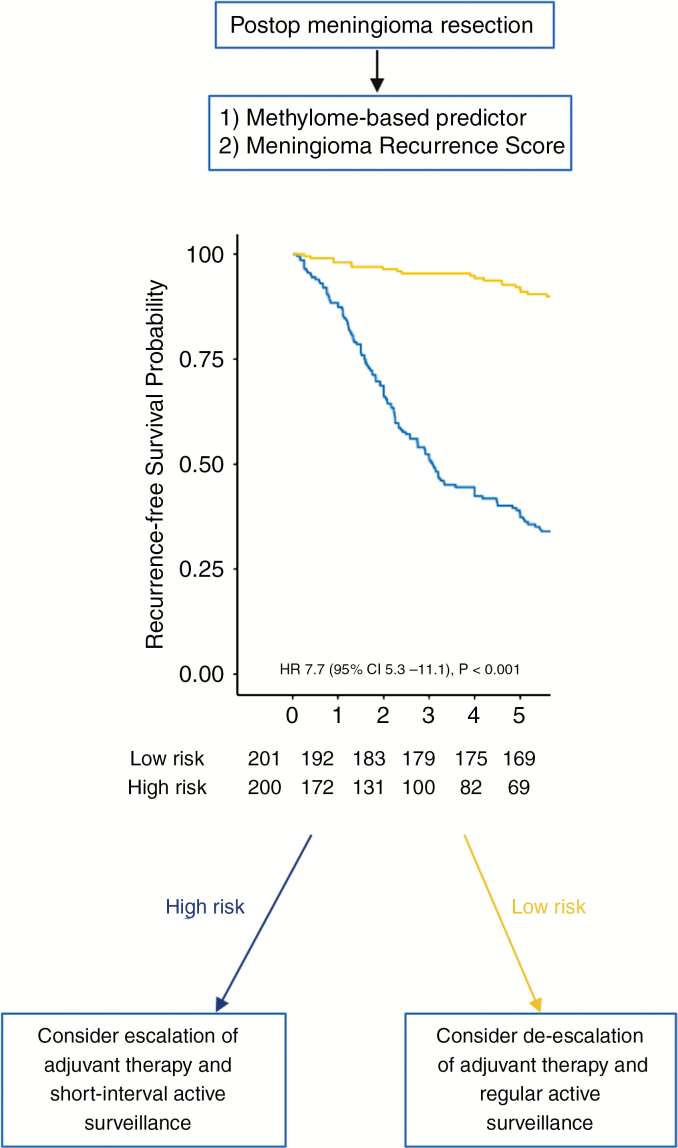
To generate and validate a meningioma recurrence score that could be translated to the clinic, we developed a nomogram to predict 5-year RFS that incorporated the validated 5-year methylome-based predictor with established prognostic covariates (WHO grade and extent of resection) using samples from the training cohort and second validation cohort (Fig. 5). Validation of the nomogram using the first validation cohort and third validation cohort independently as well as both validation cohorts combined demonstrated greater predictive performance of the nomogram with methylome predictor included compared with a nomogram using clinical factors (WHO grade and extent of resection) alone (ΔAUC = 0.24, 95% CI: 0.19–0.28; ΔAUC = 0.26, 95% CI: 0.22–0.31; ΔAUC = 0.25, 95% CI: 0.22–0.27, respectively; Fig. 5). The discriminative power of the meningioma recurrence score was approximately 82% in combined validation cohorts (AUC = 0.82, 95% CI: 0.76–0.87). The performances of the models using external validation and internal validation approaches were similar (Supplementary Fig. 7). The highest proportion of allottable points given in the nomogram is based on probabilities from the methylome-based predictor, again suggesting that the methylome predictor has greater importance in determining recurrence risk in meningiomas compared with established clinical factors. Calibration and Kaplan–Meier survival analysis of the meningioma recurrence score clearly stratify patients with high risk and low risk for 5-year recurrence (HR 7.7, 95% CI: 5.3–11.1, P < 0.001; Fig. 6). Interestingly, while histologic grades II and III are meant to predict high recurrence risk, we find that the low-risk group in total contains 39 grade II tumors (34.5% of the grade II tumors) and 4 grade III tumors (8.3% of the grade III tumors). Conversely, while a WHO grade I designation is meant to convey a low risk of recurrence, there were 35 (21.2%) patients with grade I tumors in the high-risk group. These results indicate refinement of risk estimate by the nomogram relative to current classification standards. To facilitate unrestricted global dissemination, we have created a freely available online calculator of the meningioma recurrence score at: https://meningiomas.shinyapps.io/meningioma_recurrence_score_online_calculator/.
Fig. 5.
Nomogram to predict 5-year recurrence risk in meningiomas. (A) Total points generated from scoring of methylome-based RFS predictor, WHO grade, and Simpson grade are tallied in the calculator and correlated to 5-year RFS in the nomogram in the risk nomogram. (B) Time-dependent average AUC with 95% CI as well as ΔAUC (dAUC) with 95% CI using 10 000 bootstrap resampling validation approach generated for the meningioma recurrence score and a nomogram using clinical factors alone in the first and third validation cohorts as well as both combined validation cohorts. (C) Calibration curve of the nomogram to predict RFS at 5 years in the combined validation cohort. The observed RFS is plotted on the y-axis and nomogram predicted probability is plotted on the x-axis.
Fig. 6.
The meningioma recurrence score identifies 2 risk groups (high risk vs low risk) that may help individualize adjuvant management decisions such as the need for radiation therapy in patients with meningiomas. Patient tumor samples can be interrogated for DNA methylation profile of selected 9529 probes, and a 5-year methylome-based RFS predictor score is generated. This score is combined with tumor WHO grade and Simpson grade in the meningioma recurrence score to develop an individualized probability of 5-year RFS.
Details on imputations for the online calculator can be found in the Supplementary Methods, and case examples demonstrating the power of personalized predictions are detailed in Supplementary Figures 8–10.
Discussion
In this multicenter study, we demonstrated the transformative utility of integrating clinical and molecular factors for use beyond simple classification into the realm of individualized prognostication in neuro-oncology. Our methylome-based predictor was more reliably able to predict early (5 y) RFS in comparison with histologic grading and was associated with RFS independent of established clinical and molecular factors. Combining the methylome predictor with established prognostic clinical factors (WHO grade and extent of resection) in a meningioma recurrence score refined prognostication for individual patients with meningiomas beyond established prognostic clinical and molecular factors with therapeutic implications for individualizing decision making regarding the need for adjuvant therapy after surgery in meningiomas.
Although WHO grade is associated with recurrence in populations of meningioma patients and is currently used to guide therapy, the clear within-grade variation for risk of recurrence and interrater variability makes it challenging to rely on tumor grade alone to predict recurrence and guide postoperative management decisions for individual patients. As a manifestation of this imprecision, some patients with biologically aggressive tumors may be inappropriately subsumed within the group of histologically benign tumors. With current standard of care, it is thought that up-front treatment with adjuvant radiation therapy after surgical resection offers the best chance to delay recurrence, and therefore some patients are not appropriately selected for adjuvant treatment with standard-of-care approaches.3,4 Conversely, there are also some patients with histologically defined intermediate or higher-grade tumors that in fact harbor indolent tumor behavior. Such patients may be receiving adjuvant radiation therapy in the absence of a defined need. Radiation, even when optimized to minimize adverse effects, still carries the risk for adverse radiation effects such as reactive inflammation, vasculitis, and necrosis, all of which have sequelae on patient cognition and quality of life.5 There is a clear need for a more refined predictor of recurrence patterns for individual patients with meningiomas beyond simple classifications, so that the decision for adjuvant therapy, radiotherapy, or otherwise can be appropriately selected and personalized for patients.
The burden of chromosomal alterations have repeatedly been shown to be one of the most important prognostic molecular alterations in meningioma.11,17,22 Similar to others,11,22 we found recurrent alterations in 1p, 4p, 6q, 10q, 14q, and 18q in a subset of meningiomas that were enriched in a higher risk group. However, using a previously published copy number score designed to identify meningiomas with high recurrence risk,17 we found that the burden of copy number alteration was not an independent predictor of recurrence once adjusted for methylation signature. Similarly, although TERT promoter mutations are known to be enriched in more aggressive meningiomas,10 our analysis demonstrated that TERT promoter mutation was not independently associated with RFS on multivariable analysis with the 5-year methylome predictor included in the model. Taken together, these results suggest that our 5-year methylome predictor can provide prognostic information beyond previously established molecular factors in meningioma.
Probes included in the predictor were selected based on correlations of either hypo- or hypermethylated status with respect to RFS. Interestingly, the distribution of these probes was not random, either with respect to methylation status or with respect to association with known genes. The majority (over 86%) of our included probes were all associated with unfavorable RFS when hypermethylated. This suggests that in general, hypermethylation of a set of CpG sites in meningioma correlates with clinical aggressiveness. For example, hypermethylation of the homeobox and T-box families of genes were found to be highly overrepresented in the set of relevant probes, suggesting possible involvement of this class of developmental factors in the clinical behavior of meningioma, which requires further validation.
To generate a tool that could be used by clinicians to capture the heterogeneity in recurrence risk in meningiomas, we established a 5-year meningioma recurrence score that combined our validated methylome-based predictor with well-established prognostic clinical factors (WHO grade and extent of resection). The performance of our meningioma recurrence score was improved with the methylome-based predictor included in the nomogram (ΔAUC = 0.25, 95% CI: 0.22–0.27), and the overall discrimination of our nomogram was high (AUC ~82% in 2 independent cohorts). The construction (and evaluation) of our nomogram meets the standards of reporting on nomograms in oncology and is one of the few to demonstrate robust evaluation using multiple independent validation cohorts.23 Now that we have demonstrated that our tools are robustly validated, we are well positioned to prospectively validate the use of our tools to demonstrate efficacy with adjuvant therapy strategies in high-risk patients.24 Our meningioma recurrence score informs both patients and clinicians about individualized risk of recurrence and can be used to guide clinicians regarding the need for adjuvant therapy and/or close clinical follow-up.
Our study has some limitations. First, although we have identified a group of probes with distinct epigenetic changes that in combination are predictive of recurrence risk in meningiomas for individual patients, it is unclear whether these changes may be conferring variant behavioral phenotypes or whether they are a surrogate for general cellular dysregulation. Nevertheless, the set of highly refined and selected probes in our predictor are enriched to be located on promoters of CpG islands where aberrant DNA methylation has clearly been linked to carcinogenesis.25 The correlation of methylation with gene expression in our study was exploratory and would benefit from additional investigation with matched epigenetic and transcriptomic analysis in the same samples. Moreover, although each institution conformed to a common definition for tumor recurrence and time to recurrence, there is no universally standardized definition of recurrence in meningiomas. The Response Assessment in Neuro-Oncology guidelines may offer an avenue for standardized definition of recurrence to be collected on meningiomas in future clinical trials, which will help with communication across different centers and may also help with further model refinement.26
Our predictor has been designed and validated such that it can be applied to data from fresh frozen or paraffin embedded tissues using the current commonly used standard platform for genome-wide DNA methylation profiling, facilitating immediate adoption into clinical practice. Our newly developed and retrospectively validated meningioma recurrence score combines both methylome and clinical factors and can be freely used by clinicians to personalize decision making regarding postoperative management of the most common primary intracranial brain tumor via a web-based interface.
Funding
This work was supported by the Brain Tumor Charity Quest for Cures: Collaborative Team Award, and a Canadian Institute of Health Research‒Institute of Cancer Research Operating Grant.
Supplementary Material
Conflict of interest statement. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Authorship statement. KA, GZ, FN conceived, designed, and supervised the study. GZ, KA, FS, PH, MW, MP, CHH, AvD, IGT, HKN, MT, GT collected the specimens. FN, MY, SS, SK, SM, JHB constructed the database. KA, FS, AvD, HN, JS offered pathological review. MY, FN LP, OS did the statistical and computational analyses. FN, MY, KA, GZ wrote the manuscript and all authors revised the manuscript. All authors discussed and reviewed the manuscript and approved the manuscript for publication.
The generation of the manuscript has been supported by the membership of the consortium, which at the time of supplement generation includes: Kenneth Aldape, Karolyn Au, Jill Barnhartz-Sloan, Wenya Linda Bi, Priscilla K. Brastianos, Nicholas Butowski, Carlos Carlotti, Michael D. Cusimano, Francesco DiMeco, Katharine Drummond, Ian F. Dunn, Evanthia Galanis, Caterina Giannini, Roland Goldbrunner, Brent Griffith, Rintaro Hashizume, C. Oliver Hanemann, Christel Herold-Mende, Craig Horbinski, Raymond Y. Huang, David James, Michael D. Jenkinson, Christine Jungk, Timothy J. Kaufman, Boris Krischek, Daniel Lachance, Christian Lafougère, Ian Lee, Jeff C. Liu, Yasin Mamatjan, Tathiane M. Malta, Christian Mawrin, Michael McDermott, David Munoz, Farshad Nassiri, Houtan Noushmehr, Ho-Keung Ng, Arie Perry, Farhad Pirouzmand, Laila M Poisson, Bianca Pollo, David Raleigh, Felix Sahm, Andrea Saladino, Thomas Santarius, Christian Schichor, David Schultz, Nils O. Schmidt, Warren Selman, Andrew Sloan, Julian Spears, James Snyder, Suganth Suppiah, Ghazaleh Tabatabai, Marcos Tatagiba, Daniela Tirapelli, Joerg C. Tonn, Derek Tsang, Michael A. Vogelbaum, Andreas von Deimling, Patrick Y. Wen, Tobias Walbert, Manfred Westphal, Adriana M. Workewych, Gelareh Zadeh.
Date of accession statement. Previously unpublished raw methylation files (idat files) have been submitted to the European Genome-phenome Archive (EGAS00001003490).
Contributor Information
International Consortium on Meningiomas:
Kenneth Aldape, Karolyn Au, Jill Barnhartz-Sloan, Wenya Linda Bi, Priscilla K Brastianos, Nicholas Butowski, Carlos Carlotti, Michael D Cusimano, Francesco DiMeco, Katharine Drummond, Ian F Dunn, Evanthia Galanis, Caterina Giannini, Roland Goldbrunner, Brent Griffith, Rintaro Hashizume, C Oliver Hanemann, Christel Herold-Mende, Craig Horbinski, Raymond Y Huang, David James, Michael D Jenkinson, Christine Jungk, Timothy J Kaufman, Boris Krischek, Daniel Lachance, Christian Lafougère, Ian Lee, Jeff C Liu, Yasin Mamatjan, Tathiane M Malta, Christian Mawrin, Michael McDermott, David Munoz, Farshad Nassiri, Houtan Noushmehr, Ho-Keung Ng, Arie Perry, Farhad Pirouzmand, Laila M Poisson, Bianca Pollo, David Raleigh, Felix Sahm, Andrea Saladino, Thomas Santarius, Christian Schichor, David Schultz, Nils O Schmidt, Warren Selman, Andrew Sloan, Julian Spears, James Snyder, Suganth Suppiah, Ghazaleh Tabatabai, Marcos Tatagiba, Daniela Tirapelli, Joerg C Tonn, Derek Tsang, Michael A Vogelbaum, Andreas von Deimling, Patrick Y Wen, Tobias Walbert, Manfred Westphal, Adriana M Workewych, and Gelareh Zadeh
References
- 1. Ostrom QT, Gittleman H, Xu J, et al. . CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(Suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Melamed S, Sahar A, Beller AJ. The recurrence of intracranial meningiomas. Neurochirurgia (Stuttg). 1979;22(2):47–51. [DOI] [PubMed] [Google Scholar]
- 3. Weber DC, Ares C, Villa S, et al. . Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: A phase-II parallel non-randomized and observation study (EORTC 22042–26042). Radiother Oncol. 2018;128(2):260–265. [DOI] [PubMed] [Google Scholar]
- 4. Rogers L, Zhang P, Vogelbaum MA, et al. . Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018;129(1):35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogers L, Barani I, Chamberlain M, et al. . Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toro-Moreno AC, Serna-Velez L, Gallego-González D, Jaramillo-Jaramillo LI, Martínez-Sánchez LM, Álvarez-Hernández LF. Tumores de sistema nervioso central en pediatría: Presente y futuro del abordaje diagnóstico. Rev Ecuatoriana Neurol. 2017;26(3):283–288. [Google Scholar]
- 7. Brastianos PK, Horowitz PM, Santagata S, et al. . Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark VE, Erson-Omay EZ, Serin A, et al. . Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science (80-). 2013;339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark VE, Harmancl AS, Bai H, et al. . Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. 2016;48(10):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahm F, Schrimpf D, Olar A, et al. . TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5):djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sahm F, Schrimpf D, Stichel D, et al. . DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 12. Olar A, Wani KM, Wilson CD, et al. . Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017;133(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reuss DE, Piro RM, Jones DTW, et al. . Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol. 2013;125(3):351–358. [DOI] [PubMed] [Google Scholar]
- 14. Lee Y, Liu J, Patel S, et al. . Genomic landscape of meningiomas. Brain Pathol. 2010;20(4):751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Claus EB, Park PJ, Carroll R, Chan J, Black PM. Specific genes expressed in association with progesterone receptors in meningioma. Cancer Res. 2008;68(1):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hovestadt V, Zapatka M.. conumee: enhanced copy-number variation analysis using Illumina 450k methylation arrays. R package version 0.99. R Packag 2015. [Google Scholar]
- 17. Aizer AA, Abedalthagafi M, Linda Bi W, et al. . A prognostic cytogenetic scoring system to guide the adjuvant management of patients with atypical meningioma. Neuro Oncol. 2016;18(2):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–344. [DOI] [PubMed] [Google Scholar]
- 19. Huang DW, Sherman BT, Tan Q, et al. . The DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8(9):R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25(20):3474–3486. [DOI] [PubMed] [Google Scholar]
- 21. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals published by: Biometrika Trust Stable URL: http://www.jstor.org/stable/2337123. Biometrika. 2008;81(3):515–526. [Google Scholar]
- 22. Harmancl AS, Youngblood MW, Clark VE, et al. . Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun. 2017;8:14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McShane LM, Cavenagh MM, Lively TG, et al. . Criteria for the use of omics-based predictors in clinical trials. Nature. 2013;502(7471):317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sproul D, Meehan RR. Genomic insights into cancer-associated aberrant CpG island hypermethylation. Brief Funct Genomics. 2013;12(3):174–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang RY, Bi WL, Weller M, et al. . Proposed response assessment and endpoints for meningioma clinical trials: report from the Response Assessment in Neuro-Oncology (RANO) Working Group. Neuro Oncol. 2019;21(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.