Abstract
Background
Meningioma patients are known to face cognitive deficits before and after surgery. We examined individual changes in cognitive performance over time and identified preoperative predictors of cognitive functioning 12 months after surgery in a large sample of meningioma patients.
Methods
Patients underwent neuropsychological assessment (NPA) using CNS Vital Signs 1 day before (T0) and 3 (T3) and 12 (T12) months after surgery. Patients’ sociodemographically corrected scores on 7 cognitive domains were compared with performance of a normative sample using one-sample z tests and chi-square tests of independence. Reliable change indices with correction for practice effects were calculated for individual patients. Linear mixed effects models were used to identify preoperative predictors of performance at T12.
Results
At T0, 261 patients were assessed, and 229 and 82 patients were retested at T3 and T12, respectively. Patients showed impaired cognitive performance before and after surgery, and although performance improved on the group level, cognitive scores remained significantly lower than in the normative sample up to T12. On the individual level, performance remained stable in the majority of patients. Better preoperative performance, younger age, male sex, and higher educational level predicted better late cognitive performance.
Conclusions
Meningioma patients face serious and persistent pre- and postsurgical cognitive deficits. A preoperative NPA together with sociodemographic characteristics may provide valuable information on the late cognitive outcome of individual meningioma patients. These results can help to inform patients and clinicians on late cognitive outcomes at an early stage, and emphasizes the importance of presurgical NPA and timely cognitive rehabilitation.
Keywords: cognition, individual changes, meningioma, predictors, reliable change index
Key Points.
Meningioma patients face cognitive deficits before and after surgery.
Performance improves after surgery but remains significantly lower than in controls.
Late cognitive performance is best predicted by preoperative cognitive performance.
Importance of the Study.
Meningioma patients showed significantly worse cognitive performance before and after surgery compared with healthy controls. Although performance improved over time on the level of the whole group, cognitive scores remained significantly lower than in controls up to 12 months after surgery. On the individual level, performance remained stable in the majority of meningioma patients. Preoperative cognitive performance of meningioma patients turned out to be the best predictor for late performance, and sociodemographic characteristics (ie, age, sex, and education) were predictive for cognitive outcomes in meningioma patients. These results can help to inform patients and clinicians on late cognitive status at an early stage, and emphasize the need for presurgical neuropsychological assessments and timely cognitive rehabilitation in meningioma patients who are at risk for cognitive impairment after surgery.
Tumor resection is the preferred treatment for the vast majority of patients with intracranial meningioma.1 However, meningioma patients already suffer from deficits in several cognitive domains before surgery.2 Surgical resection of the meningioma has been found to improve cognitive performance of patients, but some postoperative cognitive deficits continue to exist, as significant cognitive impairments are reported up to 4 years after surgery.2–11 However, to date, preoperative cognitive functioning has not often been examined, thereby limiting statements about changes in cognitive performance over time. The few studies that explored changes over time (following a preoperative assessment up to 9 months after surgery) predominantly demonstrated improved cognitive performance.6–10,12,13 Yet, study results were presented on the group instead of individual patient level, fairly simple measures of change in performance were adopted (eg, raw mean difference scores), and practice effects of repeated assessments were often not corrected for.14
Despite the fact that extensive cognitive deficits in meningioma patients have been described by several studies, research into predictors of cognitive performance in these patients remains limited.2 Mixed findings were reported with respect to the association between cognitive performance and, among others, tumor location and psychological factors (ie, anxiety and depression).2,3,5,13,15 Moreover, preoperative predictors of late cognitive outcomes have only minimally been addressed in meningioma patients, which is remarkable given the negative impact of cognitive deficits on, for example, returning to social and professional activities after meningioma surgery.5,16,17 Information on the sociodemographic, clinical, psychological, or cognitive characteristics of patients who are at risk for cognitive impairment in the long term after surgery may help to inform patients and clinicians at an earlier stage.
We explored cognitive functioning using a computerized neuropsychological battery in a large sample of meningioma patients one day before, and 3 and 12 months after surgery. Changes in performance were assessed at the group level, as well as at the individual level using reliable change indices (RCIs) for each of the two time intervals. Additionally, we sought to identify preoperative predictors of late (ie, 12 months postoperative) cognitive performance.
Materials and Methods
Design
The present study was part of a prospective longitudinal study in which brain tumor patients admitted for surgical resection between November 2010 and June 2017 underwent neuropsychological assessment (NPA) one day before surgery (T0) and 3 months after surgery (T3) as part of standard clinical neuro-oncological care. An (approximately) 12-month postoperative follow-up assessment (T12) was added as from January 2014 for research purposes in order to explore long-term cognitive functioning.
Patients
Cases eligible for the current study were patients who underwent initial surgical resection, and who were histopathologically diagnosed with a WHO grade I or II meningioma. We excluded patients under the age of 18 years, with a history of intracranial neurosurgery, intraosseous meningioma, a recent history (≤2 y) of severe psychiatric or neurologic disorders, other major medical illnesses in the year prior to surgery (eg, cancer), a lack of basic proficiency in Dutch, or/and the inability to undergo the NPA due to severe visual, motor, or cognitive problems. In addition, patients who participated in the cognitive rehabilitation studies that were simultaneously running were excluded from the current study if they had been randomly assigned to the intervention (ie, rehabilitation) group.18,19 The cutoff for the maximum time interval between T0 and T12 assessment was set at 21 months.
All patients provided written informed consent. The study was approved by the Medical Ethics Committee (file NL41351.008.12). There is considerable overlap between the patient sample of the current study and two previous studies.9,20
Measures and Procedure
Sociodemographic characteristics
Patients were assessed per standardized protocol at all three timepoints, including a checklist and standardized interview at T0 (ie, for obtaining and verifying sociodemographic information such as age, sex, and educational level). The highest completed level of education was classified according to the Dutch Verhage scale.21 Its 7 categories were merged into 3 categories: low educational level (Verhage 1–4: primary level education or lower), middle educational level (Verhage 5: completion of average level secondary education), and high educational level (Verhage 6, 7: high level secondary education or university degree).
Clinical characteristics
Clinical information was obtained from the electronic medical charts. A histopathological diagnosis was provided following surgery and categorized as WHO grade I or II meningioma.22 By means of a preoperative contrast-enhanced T1 weighted MRI, tumor location (ie, supratentorial vs infratentorial), and further classified as frontal (ie, frontal, frontal-temporal, and frontal-parietal) versus non-frontal involvement, hemisphere (ie, right, left, bilateral), and the number of tumors were identified. In addition, we determined preoperative total tumor volume (in mm3) of the meningioma operated on using semi-automatic segmentations performed in ITK-SNAP (www.itksnap.org23), followed by minor manual adjustments to lesion margins. The American Society of Anesthesiologists (ASA) score was considered as a physical status classification, ranging from ASA I (patient completely healthy) to ASA V (moribund patient).24 ASA score was considered dichotomous: patients within ASA categories I and II were considered healthy, whereas patients within categories III and IV were considered to have substantial comorbidities. Anti-epileptic drugs, corticosteroids, benzodiazepines, opioids, antipsychotics, stimulants, and/or antidepressants were recorded as psychotropic medications.
Psychological characteristics
Anxiety and depression were assessed using the Dutch translation of the Hospital Anxiety and Depression Scale (HADS).25,26 This self-report instrument consists of 14 items: each subscale (anxiety and depression) includes 7 items, resulting in a score of 0–21 for each subscale, with higher scores representing more symptoms. The Dutch translation of the HADS has good psychometric qualities, with Cronbach’s alphas of .81–.84 and .71–.86 for the anxiety and depression subscales, respectively.26
Cognitive performance
The formal Dutch translation of the computerized neuropsychological battery CNS Vital Signs (VS) was used to examine cognitive performance. CNS VS comprises 7 neuropsychological tests, yielding measures of performance in 11 cognitive domains.27 Since the measures of performance for some domains are largely based on scores on the same tests, we considered only the following 7 cognitive domains: Verbal Memory, Visual Memory, Processing Speed, Psychomotor Speed, Reaction Time, Complex Attention, and Cognitive Flexibility (Table 1). NPAs were performed using the CNS VSX local software app, on a laptop running a 64-bit operating system. A well-trained test technician remained present during each assessment.
Table 1.
Supplementary material on CNS Vital Signs
| Cognitive Domain | CNS VS Test | Domain Score Calculations | Description |
|---|---|---|---|
| Verbal Memory | Verbal memory test (VBM) | VBM direct correct hits + VBM direct correct passes + VBM delayed correct hits + VBM delayed correct passes | Learning a list of 15 words, with a direct recognition, and after 6 more tests a delayed recognition trial |
| Visual Memory | Visual memory test (VIM) | VIM direct correct hits + VIM direct correct passes + VIM delayed correct hits + VIM delayed correct passes | Learning a list of 15 geometric figures, with a direct recognition, and after 6 more tests a delayed recognition trial |
| Processing Speed | Symbol digit coding (SDC) | SDC correct responses – SDC errors | Number 1 to 9 correspond to different symbols. As many correct numbers as possible have to be filled out underneath the presented symbols in 90 seconds |
| Psychomotor Speed | Finger tapping test (FTT) Symbol digit coding test (SDC) |
FTT taps right hand + FTT taps left hand + SDC correct responses | Pressing the space bar with the index finger as many times in 10 s above mentioned |
| Reaction Time | Stroop test (ST) | (ST part II reaction time on correct responses + ST part III reaction time on correct responses) / 2 | In part I, pressing the space bar as soon as the words RED, YELLOW, BLUE, and GREEN appear—in part II, pressing the space bar as the color of the word matches what the word says—in part III, pressing the space bar as the color of the word does not match what the word says |
| Complex Attention | Continuous performance test (CPT) Shifting attention test (SAT) Stroop test (ST) |
Stroop commission errors + SAT errors + CPT commission errors + CPT omission errors | Responding to a target stimulus “B” but no any other letter Shifting from one instruction to another quickly and accurately (matching geometric objects either by shape or color). |
| Cognitive Flexibility | Shifting attention test (SAT) Stroop test (ST) |
SAT correct—SAT errors—ST commission errors | Above-mentioned |
After completing the battery, which takes approximately 30–40 minutes, raw scores for each domain are automatically provided by the program (Table 1). Since effects of age, education, and sex on CNS VS performance were demonstrated in a Dutch normative sample (N = 158; age 20–80 y, education 10–26 y, assessed at baseline and at 3- and 12-month follow-up), raw cognitive domain scores were converted into sociodemographically adjusted z scores (see Rijnen et al28 for a detailed description). In addition, effects of practice were found in the Dutch normative sample between T0 and T3. Test scores at T3 and T12 were therefore corrected for practice effects, in addition to the sociodemographic corrections.29
Statistical Analyses
Patients’ characteristics
Descriptive and comparative analyses (ie, one-sample z-tests, chi-square tests of independence) were performed to explore potential differences in baseline sociodemographic, clinical, and psychological variables of the different patient samples assessed at T0, T3, and T12 and the T12 dropouts.
Pre- and postoperative cognitive performance
Cognitive performance in individual patients was defined as impaired if a z score was ≤−1.50.30 The numbers and percentages of patients scoring impaired were calculated for all cognitive domains at all timepoints. In addition, a chi-square test of independence was conducted to compare the proportions of meningioma patients with impaired performance (per domain, per timepoint) with the proportions of participants in the normative sample with impaired performance (ie, to test whether such deviant scores occurred significantly more frequently in meningioma patients than in controls).
To explore mean performance on the cognitive domains of meningioma patients compared with the normative controls at T0, T3, and T12, one-tailed one-sample z-tests were performed (test values: mean [M] z = 0, standard deviation [SD] = 1). The mean z score for each domain (ie, the difference between the patient sample and the normative sample in terms of SDs) is comparable to Glass’s delta (∆) effect size (ES) when calculated according the following formula: Meanpatients − Meancontrols/SDcontrols. ES ≤0.50, between 0.51 and 0.79, and ≥0.80 respectively reflected small, medium, and large effects.31
Changes in individual and group cognitive performance over time
Changes in cognitive performance over time in individual patients were examined using RCIs. RCIs illustrate reliable changes in performance in individual patients, compared with changes in performance of controls. A standardized regression-based RCI as described by Maassen, Bossema, and Brand32 was adopted. Rijnen et al29 described details with regard to the RCI for changes in CNS VS performance specifically, based upon results on repeated testing in a Dutch normative sample from baseline (n = 158) to 3- (n = 133) and 12-month (n = 77) follow-up. In the current study, change was defined as RCI values exceeding ± 1.645 (corresponding with a two-tailed alpha of 0.10%, 90% confidence interval), with positive values indicating improved performance and negative values representing declines. The numbers of patients with improved, stable, and declined performance were counted for each domain at the two time intervals. Additionally, chi-square tests of independence were conducted for the separate domains to compare the proportion of “changers” (improvers or decliners) in the meningioma patients to the proportion of “changers” in the normative sample (ie, again to test whether changes occurred significantly more frequently in patients than in controls). In case of statistical significance, standardized residuals were used to interpret chi-square tables as to which cells (ie, which change category: improved, stable, declined) contributed to the significant result. We assessed the amount of overlap between patients with solely improved or declined performance between both T0–T3 and T3–T12 (ie, to determine whether changes comprise further improvements/deterioration in the same patients, or “new” changes in patients who showed stable performance over the other time interval).
Changes over time (ie, between T0–T3 and T3–T12) on the mean group level were assessed using the linear mixed effects models (LMEMs) (described in detail below).
Predictors of late cognitive impairment
LMEMs for repeated measurements were fitted to examine preoperative predictors of late (T12) cognitive functioning in meningioma patients for each domain. To estimate the model parameters, the restricted maximum likelihood estimate method was used. The Akaike information criterion and Bayesian information criterion were used to estimate model fit. A heterogeneous first-order autoregressive covariance structure was selected, the random effect was subject ID, and predictors were entered as fixed effects into the model. Outcome measures were the z scores for the separate cognitive domains at T12. Predictors included timepoint (T0, T3, T12), the number of months between T0 and T12 (ranging 8–21 months), sociodemographic (age, sex, educational level), clinical (tumor location: hemisphere, supra- versus infratentorial, frontal versus nonfrontal, number of meningioma, tumor volume, ASA score, medication use), and psychological (anxiety and depression) variables. In addition, T0 scores of all domains were included in the LMEMs as cognitive predictors except the T0 scores of the predicted domain itself. Including T0 performance of a specific domain itself as a predictor (in addition to the inclusion of T0 performance by assessing the fixed effect of timepoint) would result in the factor being incorporated twice in the model. All other individual domains were entered into the model to examine if there were domains that were consistently predictive for multiple domains (if so, in the future a subset of domains could be assessed prior to surgery to minimize patient burden). A variance inflation factor of over 10 was used as cutoff for multicollinearity in the final models.33
Statistical analyses were performed using the Statistical Package for the Social Sciences version 24.0 (IBM), except for the LMEMs, for which we used the nmle34 package in R. To reduce false discovery rate due to multiple statistical testing, P-values were set against a corrected alpha, using the Benjamini–Hochberg (BH) procedure (leaving greater power than the Bonferroni technique).35 When performing the BH procedure, individual P-values (per hypothesis) were ranked from smallest to largest: the smallest P-value has the rank i = 1, the next smallest has i = 2, etc. Adjusted P-values were calculated by multiplying the original P-value by (m/i), where m is the number of tests, and i the rank of the specific P-value. BH-adjusted P-values are then compared with the original alpha of 0.05, and the rank of the largest adjusted P-value that is smaller than 0.05 is used to calculate an adjusted alpha level by following the formula (i/m)*0.05.35
Results
Patients’ Characteristics
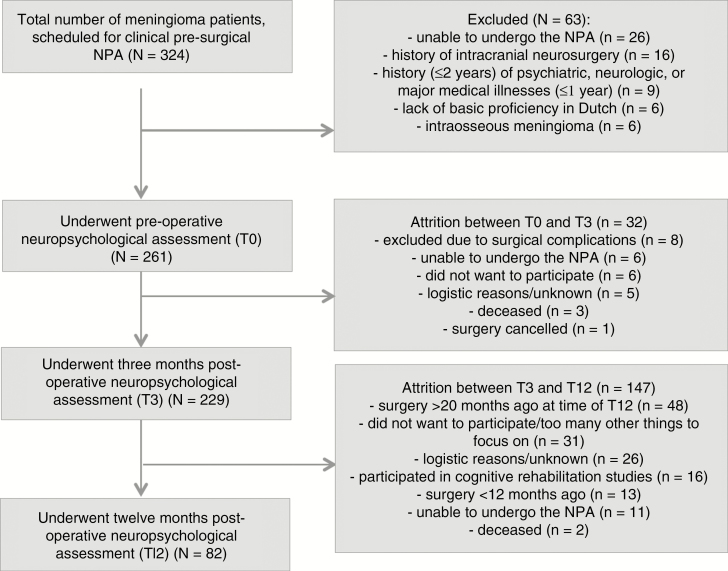
Fig. 1 shows the flow of meningioma patients through the current study. At T0, 261 patients were included. Thirty-two patients (12%) did not complete T3, resulting in 229 patients with both T0 and T3. Sixty-four percent of these patients did not undergo T12, mainly (in 33%) due to the later implementation of this measurement. Eventually, 82 patients underwent all three NPAs. The median time interval between T0 and T3 was 2.83 months (range 1.00–5.75 months). Median time interval between T0 and T12 was 12.42 months (range 8.51–20.40 months).
Fig. 1.
Flowchart of meningioma patients eligible for inclusion and follow-up.
Table 2 presents characteristics of the meningioma patients. There were no significant differences between the T0, T3, and T12 samples regarding sociodemographic, clinical, and psychological characteristics. In addition, no significant differences were found between the patients who underwent T12 and those who did not with regard to baseline patient characteristics (Table 2), and T3 cognitive performance (ie, the timepoint at which patients decided whether or not to participate in T12; data not shown) (P values > 0.134).
Table 2.
Baseline sociodemographic, clinical, and psychological characteristics of samples of meningioma patients at each timepoint
| Baseline Characteristics | T0 (n = 261) | T3 (n = 229) | T12 (n = 82) | Dropout T12 (n = 147) |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age, y: mean ± SD (range) | 57.8 ± 11.7 (23–82) | 57.1 ± 11.7 (23–82) | 55.9 ± 10.7 (32–75) | 58.7 ± 12.0 (23–82) |
| Education, y: mean ± SD (range) | 13.7 ± 3.7 (6–26) | 14.0 ± 3.7 (6–26) | 14.4 ± 3.7 (8–26) | 13.5 ± 3.7 (6–22) |
| Sex: female/male n(%) | 189(72)/72(28) | 167(73) / 62(27) | 59(72) / 23(28) | 130(73)/49(27) |
| Clinical characteristics | ||||
| WHO grade: I/II n(%) | 240(92) / 21(8) | 211(92) / 18(8) | 76(93) / 6(7) | 164(92)/15(8) |
| Number of meningioma: 1/≥2 n(%) | 244(94) / 17(6) | 217(95) / 12(5) | 79(96) / 3(4) | 168(94)/11(6) |
| Hemisphere: Left/right/bilateral n(%) | 106(41) / 124(48) / 31(11) | 94(41) / 107(47) / 28(12) | 32(39) / 39(48) / 11(13) | 74(41)/85(48)/20(11) |
| Supratentorial/infratentorial n(%) | 238(91) / 23(9) | 208(91) / 21(9) | 75(91) / 7(9) | 163(91) / 16(9) |
| Frontal/nonfrontal n(%) | 154(59) / 107(41) | 135(59) / 94(41) | 46(56) / 36(44) | 108 (60)/71(40) |
| Tumor volume (cm3): median (range)a | 33.5 (0.45–150.2) | 32.0 (0.45–150.2) | 34.5 (0.45–144.8) | 32.0 (0.62–150.2) |
| ASA score: I, II/III, IV n(%) | 225(86) / 36(14) | 202(88) / 27(12) | 71(87) / 11(13) | 154(86) / 25 (14) |
| Psychotropic medication: yes/no n(%)b | 142(56) / 112(44) | 122(54) / 102(46) | 45(56) / 35(44) | 97 (56) / 77 (44) |
| Psychological characteristics | ||||
| Anxiety HADS: mean ± SD (range)c | 7.2 ± 4.2 (0–20) | 7.0 ± 4.2 (0–19) | 7.0 ± 4.0 (0–17) | 7.3 ± 4.3 (0–20) |
| Depression HADS: mean ± SD (range)c | 5.9 ± 4.6 (0–21) | 5.7 ± 4.5 (0–21) | 6.0 ± 4.9 (0–21) | 5.8 ± 4.5 (0–19) |
a data missing T0 n = 30; T3 = 24; T12 = 6; b data missing T0 n = 7; T3 n = 5; T12 n = 2; c data missing T0 = 32; T3 = 25; T12 = 7.
Pre- and Postoperative Cognitive Performance
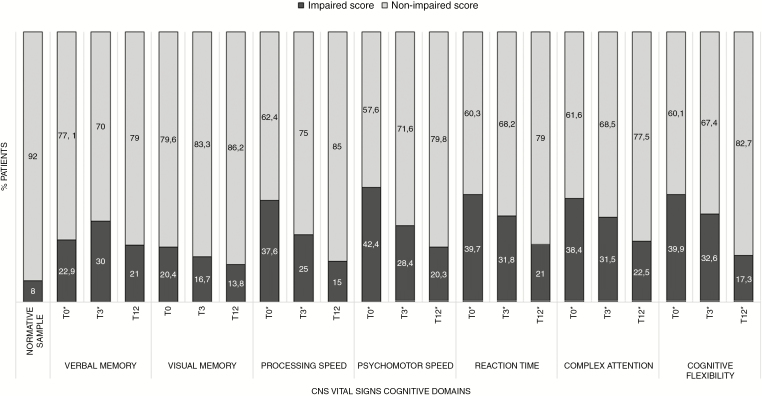
At T0, percentages of patients with impaired scores ranged from 20.4% to 42.4% over domains (Fig. 2). At T3 and T12 the percentages of patients scoring impaired on the cognitive domains respectively ranged from 16.7% to 32.6%, and from 13.8% to 22.5%. For 6 out of 7 domains at T0 and T3, and still 3 out of 7 domains at T12 after surgery, impairments were significantly more common in meningioma patients than in normative controls (P-values < BH-corrected alpha 0.04; Fig. 2).
Fig. 2.
Percentages of meningioma patients with impaired or non-impaired performance over CNS VS cognitive domains at all timepoints. The asterisk (*) indicates impairments that were significantly more common in meningioma patients compared with normative controls.
We found significantly lower mean performance in patients compared with the normative sample on all domains at T0 (ES ranging from −0.54 to −1.53), T3 (ES from −0.37 to −1.22), and T12 (ES from −0.35 to −0.76) (P values< BH-corrected alpha 0.05; Table 3). At T0 and T3, lowest mean z scores were found for Complex Attention (respectively −1.43 and −1.22) and Reaction Time (respectively −1.53 and −1.19). At T12, lowest mean scores were found for Psychomotor Speed (−0.76) and, again, Reaction Time (−0.65).
Table 3.
Cognitive performance on CNS VS domains of meningioma patients compared with the normative sample
| T0 | T3 | T12 | ||||
|---|---|---|---|---|---|---|
| Domain | z Scorea M(SD) | z test | z Scorea M(SD) | z test | z Scorea M(SD) | z-Test |
| Verbal Memory | −0.67(1.30) | −10.38* | −0.90(1.35) | −13.50* | −0.59(1.19) | −5.29* |
| Visual Memory | −0.54(1.23) | −8.35* | −0.37(1.26) | −5.52* | −0.35(1.14) | −3.09* |
| Processing Speed | −1.11(1.36) | −17.39* | −0.85(1.22) | −12.75* | −0.58(0.99) | −5.22* |
| Psychomotor Speed | −1.31(1.66) | −20.45* | −0.93(1.36) | −13.95* | −0.76(1.14) | −6.72* |
| Reaction Time | −1.53(2.38) | −23.79* | −1.19(2.02) | −17.83* | −0.65(1.61) | −5.81* |
| Complex Attention | −1.43(2.60) | −21.61* | −1.22(2.29) | −18.06* | −0.61(1.92) | −5.43* |
| Cognitive Flexibility | −1.34(2.28) | −20.69* | −1.12(1.79) | −16.62* | −0.48(1.46) | −4.34* |
*P < BH-corrected alpha 0.05.
a Equals Glass’s ∆ effect sizes where ≤0.50 = small, 0.51–0.79 = medium, ≥0.80 = large28
Changes in Individual and Group Cognitive Performance over Time
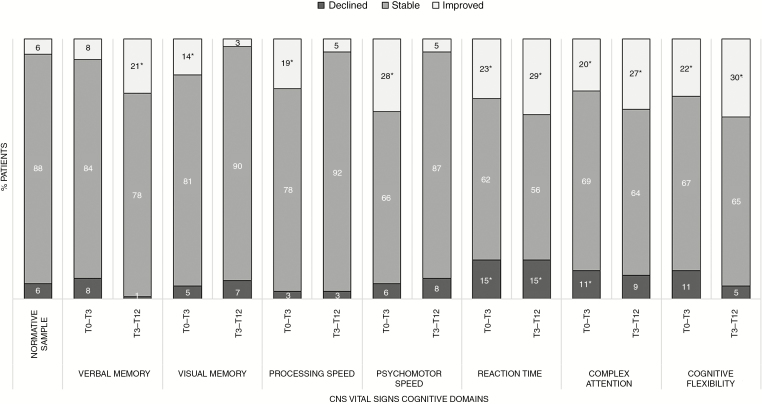
On the individual level, patients demonstrated improvements more often (ranging 8–28% between T0–T3 and 3–30% between T3–T12 over different cognitive domains) than declines (ranging 3–15% between T0 and T3 and 1%–15% between T3 and T12) over time (Fig. 3). To compare, 5–6% of the normative sample showed declined performance, and another 5–6% showed improved performance over time (ie, RCIs exceeding ± 1.645). Declined performance in patients was for none of the domains, neither over the first nor over the second interval, significantly more frequent than in the normative sample, except for Reaction Time between T0 and T3 (χ2 (2) = 26.84, P ≤ 0.001) and T3 and T12 (χ2 (2) = 28.45, P ≤ 0.001). However, improved performance occurred significantly more frequently on 6 out of 7 domains over the first interval (all domains but Verbal Memory), and 4 out of 7 domains (ie, Verbal Memory, Reaction Time, Complex Attention, and Cognitive Flexibility) over the second interval. Of the 28 patients who improved between T3 and T12 (ie, improved on at least one cognitive domain, with stable performance on the remaining domains), 36% already showed improved performance between T0 and T3. Of the 9 patients who showed declined performance (ie, declined on at least one cognitive domain, with stable performance on the remaining domains) between T3 and T12, 11% had already declined between T0 and T3.
Fig. 3.
Changes in cognitive performance of individual meningioma patients. The asterisk (*) indicates changes that were significantly more common in meningioma patients compared with normative controls.
The final LMEMs demonstrated changes in cognitive performance on the group level, as shown in Table 4. Patients’ performance improved significantly over the first time interval on 3 domains, namely Processing Speed, Psychomotor Speed, and Reaction Time (respectively B = 0.22, SE = 0.07, P = 0.002; B = 0.42, SE = 0.08, P < 0.001; B = 0.33, SE = 0.13, P = 0.013). Verbal Memory performance was found to decline significantly (B = −0.22, SE = 0.09, P = 0.018), and no significant changes were found for Visual Memory, Complex Attention, and Cognitive Flexibility (respectively B = 0.19, SE = 0.09, P = 0.038; B = 0.25, SE = 0.15, P = 0.095; and B = 0.21, SE = 0.12, P = 0.079). Over the second interval, significantly improved performance was found for 4 out of 7 domains. No changes were found between T3 and T12 on Verbal Memory (B = 0.26, SE = 0.13, P = 0.044), Visual Memory (B = −0.10, SE = 0.12, P = 0.398), and Psychomotor Speed (B = 0.02, SE = 0.09, P = 0.864).
Table 4.
Parameter estimates of the LMEMs for preoperative predictors of late cognitive performance (at T12) on CNS VS domains
| Verbal Memory | Visual Memory | Processing Speed | Psychomotor Speed | Reaction Time | Complex Attention | Cognitive Flexibility | |
|---|---|---|---|---|---|---|---|
| Β (SE) | Β (SE) | Β (SE) | Β (SE) | Β (SE) | Β (SE) | Β (SE) | |
| Interval T0-T3a | −0.22 (0.09) | 0.19 (0.09) | 0.22 (0.07) | 0.42 (0.08) | 0.33 (0.13) | 0.25 (0.15) | 0.21 (0.12) |
| Interval T3-T12a | 0.26 (0.13) | −0.10 (0.12) | 0.21 (0.07) | 0.01 (0.09) | 0.49 (0.18) | 0.49 (0.18) | 0.53 (0.10) |
| Months T0-T12b | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Sociodemographic variables | |||||||
| Ageb | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) | −0.01 (0.01) | −0.02 (0.01) | −0.02 (0.01) | −0.00 (0.01) |
| Sexb | |||||||
| female (vs men) | 0.15 (0.20) | −0.46 (0.18) | −0.53 (0.15) | 0.05 (0.16) | 0.37 (0.30) | 0.05 (0.19) | 0.24 (0.14) |
| Educationb | |||||||
| middle (vs low) | −0.02 (0.20) | 0.16 (0.18) | 0.06 (0.16) | −0.21 (0.17) | 0.22 (0.31) | −0.06 (0.20) | 0.01 (0.14) |
| high (vs low) | 0.44 (0.21) | 0.08 (0.19) | −0.03 (0.17) | 0.53 (0.17) | 0.40 (0.33) | 0.41 (0.21) | −0.27 (0.15) |
| Clinical variables | |||||||
| Hemisphereb | |||||||
| right (vs left) | −0.08 (0.17) | −0.15 (0.15) | −0.14 (0.13) | −0.26 (0.14) | −0.14 (0.25) | 0.03 (0.16) | 0.20 (0.12) |
| Supratentorialb | |||||||
| yes (vs no) | 0.16 (0.29) | 0.50 (0.26) | −0.27 (0.23) | −0.37 (0.24) | 0.24 (0.45) | 0.13 (0.29) | −0.13 (0.21) |
| Frontalb | |||||||
| yes (vs no) | −0.11 (0.17) | −0.40 (0.16) | −0.19 (0.14) | −0.11 (0.14) | 0.13 (0.27) | 0.27 (0.17) | 0.03 (0.13) |
| Multipleb | |||||||
| yes (vs no) | 0.10 (0.35) | 0.41 (0.32) | 0.18 (0.28) | −0.48 (0.30) | 0.25 (0.54) | −0.01 (0.34) | 0.15 (0.25) |
| Volume mmbc | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| ASA scoreb | |||||||
| >3 (vs 1 or 2) | 0.43 (0.24) | −0.23 (0.22) | 0.07 (0.19) | −0.51 (0.20) | −0.11 (0.38) | −0.19 (0.24) | 0.36 (0.17) |
| Medicationbd | |||||||
| yes (vs no) | −0.06 (0.17) | −0.25 (0.15) | −0.24 (0.13) | 0.01 (0.14) | 0.03 (0.26) | −0.22 (0.17) | 0.21 (0.12) |
| Psychological variables | |||||||
| HADS Abe | 0.01 (0.02) | −0.05 (0.02) | −0.03 (0.02) | −0.04 (0.02) | 0.01 (0.04) | −0.01 (0.02) | −0.00 (0.02) |
| HADS Dbe | −0.01 (0.02) | 0.02 (0.02) | 0.03 (0.02) | 0.03 (0.02) | −0.04 (0.03) | 0.01 (0.02) | −0.01 (0.02) |
| Cognitive variables | |||||||
| Verbal Memoryb | − | 0.31 (0.06) | 0.04 (0.06) | 0.02 (0.06) | 0.09 (0.11) | 0.04 (0.07) | −0.07 (0.05) |
| Visual Memoryb | 0.40 (0.07) | — | −0.09 (0.06) | 0.05 (0.06) | 0.02 (0.12) | 0.07 (0.08) | 0.06 (0.06) |
| Processing Speedb | 0.01 (0.09) | −0.09 (0.08) | — | 0.34 (0.06) | 0.17 (0.14) | 0.05 (0.09) | 0.15 (0.07) |
| Psychomotor Speedb | 0.12 (0.08) | 0.09 (0.08) | 0.39 (0.05) | — | 0.18 (0.13) | −0.16 (0.08) | 0.05 (0.06) |
| Reaction Timeb | −0.01 (0.05) | −0.03 (0.04) | 0.01 (0.04) | 0.02 (0.04) | — | −0.04 (0.05) | 0.11 (0.03) |
| Complex Attentionb | 0.08 (0.09) | 0.05 (0.08) | 0.02 (0.07) | −0.10 (0.07) | −0.09 (0.13) | — | 0.59 (0.03) |
| Cognitive Flexibilityb | −0.08 (0.11) | 0.06 (0.10) | 0.18 (0.09) | 0.23 (0.09) | 0.44 (0.17) | 1.05 (0.05) | — |
a in bold: P < BH-corrected alpha .03.
b in bold: P < BH-corrected alpha .005.
c data missing for n = 6; d data missing for n = 6; e data missing for n = 7.
Estimates (B) are positive or negative depending on whether they are predicting higher (+) or lower (-) cognitive performance at T12.
Predictors of Late Cognitive Impairment
Table 4 shows the final LMEMs for the cognitive domains. Older age and Cognitive Flexibility score at T0 were significantly associated with a lower z score on Complex Attention at T12. Male sex and higher T0 Psychomotor Speed performance were significantly predictive for higher scores on Processing Speed. A high educational level and better T0 Processing Speed performance significantly predicted a higher Psychomotor Speed score. Higher T0 Verbal Memory performance significantly predicted higher Visual Memory scores, and vice versa, higher T0 Visual Memory performance predicted higher Verbal Memory z scores. Higher performance on Reaction Time and Complex Attention at T0 were significantly predictive of higher Cognitive Flexibility scores (P values < BH-corrected alpha 0.005). None of the preoperatively known clinical or psychological variables, nor the number of months between T0 and T12, were found to significantly predict performance at T12 (P values > BH-corrected alpha 0.005).
The variance explained (ie, marginal R2) ranged from 3% to 14% when only sociodemographic variables were included in the LMEM as fixed effects, from 5% to 22% when clinical variables were added, from 7% to 22% when psychological variables were added, and from 24% up to 85% when the cognitive variables were added to the models (data not shown).
Discussion
In general, preoperative cognitive deficits have been documented in patients with meningioma, and a number of studies suggested that patients also show postoperative impairments.2–11 Prospective studies including preoperative assessments as well as analyses on the individual level, however, are still lacking.
We found extensive preoperative as well as 3- and 12-month postoperative cognitive deficits in our large sample of meningioma patients: mean performance of patients was significantly lower on all cognitive domains at all three timepoints compared to the normative sample with predominantly large, but also medium, effect sizes. On the individual patient level, impairments were significantly more common in meningioma patients compared with normative controls on 6 out of 7 domains at T0 and T3, and on 3 out of 7 domains at T12. Performance on Psychomotor Speed, Reaction Time, and Complex Attention was most frequently, as well as most severely, impaired. A prior study demonstrated significant predictive value of cognitive functioning with regard to functional independence in patients with glioma, providing evidence that cognitive functioning, assessed with neuropsychological tests, can be translated into “real-world” functions and activities.36 The patients in our study may, for example, have difficulty with the agility and adequacy of movements (related to Psychomotor Speed), may respond slowly to stimuli (related to Reaction Time), and may struggle to adapt behaviors and thoughts to new, changing, or unexpected events (related to Complex Attentions, but also to higher order executive functions). These problems complicate (and might even endanger) the ability to perform everyday activities, such as driving a car or preparing dinner. The results of the current study add support for the implementation of routine NPAs in the clinical management of meningioma patients to monitor and deal with common and serious cognitive deficits in order to maximize quality of life and functional independence in home, work, and social settings.
The results indicate that cognitive performance on the group level improves over time. Over the first time interval we found significant improvements on Processing Speed, Psychomotor Speed, and Reaction Time. Further improved performance was found for Processing Speed, Reaction Time, Complex Attention, and Cognitive Flexibility over the second time interval. Yet, as it is expected that some patients show improved and other patients show declined performance, mean group results may mask changes in individual patients. RCIs showed respectively declined and improved performance in 1‒15% and 3‒30% of the patients over the different cognitive domains over the two time intervals. The overlap of patients with solely improved or declined performance between both T0–T3 and T3–T12 was rather small (ie, respectively 36% and 11% of the patients improving and declining over both intervals), suggesting that changes over the second interval in most cases are not continuations of changes in the previous interval. Furthermore, the proportion of “decliners” was for none of the domains, nor for the two intervals, significantly larger than in the normative sample, except for Reaction Time over both intervals. In contrast, improvements were significantly more common in meningioma patients (compared with the normative sample) for most domains over the first interval, and over half of the domains over the second interval. Improvements of performance over time were most frequent and largest for the domains that were most frequently and severely impaired at T0, namely Reaction Time, Psychomotor Speed, and Complex Attention. It should be recognized that very low baseline performance leaves the most room for improvement. However, as the far majority of patients shows stable performance over time and regression to the mean is controlled for by using RCIs, it is likely that an overall small to moderate improvement among many patients explains the significant group-level improvements on these domains, instead of a small group of patients with large improvements. Furthermore, it should be noted that postoperative improvements do not imply that performance of patients returns to unimpaired levels: group performance was still significantly lower on all domains compared with the normative sample; and in addition, about 13% up to almost a quarter of the patients showed impaired performance over different domains at T12.
The LMEMs showed that preoperative cognitive performance was the best predictor for late cognitive performance, and sociodemographic characteristics were predictive for cognitive outcomes. Younger age, male sex, and a higher education were predictors of better performance on some domains 12 months after surgery, while sociodemographically corrected z scores28 were used. These findings can be partly related to the concept of cognitive reserve. Cognitive reserve posits cognitive processes—consisting of differences in cognitive efficiency, capacity or flexibility shaped by, for example, education, socioeconomic status, and life experiences—as explanation of differences between patients who are functionally impaired and patients who are not, despite equal brain pathologies.37–39 The finding of additional predictive effects of age and education, factors that are both associated with cognitive reserve,39 suggests that these variables play a larger role in meningioma patients than in healthy controls. Neither the clinical nor the psychological variables appeared to have significant predictive value for late cognitive performance. Mixed results have been demonstrated in previous studies with regard to the location and volume of meningioma in relation to cognitive performance; whereas some studies demonstrated no significant effects,2 others demonstrated, for example, more deficits in patients with frontal meningioma.12,13,15,20 Meningioma indeed yield local effects because of its mass effect on the surrounding healthy brain.2,20 Consequently, this will likely also reduce the functional integrity of remote brain regions, as locally compressed brain areas and white matter pathways are densely connected to other parts of the brain.40 This would explain why tumor location and volume are not very strong predictors of long-term cognition, as cognition is considered to be globally rather than locally represented in the brain. Results of the current study also suggest that preoperative mood is not a predictor for cognitive outcome. Yet, some (small) associations between mood and cognitive performance after surgery in (partly) the same sample were demonstrated before.9 A perioperative increase of anxiety and depression can be a very normal reaction to the diagnosis with and major treatment of a meningioma; however, it is not very likely that this increase is also related to late cognitive deficits.
Our psychometric studies on CNS VS in healthy controls have led us to develop and use sociodemographically adjusted z scores, as well as RCIs with correction for practice effects when interpreting performance on CNS VS.28,29 However, CNS VS was assumed to be suitable for serial administration without inducing practice effects at time of the previous study in meningioma patients.9,27 It should therefore be noted that the observed severity of cognitive deficits of meningioma patients in that previous study was possibly underestimated. In addition, improvement due to practice effects may have overwhelmed effects of “true” change, and may have contributed to the observed improvement in test performance in the former patient group.9
The current study has some limitations that should be noted. We solely included patients who were considered acceptable candidates for surgery and capable of undergoing the preoperative NPA. Consequently, results may be biased towards an overestimation of cognitive performance in meningioma patients in general. Also, one should take into account that T12 was (as opposed to T0 and T3) no longer part of clinical neuro-oncological care. As this assessment was implemented about 4 years after the start of the study, for a significant proportion of the T3 sample (ie, 33%) more than 21 months had already passed since surgery for these patients, and those were excluded. A considerably smaller number of patients (21%) dropped out because they were not motivated to participate. Comparisons of baseline characteristics of the samples included at T0, T3, and T12 suggest that there were no differences between patients who completed the assessments. It is therefore unlikely that the patients we evaluated formed a very specific group. Furthermore, it would have been interesting to predict group membership (ie, declined, stable, improved) instead of predicting late performance. However, as the percentages of patients with declined or improved performance ranged from 1% to 15% at most across the different domains, these numbers were not sufficient to carry out statistical prediction analysis on group membership at T12. We chose to predict late performance, as this also provides clinically meaningful information of longer-term cognitive outcome at an early stage. Finally, it is important to mention that CNS VS is somewhat limited in terms of covering all cognitive functions: language and visuospatial abilities, for example, are not assessed. Yet, in order to systematically assess and not overburden patients who are part of a carefully defined clinical path, we designated CNS VS as a good alternative to extensive (and therefore costly and time-consuming) NPA.
Increasing attention is being paid to rehabilitation in meningioma patients. A former randomized controlled trial (RCT) by Zucchella et al41 showed immediate positive effects of cognitive rehabilitation in the first weeks after surgery on cognitive performance in a sample of neuro-oncological patients, including meningioma patients. However, as this study did not include a longer-term follow-up, it remains unknown whether these effects persist. More recently, feasibility of and patient satisfaction with an iPad-based cognitive rehabilitation program 3 months after surgery was demonstrated in a small and heterogeneous group of patients with glioma and meningioma.18 An RCT on the effects of this program on (among others) cognitive performance in a larger sample is currently ongoing.19 The increasing opportunities for rehabilitation of cognition demand knowledge of characteristics of meningioma patients who are at high risk of cognitive deficits after surgery, as presented by the current study. We found deficits in a broad range of cognitive domains in many patients; therefore, we recommend that cognitive rehabilitation should at least offer psychoeducation about cognitive functioning (which can be easily accessible for many patients) and teaching of compensatory skills (including strategies and exercises—for example, to try to focus on one task at a time and to outline steps required to complete a task before beginning it) in order to effectively address the widespread deficits.
To conclude, although performance improved over time on the group level in our large sample of meningioma patients, the majority of individual patients showed stable cognitive functioning, and cognitive scores still remained significantly lower than in healthy controls up to 12 months after surgery. Our study indicates that a preoperative NPA, together with easily available sociodemographic information, may provide valuable information on the late cognitive outcome of individual meningioma patients. This knowledge can help to inform patients and clinicians on late cognitive status at an early stage. In addition, it emphasizes the importance of presurgical NPA, and of timely rehabilitation in meningioma patients who are at risk for cognitive impairment.
Funding
This study is funded by ZonMw, a Dutch national organization for health research and development (project number 842003007), and CZ Group, a Dutch non-profit health insurer’s foundation (project number 201300447).
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authorship statement
Experimental design and implementation: MM Sitskoorn, K Gehring, GJM Rutten, I Meskal, SJM Rijnen, Analyses and interpretation of the data: all authors, Writing manuscript at draft and revision stages: all authors, Read and approved final version: all authors
References
- 1. Goldbrunner R, Minniti G, Preusser M, et al. . EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 2. Meskal I, Gehring K, Rutten GJ, Sitskoorn MM. Cognitive functioning in meningioma patients: a systematic review. J Neurooncol. 2016;128(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Nieuwenhuizen D, Klein M, Stalpers LJ, Leenstra S, Heimans JJ, Reijneveld JC. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J Neurooncol. 2007;84(3):271–278. [DOI] [PubMed] [Google Scholar]
- 4. Dijkstra M, van Nieuwenhuizen D, Stalpers LJ, et al. . Late neurocognitive sequelae in patients with WHO grade I meningioma. J Neurol Neurosurg Psychiatry. 2009;80(8):910–915. [DOI] [PubMed] [Google Scholar]
- 5. Waagemans ML, van Nieuwenhuizen D, Dijkstra M, et al. . Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery. 2011;69(1):72–78; discussion 78. [DOI] [PubMed] [Google Scholar]
- 6. Tucha O, Smely C, Lange KW. Effects of surgery on cognitive functioning of elderly patients with intracranial meningioma. Br J Neurosurg. 2001;15(2):184–188. [DOI] [PubMed] [Google Scholar]
- 7. Tucha O, Smely C, Preier M, Becker G, Paul GM, Lange KW. Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. J Neurosurg. 2003;98(1):21–31. [DOI] [PubMed] [Google Scholar]
- 8. Yoshii Y, Tominaga D, Sugimoto K, et al. . Cognitive function of patients with brain tumor in pre- and postoperative stage. Surg Neurol. 2008;69(1):51–61; discussion 61. [DOI] [PubMed] [Google Scholar]
- 9. Meskal I, Gehring K, van der Linden SD, Rutten GJ, Sitskoorn MM. Cognitive improvement in meningioma patients after surgery: clinical relevance of computerized testing. J Neurooncol. 2015;121(3):617–625. [DOI] [PubMed] [Google Scholar]
- 10. Di Cristofori A, Zarino B, Bertani G, et al. . Surgery in elderly patients with intracranial meningioma: neuropsychological functioning during a long term follow-up. J Neurooncol. 2018;137(3):611–619. [DOI] [PubMed] [Google Scholar]
- 11. Krupp W, Klein C, Koschny R, Holland H, Seifert V, Meixensberger J. Assessment of neuropsychological parameters and quality of life to evaluate outcome in patients with surgically treated supratentorial meningiomas. Neurosurgery. 2009;64(1):40–47; discussion 47. [DOI] [PubMed] [Google Scholar]
- 12. Liouta E, Koutsarnakis C, Liakos F, Stranjalis G. Effects of intracranial meningioma location, size, and surgery on neurocognitive functions: a 3-year prospective study. J Neurosurg. 2016;124(6):1578–1584. [DOI] [PubMed] [Google Scholar]
- 13. Hendrix P, Hans E, Griessenauer CJ, Simgen A, Oertel J, Karbach J. Neurocognitive status in patients with newly-diagnosed brain tumors in good neurological condition: the impact of tumor type, volume, and location. Clin Neurol Neurosurg. 2017;156:55–62. [DOI] [PubMed] [Google Scholar]
- 14. Calamia M, Markon K, Tranel D. Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychol. 2012;26(4):543–570. [DOI] [PubMed] [Google Scholar]
- 15. Bommakanti K, Somayajula S, Suvarna A, et al. . Pre-operative and post-operative cognitive deficits in patients with supratentorial meningiomas. Clin Neurol Neurosurg. 2016;143:150–158. [DOI] [PubMed] [Google Scholar]
- 16. Zamanipoor Najafabadi AH, Peeters MCM, Dirven L, et al. . Impaired health-related quality of life in meningioma patients-a systematic review. Neuro Oncol. 2017;19(7):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benz LS, Wrensch MR, Schildkraut JM, et al. . Quality of life after surgery for intracranial meningioma. Cancer. 2018;124:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Linden SD, Sitskoorn MM, Rutten GM, Gehring K. Feasibility of the evidence-based cognitive telerehabilitation program remind for patients with primary brain tumors. J Neurooncol. 2018;137(3):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van der Linden SD, Sitskoorn MM, Rutten GM, Gehring K. Study protocol for arandomized controlled trial evaluating the efficacy of an evidence-based iPad-app for cognitive rehabilitation in patients with primary brain tumors. Neurosurgery. 2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Baene W, Rijnen SJM, Gehring K, Meskal I, Rutten GJM, Sitskoorn MM. Lesion symptom mapping at the regional level in patients with a meningeoma. Neuropsychol. 2018;33(1):103–110. [DOI] [PubMed] [Google Scholar]
- 21. Verhage F. Intelligence and Age: Research Study in Dutch Individuals Aged Twelve to Seventy-Seven. Assen. Amsterdam, Netherlands: Van Gorcum/Prakke & Prakke; 1964. [Google Scholar]
- 22. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 23. Yushkevich PA, Gerig G. ITK-SNAP: an intractive medical image segmentation tool to meet the need for expert-guided segmentation of complex medical images. IEEE Pulse. 2017;8(4):54–57. [DOI] [PubMed] [Google Scholar]
- 24. Dripps RD. New classification of physical status. Anesthesiology. 1963:24(111). [Google Scholar]
- 25. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 26. Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370. [DOI] [PubMed] [Google Scholar]
- 27. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Arch Clin Neuropsychol. 2006;21(7):623–643. [DOI] [PubMed] [Google Scholar]
- 28. Rijnen SJM, Meskal I, Emons WHM, et al. . Evaluation of normative data of a widely used computerized neuropsychological battery: applicability and effects of sociodemographic variables in a Dutch sample. Assessment. 2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rijnen SJM, van der Linden SD, Emons WHM, Sitskoorn MM, Gehring K. Test-retest reliability and practice effects of a computerized neuropsychological battery: A solution-oriented approach. Psychol Assess. 2018;30(12):1652–1662. [DOI] [PubMed] [Google Scholar]
- 30. Lezak MD, Howieson DB, Bigler ED, Tranel D (ed). Neuropsychological Assessment. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 31. Glass GV, McGaw B, Smith ML.. Meta-Analysis in Social Research. Beverly Hills, CA: Sage Publications; 1981. [Google Scholar]
- 32. Maassen GH, Bossema E, Brand N. Reliable change and practice effects: outcomes of various indices compared. J Clin Exp Neuropsychol. 2009;31(3):339–352. [DOI] [PubMed] [Google Scholar]
- 33. Myers R. Classical and modern regression with applications. 2nd ed. Boston, MA: Duxbury; 1990. In Field A, Discovering Statistics Using SPSS. 3rd ed. London: SAGE Publications Ltd; 2009:224. [Google Scholar]
- 34. Pinheiro J, Bates D, DebRoy S, Sarkar D, and R Core Team nmle: linear and nonlinear mixed effect models. R package version 3.1–137. 2018. https://CRAN.R-project.org/package=nlme. [Google Scholar]
- 35. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc series B. 1995;57(1):189–300. [Google Scholar]
- 36. Noll KR, Bradshaw ME, Weinberg JS, Wefel JS. Neurocognitive functioning is associated with functional independence in newly diagnosed patients with temporal lobe glioma. Neurooncol Pract. 2018;5(3):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3(4):369–382. [DOI] [PubMed] [Google Scholar]
- 38. Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17(10):502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363(9420):1535–1543. [DOI] [PubMed] [Google Scholar]
- 41. Zucchella C, Bartolo M, Di Lorenzo C, Villani V, Pace A. Cognitive impairment in primary brain tumors outpatients: a prospective cross-sectional survey. J Neurooncol. 2013;112(3):455–460. [DOI] [PubMed] [Google Scholar]