Abstract
The finding that most grades II and III gliomas harbor isocitrate dehydrogenase (IDH) mutations conveying a relatively favorable and fairly similar prognosis in both tumor grades highlights that these tumors represent a fundamentally different entity from IDH wild-type gliomas exemplified in most glioblastoma. Herein we review the most recent developments in molecular neuropathology leading to reclassification of these tumors based upon IDH and 1p/19q status, as well as the potential roles of methylation profiling and deletional analysis of cyclin-dependent kinase inhibitor 2A and 2B. We discuss the epidemiology, clinical manifestations, benefit of surgical resection, and neuroimaging features of lower-grade gliomas as they relate to molecular subtype, including advanced imaging techniques such as 2-hydroxyglutarate magnetic resonance spectroscopy and amino acid PET scanning. Recent, ongoing, and planned studies of radiation therapy and both cytotoxic and targeted chemotherapies are summarized, including both small molecule and immunotherapy approaches specifically targeting the mutant IDH protein.
Keywords: astrocytoma, glioma, IDH mutation, lower-grade, oligodendroglioma
In neuro-oncology vernacular, low-grade glioma has long been taken to denote grade II diffuse gliomas, whereas the term high-grade glioma encompassed grades III and IV tumors. However, recent developments suggest this division is wanting and have led to the term “lower-grade glioma” to designate both grades II and III gliomas. One factor behind this is the blurry dividing line between grades II and III tumors on the basis of mitotic activity reflected in the low concordance rates among expert neuropathologists.1 A second factor has been the recognition that isocitrate dehydrogenase (IDH) mutations characterize the great majority of grades II and III gliomas but are distinctly uncommon in grade IV tumors. Additionally, when analysis is restricted to IDH-mutated tumors and controlled for 1p/19q deletional status, the prognoses of grade II and grade III gliomas are within a similar range.2,3 Herein we review recent developments in our understanding of lower-grade gliomas with emphasis on how these discoveries will inform future efforts to improve the outcome of these tumors.
Pathology
While histological diagnosis of lower-grade gliomas has had a long history, each iteration of disease classification suffered from continued intra- and interobserver variability, with detrimental consequences on prognostic precision and treatment decision making.1,4 Indeed, the microscopic discrimination between astrocytomas and oligodendrogliomas has long proved difficult, even for the most experienced diagnostic neuropathologists, many of whom also doubted the existence of mixed gliomas as separate clinicopathological entities.5,6
Since the 1990s, oligodendrogliomas have been known to feature a recurrent genetic mutation, loss of chromosomes 1p and 19q, the etiology of which was subsequently found to be an unbalanced translocation.7,8 Over the ensuing 15 years, molecular features such as 1p/19q codeletion served as ancillary testing in support of a diagnosis based on established histological and immunohistochemical evidence.
The 2007 World Health Organization (WHO) classification was the last to rely solely on microscopic morphology. It recognized 7 diffuse gliomas, each with cytological and immunohistochemical evidence of differentiation along astrocytic, oligodendroglial, or both lineages. Histological grading based on mitoses, microvascular proliferation, and necrosis, morphological features correlating with more aggressive biology, permitted further refinement into distinct prognostic entities.9
More recently, the power of comprehensive molecular analysis techniques, including widespread use of gene expression and copy number profiling with microarray technologies as well as mutational profiling via Sanger sequencing, has transformed tumor classification from a morphological to a molecular basis. Studies have clearly established that significant intertumoral molecular heterogeneity exists among each of the histologically defined diffuse gliomas.10,11
The development of massively parallel (next-generation) sequencing technologies in the 2000s facilitated genome-wide mutational profiling of tumors. One of the first studies to use this new technology in gliomas sequenced 20 661 protein coding genes in 22 glioblastoma patient samples.12 In addition to known mutational drivers of glioblastoma, they identified mutations in the IDH1 gene, encoding isocitrate dehydrogenase, a metabolic enzyme involved in the tricarboxylic acid cycle. This discovery led to an explosion in research activity focused on the role of metabolism in cancer, particularly gliomas.13 But it also significantly altered the trajectory of diagnostic neuropathology and laid the groundwork for the subsequent 2016 WHO classification update, particularly for lower-grade (WHO grades II and III) gliomas. Indeed, a year later (2009), the same group screened for mutations in IDH1 and the related IDH2 gene in hundreds of CNS and non-CNS tumors.14 They identified mutations affecting IDH1 codon 132 or the analogous codon 172 in IDH2 in over 70% of lower-grade gliomas, as well as a subset of secondary glioblastomas that evolved from lower-grade precursors. Importantly, IDH1/2 mutations occurred independently of glioma histology, as they were found in both astrocytomas and oligodendrogliomas. IDH1/2-mutant (mt) tumors were associated with distinct genetic and clinical characteristics, portending a better outcome than IDH1/2 wild-type (wt) tumors.
Comprehensive genomics and integrative bioinformatics studies from 2008–2015 radically changed the diagnostic landscape for gliomas. Two landmark papers in 2015 proved pivotal: One was a population-based study of 1087 diffuse gliomas that analyzed the mutation status of 3 molecular markers (1p/19q, IDH1/2, and telomerase reverse transcriptase [TERT] promoter). It showed that classification based upon these 3 markers stratified grades II and III gliomas into 5 molecular subgroups that independently associated with clinical outcomes.15 The second, a study by The Cancer Genome Atlas (TCGA) of 293 lower-grade gliomas, utilized comprehensive data from multiple omics platforms, including exome and RNA sequencing, DNA copy number and methylation, microRNA, and targeted protein expression analyses. Unbiased integrative bioinformatics analysis identified 3 molecular subtypes that stratified lower-grade gliomas based on the status of 2 molecular markers, 1p/19q codeletion and IDH1/2 mutations.16 Importantly, each of these 3 subtypes—IDHmt lacking 1p/19q codeletion, IDHmt with1p/19q codeletion, and IDHwt—had non-overlapping survival curves and conveyed prognostic significance. The best outcomes were in patients with 1p/19q codeleted tumors with a median survival of 8.0 years, compared with 6.3 years for IDHmt, non-codeleted tumors and 1.7 years for IDHwt tumors. The majority of IDHmt tumors without codeletion were astrocytomas histologically, and nearly all featured mutations in both tumor protein 53 (TP53; 94%) and alpha thalassemia/mental retardation syndrome X-linked (ATRX; 86%). The majority of IDHmt tumors with codeletion manifested oligodendroglial histology and harbored CIC (capicua), FUBP1 (far upstream element binding protein 1), Notch1, and TERT promoter mutations. These data confirmed previous reports identifying CIC and FUBP1 as candidate oligodendroglioma tumor suppressor genes lost on chromosomes 1p and 19q, respectively.17 Other large studies have reported similar findings.18,19
Approximately 20% of lower-grade gliomas lack an IDH mutation; this is particularly common in grade III tumors and tumors with astrocytic histology.20 Such tumors commonly manifest molecular alterations typically seen in glioblastoma, including chromosome 7 gains, chromosome 10 deletions, amplification of epidermal growth factor receptor (EGFR), TERT promoter mutations, and deletions of cyclin-dependent kinase inhibitor 2A (CDKN2A) and retinoblastoma protein (RB1). Overall, the prognosis of these IDHwt tumors is much worse than their corresponding IDHmt counterparts. The unfavorable prognosis is particularly strong for IDHwt anaplastic gliomas (median survival 1.3 years in one large study, compared with 8.4 years for IDHwt low-grade gliomas).20 In this study, IDHwt tumors were further divided into a molecularly unfavorable group (those having either EGFR amplification, H3F3A mutation, or TERT promoter mutation) and a favorable group lacking those alterations: median overall survival (OS) was 1.2 versus 7.6 years. A subsequent TCGA analysis of 1122 glioblastoma and lower-grade glioma datasets showed that IDHwt lower-grade gliomas segregated into 3 DNA methylation subtypes.21 Two of these shared the classical and mesenchymal gene expression signatures of glioblastoma and harbored glioblastoma-like mutations, including EGFR, phosphatase and tensin homolog (PTEN), and neurofibromatosis type 1 (NF1). The third methylation subtype shared mutational similarities to the non-diffuse glioma, pilocytic astrocytoma (WHO grade I), and portended a similarly favorable prognosis.
Based largely on these studies, the WHO working group met from 2014 to 2016 to discuss future classification of CNS tumors, including gliomas.22,23 Their work culminated in the updated 2016 WHO classification of gliomas, which represented a dramatic nosological shift in focus away from diagnoses based solely on morphological criteria to one of integrative diagnoses based on both phenotype and genotype.24 Six diagnostic entities were codified, each with a requisite molecular finding (Table 1, Figures 1–3).
Table 1.
Diffuse glioma classification, WHO 2016
| Diagnostic Entity | Diagnostic Molecular Feature(s) | Grade | Ancillary Molecular Tests |
|---|---|---|---|
| Diffuse astrocytoma | IDH-mutant | II | TP53, ATRX |
| Anaplastic astrocytoma | IDH-mutant | III | TP53, ATRX |
| Glioblastoma | IDH-mutant | IV | TP53, ATRX, PDGFRA |
| Glioblastoma | IDH wild-type | IV | TP53, EGFR, PTEN, NF1 |
| Oligodendroglioma | IDH-mutant 1p/19q-codeleted | II | TERT |
| Anaplastic oligodendroglioma | IDH-mutant 1p/19q-codeleted | III | TERT |
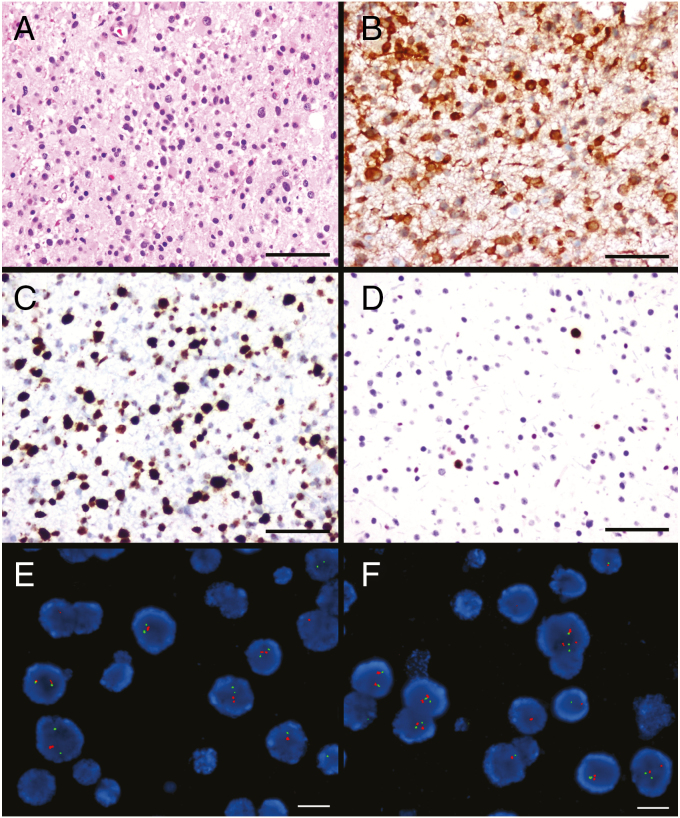
Fig. 1.
Astrocytoma. H&E shows a hypercellular tumor composed of pleomorphic tumor cells (A). This tumor is immunoreactive for IDH1 R132H (B) and TP53 (C), but has lost ATRX expression (D). Fluorescence in situ hybridization (FISH) showed retention of 1p (E) and 19q (F). Scale bar A–D = 100 microns; scale bar E–F = 20 microns. FISH images courtesy of Kathleen A. Kaiser-Rogers, PhD.
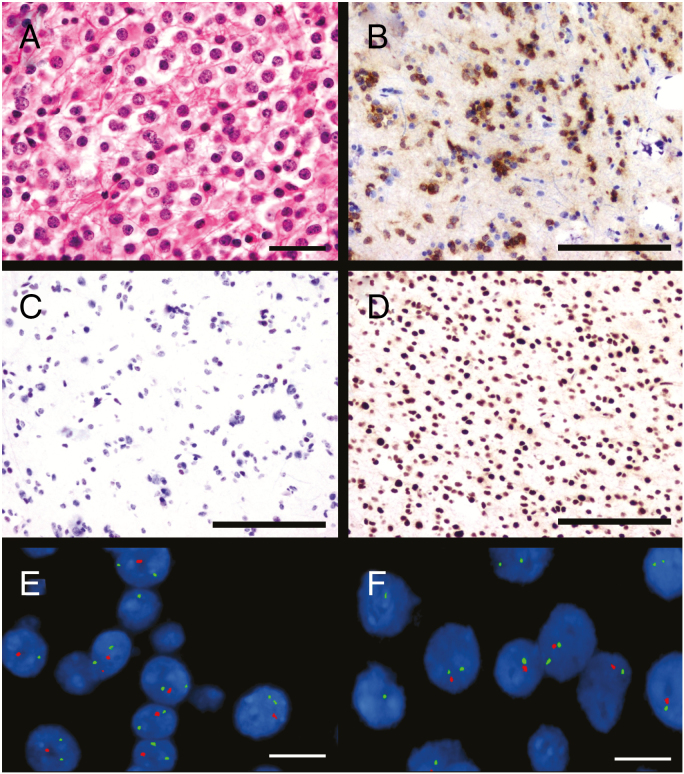
Fig. 2.
Oligodendroglioma. H&E shows a hypercellular tumor composed of atypical cells with round, regular nuclei and perinuclear halos (A). This tumor is immunoreactive for IDH1 R132H (B) and ATRX (D), but not TP53 (C). Fluorescence in situ hybridization (FISH) showed deletion of both 1p (E) and 19q (F). Scale bar A–D = 100 microns; scale bar E–F = 20 microns. FISH images courtesy of Kathleen A. Kaiser-Rogers, PhD.
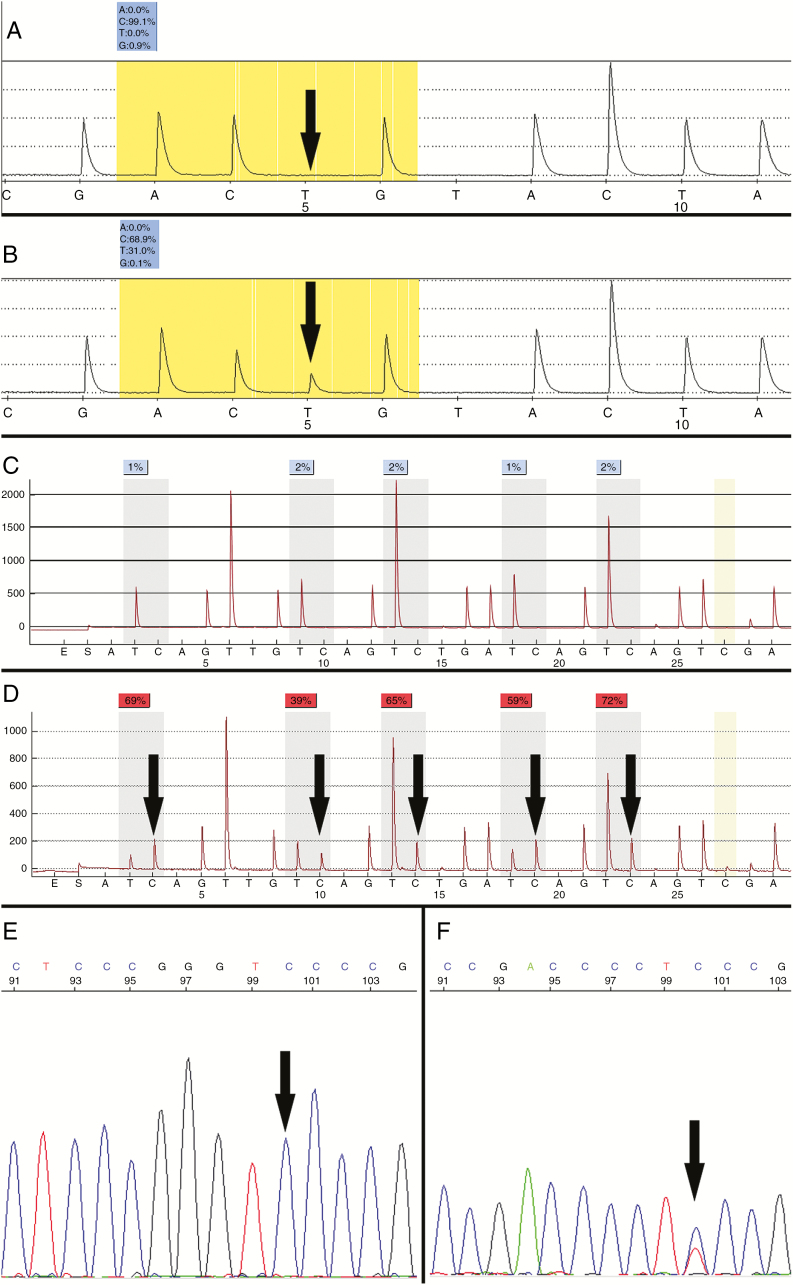
Fig. 3.
Molecular diagnostics of diffuse gliomas. Use of custom pyrosequencing to detect wild-type (A) and R132H mutant (B, C > T) IDH1 on reverse strand pyrograms. Bisulfite pyrosequencing detects CpG methylation within the MGMT promoter as increased C peaks (≥10%) at five indicated sites (C, negative; D, positive). Lack of the C peak highlighted in gold indicates complete bisulfite conversion. Sanger sequencing detects the 124 C > T (C228T) and 146 C > T (C250T) mutations in the TERT promoter. Only C is present at the indicated position in samples with wild-type TERT promoter (E); a T peak at this site (146) indicates mutation (courtesy of Jonathan Galeotti and Karen Weck).
While the 2016 WHO classification of lower-grade gliomas significantly improved diagnostic precision and prognostic accuracy, it continued to harbor several limitations. Chief among these was the continued reliance on morphological criteria, specifically mitotic activity, to separate WHO grade II from WHO grade III gliomas. Identifying “brisk” or “high” mitotic activity has had a long history in glioma diagnostic neuropathology. Common methods for enumerating mitotic indices include subjective counting of mitoses on hematoxylin and eosin (H&E)–stained sections and immunohistochemical staining of surrogate markers such as MIB-1. However, lack of clarity regarding reproducible methods and defined cutoffs plagued multiple iterations of the WHO classification, including 1993, 2000, 2007, and 2016. In the United States, adoption of 6 mitoses in 10 high-powered fields became a widely used cutoff for anaplastic oligodendroglioma (WHO grade III) diagnosis, based largely on work from the Mayo Clinic.25 However, no clear cutoffs were defined for anaplastic astrocytomas (WHO grade III). This lack of clarity significantly impacted diagnostic accuracy and intra- and interobserver reproducibility, with detrimental effects on prognostic precision.
In the era of molecular testing for glioma classification, this lack of clarity proved problematic, as preliminary studies suggested that mitotic activity lost its significance particularly in IDHmt tumors.2,3 To address this issue, a recent study from an international group of neuro-oncology researchers developed a novel grading system for IDHmt astrocytic gliomas (ie, those lacking 1p/19q codeletions).26 This retrospective cohort study utilized a discovery set of 211 IDHmut, astrocytic gliomas (WHO grades II–IV) and 3 separate validation sets, all characterized for their genome-wide DNA methylation and copy number variation (CNV) profiles. They found that stratification based on both morphological (necrosis) and molecular (homozygous deletion of CDKN2A/2B and CNV status) harbored significantly improved prognostic accuracy relative to 2016 WHO criteria. Collectively, these studies challenge the notion that histologically defined proliferation biomarkers retain their importance in contemporary glioma neuropathology, particularly for IDHmt gliomas.
Epidemiology
Data in currently available brain tumor registries do not take into account molecular subtyping of lower-grade gliomas on the basis of IDH and 1p/19q status. Based on pre-2016 WHO classification, the predicted yearly incidence in 2018 of grade II astrocytomas, oligodendroglioma, and mixed gliomas in the United States is 0.48, 0.24, and 0.19 per 100 000 per year, for estimated totals of 1400, 660, and 440, respectively.27 (As noted earlier, in the future the mixed glioma category will shrink dramatically with resolution into astrocytoma and oligodendroglioma with molecular testing.) Corresponding figures for grade III astrocytomas and oligodendrogliomas are 0.40 (1630 cases) and 0.11 (390 cases).
Risk factors for development of lower-grade gliomas are poorly understood. The only recognized environmental risk factor is a remote history of prior ionizing radiation, such as in long-term survivors of childhood leukemia. A prior history of allergies or asthma appears somewhat protective against gliomas in general, perhaps suggesting a role for immune surveillance. Well-defined inherited tumor predisposition syndromes (NF1, Li–Fraumeni, Lynch, Ollier, Maffucci, and melanoma-neural tumor syndromes) account for a miniscule proportion of cases, but the 5–10% of patients with glioma with a positive glioma family history and studies consistently demonstrating a 2-fold glioma risk in first-degree relatives of glioma patients point to other complex hereditary factors. In the last several years, genome-wide association studies have identified several gene variants that confer increased risk of developing gliomas, including lower-grade gliomas. Most of these variants, some of which are polymorphisms of biologically relevant genes such as TP53 and genes involved in telomere maintenance, are low penetrance and only modestly increase risk.28 However, one risk allele on chromosome 8 near CCDC26 increases the risk of specifically developing an oligodendroglioma or IDHmt glioma 6-fold.29 Approximately 40% of patients with oligodendrogliomas or IDH-mutated astrocytomas carry at least one copy of this allele compared with 8% of controls. The function of this inherited variant remains unknown.
Clinical Features
Although lower-grade gliomas may present in various ways, the most common manifestation is seizures, and the development of seizures during the course of the disease may herald tumor progression.30 In grade II gliomas, seizures are the initial manifestation in more than 70%31,32; as many as 90% of patients with oligodendrogliomas eventually develop tumor-related epilepsy.32 Among grade II tumors, the incidence of epilepsy is higher with IDH mutation,31,33 and it has been posited that the oncometabolite 2-hydroxyglutarate (2-HG) produced by the mutated enzyme mediates this tendency through excitatory effects on the N-methyl-D-aspartate receptor. IDH mutation remains a strong risk factor for seizures even when controlling for tumor location, grade, and 1p/19q status.33 Neuroimaging in workup of unrelated symptoms or disorders accounts for discovery of 4–10% of low-grade gliomas.32,34,35 Anaplastic gliomas are somewhat less likely to manifest with seizures (57%) and are more likely than grade II gliomas to produce mental status, vision, and motor deficits.36 Neurocognitive function (NCF) is impaired in a substantial minority of lower-grade glioma patients when formally tested, with executive function particularly vulnerable.37
Neuroimaging Features
Standard MRI provides detailed anatomic characterization of lower-grade gliomas; most tumors are T2-hyperintense with no to mild contrast enhancement. However, several important diagnostic challenges remain. Foremost among these are (1) accurately assessing therapy response in slower growing tumors, which often requires differentiating tumor from treatment change and (2) early identification of transformation into glioblastoma.
The IDH1 and IDH2 mutations result in the production of 2-HG, which accumulates in mutant tumor cells in high concentrations (5–35 mM) but is essentially absent in non-mutant tumors.38 Levels of 2-HG correspond to tumor cellularity, but not to mitotic index or tumor grade.39 One new avenue with potential to assess therapy response is the use of MR spectroscopy (MRS) to quantify 2-HG. Levels of 2-HG decrease in IDH1 mutant glioma after chemotherapy and radiation, and the volume of decreased 2-HG correlates with improved clinical status.40 Interestingly, changes in FLAIR volume do not correlate with change in functional status, suggesting MRS adds value to standard anatomic imaging in these patients. Measurements of 2-HG may improve assessment of tumor burden, which is particularly relevant to posttreatment patients whose scans can demonstrate pseudoresponse or pseudoprogression with non-specific fluid attenuated inversion recovery (FLAIR) signal and/or enhancement. Since inhibitors of the mutated IDH protein are being tested in clinical trials, 2-HG measurements may also be a means of testing drug targeting and efficiency.41 Technical improvements in quantifying 2-HG by MRS are needed to overcome inaccuracies introduced by overlying metabolite spectra, in particular those due to the structurally related molecules glutamate and glutamine.42,43 Measurements of 2-HG in smaller tumors can also be problematic due to partial-volume effects, a particularly important limitation following surgery when volumes of residual tumor can be quite small.39 Overcoming these challenges may allow 2-HG measurements by MRS to serve as a versatile and non-invasive biomarker of glioma diagnosis, prognosis, and therapy response.
Positron emission tomography (PET) provides a non-invasive method to image tissue metabolism and has been extensively studied in brain tumors. Although traditionally the glucose analog 2-deoxy-2-(18F)fluoro-D-glucose (FDG) has been used for PET, non-FDG-PET tracers have recently been gaining ascendency. Gliomas of all grades accumulate amino acid tracers with little background activity, alleviating the problem of nonspecific cerebral cortex uptake that hampers the use of FDG-PET. Amino acid PET tracers undergo active transport, and therefore do not rely on blood–brain barrier disruption. Therefore, they provide a different form of contrast than gadolinium-based agents, and can be used to label tumors that are non-enhancing on standard contrast-enhanced MRI. Indeed, in one study, amino acid PET has been reported to be superior to MRI for identifying treatment response in grade II gliomas following temozolomide (TMZ) therapy.44 This and other results suggest that amino acid PET may improve the ability to accurately quantify tumor burden as well as refine patient prognosis and reduce diagnostic challenges associated with pseudoprogression.45 The widespread use of amino acid PET tracers for glioma assessment has been slow, likely due to a number of issues, including limited access to newer PET tracers, lack of officially approved indications in patients with brain tumors, and difficulty receiving reimbursement from insurance companies. However, the use of amino acid PET imaging for gliomas has been recently promulgated by the Response Assessment in Neuro-Oncology working group, potentially leading to increased momentum for its adoption in routine patient care.46
Lower-grade glioma can transform into glioblastoma, leading to faster growth, treatment resistance, and short survival. Non-invasive markers of this transformation could yield gains in patient care. Traditionally the development of contrast enhancement in previously non-enhancing tumors was thought to indicate transformation into glioblastoma, but accuracy of this metric is limited,47 particularly as approximately half of anaplastic astrocytoma are enhancing at initial presentation.48 Advanced MR and PET imaging may be more specific for this process. For instance, higher perfusion in both grade II and grade III glioma portends shorter survival.49,50 For grade II glioma, relative cerebral blood volume (rCBV) under 1.75 is associated with nonsignificant growth and time to progression that is 19 times longer than tumor with high rCBV. Further, rCBV is low and stable in non-transformers (around 1.5) but rises to a mean of 5.4 in transformers. Importantly, substantial increases in rCBV can be detected up to 12 months prior to the development of contrast enhancement.50,51
Growth rates may be dependent on molecular status. For instance, slower growth has been reported for 1p/19q codeleted tumors as well as tumors that do not overexpress p53.52 Additionally, time to malignant progression and OS are longer for IDH1mt versus IDHwt tumors.53 TMZ treatment appears to reduce growth rates in almost all lower-grade glioma patients (>90%), with subsequent resumption of faster growth rates following cessation of TMZ therapy.52 Interestingly, 1p/19q codeleted tumors have a lower rate of relapse following TMZ treatment compared with intact tumors.
Regardless of molecular status, growth rates may be a reliable marker of malignant transformation in grade II glioma, as average growth rates are substantially lower in non-transformers compared with transformers.54 Further, it has been demonstrated that growth rates increase significantly in the 6 months before transformation,55,56 providing another early warning sign that more aggressive treatment options may need to be pursued. These reports along with studies of MRS and amino acid PET discussed above demonstrate how metabolic and quantitative imaging are becoming an important and necessary adjunct to standard anatomic imaging in the evaluation of patients with lower-grade gliomas.
Surgery for Lower-Grade Gliomas
As noted above, the release of the 2016 WHO diagnostic criteria for gliomas has made the requirement for a tissue-based diagnosis even more central to the management of lower-grade gliomas. In the past it had been acceptable to monitor some lesions with neuroimaging alone; now, the advent of new diagnostic markers provides more definitive information about prognosis and potential benefit from treatment. Consequently, observation is relegated to the increasingly rare situations where the acquisition of tissue would be considered unreliable or inappropriate due to medical comorbidities. Diagnostic tissue can be obtained via a stereotactic biopsy or in the course of an open surgical resection of the tumor mass. The choice of approach is influenced mostly by tumor-specific factors, with biopsy alone usually reserved to situations in which the tumor is deep and an approach to it would be associated with significant risk of morbidity, or when the tumor is so diffuse that an extensive resection would not be feasible.
Complete surgical resection of a suspected lower-grade glioma is the currently favored approach, when feasible.57 It is important to understand how a complete resection is defined, as the definitions of complete resection vary based upon tumor grade and whether or not contrast enhancement is present.58 Diffuse gliomas may have different patterns of infiltration on MRI that can lead to uncertainty as to what is, or is not, considered to be tumor tissue, and hence evaluations of the completeness of resection may be subjective. Imaging-complete resection remains a goal of surgery as there is accumulating evidence relating the completeness of resection to favorable outcome. Surgical resection can also provide a more accurate pathological diagnosis, as tumors tend to be heterogeneous and the grade could be incorrect as a result of sampling error.59 This goal of complete resection must be balanced, however, against the reality that gliomas tend to be infiltrative and often involve cortical and subcortical regions of discrete neurological functioning. Loss of neurological function that may occur if these regions are violated is associated with worsened survival in high-grade gliomas,60 and the impact is likely the same in lower-grade gliomas; hence, a careful balance between maximizing extent of resection (EOR) with minimization of neurological complications must be observed by the surgeon. There are multiple tools that help neurosurgical oncologists achieve this balance, including image guidance, intraoperative MRI, functional MRI with diffusion tensor imaging, navigated transcranial magnetic stimulation, and awake mapping/monitoring with electrocorticography and direct cortical/subcortical stimulation, the details of which are beyond the scope of this review and are well described elsewhere.61–64
There are no randomized studies that provide definitive evidence of a causal link between EOR and survival in lower-grade gliomas.65 It is unlikely that any such study will be performed due to the ethical and equipoise concerns associated with randomization to an intended subtotal resection. Nonetheless, over 2 dozen studies have shown an association between EOR and survival in grade II gliomas.66 The most recent studies, which used volumetric tools to quantitatively evaluate the impact of EOR, showed that higher EOR (typically >90%) was associated with longer 5-year survival, better seizure control, and longer time to malignant transformation.67–69 The retrospective design of these studies has raised concerns about selection bias; that is, some tumors are more inherently resectable than others, and these tumors also may be inherently less aggressive, and hence the impact of surgery is an epiphenomenon. This concern is illustrated by the observation in grade III and IV gliomas that those carrying the IDH1 mutation are more inherently resectable.70 For IDHmt gliomas maximal resection of FLAIR volume is associated with increased survival, whereas for IDHwt grades III and IV gliomas, resection of contrast enhancement is associated with improved survival but additional resection of FLAIR volume does not provide further benefit. For grade II gliomas, the impact of IDH1 mutation on OS was shown on multivariate analysis to be greater than that of EOR for a series of patients who underwent extensive resection (median EOR of 90.4%).71 Interestingly, however, for patients with IDH1 mutated tumors, a smaller residual tumor volume (RTV) was associated with a longer time to progression, and when minimal resections (<40%) were removed from the analysis, EOR had an independent positive effect on OS even after adjustment for IDH1 mutation status.71 These results support the relationship observed in high-grade gliomas that minimization of RTV produces an OS benefit.72 Other studies in lower-grade gliomas appear to confirm the impact of EOR and minimization of RTV, independent of molecular status,73 and one study appears to suggest that in IDH1 mutated tumors, even a small RTV has a negative impact on OS.74 There has been speculation as to whether the most favorable of the lower-grade gliomas, namely those with IDH1 mutation and 1p/19q codeletion, could be subjected to a surgical biopsy only followed by adjuvant chemoradiation or observation alone.75 However, this strategy has not been prospectively tested, and since EOR appears to have an impact on survival of even this most favorable group of patients, this speculation seems dubious.74,76
The closest we have come to a randomized trial is a population-based parallel cohort study that evaluated the outcomes of a series of consecutive patients with lower-grade gliomas who underwent biopsy alone or resection.77 The 5-year OS for the resection group was 82%, as opposed to 54% for the biopsy only group, and the majority of survival benefit was observed in those patient who had an RTV of 15 cm3 or less.77 Similar results were obtained from a parallel cohort Norwegian study in which one surgical center pursued biopsy followed by watchful waiting while another center performed maximal safe resection.78 Some neurosurgeons advocate going beyond the imaging target to perform a “supramaximal” resection as a strategy, but to date the reports are limited to a few centers without independent verification of survival benefit and functional risk.79,80 Despite the lack of randomized data, the accumulated evidence strongly supports the practice of maximal function-based resection of the MRI-visible portion of a lower-grade glioma when feasible, as defined by the avoidance of risk of new or permanently worsened neurological deficits. While the management of recurrent lower-grade gliomas is beyond the scope of this review, re-resection is often an option, subject to the same considerations regarding proximity to functional cortex and white matter and diffuseness, as is the case for newly diagnosed tumors.
Radiotherapy
The role of radiotherapy for patients with grade III gliomas was established with older randomized trials for high-grade gliomas that found a significant survival benefit for postoperative radiotherapy compared with no radiotherapy. However, these trials enrolled both grade III and grade IV gliomas, with the majority of the patients having grade IV tumors. Nonetheless, it is generally accepted that radiotherapy is standard of care for newly diagnosed grade III gliomas.
For grade II gliomas, timing of radiation (postoperative vs salvage) typically depends on several variables such as age and EOR.81 The European Organisation for Research and Treatment of Cancer (EORTC) 22845 trial randomized adults with grade II gliomas to radiotherapy (54 Gy) versus deferred radiation until the time of progression.82 The 5-year rate of progression-free survival (PFS) was significantly better for patients receiving initial radiation therapy (55% vs 35%). However, median OS was not different (7.4 vs 7.2 y), which suggests that immediate radiotherapy is not superior to the same radiotherapy given at the time of progression. There were no differences in the rate of malignant transformation between the study arms at the time of progression, but at 1 year there were significantly fewer seizures in the immediate versus deferred radiotherapy group (25% vs 41%, respectively; P = 0.0329),
Given the lack of proven benefit of early postoperative versus delayed radiation for grade II gliomas, and the similarity in prognosis of grade II and grade III IDHmt astrocytomas, the EORTC is planning a phase III trial examining treatment timing in IDHmt lower-grade gliomas. EORTC 1635, also known as the I-WOT study (for “IDH Mutated 1p/19q Intact Lower-Grade Glioma Following Resection”), proposes to randomize between early and delayed radiotherapy followed by 12 cycles of adjuvant TMZ.83 Since the early treatment group will have a longer time to first progression than the observation group, the primary endpoint will be time from randomization to the second treatment intervention.
For grade II gliomas the recommended radiation dosing is between 45 and 54 Gy in 1.8 to 2.0 Gy fractions. Two prospective randomized clinical trials (EORTC 22844 and North Central Cancer Treatment Group [NCCTG] 86-72-51) failed to show improved outcome with higher radiation therapy doses (59.4 Gy and 64.8 Gy, respectively).84,85 Analyses of failure patterns in this population revealed that tumor progression occurs most frequently at the primary site. Tumor volumes are best defined using FLAIR and/or T2 signal abnormality on MRI and generally treated with a 1 to 2 cm anatomically constrained margin.
For grade III gliomas, common fractionation schedules include 59.4 Gy in 33 fractions or 57 Gy in 30 fractions delivered to higher-risk regions (eg, surgical bed, contrast enhancement, FLAIR/T2 signal abnormality) with little to no margin expansion. For both of these fractionation schedules a 1–2 cm clinical target volume (CTV) expansion on the high-risk region is often treated to 50.4 Gy in 28 fractions (followed by a sequential boost of 9 Gy to the higher-risk region with little to no margin to total dose of 59.4 Gy in 33 fractions) or to 51 Gy, delivered in 30 fractions, respectively, using simultaneous boost technique (while treating the higher-risk region to 57 Gy in 30 fractions). As with grade II gliomas, the margin expansion is anatomically constrained. Because of the better prognosis of grade III gliomas and concerns about radiotherapy late effects, glioblastoma dose regimes such as 60 Gy in 30 fractions are not typically used, although they would be a reasonable consideration for IDHwt anaplastic astrocytomas.
Acute toxicities occur during radiation and generally resolve. Typical acute toxicities include partial (often temporary) alopecia, fatigue, and skin erythema. Late toxicities such as radiation necrosis can occur several months to a few years after radiotherapy. In general the risk of radiation necrosis is 5% or less with doses typically used for grades II and III gliomas.85
The primary rationale for delaying or avoiding radiation is concern over radiation-induced cognitive deterioration. Some retrospective studies have found increased cognitive difficulties after cranial radiotherapy. However, these retrospective studies have several deficiencies, most significant being the lack of baseline testing, as the tumor itself may cause cognitive impairment.86 In general, studies that prospectively assess cognitive function before (ie, at baseline) and after radiotherapy have not found significant cognitive decline after focal radiotherapy. For example, a substudy of 20 of the 203 adult patients with grade II gliomas enrolled on NCCTG 86-72-51 prospectively underwent cognitive testing before and up to 5 years after localized radiation therapy.87 In this study, no significant losses in new learning, memory, or general intellectual function were seen.
Another trial prospectively assessed cognitive function for 17 patients with grade II and III gliomas before radiotherapy (54–55.8 Gy delivered with 1.8 Gy fractions) and serially over time up to 48 months.88 A “non-irradiated” control group of 14 patients with grade II gliomas also prospectively underwent cognitive testing overtime. Besides a transient decrease of performance in the Reaction Time test at 6 months post-radiotherapy there were no other significant changes over time in the 2 groups. In addition, there were no significant differences in cognitive function over time between the irradiated cohort and the non-irradiated cohort. The results of these trials are consistent with other prospective studies that find a low incidence of cognitive decline in adults after moderate dose (ie, 45 to 54 Gy), conventionally fractionated (ie, 1.8 to 2 Gy), focal radiation using modern techniques.86 However, one prospective series on patients with low-grade glioma who received radiotherapy showed a progressive decline in attentional functioning, even in those who received fraction doses that are regarded as safe (≤2 Gy), recognizing this trial suffered the limitation of lacking baseline (ie, prior to radiotherapy) cognitive testing.89
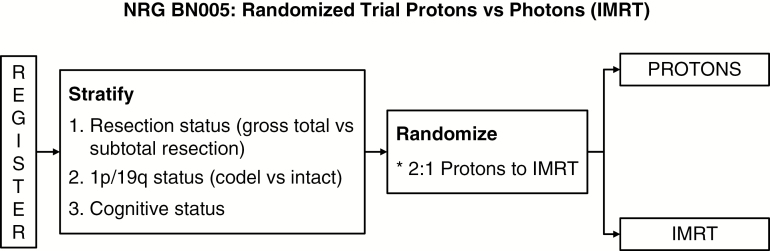
Protons are charged particles with favorable physical properties such that most of the dose is deposited into the target and the dose to the surrounding normal tissues is markedly reduced. Given the relatively good prognosis of IDH mutant grade II and grade III gliomas, there is growing interest in the use of protons to decrease dose to surrounding tissues and therefore potentially decrease late toxicities. A prospective trial of 20 patients with grade II glioma treated with protons (54 Gy in 30 fractions) found no decline in cognitive functioning or quality of life over time (median follow-up 5.1 y).90 Based on the results of this trial and other studies, NRG-BN005 (NCT03180502) is currently randomizing patients with IDHmt grade II and III gliomas to protons versus photons (Figure 4) with cognitive function being the primary endpoint. Patients in both study arms receive adjuvant TMZ after radiotherapy.
Fig. 4.
Schema of NRG BN005.
Chemotherapy in Lower-Grade Gliomas
The activity of blood–brain barrier penetrating, alkylator-based chemotherapy in lower-grade gliomas with regimens like PCV (procarbazine, CCNU [lomustine], and vincristine) and TMZ chemotherapy was first seen in the recurrent disease setting, most notably in tumors with oligodendroglial histology (oligodendroglioma or oligoastrocytoma) or 1p/19q codeletion.91–96 More recently, randomized controlled trials have demonstrated improvements in both PFS and OS in newly diagnosed lower-grade gliomas with the addition of chemotherapy to radiation therapy (Table 2). Three of these trials, initiated in the 1990s, investigated the addition of PCV to radiation in study populations defined by standard histology: 2 in anaplastic oligodendroglial tumors,97,98 1 in grade II gliomas.81 The fourth trial evaluated the addition of TMZ in 1p/19q non-codeleted anaplastic gliomas.99 Each of these trials reported that the addition of chemotherapy to radiation therapy increased OS despite high crossover rates (56–79%) at tumor progression to salvage chemotherapy in the radiation-only arm.81,97–99 Although initial reports of the trials on PCV chemotherapy in anaplastic oligodendroglial tumors noted maximal survival benefit in 1p/19q codeleted tumors, subsequent analyses suggested 3 related candidate markers predicting benefit from adjuvant PCV: IDH mutation, cytosine-phosphate-guanine (CpG) island methylated phenotype, and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation.100,101 In the EORTC 26951 study, MGMT promoter methylation assessed with a genome-wide methylation assay was the best predictor for benefit from PCV101; the Radiation Therapy Oncology Group (RTOG) 9402 study identified IDH mutational status as a predictive factor.100 Two other European studies compared initial chemotherapy with radiation therapy: in one, either PCV or TMZ was compared with radiotherapy in anaplastic glioma; in the second, dose-dense TMZ was compared with radiotherapy in grade II gliomas.102,103 Neither study demonstrated benefit with initial chemotherapy alone, with the suggestion of a worse outcome after initial chemotherapy in some analyses in patients with astrocytoma.102,103 Even in the favorable 1p/19q codeleted group the median PFS with TMZ alone was limited (in most reports approximately 5 y); in contrast, for IDHmt astrocytoma PFS is only 2.5–3.5 years with this approach.102–105 Data on upfront PCV in molecularly defined oligodendroglioma are more limited; reports suggest 5.5 to 8 years PFS.102,106 Another trial explored TMZ induction therapy followed by thiotepa and busulfan myeloablative treatment with stem cell rescue. Among 1p/19q codeleted patients, 5-year PFS was 50% and 5-year OS 93%, apparently not superior to TMZ or PCV alone.107 A single-arm study of initial treatment with TMZ alone in grade II gliomas reported a PFS of 3.6 years in IDHmt tumors and 4.9 years in 1p/19q codeleted tumors; with median OS of 11.2 and 9.7 years, respectively.104 This compares unfavorably with the 13 to 14 years reported in RTOG 9802 in IDHmt grade II gliomas and 1p/19q codeleted anaplastic oligodendrogliomas in the adjuvant PCV trials.81,98 Thus, while a formal comparison of initial treatment with chemotherapy alone to initial treatment with both chemotherapy with radiotherapy is unavailable, the currently available data suggest that combining radiotherapy with chemotherapy in newly diagnosed glioma patients increases survival compared with single modality treatment (either radiation or chemotherapy alone). A potential benefit of a chemotherapy-alone approach is the possibility of deferring the risk of radiation-induced cognitive effects; however, the current data suggest this approach is likely to compromise OS. Although better tolerated than PCV, TMZ has been associated with the development of a hypermutated tumor phenotype at progression, mediated by TMZ-induced mutations in mismatch repair pathway genes.108 While the hypermutated state confers resistance to TMZ, from a clinical perspective the DNA mutational pattern at progression is less important than the total duration of treatment response and OS. For the subgroup of IDHwt lower-grade gliomas, which resemble glioblastoma both at the molecular level and in prognosis, the combination of radiation and TMZ as in glioblastoma should be considered.20,109
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) reported in trials on adjvuant chemotherapy in grade II and III glioma
| Histology | Trial Question | n | Median OS, y | HR [95% CI] for OS |
|---|---|---|---|---|
| Anaplastic oligodendroglioma98 | RT/PCV vs RT | 368 | 3.5 vs 2.5 | 0.75 [0.60, 0.95] |
| Anaplastic oligodendroglioma97 | RT/PCV-i vs RT | 291 | 4.6 vs 4.7 | 0.79 [0.60, 1.04] |
| Low-grade glioma81 | RT/PCV vs RT | 251 | 13.3 vs 7.8 | 0.59 [0.42, 0.83] |
| Anaplastic astrocytoma110 | RT/carmustine + DBD vs RT | 193 | 2.3 vs 2.0 | 0.77 [0.56, 1.06] |
| Anaplastic glioma, 1p/19q intact99 | RT/TMZ vs RT | 745 | NR vs 3.4 | 0.65* (0.45, 0.93 |
| Anaplastic glioma102 | TMZ or PCV vs RT | 318 | 6.9 vs 6.0 | 1.11 [0.8, 1.55] |
| Low-grade glioma103 | RT vs TMZ | 447 | 3.3 vs 3.8 (PFS) | 1.16 [0.9, 1.5] |
| Anaplastic astrocytoma111 | RT/TMZ vs RT/lomustine or carmustine | 197 | 3.9 vs 3.8 | 0.94 [0.67, 1.32] |
*99.145 % confidence interval; °Primary endpoint: PFS; DBD: dibromodulciterol; RT: radiotherapy; NR: not reached.
Adapted with permission from: Martin J. van den Bent, Susan M. Chang. “Grade II and III Oligodendroglioma and Astrocytoma”, Neurologic Clinics, 2018, Elsevier.
Temozolomide versus Nitrosoureas
A debated issue is the relative efficacy of TMZ versus nitrosourea-based chemotherapy. PCV was the chemotherapy utilized in the initial lower-grade glioma studies showing benefit from the addition of adjuvant chemotherapy to radiation therapy.81,97,98 The more recent results of the CATNON trial show that adjuvant TMZ results in prolonged survival in patients with grade III 1p/19q non-codeleted tumors. RTOG 9813 compared adjuvant TMZ with adjuvant BCNU or CCNU following radiotherapy in anaplastic astrocytomas and observed no survival difference; however, myelosuppression in the nitrosourea-treated patients led to more frequent treatment discontinuation.111 TMZ was also clearly better tolerated in comparison to PCV in the NOA-04 anaplastic glioma trial.102 Nonetheless, several retrospective analyses have suggested better survival results with PCV compared with TMZ in 1p/19q codeleted tumors.102,112,113 For this reason, some experts recommend PCV with radiation therapy for codeleted tumors and reserve TMZ for 1p/19q-intact astrocytic ones.114 The ongoing CODEL study (NCT00887146) comparing adjuvant PCV with combined chemo-irradiation with TMZ in codeleted grades II and III tumors should eventually answer this question. Notably, some data suggest vincristine does not cross the blood–brain barrier well.115 Consequently, some clinicians omit vincristine from the PCV regimen.
Bevacizumab
Bevacizumab has an established role in the management of recurrent glioblastoma. Initial uncontrolled studies reported similar outcome of treatment with bevacizumab in relapsing grade III tumors compared with those obtained in recurrent glioblastoma.116–119 Radiographic response rates have ranged from 50% to 70%, with 6-month PFS 40–70%, and median OS 9–15 months. In glioblastoma, it is clear that despite improving PFS, bevacizumab does not improve OS in either the newly diagnosed or recurrent disease setting.120–122 Similarly, the randomized phase II TAVAREC trial in relapsing 1p/19q-intact lower-grade gliomas with enhancing disease showed that the combination of bevacizumab and TMZ improves neither PFS nor OS in comparison to treatment with TMZ alone.123 In view of this, the role of bevacizumab in recurrent lower-grade gliomas should be selective and restricted to palliate symptoms related to vasogenic edema; a true antitumoral effect has not been demonstrated in glioma.
Second Line (and Beyond) Therapies
The treatment of progressive tumors after prior chemotherapy (either as part of initial management or after first progression) represents an unmet clinical need. Several trials in recurrent lower-grade glioma have shown activity to agents that are now used in the front-line setting during or immediately following radiotherapy (PCV and TMZ).92,95,124 Data on second-line treatment with nitrosoureas or TMZ are limited and suggest only modest activity. Limited studies on second-line treatment with PCV, nitrosoureas, and TMZ suggest modest activity. PCV has some activity in oligodendroglial tumors following prior TMZ and vice versa.125–127 In TMZ-refractory anaplastic astrocytoma, lomustine has a partial response rate of 6% and 40% 6-month PFS, suggesting slight activity.128 Everolimus showed a high 6-month PFS but no radiographic responses in a prospective uncontrolled trial of everolimus in progressive grade II astrocytoma; interpretation of these results is difficult due to the heterogeneous patient population and the lack of a control arm.129 The ongoing STELLAR phase III trial randomizes patients with anaplastic astrocytoma, both IDHmt and IDHwt, to lomustine ± the ornithine decarboxylase inhibitor eflornithine; this agent blocks the synthesis of polyamines, thought to play an important role in glioma initiation and progression.130 Data suggest that IDHmt tumors that relapse after TMZ may show microsatellite instability (MSI) and a hypermutated phenotype due to TMZ-induced mutations in mismatch repair genes. In the USA pembrolizumab and nivolumab have been registered for use in MSI high tumors; systematic studies using anti–programmed death 1 (PD-1) and anti–PD-ligand L1 antibodies in MSI high recurrent grade II and III gliomas are ongoing but so far no results have been reported.
There have been some reports on the use of re-irradiation in the treatment of lower-grade gliomas. A study of 63 patients treated with fractionated stereotactic re-irradiation (median total dose of 36 Gy with median interval between the first radiation therapy and re-irradiation of 50 mo) found the treatment to be well tolerated with no severe side effects noted.131 From the time point of re-irradiation, median survival was 23 months and median PFS was 12 months. In general re-irradiation is typically recommended if the new lesion is outside the target of the prior radiotherapy. Re-irradiation is considered if PFS is greater than 2 years after the prior radiotherapy, especially if systemic therapy options are limited, or if the tumor has transformed into a higher-grade tumor.
Novel Therapies Specifically Targeting IDH Mutations
Despite the fact that IDH mutations confer a favorable prognosis15,16,132 and higher sensitivity to therapy, the mutated IDH protein has been regarded as a potential target for both specific inhibitors based on preclinical data133,134 and immunotherapies135 targeting the neoantigen.
As noted earlier, IDH1 and IDH2 mutations almost uniformly occur in critical residues in the catalytic site resulting in the inhibition of wild-type enzymatic activity. Whereas wild-type IDH catalyzes the production of α-ketoglutarate (α-KG) from isocitrate, mutant IDH has a neomorphic enzymatic function and catalyzes the conversion of α-KG to 2-HG,136 which is structurally similar to α-KG. It is also believed to play a crucial role in mutant IDH-mediated malignant cellular transformation by inhibiting α-KG dependent enzymes, effectively leading to hypermethylation of chromatin and antiproliferative effects on glioma infiltrating lymphocytes, thereby preventing relevant immune responses.137
Recent data suggest that 2-HG, despite its activity as an oncometabolite in IDHmt tumors, has antitumor activity in leukemia and glioma.138 This is mediated through inhibition of the enzymatic activity of fat mass and obesity associated protein (FTO). FTO effectively demethylates internal N6-methyladenosine (m6A). Inhibitors of mutant IDH and FTO inhibitors may be attractive for IDHmt tumors.
Although data for FTO inhibition are not yet available, initial data with IDH inhibitors indicate that AG-120 (ivosidenib) is safe in IDH1mt cancer patients and efficacious in advanced IDH1mt acute myelogenous leukemia (AML). There has been some controversy regarding the potential benefit of IDH inhibitors in gliomas, with some concerns raised regarding the possibility that once epigenetic changes develop in IDH mutated tumors, inhibiting mutant IDH may be ineffective. However, in a phase I study that included 66 glioma patients, AG120 (ivosidenib) did not have significant activity in high-grade recurrent tumors but was reported to stabilize the growth of lower-grade gliomas with non-enhancing tumors.139 Other IDH1mt inhibitors in clinical development (Table 3) are AG-881 (vorasidenib), BAY 1436032, and IDH305. AG-881 (vorasidenib) is a potent inhibitor of both IDH1 and 2 with good brain penetration. In a phase I study140 the drug was reasonably well tolerated below 100 mg/day and, as with AG120 (ivosidenib), appeared to slow the growth of some patients with non-enhancing lower-grade gliomas. For AML, the IDH2 inhibitor enasidenib (AG-221) has demonstrated efficacy and is FDA approved.141 A study of BAY 1436032 in contrast-enhancing recurrent gliomas was recently stopped for futility. Human studies in IDH2mt gliomas are not yet started.
Table 3.
Clinical trials of IDHmt-targeting drugs
| Drug | Phase | Target | Completion | Histology | Inclusion | Goal |
|---|---|---|---|---|---|---|
| AG-120 | I | IDH1X | Completed | nd | Progressive | safety, MTD |
| AG-881 NCT02481154 | I | IDH1/2X | Q3 2018 | nd | Progressive | safety, MTD |
| AG-120 & AG-881 NCT03343197 | I | IDH1R132H | Q3 2020 | °II-III | Progressive, preoperative | 2HG -MRS |
| IDH305 NCT02381886 | I | IDH1X132X | Completed | °III-IV | Recurrent after RCTX | safety, MTD |
| BAY1436032 NCT02746081 | Phase I/expansion | IDH1R132X | Closed early after futility analysis | °III-IV | Recurrent after RCTX | safety, MTD |
Abbreviations: 2HG-MRS, 2-hydroxyglutarate magnetic resonance spectroscopy; nd, not detailed; MTD, maximum tolerated dose; RCTX, radiochemotherapy; Q, quarter.
Recently, there are increasing data that in IDH mutated tumors, the 2HG inhibits KDM4A/B, suppressing homologous recombination and rendering these tumors sensitive to poly(ADP-ribose) polymerase (PARP) inhibition.142,143 As a result of these data, the Adult Brain Tumor Consortium is planning a study combining the PARP inhibitor pamiparib (BGB-290) with TMZ for recurrent IDH mutated gliomas. Other agents being evaluated for IDH mutated tumors include the glutaminase inhibitor CB-839 in combination with radiation therapy, and demethylating agents. Several other agents may have therapeutic potential for IDH mutated tumors,144 including nicotinamide phosphoribosyltransferase (NAMPT) inhibitors.145 Mutant IDH1 lowers NAD+ levels by downregulating the NAD+ salvage pathway enzyme nicotinate phosphoribosyltransferase (NARPT1), with the result that the tumor is sensitive to additional NAD+ depletion via concomitant NAMPT inhibition. Unfortunately the currently available NAMPT inhibitors have significant ocular toxicity, limiting their use clinically when administered systemically. However, local delivery may potentially overcome this hurdle.146
Neoepitope-specific vaccines are of considerable interest in glioma. IDH1 R132H was previously identified to contain a neoepitope.135 The peptide vaccine trial Neurooncology Working Group of the German Cancer Society (NOA)-16, a phase I first-in-man multicenter clinical study targeting this epitope, was recently completed and met the primary endpoints of safety and immunogenicity.147
It has recently been shown that 2-HG is taken up by T cells in the glioma microenvironment, where it suppresses T-cell immunity. In WHO grades II and III gliomas, IDHmt tumors display reduced T-cell abundance and altered calcium signaling. Experimentally, antitumor immunity is improved by inhibition of the neomorphic enzymatic function of mutant IDH1,137 which may guide future clinical development. Current trials utilizing immunotherapy to target mutated IDH protein are summarized in Table 4.
Table 4.
Clinical trials of vaccines targeting IDHmt
| Trial | Drug | Phase | Target | Status | Histo. | Inclusion | Target |
|---|---|---|---|---|---|---|---|
| NOA-16 NCT02454634 | 20-mer, Montanide, Imiquimod | Phase I | IDH1R132H | LPO Q3/2017 | °III-IV | Newly diagnosed with RCTX | Safety, Immune |
| AMPLIFY-NEOVAC (NOA-21) NCT pending | 20-mer, Montanide, Imiquimod ± Avelumab | Phase I | IDH1R132H | FPI Q2 2018 | °II-IV | Recurrent after RCTX | Safety, Immune |
| IDH1R132H-DC NCT02771301 | Peptide on patient-autologous DCs | Phase I | IDH1R132H | Q1 2019 | °II-IV | ND with RCTX | Safety |
| RESIST NCT02193347 | 25-mer, Tetanus-Diphtheria-Toxoid | Phase 1 | IDH1R132H | Q2 2019 | °II | Progressive with TMZ | Safety, Immune |
Abbreviations: FPI, first patient in; LPO, last patient out; RCTX, radiochemotherapy; Q, quarter.
Neurocognitive Functioning, Symptom Burden, and Health-Related Quality of Life
For patients with IDHmt grade II or III gliomas, who have a generally far more protracted course of disease than glioblastoma patients, function and well-being following initial treatment are extremely important. Some of these patients have a median survival as long as 15 years, in sharp contrast with the less than 2-year median survival in glioblastoma.81,148 Living independently as long as possible is an important goal of treatment for both patient groups. However, despite stability of disease, long-term survivors may suffer from cognitive and neurologic deficits that preclude an independent life.149
Clinical outcome assessments (COAs) that measure the patient’s functioning and well-being are important to include not only in clinical trials in glioma to determine the net clinical benefit of (new) treatments, but also in daily clinical practice to facilitate symptom control and monitor functioning in these patients. COAs may assess disease-specific or treatment-associated effects, with options including performance-based measures such as questionnaires that measure symptoms or various aspects of health-related quality of life (HRQoL).
Both the tumor itself as well as tumor treatment and supportive treatment may have impact on HRQoL, symptoms, and NCF. Compared with healthy controls, glioma patients at baseline already have focal and generalized symptoms leading to a compromised HRQoL.150,151 The percentage of glioma patients with NCF deficit at baseline depends on tumor grade, location, and extent of testing. Treatment may also affect these outcomes, both positively and negatively.152 In glioblastoma, for example, several clinical studies have demonstrated that the disease itself has a far more negative impact on HRQoL than initial tumor treatment, given that HRQoL during and following treatment remains stable, until progressive disease occurs.122,153
The absence of a clear negative impact on outcomes like NCF and HRQoL also holds true for early treatment effects of radiation and chemotherapy in most studies of grades II and III gliomas. The cognitive impact of radiotherapy has already been discussed. Nor were differences found in NCF measured by Mini-Mental State Examination (MMSE) between grade II glioma patients treated with radiotherapy only versus radiotherapy followed by PCV chemotherapy.154 A more recent EORTC study comparing radiotherapy with initial TMZ for high-risk grade II glioma patients showed a significant difference in neither HRQoL nor MMSE during 3 years follow-up.155
Clinical studies in anaplastic glioma show similar results. In RTOG 9402, patients with anaplastic glioma were treated with either radiotherapy alone or with PCV and radiotherapy. NCF measured with MMSE as well as HRQoL remained stable in both arms over time for surviving patients, while patients who died (rapidly) declined on both outcomes, due to progressive disease.156 A similar EORTC trial demonstrated a transient negative impact of PCV chemotherapy on HRQoL in terms of increased nausea/vomiting, loss of appetite, and drowsiness during and shortly after treatment, but no lasting negative effects up to 2.5 years following treatment.157 More importantly, a subset of 32 long-term surviving patients of the EORTC study (median survival 147 mo) underwent measurement of both NCF with a cognitive test battery and HRQoL with EORTC questionnaires. In 27 patients without progression, HRQoL was similar at 2.5 years following treatment. While most patients lived independently, 8/27 had severe cognitive impairment precluding an independent life, with 5 patients requiring institutionalized care.149 There was no difference in cognitive impairment between the radiation/PCV and radiation-alone groups.
Late effects of treatment, particularly delayed radiation damage, represent a dreaded potential cause of compromised NCF and HRQoL. Indeed, compared with non-irradiated non-progressing grade II glioma patients, those who had been treated with focal radiotherapy developed after a mean of 12 years cognitive deficits mainly in the attention domain, even with daily fraction doses of 1.8 Gy.89 However, one should also consider supportive medication (anti-epileptic medication, dexamethasone, antidepressant or anxiolytic medication) to have a possibly negative impact on NCF and HRQoL in long-term survivors.158
Thus, although decline in outcomes like NCF, symptom burden, and overall HRQoL is not commonly seen in lower-grade glioma early in the course of disease, it may develop relatively late, either due to progressive disease or in long-term stable survivors. The risk of late treatment toxicity should therefore be carefully weighed in patients with good prognosis. Although challenging, it is important to prospectively study lower-grade glioma patients with COAs not only until tumor progression, but also following progression until further decline. This should provide a better understanding of the reasons for and the timing of decline. Additional measures on epilepsy burden and instrumental activity of daily life functioning in these patients might be helpful.159
Conclusion
The discovery of the critical role of IDH mutation in genesis of lower-grade gliomas has redefined the landscape of these tumors. Their molecular classification has already undergone a sea change, and this will undoubtedly further evolve in the next few years as prognostic markers more accurate than mitotic activity are brought into clinical practice. When MRS for 2-HG determination develops further, we may have a new clinical tool for assessing tumor activity. Maximal safe surgical resection of tumor on FLAIR sequence is recommended. The benefit of chemoradiation with lipid-soluble alkylating agents has recently been proven. Novel therapies targeting the mutated IDH protein and its metabolic effects carry the potential for improved outcomes in this disease.
Funding
None.
Conflict of interest statement. David Schiff has received research funding from Bayer Pharmaceutics and serves on the IDMC for Orbus Pharmaceuticals. Patrick Wen has received research support and consulted for Agios Pharmaceuticals. Martin van den Bent has received honoraria from Agios Pharmaceuticals. Michael Platten has received research support and served on advisory board for Bayer Pharmaceutics; he has a patent: Means and methods for treating or diagnosing IDH1 R132H mutant-positive cancers (WO 2013/102641 A1, PCT/EP2013/050048) All other co-authors report no conflicts.
References
- 1. van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol. 2010;120(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olar A, Wani KM, Alfaro-Munoz KD, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015;129(4):585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reuss DE, Mamatjan Y, Schrimpf D, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: grading problem for WHO. Acta Neuropathol. 2015;129(6):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79(7):1381–1393. [DOI] [PubMed] [Google Scholar]
- 5. Burger PC. What is an oligodendroglioma? Brain Pathol. 2002;12(2):257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sahm F, Reuss D, Koelsche C, et al. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014;128(4):551–559. [DOI] [PubMed] [Google Scholar]
- 7. Griffin CA, Burger P, Morsberger L, et al. Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol. 2006;65(10):988–994. [DOI] [PubMed] [Google Scholar]
- 8. Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66(20):9852–9861. [DOI] [PubMed] [Google Scholar]
- 9. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vitucci M, Hayes DN, Miller CR. Gene expression profiling of gliomas: merging genomic and histopathological classification for personalised therapy. Br J Cancer. 2011;104(4):545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 12. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venneti S, Thompson CB. Metabolic reprogramming in brain tumors. Annu Rev Pathol. 2017;12:515–545. [DOI] [PubMed] [Google Scholar]
- 14. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brat DJ, Verhaak RG, Aldape KD, et al. ; Cancer Genome Atlas Research Network Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333(6048):1453–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiestler B, Capper D, Sill M, et al. Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128(4):561–571. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–468. [DOI] [PubMed] [Google Scholar]
- 20. Aibaidula A, Chan AK, Shi Z, et al. Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol. 2017;19(10):1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ceccarelli M, Barthel FP, Malta TM, et al. ; TCGA Research Network Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Louis DN, Perry A, Burger P, et al. ; International Society Of Neuropathology–Haarlem International society of neuropathology–haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 24. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.. WHO Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 25. Giannini C, Scheithauer BW, Weaver AL, et al. Oligodendrogliomas: reproducibility and prognostic value of histologic diagnosis and grading. J Neuropathol Exp Neurol. 2001;60(3):248–262. [DOI] [PubMed] [Google Scholar]
- 26. Shirahata M, Ono T, Stichel D, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136(1):153–166. [DOI] [PubMed] [Google Scholar]
- 27. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rice T, Lachance DH, Molinaro AM, et al. Understanding inherited genetic risk of adult glioma - a review. Neurooncol Pract. 2016;3(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jenkins RB, Xiao Y, Sicotte H, et al. A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet. 2012;44(10):1122–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruda R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14(Suppl 4):iv55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhong Z, Wang Z, Wang Y, You G, Jiang T. IDH1/2 mutation is associated with seizure as an initial symptom in low-grade glioma: a report of 311 Chinese adult glioma patients. Epilepsy Res. 2015;109:100–105. [DOI] [PubMed] [Google Scholar]
- 32. Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54(7):1442–1448. [DOI] [PubMed] [Google Scholar]
- 33. Chen H, Judkins J, Thomas C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pallud J, Fontaine D, Duffau H, et al. Natural history of incidental World Health Organization grade II gliomas. Ann Neurol. 2010;68(5):727–733. [DOI] [PubMed] [Google Scholar]
- 35. Potts MB, Smith JS, Molinaro AM, Berger MS. Natural history and surgical management of incidentally discovered low-grade gliomas. J Neurosurg. 2012;116(2):365–372. [DOI] [PubMed] [Google Scholar]
- 36. Chang SM, Parney IF, Huang W, et al. ; Glioma Outcomes Project Investigators Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293(5):557–564. [DOI] [PubMed] [Google Scholar]
- 37. van Kessel E, Baumfalk AE, van Zandvoort MJE, Robe PA, Snijders TJ. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de la Fuente MI, Young RJ, Rubel J, et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol. 2016;18(2):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andronesi OC, Loebel F, Bogner W, et al. Treatment response assessment in IDH-mutant glioma patients by noninvasive 3D functional spectroscopic mapping of 2-Hydroxyglutarate. Clin Cancer Res. 2016;22(7):1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andronesi OC, Arrillaga-Romany IC, Ly KI, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun. 2018;9(1):1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4(116):116ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roelcke U, Wyss MT, Nowosielski M, et al. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro Oncol. 2016;18(5):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view - What is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17(11):1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Albert NL, Weller M, Suchorska B, et al. Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Narang AK, Chaichana KL, Weingart JD, et al. Progressive low-grade glioma: assessment of prognostic importance of histologic reassessment and MRI findings. World Neurosurg. 2017;99:751–757. [DOI] [PubMed] [Google Scholar]
- 48. Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26(10):2466–2474. [PMC free article] [PubMed] [Google Scholar]
- 49. Hirai T, Murakami R, Nakamura H, et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol. 2008;29(8):1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caseiras GB, Chheang S, Babb J, et al. Relative cerebral blood volume measurements of low-grade gliomas predict patient outcome in a multi-institution setting. Eur J Radiol. 2010;73(2):215–220. [DOI] [PubMed] [Google Scholar]
- 51. Danchaivijitr N, Waldman AD, Tozer DJ, et al. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology. 2008;247(1):170–178. [DOI] [PubMed] [Google Scholar]
- 52. Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61(5):484–490. [DOI] [PubMed] [Google Scholar]
- 53. Gozé C, Blonski M, Le Maistre G, et al. Imaging growth and isocitrate dehydrogenase 1 mutation are independent predictors for diffuse low-grade gliomas. Neuro Oncol. 2014;16(8):1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pallud J, Blonski M, Mandonnet E, et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol. 2013;15(5):595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rees J, Watt H, Jäger HR, et al. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009;72(1):54–64. [DOI] [PubMed] [Google Scholar]
- 56. Brasil Caseiras G, Ciccarelli O, Altmann DR, et al. Low-grade gliomas: six-month tumor growth predicts patient outcome better than admission tumor volume, relative cerebral blood volume, and apparent diffusion coefficient. Radiology. 2009;253(2):505–512. [DOI] [PubMed] [Google Scholar]
- 57. Soffietti R, Baumert BG, Bello L, et al. ; European Federation of Neurological Societies Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–1133. [DOI] [PubMed] [Google Scholar]
- 58. Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) working group. Neurosurgery. 2012;70(1):234–243; discussion 243. [DOI] [PubMed] [Google Scholar]
- 59. Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3(3):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463–469; discussion 469–470. [DOI] [PubMed] [Google Scholar]
- 61. Hervey-Jumper SL, Li J, Lau D, et al. Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg. 2015;123(2):325–339. [DOI] [PubMed] [Google Scholar]
- 62. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 63. Sollmann N, Wildschuetz N, Kelm A, et al. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach. J Neurosurg. 2018;128(3):800–810. [DOI] [PubMed] [Google Scholar]
- 64. Mohammadi AM, Sullivan TB, Barnett GH, et al. Use of high-field intraoperative magnetic resonance imaging to enhance the extent of resection of enhancing and nonenhancing gliomas. Neurosurgery. 2014;74(4):339–348; discussion 349; quiz 349. [DOI] [PubMed] [Google Scholar]
- 65. Jiang B, Chaichana K, Veeravagu A, Chang SD, Black KL, Patil CG. Biopsy versus resection for the management of low-grade gliomas. Cochrane Database Syst Rev. 2017;4:CD009319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130(2):269–282. [DOI] [PubMed] [Google Scholar]
- 67. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 68. Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012;70(4):921–928; discussion 928. [DOI] [PubMed] [Google Scholar]
- 69. Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg. 2011;115(2):240–244. [DOI] [PubMed] [Google Scholar]
- 70. Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jungk C, Scherer M, Mock A, et al. Prognostic value of the extent of resection in supratentorial WHO grade II astrocytomas stratified for IDH1 mutation status: a single-center volumetric analysis. J Neurooncol. 2016;129(2):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 73. Grabenbauer GG, Roedel CM, Paulus W, et al. Supratentorial low-grade glioma: results and prognostic factors following postoperative radiotherapy. Strahlenther Onkol. 2000;176(6):259–264. [DOI] [PubMed] [Google Scholar]
- 74. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018;20(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Buckner J, Giannini C, Eckel-Passow J, et al. Management of diffuse low-grade gliomas in adults - use of molecular diagnostics. Nat Rev Neurol. 2017;13(6):340–351. [DOI] [PubMed] [Google Scholar]
- 76. Snyder LA, Wolf AB, Oppenlander ME, et al. The impact of extent of resection on malignant transformation of pure oligodendrogliomas. J Neurosurg. 2014;120(2):309–314. [DOI] [PubMed] [Google Scholar]
- 77. Roelz R, Strohmaier D, Jabbarli R, et al. Residual tumor volume as best outcome predictor in low grade glioma - a nine-years near-randomized survey of surgery vs. biopsy. Sci Rep. 2016;6:32286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 79. Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. Clinical article. J Neurosurg. 2011;115(2):232–239. [DOI] [PubMed] [Google Scholar]
- 80. de Leeuw CN, Vogelbaum MA. Supratotal resection in glioma: a systematic review. Neuro Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van den Bent MJ, Afra D, de Witte O, et al. ; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 83. Le Rhun E, Gorlia T. Farewell to monomodality treatment in patients with WHO lower grade glioma? Eur J Cancer. 2018;88:109–114. [DOI] [PubMed] [Google Scholar]
- 84. Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–556. [DOI] [PubMed] [Google Scholar]
- 85. Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20(9):2267–2276. [DOI] [PubMed] [Google Scholar]
- 86. Brown PD, Buckner JC, Uhm JH, Shaw EG. The neurocognitive effects of radiation in adult low-grade glioma patients. Neuro Oncol. 2003;5(3):161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Laack NN, Brown PD, Ivnik RJ, et al. ; North Central Cancer Treatment Group Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys. 2005;63(4):1175–1183. [DOI] [PubMed] [Google Scholar]
- 88. Vigliani MC, Sichez N, Poisson M, Delattre JY. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4-year results. Int J Radiat Oncol Biol Phys. 1996;35(3):527–533. [DOI] [PubMed] [Google Scholar]
- 89. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 90. Shih HA, Sherman JC, Nachtigall LB, et al. Proton therapy for low-grade gliomas: results from a prospective trial. Cancer. 2015;121(10):1712–1719. [DOI] [PubMed] [Google Scholar]
- 91. Cairncross G, Macdonald D, Ludwin S, et al. Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994;12(10):2013–2021. [DOI] [PubMed] [Google Scholar]
- 92. Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal brain tumor group. J Clin Oncol. 1999;17(9):2762–2771. [DOI] [PubMed] [Google Scholar]
- 93. van den Bent MJ, Taphoorn MJ, Brandes AA, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Group Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol. 2003;21(13):2525–2528. [DOI] [PubMed] [Google Scholar]
- 94. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 95. Taal W, Dubbink HJ, Zonnenberg CB, et al. ; Dutch Society for Neuro-Oncology First-line temozolomide chemotherapy in progressive low-grade astrocytomas after radiotherapy: molecular characteristics in relation to response. Neuro Oncol. 2011;13(2):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Soffietti R, Rudà R, Bradac GB, Schiffer D. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998;43(5):1066–1073. [DOI] [PubMed] [Google Scholar]
- 97. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 99. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic Oligodendrogliomas and Oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513–5522. [DOI] [PubMed] [Google Scholar]
- 102. Wick W, Roth P, Hartmann C, et al. ; Neurooncology Working Group (NOA) of the German Cancer Society Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18(11):1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wahl M, Phillips JJ, Molinaro AM, et al. Chemotherapy for adult low-grade gliomas: clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro Oncol. 2017;19(2):242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Izquierdo C, Alentorn A, Idbaih A, et al. Long-term impact of temozolomide on 1p/19q-codeleted low-grade glioma growth kinetics. J Neurooncol. 2018;136(3):533–539. [DOI] [PubMed] [Google Scholar]
- 106. Taal W, van der Rijt CC, Dinjens WN, et al. Treatment of large low-grade oligodendroglial tumors with upfront procarbazine, lomustine, and vincristine chemotherapy with long follow-up: a retrospective cohort study with growth kinetics. J Neurooncol. 2015;121(2):365–372. [DOI] [PubMed] [Google Scholar]
- 107. Thomas AA, Abrey LE, Terziev R, et al. Multicenter phase II study of temozolomide and myeloablative chemotherapy with autologous stem cell transplant for newly diagnosed anaplastic oligodendroglioma. Neuro Oncol. 2017;19(10):1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wijnenga MMJ, Dubbink HJ, French PJ, et al. Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathol. 2017;134(6):957–959. [DOI] [PubMed] [Google Scholar]
- 110. Hildebrand J, Gorlia T, Kros JM, et al. ; EORTC Brain Tumour Group investigators Adjuvant dibromodulcitol and BCNU chemotherapy in anaplastic astrocytoma: results of a randomised European Organisation for Research and Treatment of Cancer phase III study (EORTC study 26882). Eur J Cancer. 2008;44(9):1210–1216. [DOI] [PubMed] [Google Scholar]
- 111. Chang S, Zhang P, Cairncross JG, et al. Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: results of NRG Oncology RTOG 9813. Neuro Oncol. 2017;19(2):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lassman AB, Iwamoto FM, Cloughesy TF, et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13(6):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Figarella-Branger D, Mokhtari K, Dehais C, et al. ; POLA Network Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro Oncol. 2014;16(9):1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wick W, Winkler F. Regimen of procarbazine, lomustine, and vincristine versus temozolomide for gliomas. Cancer. 2018;124(13):2674–2676. [DOI] [PubMed] [Google Scholar]
- 115. Boyle FM, Eller SL, Grossman SA. Penetration of intra-arterially administered vincristine in experimental brain tumor. Neuro Oncol. 2004;6(4):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Desjardins A, Reardon DA, Herndon JE 2nd, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2008;14(21):7068–7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chamberlain MC, Johnston S. Bevacizumab for recurrent alkylator-refractory anaplastic oligodendroglioma. Cancer. 2009;115(8):1734–1743. [DOI] [PubMed] [Google Scholar]
- 118. Chamberlain MC, Johnston S. Salvage chemotherapy with bevacizumab for recurrent alkylator-refractory anaplastic astrocytoma. J Neurooncol. 2009;91(3):359–367. [DOI] [PubMed] [Google Scholar]
- 119. Taillibert S, Vincent LA, Granger B, et al. Bevacizumab and irinotecan for recurrent oligodendroglial tumors. Neurology. 2009;72(18):1601–1606. [DOI] [PubMed] [Google Scholar]
- 120. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 122. Wick W, Gorlia T, Bendszus M, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 123. van den Bent MJ, Klein M, Smits M, et al. Bevacizumab and temozolomide in patients with first recurrence of WHO grade II and III glioma, without 1p/19q co-deletion (TAVAREC): a randomised controlled phase 2 EORTC trial. Lancet Oncol. 2018;19(9):1170–1179. [DOI] [PubMed] [Google Scholar]
- 124. Cairncross JG, Macdonald DR. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann Neurol. 1988;23(4):360–364. [DOI] [PubMed] [Google Scholar]
- 125. van den Bent MJ, Chinot O, Boogerd W, et al. Second-line chemotherapy with temozolomide in recurrent oligodendroglioma after PCV (procarbazine, lomustine and vincristine) chemotherapy: EORTC Brain Tumor Group phase II study 26972. Ann Oncol. 2003;14(4):599–602. [DOI] [PubMed] [Google Scholar]
- 126. Triebels VH, Taphoorn MJ, Brandes AA, et al. Salvage PCV chemotherapy for temozolomide-resistant oligodendrogliomas. Neurology. 2004;63(5):904–906. [DOI] [PubMed] [Google Scholar]
- 127. Kouwenhoven MC, Kros JM, French PJ, et al. 1p/19q loss within oligodendroglioma is predictive for response to first line temozolomide but not to salvage treatment. Eur J Cancer. 2006;42(15):2499–2503. [DOI] [PubMed] [Google Scholar]
- 128. Chamberlain MC. Salvage therapy with lomustine for temozolomide refractory recurrent anaplastic astrocytoma: a retrospective study. J Neurooncol. 2015;122(2):329–338. [DOI] [PubMed] [Google Scholar]
- 129. Wahl M, Chang SM, Phillips JJ, et al. Probing the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in gliomas: a phase 2 study of everolimus for recurrent adult low-grade gliomas. Cancer. 2017;123(23):4631–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Levin VA, Ictech SE, Hess KR. Clinical importance of eflornithine (α-difluoromethylornithine) for the treatment of malignant gliomas. CNS Oncol. 2018;7(2):CNS16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Combs SE, Ahmadi R, Schulz-Ertner D, Thilmann C, Debus J. Recurrent low-grade gliomas: the role of fractionated stereotactic re-irradiation. J Neurooncol. 2005;71(3):319–323. [DOI] [PubMed] [Google Scholar]
- 132. Bralten LB, Kloosterhof NK, Balvers R, et al. IDH1 R132H decreases proliferation of glioma cell lines in vitro and in vivo. Ann Neurol. 2011;69(3):455–463. [DOI] [PubMed] [Google Scholar]
- 133. Pusch S, Krausert S, Fischer V, et al. Pan-mutant IDH1 inhibitor BAY 1436032 for effective treatment of IDH1 mutant astrocytoma in vivo. Acta Neuropathol. 2017;133(4):629–644. [DOI] [PubMed] [Google Scholar]
- 134. Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340(6132):626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. [DOI] [PubMed] [Google Scholar]
- 136. Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite ®-2-hydroxyglutarate. Nat Med. 2018;24(8):1192–1203. [DOI] [PubMed] [Google Scholar]
- 138. Su R, Dong L, Li C, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell. 2018;172(1–2):90–105 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mellinghoff IK, Touat M, Maher E, et al. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro-Oncology. 2017;19(Suppl 6):vi10–vi11. [Google Scholar]
- 140. Mellinghoff IK, Penas-Prado M, Peters KB, et al. Phase 1 study of AG-881, an inhibitor of mutant IDH1/IDH2, in patients with advanced IDH-mutant solid tumors, including glioma. J Clin Oncol. 2018; 36(Suppl):abstract 2002. [Google Scholar]
- 141. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sulkowski PL, Corso CD, Robinson ND, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017;9(375). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Waitkus MS, Diplas BH, Yan H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell. 2018;34(2):186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tateishi K, Wakimoto H, Iafrate AJ, et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell. 2015;28(6):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shankar GM, Kirtane AR, Miller JJ, et al. Genotype-targeted local therapy of glioma. Proc Natl Acad Sci USA. 2018;115(36):E8388–E8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Platten M, Schilling D, Bunse L, et al. A mutation-specific peptide vaccine targeting IDH1R132H in patients with newly diagnosed malignant astrocytomas: A first-in-man multicenter phase I clinical trial of the German neurooncology working group (NOA-16). J Clin Oncol. 2018;36(Suppl):abstract 2001. [Google Scholar]
- 148. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 149. Habets EJ, Taphoorn MJ, Nederend S, et al. Health-related quality of life and cognitive functioning in long-term anaplastic oligodendroglioma and oligoastrocytoma survivors. J Neurooncol. 2014;116(1):161–168. [DOI] [PubMed] [Google Scholar]
- 150. Boele FW, Douw L, Reijneveld JC, et al. Health-related quality of life in stable, long-term survivors of low-grade glioma. J Clin Oncol. 2015;33(9):1023–1029. [DOI] [PubMed] [Google Scholar]
- 151. Taphoorn MJ, Stupp R, Coens C, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour Group; EORTC Radiotherapy Group; National Cancer Institute of Canada Clinical Trials Group Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(12):937–944. [DOI] [PubMed] [Google Scholar]
- 152. Dirven L, Aaronson NK, Heimans JJ, Taphoorn MJ. Health-related quality of life in high-grade glioma patients. Chin J Cancer. 2014;33(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Taphoorn MJ, Henriksson R, Bottomley A, et al. Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol. 2015;33(19):2166–2175. [DOI] [PubMed] [Google Scholar]
- 154. Prabhu RS, Won M, Shaw EG, et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: secondary analysis of RTOG 98-02. J Clin Oncol. 2014;32(6):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Reijneveld JC, Taphoorn MJB, Coens C, et al. Health-related quality of life in patients with high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1533–1542. [DOI] [PubMed] [Google Scholar]
- 156. Wang M, Cairncross G, Shaw E, et al. ; Radiation Therapy Oncology Group (RTOG); North Central Cancer Treatment Group (NCCTG); Southwest Oncology Group (SWOG); National Cancer Institute of Canada Clinical Trials Group (NCIC CTG); Eastern Cooperative Oncology Group (ECOG) Cognition and quality of life after chemotherapy plus radiotherapy (RT) vs. RT for pure and mixed anaplastic oligodendrogliomas: radiation therapy oncology group trial 9402. Int J Radiat Oncol Biol Phys. 2010;77(3):662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Taphoorn MJ, van den Bent MJ, Mauer ME, et al. ; European Organisation for Research and Treatment of Cancer Health-related quality of life in patients treated for anaplastic oligodendroglioma with adjuvant chemotherapy: results of a European organisation for research and treatment of cancer randomized clinical trial. J Clin Oncol. 2007;25(36):5723–5730. [DOI] [PubMed] [Google Scholar]
- 158. Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 159. Oort Q, Dirven L, Meijer W, et al. Development of a questionnaire measuring instrumental activities of daily living (IADL) in patients with brain tumors: a pilot study. J Neurooncol. 2017;132(1):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]