Abstract
Endothelial cell (EC) dysfunction is a crucial initiation event in the development of atherosclerosis and is associated with diabetes mellitus, hypertension, and heart failure. Both digestive and oxidative inflammatory conditions lead to the endogenous formation of nitrated derivatives of unsaturated fatty acids (FAs) upon generation of the proximal nitrating species nitrogen dioxide (·NO2) by nitric oxide (·NO) and nitrite-dependent reactions. Nitro-FAs (NO2-FAs) such as nitro-oleic acid (NO2-OA) and nitro-linoleic acid (NO2-LA) potently inhibit inflammation and oxidative stress, regulate cellular functions, and maintain cardiovascular homeostasis. Recently, conjugated linoleic acid (CLA) was identified as the preferential FA substrate of nitration in vivo. However, the functions of nitro-CLA (NO2-CLA) in ECs remain to be explored. In the present study, a distinct transcriptome regulated by NO2-CLA was revealed in primary human coronary artery endothelial cells (HCAECs) through RNA sequencing. Differential gene expression and pathway enrichment analysis identified numerous regulatory networks including those related to the modulation of inflammation, oxidative stress, cell cycle, and hypoxic responses by NO2-CLA, suggesting a diverse impact of NO2-CLA and other electrophilic nitrated FAs on cellular processes. These findings extend the understanding of the protective actions of NO2-CLA in cardiovascular diseases and provide new insight into the underlying mechanisms that mediate the pleiotropic cellular responses to NO2-CLA.
Keywords: conjugated linoleic acid, endothelial cell, nitro-fatty acid, nitroalkene, RNA-Seq
INTRODUCTION
The vascular endothelium maintains vessel structure and adapts to hemodynamic alterations and chemical signals by the production of diverse factors involved in vascular homeostasis, cellular adhesion, thromboresistance, and vessel wall inflammation. Numerous risk factors such as smoking, hypercholesterolemia, and diabetes mellitus lead to endothelial cell (EC) dysfunction (56, 63), which is an early mediator in the development of atherosclerosis.
Nitro-fatty acids (NO2-FAs) contribute to the maintenance of cell homeostasis. Inflammatory conditions lead to nitric oxide (NO) and nitrite ()-dependent unsaturated FA nitration, resulting in the endogenous formation of both free- and esterified-FA nitroalkene derivatives (4, 12, 17, 59) of linoleic acid (NO2-LA) and oleic acid (NO2-OA). NO2-FAs protect against cardiac ischemic injury (47), angioplasty-induced restenosis (8), cardiac ischemia-reperfusion (I/R) injury (39), angiotensin II-induced hypertension (69), and atherosclerosis (46). The specific regioisomer 10-nitro-OA (CXA-10) is currently being evaluated in Phase II clinical trials in pulmonary arterial hypertension (NCT03449524) and primary focal segmental glomerulosclerosis (NCT03422510). NO2-FA adduction to nucleophilic amino acids (His and Cys) in redox-sensitive enzymes and signaling mediators alters protein functions, including inactivation of the oxidant-generating enzyme xanthine oxidoreductase (XOR) (27), activation of nuclear factor 2-related factor (Nrf2)-dependent phase 2 gene expression via S-nitroalkylation of Keap-1 (24) and S-nitro-alkylation of the NF-κB p65 subunit (10). Posttranslational protein modifications induced by NO2-FAs alter the target protein structure and functions, thereby regulating critical biological processes in cells (28, 58).
Recently, conjugated linoleic acid (CLA) was identified as a preferential FA substrate for nitration in vivo (4, 12, 49). Endogenously occurring nitro-CLA (NO2-CLA) exists as two predominant positional isomers: 9- and 12-NO2-CLA (4, 55). Oral consumption of CLA in combination with dietary sources of leads to NO2-CLA formation that reaches clinically relevant plasma levels in mice (4) and humans (12, 20). The abundance of CLA in vivo and the preferential nitrogen dioxide reaction with conjugated double bond configurations result in NO2-CLA being readily produced endogenously and an attractive target for potential clinical applications (12, 20, 55). However, the biological signaling actions of NO2-CLA in ECs remain to be investigated.
In the present study, we revealed a distinct transcriptome regulated by NO2-CLA in primary human coronary artery endothelial cells (HCAECs) with RNA sequencing (RNA-Seq). Analysis of differential gene expression and the engagement of critical signaling pathways reveals that NO2-CLA mediates diverse effects on multiple endothelial cell functions and regulatory networks.
MATERIALS AND METHODS
Cell culture.
HCAECs isolated from a male, 37 yr old, nonsmoking donor were purchased from Lonza (#CC-2585) and cultured in the EGM-2MV microvascular endothelial cell growth medium-2 containing 5% fetal bovine serum (Lonza, #CC-3202) at a 37°C/5% CO2-humidified incubator. HCAECs for the treatments and analysis in this study were used at passages 3–5.
Cell treatment.
For the RNA-Seq experiment, two sets of HCAECs at 90% confluence were treated with DMSO, CLA (10 µM), or NO2-CLA (10 µM) for 1 h, followed by addition to one of the sets of either ethanol (Control) or palmitic acid (200 µM) (Cayman Chemical) for the subsequent 6 h. For RNA-Seq analysis, six groups were considered: DMSO + control (ethanol), CLA (10 µM) + control, NO2-CLA (10 µM) + control, DMSO + palmitic acid, CLA (10 µM) + palmitic acid, and NO2-CLA (10 µM) + palmitic acid. Each group had four biological replicates. Next, quantitative real-time PCR (qPCR) was applied to validate the genes identified from RNA-Seq and establish the dose-dependent gene regulation in response to NO2-CLA treatment as follows: HCAECs at 90% confluence were treated with DMSO, different concentrations of CLA (1, 5, 10 µM), or NO2-CLA (1, 5, 10 µM) for 1 h, followed by addition of either ethanol or palmitic acid (200 µM) for subsequent 6 h. NO2-CLA was synthesized as described previously (65).
RNA extraction.
Total RNA from HCAECs was extracted with RNeasy Mini Kit (Qiagen) and treated on-column with RNase-free DNase I following the manufacturer’s protocol (Qiagen). Total RNA samples were assessed for quality with the BioAnalyzer (Agilent).
RNA-Seq.
RNA library preparation and sequencing were performed by the DNA sequencing core of the University of Michigan. Briefly, the RNA library was prepared with TruSeq RNA Library Prep Kit v2 (Illumina, WI) and sequenced on a HiSeq 4000 platform (Illumina) to generate nonstranded single end 51 bp reads according to the manufacturer’s protocols (35). In total, ~1,030 million reads were generated with the HiSeq 4000 platform (average 43 million reads per sample). The raw data from this study have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE124522.
Read mapping and gene expression analysis.
The quality control of the sequencing reads from each sample was assessed with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). One sample from the control group was excluded from the subsequent analysis because the quality control analysis of this sample showed low mapping rate due to low library quality. The human reference cDNA sequences were downloaded from the Ensembl database (GRCh38 ftp://ftp.ensembl.org/pub/release-93/fasta/homo_sapiens/cdna/). The reference was indexed with Salmon, and gene expression quantification was performed with Salmon in its nonalignment-based mode (42). Differential expression analysis was performed with the DEseq2 package in R (37). The log2 fold change was calculated by default settings with the DESeq2 package, in which the log2 fold change was estimated using the empirical Bayes shrinkage strategy that shrinks log2 fold changes (LFC) estimates toward zero when the gene expression is low (37). Differentially expressed genes (DEGs) are defined as >1.3-fold change in expression level and false discovery rate <0.05. principal component analysis (PCA), volcano plot, and heat maps were generated with an in-house R script.
Gene ontology and KEGG pathway enrichment analysis.
The Gene Ontology and KEGG pathway enrichment analyses were performed with the ClueGO package in Cytoscape (3). Multiple testing correction was performed using Benjamini and Hochberg’s approach (1).
qPCR.
Total RNAs were reverse-transcribed into cDNA with random hexamers used as primers (SuperScript III RT-PCR kit, ThermoFisher). qPCR was performed with iQ SYBR Green Supermix (Bio-Rad) as a validation set. Gene expression was normalized against the internal control glyceraldehyde 3-phosphate dehydrogenase. The primer sequences are shown in Supplemental Table S1. The supplemental tables are available at https://zenodo.org/record/2530146#.XOKvu0hKiUl.
Statistical analysis.
Statistical analyses for qPCR were performed by one-way ANOVA followed by Dunnett test for multiple comparisons with GraphPad Prism version 6.0. Quantitative data are expressed as means ± SE. A P value of < 0.05 was considered statistically significant.
RESULTS
DEGs in response to NO2-CLA in HCAECs.
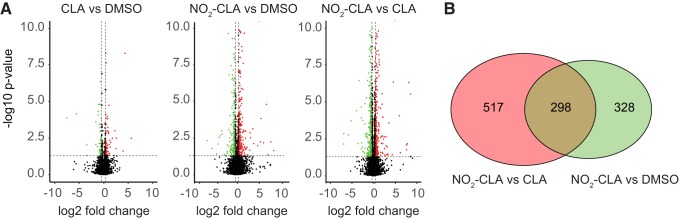
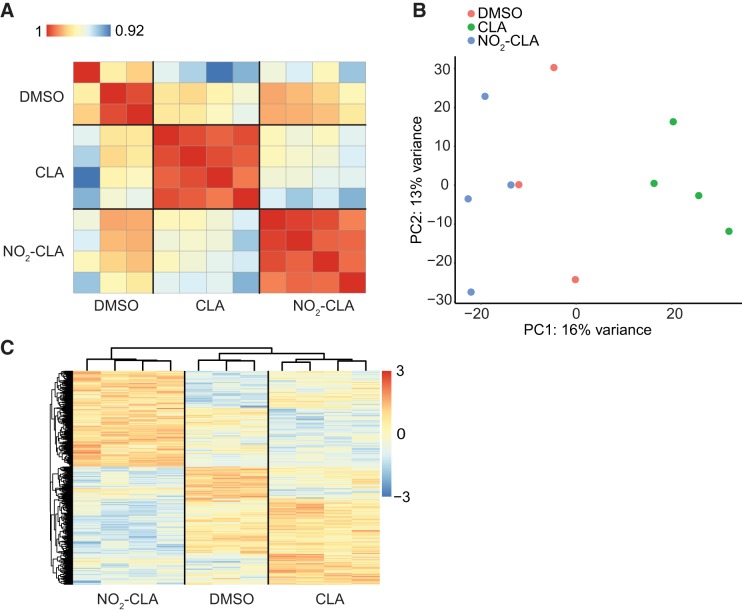
To investigate the effect of NO2-CLA on the EC transcriptome, we treated the HCAECs with electrophilic NO2-CLA (10 µM), nonelectrophilic CLA (10 µM), or DMSO. Differential gene expression was pairwise compared between CLA versus DMSO, NO2-CLA versus DMSO, and NO2-CLA versus CLA (Fig. 1A). Notably, only 150 genes show significant changes between CLA versus DMSO (Supplemental Table S2). In sharp contrast, a total of 1,143 DEGs were identified in response to NO2-CLA treatment (Fig. 1B) with 298 genes overlapping between the comparisons of NO2-CLA versus DMSO and NO2-CLA versus CLA (Supplemental Tables S3 and S4). Pearson’s correlation coefficient matrix (Fig. 2A), PCA (Fig. 2B), and heat map (Fig. 2C) based on the DEGs of each group consistently show that NO2-CLA induces a pattern of gene expression profile distinct from DMSO and CLA treatment.
Fig. 1.
Genes regulated by nitro-conjugated linoleic acid (NO2-CLA) in human coronary artery endothelial cells (HCAECs). A: pairwise volcano plot of differentially expressed genes (DEGs) between different treatments. B: Venn diagram showing overlapping genes between NO2-CLA versus DMSO and NO2-CLA versus CLA. DEGs are defined as >1.3-fold change (upregulated, red; downregulated, green) and false discovery rate < 0.05, n = 4 per group.
Fig. 2.
Differentially expressed genes (DEGs) in HCAECs. A: correlation matrix (Pearson correlation coefficients) of all samples. B: principal component analysis of the DEGs. C: heat map and hierarchical clustering of the DEGs between different treatments. Color bar [red to blue (3 to −3) vertical bar] denotes the row-scaled transcripts per kilobase million (TPM) value, representing the Z score of gene expression across samples.
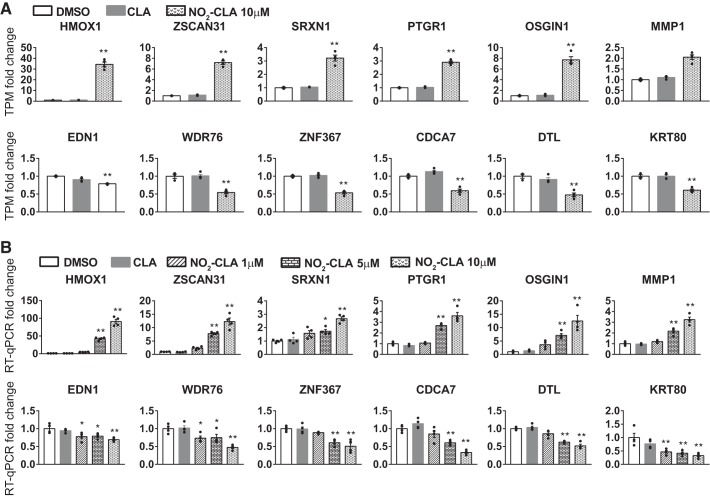
qPCR validation of RNA-Seq results.
To validate the results from RNA-Seq in HCAECs, we chose the top genes (6 upregulated and 6 downregulated) with the lowest P value from the overlapping DEGs between NO2-CLA versus DMSO and NO2-CLA versus CLA (Fig. 3A) and used qPCR to determine their expression in the different groups in the different groups and in response to increasing doses of NO2-CLA (Fig. 3B). The qPCR results validated the gene expression changes. Moreover, these genes responding to NO2-CLA were expressed in a dose-dependent manner in ECs (Fig. 3). In the DEGs, heme oxygenase 1 (HMOX1), oxidative stress-induced growth inhibitor 1 (OSGIN1), and matrix metallopeptidase 1 (MMP1) were increased by 91 ± 6.4-, 12.5 ± 2.1-, and 3.2 ± 0.23-fold, respectively, after NO2-CLA treatment (10 µM). Endothelin-1 (EDN1) was decreased by 31 ± 2.5% in NO2-CLA (10 µM)-treated ECs.
Fig. 3.
Validation of the RNA sequencing (RNA-Seq) by quantitative real-time PCR (qPCR). A: expression of the six most significantly upregulated and downregulated genes as determined by RNA-Seq. Data are shown as fold change in TPM. B: HCAECs were treated with DMSO, CLA, and NO2-CLA at different doses (1, 5, or 10 μM). Fold change in mRNA levels of the indicated genes was determined by qPCR relative to the DMSO control set as 1. qPCR data shown are representative of 3 independent experiments and normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are presented as means ± SE, n = 4 per group. *P < 0.05; **P < 0.01 by one-way ANOVA followed by Dunnett test, compared with the DMSO group.
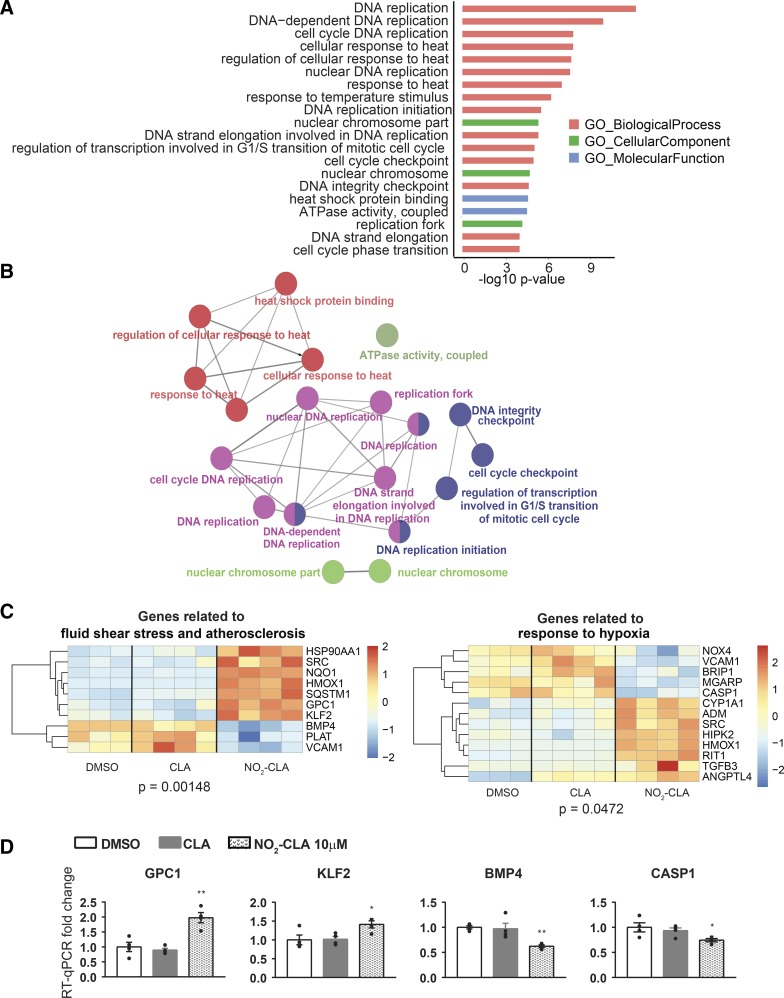
Pathway enrichment analysis of DEGs.
To define the biological actions of NO2-CLA in ECs and identify novel underlying molecular mechanisms, we pursued the pathway enrichment analysis of DEGs. Based on the overlapping DEGs from the two comparisons NO2-CLA versus DMSO and NO2-CLA versus CLA, we identified the overrepresented pathways and novel NO2-CLA-regulated genes. The top enriched pathways in response to NO2-CLA were related to cell cycle regulation, ATPase activity, heat shock protein, and nuclear chromosome (Fig. 4, A and B; Supplemental Table S5). Furthermore, we revealed two overrepresented pathways including fluid shear stress and atherosclerosis (P = 0.00148) and response to hypoxia (P = 0.0472) that are known to be crucial for endothelial function (Fig. 4C). Among the components of these pathways, we identified novel NO2-CLA-responsive genes and validated them by qPCR (Fig. 4D). NO2-CLA increased the expression of glypican 1 (GPC1) and Krüppel-like factor 2 (KLF2) by an average of 1.98- or 1.31-fold, respectively. Also, NO2-CLA decreased bone morphogenic protein-4 (BMP4) and caspase-1 expression by an average of 38 or 26%, respectively.
Fig. 4.
Function and pathway enrichment of DEGs in the groups treated with DMSO, CLA (10 μM), or NO2-CLA (10 μM). A: top GO terms and KEGG pathways in the overlapping DEGs identified from the comparisons of NO2-CLA, DMSO, and CLA. B: network of the top GO terms and pathways. C: heat map of the DEGs in two pathways essential to endothelial cell (EC) function. D: qPCR validation of the novel NO2-CLA responsive genes in the pathways in C. data are presented as means ± SE, n = 4 per group. *P < 0.05; **P < 0.01 by one-way ANOVA followed by Dunnett test, compared with the DMSO group.
NO2-CLA prevents palmitic acid-induced inflammatory responses in HCAECs.
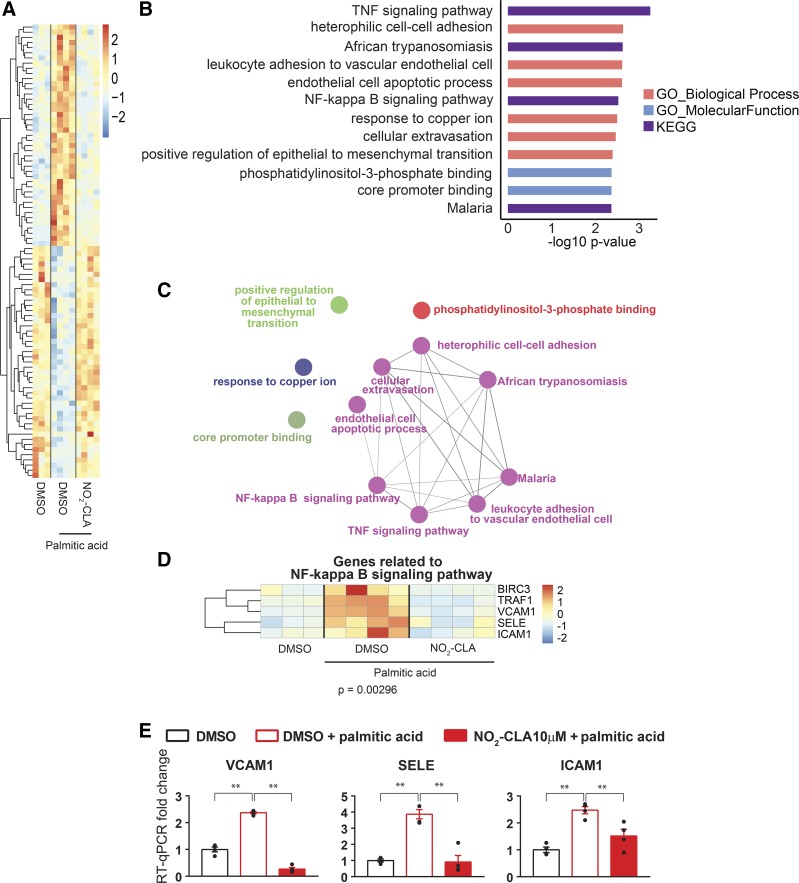
The high proportion of saturated FA consumption is associated with insulin resistance, liver steatosis, and cardiovascular disease (CVD) (5, 52). As a saturated FA, palmitic acid can induce inflammatory responses in vascular ECs (23, 33). To determine whether NO2-CLA antagonizes adverse palmitic acid-induced effects on HCAECs, we compared the response to NO2-CLA and DMSO in cells subjected to palmitic acid (200 μM). We identified a distinct transcriptomic pattern of 87 genes that were influenced by palmitic acid treatment and in turn modulated by concurrent NO2-CLA treatment (Fig. 5A, Supplemental Table S6). Pathway enrichment analysis revealed that some of these genes belong to proinflammatory pathways such as TNF signaling pathway, leukocyte adhesion to vascular endothelial cell, NF-κB signaling pathway, and cellular extravasation. These proinflammatory pathways are among the top pathways with the lowest P value (Fig. 5, B and C). For example, in the NF-κB pathway (P = 0.00296), NO2-CLA inhibited palmitic acid-induced proinflammatory adhesion molecules such as VCAM-1, SELE, and ICAM-1 expression (Fig. 5, D and E).
Fig. 5.
NO2-CLA prevents palmitic acid (PA)-induced endothelial inflammation. HCAECs were pretreated with DMSO or NO2-CLA (10 μM) for 1 h and then treated with PA (200 μM) or ethanol control for another 6 h. A: heat map of PA-regulated genes protected by NO2-CLA. B: top GO terms and KEGG pathways in the NO2-CLA reversed genes. C: group of the top GO terms. D: heat map of the effects of NO2-CLA on the NF-κB pathway response to palmitic acid in ECs. E: fold change in mRNA levels of the selected proinflammatory adhesion molecules in the indicated experimental conditions. mRNA levels were determined by qPCR and normalized by GAPDH with fold change expressed relative to the DMSO group, set as 1. The mRNA abundance of these proinflammatory adhesion molecules was determined by qPCR. Data are presented as means ± SE, n = 4 per group. *P < 0.05; **P < 0.01 by one-way ANOVA followed by Dunnett test, compared with the DMSO group.
DISCUSSION
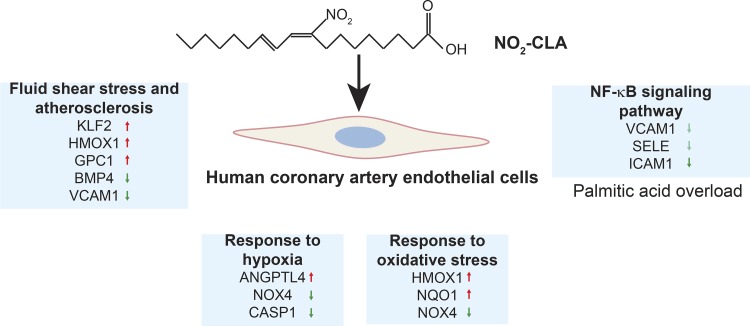
Electrophilic NO2-FAs display signaling responses following Michael addition with kinetically susceptible nucleophilic amino acids in target proteins, resulting in a broad array of responses in diverse cell types and tissues (58). NO2-OA and NO2-LA mediate adaptive anti-inflammatory and metabolic effects in ECs, macrophages, and vascular smooth muscle cells (51, 58). NO2-CLA constitutes a new class of NO2-FAs in addition to NO2-OA and NO2-LA. To better define potential mechanisms accounting for endogenously generated NO2-CLA actions in CVDs, we performed RNA-Seq analysis in primary human ECs. Our data indicate that NO2-CLA induces profound changes in the EC transcriptome and thus provides insight into the pathways and networks responding to this and other electrophilic fatty acid nitroalkene derivatives that appear to maintain and restore cellular homeostasis (Fig. 6). In the present study, we found that NO2-CLA also regulated critical signaling pathways such as inflammation, angiogenesis, and antioxidant and heat shock responses that can be observed in the ECs treated with NO2-OA or NO2-LA. Noteworthy, we further identified novel genes and signaling pathways regulated by NO2-CLA in ECs (Fig. 3 and Fig. 4, C and D), especially the pathways related to the response to hypoxia, fluid shear stress, and to atherosclerosis.
Fig. 6.
Summary of overrepresented pathways of DEGs regulated by NO2-CLA in ECs. NO2-CLA has unique effects on signaling pathways that are involved in EC homeostasis such as redox signaling, angiogenesis, response to hypoxia, and fluid shear stress. Furthermore, NO2-CLA inhibits inflammatory gene expression in ECs under conditions of palmitic acid overload.
In ECs, NO2-FAs enhance NO bioavailability (29, 31), inhibit inflammation (21, 57) and oxidative inflammatory reactions (19, 25, 27, 29, 67), enhance the heat shock response (25), and promote angiogenesis (45). NO2-OA and NO2-LA inhibit NF-κB activation and TNF-α-stimulated proinflammatory cytokine expression (10, 21). Fatty acid nitroalkenes also inhibit LPS-induced inflammation by suppressing the recruitment of TLR4 and TNF receptor-associated factor 6 (TRAF6) to EC lipid rafts (57). Elevated saturated palmitic acid levels are positively associated with metabolic disease and CVD (5, 52). Partial replacement of saturated FA-rich foods with those rich in cis-polyunsaturated FAs lowers the risk of hypertension and coronary heart disease (6, 40). Our transcriptome analysis further suggests that NO2-CLA potently inhibits inflammation including the activation of NF-κB, TNF-α, and leukocyte-EC binding pathways that are activated by palmitic acid (Fig. 5, B and C).
Oxidative inflammatory reactions that are induced by dysregulated generation and scavenging of reactive species promote endothelial dysfunction and CVD (19). NO2-LA and NO2-OA dramatically induce the expression of HMOX1, a heme catabolism enzyme responsive to inflammatory stimuli and oxidative stress (29, 67). NO2-OA upregulates HMOX1 expression in both NF-E2-related factor 2 (NRF2)-dependent and -independent mechanisms (25, 66). Also, NO2-OA upregulates the antioxidant enzyme NAD(P)H quinone dehydrogenase 1 (NQO1) (25) and inhibits the activity of the pro-oxidant enzyme XOR in ECs (27). In the present study, we found that NO2-CLA is not only a potent inducer of the antioxidant genes HMOX1 and NQO1 but also a suppressor of the pro-oxidant gene NADPH oxidase 4, emphasizing a critical role of NO2-CLA in preserving EC redox homeostasis (Fig. 4C). Notably, NO2-CLA also upregulates sequestosome 1/p62, which interacts with Kelch-like ECH-associated protein 1 and further activates NRF2 in response to oxidative stress (22) (Fig. 4C). Thus, these data significantly expand the understanding of how electrophilic NO2-FA can modulate transcriptional responses of proteins critical to the preservation of vascular EC metabolic and adaptive signaling responses.
Dysregulated endothelin signaling is involved in the pathogenesis of various CVDs such as atherosclerosis, hypertension, and restenosis (50). NO2-OA increases the NRF2-dependent expression of endothelin receptor type B in ECs and in turn reduces the extracellular concentration of EDN1 (26). We found that NO2-CLA also significantly downregulates EDN1 gene expression (Fig. 3, A and B), suggesting that NO2-FA can potentially beneficially impact endothelin signaling in various CVDs. NO2-OA and NO2-LA promote EC migration, sprouting, and angiogenesis (45). Among the genes validated in Fig. 3, MMP1 was significantly upregulated by NO2-CLA in ECs. MMP1 is a protease that degrades collagen in the extracellular matrix and enhances vascular remodeling (62). In ECs, MMP1 upregulates VEGFR2 (38) and promotes angiogenesis (48). Our data suggest a potential mechanism that mediates the NO2-FA-induced angiogenesis. OSGIN1 is an oxidative stress-induced gene that is upregulated by the transcription factor Nrf2 (34). Recent studies revealed that OSGIN1 enhances autophagy in the human airway epithelium (54, 61). Given NO2-CLA-dependent upregulation of OSGIN1, there may be a potential role of NO2-CLA in autophagy in ECs.
Cell cycle regulatory pathways such as cell cycle DNA replication, cell cycle checkpoint, and cell cycle phase transition are among the top enriched pathways in response to NO2-CLA (Fig. 4, A and B). The accurate cell cycle transition from G1 phase to S phase is crucial for the control of cell proliferation (2). In smooth muscle cells, NO2-LA inhibits cell proliferation via activation of NRF2 and upregulation of p27 (60). Moreover, in cultured pulmonary artery smooth muscle cells, NO2-OA also suppresses cell proliferation (30). However, the role of NO2-FAs in EC proliferation and the underlying mechanisms remain to be explored. The heat shock response is another major pathway activated by NO2-OA, in which numerous heat shock genes were dramatically increased in ECs (25). Enriched pathway analysis revealed that NO2-CLA consistently enhances the heat shock response (Fig. 4A), reinforcing the notion that heat shock response modulation contributes to the protective effect of NO2-FAs in ECs.
Hypoxia leads to abnormal cell metabolism (13), impaired cell growth and survival, and angiogenesis (64). NO2-OA and NO2-LA promote EC migration, tube formation, and angiogenesis in a NO-hypoxia inducible factor-1α (HIF-1α)-dependent manner (45). The present data affirm that NO2-CLA also regulates hypoxic responses via increased angiopoietin-like 4 (ANGPTL4) expression in ECs (Fig. 4C). ANGPTL4, in turn, promotes angiogenesis (32) and protects against myocardial infarction (18). CASPASE 1 (CASP1), a critical component of the inflammasome, was also identified as a novel gene downregulated by NO2-CLA (Fig. 4C). Activated caspase-1 cleaves prointerleukin-1β (IL-1β) and pro-IL-18 into mature proinflammatory cytokines IL-1β and IL-18, respectively. The inhibition of caspase-1 increased postischemic angiogenesis in mice (36). Thus, the present data support a role for NO2-CLA in modulating hypoxic responses associated with CVDs.
Additional novel pathways being regulated by NO2-CLA were identified in the present study. Different shear stress patterns induce distinct phenotypes and functions associated with resistance/susceptibility to vascular pathology in the arterial ECs (11). Disturbed flow is proatherogenic by impairing redox homeostasis and promoting inflammation, whereas steady laminar blood flow is vasoprotective by regulating EC redox homeostasis and inflammation in the vascular wall (7). Indeed, different patterns of blood flow regulate distinct transcriptomes through mechanical sensors and the modulation of signaling pathways in ECs (41, 43). The current data reveal that NO2-CLA regulates a cluster of genes that are responsive to fluid shear stress (Fig. 4, C and D). For example, laminar shear stress dramatically induces transcription factor KLF2, which upregulates the expression of anti-inflammatory, antioxidant, and antithrombotic genes that act to maintain vascular homeostasis (16). BMPs have been implicated in disorders ranging from hereditary hemorrhagic telangiectasia and peripheral arterial hypertension to atherosclerosis (14). Laminar flow inhibits BMP4, and in contrast, disturbed flow induces BMP4 expression in ECs (9, 53). Notably, similar to the effect of laminar shear stress, NO2-CLA upregulates KLF2 and downregulates BMP4 in ECs. Also, these data suggest that NO2-CLA upregulates GPC1 expression in ECs (Fig. 4, C and D). GPC1 acts as an FGF co-receptor to facilitate the binding of FGF to the FGF receptor (FGFR), subsequently activating FGF-FGFR signaling (44, 70). In ECs, GPC1 mediates the laminar flow-induced activation of the endothelial NO synthase (15, 68), supporting a potentially protective role of GPC1 in atherosclerosis. The mechanisms mediating the regulation of these novel targets by NO2-CLA remain to be explored.
In summary, our study presents a comprehensive and systematic analysis of the endothelial cell transcriptome that responds uniquely to NO2-CLA under basal and saturated FA-induced stress responses. NO2-CLA beneficially regulates critical cellular pathways and responses including cell cycle, hypoxic, anti-inflammatory, and antioxidant responses. We also reveal novel NO2-CLA-responsive genes and pathways, thereby expanding our understanding of potential NO2-CLA effects in CVD, motivating new experimental pursuits and providing new insight into the underlying mechanisms that account for the effects of fatty acid nitroalkenes in the regulation of EC function.
GRANTS
This work was supported in part by National Institutes of Health Grants R01HL-138094 (to Y. Fan), R01HL-068878 (to Y. E. Chen), R01HL-123333 (to L. Villacorta), R01HL-64937, R01HL-132550, and R01HL-103455 (to B.A. Freeman) and GM-125944 and R01DK-112854 (to F.J. Schopfer), R01HL-138139 (to J. Zhang) and American Heart Association Grants 17GRN33660955 (to F.J. Schopfer), 17PRE33400179 (to H. Lu), and 18PRE34000005 (to W. Liang).
DISCLOSURES
B. A. Freeman, F. J. Schopfer, and Y. E. Chen acknowledge an interest in Complexa, Inc. The remaining authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
H.L. and Y.F. conceived and designed research; H.L., J.S., and Y.F. performed experiments; H.L., J.S., W.L., J.Z., and Y.F. analyzed data; H.L., J.S., W.L., J.Z., S.L., L.V., F.J.S., B.A.F., Y.E.C., and Y.F. interpreted results of experiments; H.L. and J.S. prepared figures; H.L. and Y.F. drafted manuscript; H.L., J.S., W.L., J.Z., O.R., M.T.G.-B., S.L., L.V., F.J.S., B.A.F., Y.E.C., and Y.F. approved final version of manuscript; J.Z., O.R., M.T.G.-B., S.L., L.V., F.J.S., B.A.F., Y.E.C., and Y.F. edited and revised manuscript.
REFERENCES
- 1.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 2.Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14: 518–528, 2013. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman W-H, Pagès F, Trajanoski Z, Galon J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25: 1091–1093, 2009. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonacci G, Baker PR, Salvatore SR, Shores D, Khoo NK, Koenitzer JR, Vitturi DA, Woodcock SR, Golin-Bisello F, Cole MP, Watkins S, St Croix C, Batthyany CI, Freeman BA, Schopfer FJ. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J Biol Chem 287: 44071–44082, 2012. doi: 10.1074/jbc.M112.401356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briggs MA, Petersen KS, Kris-Etherton PM. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare (Basel) 5: E29, 2017. doi: 10.3390/healthcare5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, Morisseau C, Hammock BD, Freeman BA, Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc Natl Acad Sci USA 111: 8167–8172, 2014. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387, 2011. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole MP, Rudolph TK, Khoo NK, Motanya UN, Golin-Bisello F, Wertz JW, Schopfer FJ, Rudolph V, Woodcock SR, Bolisetty S, Ali MS, Zhang J, Chen YE, Agarwal A, Freeman BA, Bauer PM. Nitro-fatty acid inhibition of neointima formation after endoluminal vessel injury. Circ Res 105: 965–972, 2009. doi: 10.1161/CIRCRESAHA.109.199075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csiszar A, Lehoux S, Ungvari Z. Hemodynamic forces, vascular oxidative stress, and regulation of BMP-2/4 expression. Antioxid Redox Signal 11: 1683–1697, 2009. doi: 10.1089/ars.2008.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem 281: 35686–35698, 2006. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delmastro-Greenwood M, Hughan KS, Vitturi DA, Salvatore SR, Grimes G, Potti G, Shiva S, Schopfer FJ, Gladwin MT, Freeman BA, Gelhaus Wendell S. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic Biol Med 89: 333–341, 2015. doi: 10.1016/j.freeradbiomed.2015.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng F, Wang S, Xu R, Yu W, Wang X, Zhang L. Endothelial microvesicles in hypoxic hypoxia diseases. J Cell Mol Med 22: 3708–3718, 2018. doi: 10.1111/jcmm.13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer LA, Pi X, Patterson C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol Metab 25: 472–480, 2014. doi: 10.1016/j.tem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebong EE, Lopez-Quintero SV, Rizzo V, Spray DC, Tarbell JM. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr Biol 6: 338–347, 2014. doi: 10.1039/C3IB40199E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Y, Lu H, Liang W, Hu W, Zhang J, Chen YE. Krüppel-like factors and vascular wall homeostasis. J Mol Cell Biol 9: 352–363, 2017. doi: 10.1093/jmcb/mjx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fazzari M, Vitturi DA, Woodcock SR, Salvatore SR, Freeman BA, Schopfer FJ. Electrophilic fatty acid nitroalkenes are systemically transported and distributed upon esterification to complex lipids. J Lipid Res 60: 388–399, 2019. doi: 10.1194/jlr.M088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, Teillon J, Le Jan S, Bouleti C, Briois G, Philippe J, Pons S, Martin V, Assaly R, Bonnin P, Ratajczak P, Janin A, Thurston G, Valenzuela DM, Murphy AJ, Yancopoulos GD, Tissier R, Berdeaux A, Ghaleh B, Germain S. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation 125: 140–149, 2012. doi: 10.1161/CIRCULATIONAHA.111.049072. [DOI] [PubMed] [Google Scholar]
- 19.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J 73: 411–418, 2009. doi: 10.1253/circj.CJ-08-1102. [DOI] [PubMed] [Google Scholar]
- 20.Hughan KS, Wendell SG, Delmastro-Greenwood M, Helbling N, Corey C, Bellavia L, Potti G, Grimes G, Goodpaster B, Kim-Shapiro DB, Shiva S, Freeman BA, Gladwin MT. Conjugated Linoleic Acid Modulates Clinical Responses to Oral Nitrite and Nitrate. Hypertension 70: 634–644, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang J, Lee KE, Lim JY, Park SI. Nitrated fatty acids prevent TNFalpha-stimulated inflammatory and atherogenic responses in endothelial cells. Biochem Biophys Res Commun 387: 633–640, 2009. doi: 10.1016/j.bbrc.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285: 22576–22591, 2010. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H, Liang C, Liu X, Jiang Q, He Z, Wu J, Pan X, Ren Y, Fan M, Li M, Wu Z. Palmitic acid promotes endothelial progenitor cells apoptosis via p38 and JNK mitogen-activated protein kinase pathways. Atherosclerosis 210: 71–77, 2010. doi: 10.1016/j.atherosclerosis.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Ylä-Herttuala S, Freeman BA, Levonen AL. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J Biol Chem 286: 14019–14027, 2011. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kansanen E, Jyrkkänen HK, Volger OL, Leinonen H, Kivelä AM, Häkkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Ylä-Herttuala S, Freeman BA, Levonen AL. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem 284: 33233–33241, 2009. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kansanen E, Kuosmanen SM, Ruotsalainen AK, Hynynen H, Levonen AL. Nitro-Oleic Acid Regulates Endothelin Signaling in Human Endothelial Cells. Mol Pharmacol 92: 481–490, 2017. doi: 10.1124/mol.117.109751. [DOI] [PubMed] [Google Scholar]
- 27.Kelley EE, Batthyany CI, Hundley NJ, Woodcock SR, Bonacci G, Del Rio JM, Schopfer FJ, Lancaster JR Jr, Freeman BA, Tarpey MM. Nitro-oleic acid, a novel and irreversible inhibitor of xanthine oxidoreductase. J Biol Chem 283: 36176–36184, 2008. doi: 10.1074/jbc.M802402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoo NK, Freeman BA. Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Curr Opin Pharmacol 10: 179–184, 2010. doi: 10.1016/j.coph.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoo NK, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, Batthyany C, Freeman BA. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radic Biol Med 48: 230–239, 2010. doi: 10.1016/j.freeradbiomed.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klinke A, Möller A, Pekarova M, Ravekes T, Friedrichs K, Berlin M, Scheu KM, Kubala L, Kolarova H, Ambrozova G, Schermuly RT, Woodcock SR, Freeman BA, Rosenkranz S, Baldus S, Rudolph V, Rudolph TK. Protective effects of 10-nitro-oleic acid in a hypoxia-induced murine model of pulmonary hypertension. Am J Respir Cell Mol Biol 51: 155–162, 2014. doi: 10.1165/rcmb.2013-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koudelka A, Ambrozova G, Klinke A, Fidlerova T, Martiskova H, Kuchta R, Rudolph TK, Kadlec J, Kuchtova Z, Woodcock SR, Freeman BA, Kubala L, Pekarova M. Nitro-Oleic Acid Prevents Hypoxia- and Asymmetric Dimethylarginine-Induced Pulmonary Endothelial Dysfunction. Cardiovasc Drugs Ther 30: 579–586, 2016. doi: 10.1007/s10557-016-6700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Jan S, Amy C, Cazes A, Monnot C, Lamandé N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P, Germain S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol 162: 1521–1528, 2003. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CH, Lee SD, Ou HC, Lai SC, Cheng YJ. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int J Mol Sci 15: 10334–10349, 2014. doi: 10.3390/ijms150610334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R, Chen W, Yanes R, Lee S, Berliner JA. OKL38 is an oxidative stress response gene stimulated by oxidized phospholipids. J Lipid Res 48: 709–715, 2007. doi: 10.1194/jlr.M600501-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Chang Z, Zhu T, Villacorta L, Li Y, Freeman BA, Chen YE, Zhang J. Transcriptomic sequencing reveals diverse adaptive gene expression responses of human vascular smooth muscle cells to nitro-conjugated linoleic acid. Physiol Genomics 50: 287–295, 2018. doi: 10.1152/physiolgenomics.00090.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Pastrana J, Ferrer LM, Li YF, Xiong X, Xi H, Cueto R, Nelson J, Sha X, Li X, Cannella AL, Imoukhuede PI, Qin X, Choi ET, Wang H, Yang XF. Inhibition of Caspase-1 Activation in Endothelial Cells Improves Angiogenesis: A NOVEL THERAPEUTIC POTENTIAL FOR ISCHEMIA. J Biol Chem 290: 17485–17494, 2015. doi: 10.1074/jbc.M115.641191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazor R, Alsaigh T, Shaked H, Altshuler AE, Pocock ES, Kistler EB, Karin M, Schmid-Schönbein GW. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J Biol Chem 288: 598–607, 2013. doi: 10.1074/jbc.M112.417451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadtochiy SM, Baker PR, Freeman BA, Brookes PS. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res 82: 333–340, 2009. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nettleton JA, Brouwer IA, Geleijnse JM, Hornstra G. Saturated Fat Consumption and Risk of Coronary Heart Disease and Ischemic Stroke: A Science Update. Ann Nutr Metab 70: 26–33, 2017. doi: 10.1159/000455681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nigro P, Abe J, Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid Redox Signal 15: 1405–1414, 2011. doi: 10.1089/ars.2010.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14: 417–419, 2017. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao C, Meng F, Jang I, Jo H, Chen YE, Zhang J. Deep transcriptomic profiling reveals the similarity between endothelial cells cultured under static and oscillatory shear stress conditions. Physiol Genomics 48: 660–666, 2016. doi: 10.1152/physiolgenomics.00025.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao D, Meyer K, Mundhenke C, Drew SA, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J Biol Chem 278: 16045–16053, 2003. doi: 10.1074/jbc.M211259200. [DOI] [PubMed] [Google Scholar]
- 45.Rudnicki M, Faine LA, Dehne N, Namgaladze D, Ferderbar S, Weinlich R, Amarante-Mendes GP, Yan CY, Krieger JE, Brüne B, Abdalla DS. Hypoxia inducible factor-dependent regulation of angiogenesis by nitro-fatty acids. Arterioscler Thromb Vasc Biol 31: 1360–1367, 2011. doi: 10.1161/ATVBAHA.111.224626. [DOI] [PubMed] [Google Scholar]
- 46.Rudolph TK, Rudolph V, Edreira MM, Cole MP, Bonacci G, Schopfer FJ, Woodcock SR, Franek A, Pekarova M, Khoo NK, Hasty AH, Baldus S, Freeman BA. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 30: 938–945, 2010. doi: 10.1161/ATVBAHA.109.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res 85: 155–166, 2010. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9: 267–285, 2005. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvatore SR, Vitturi DA, Baker PR, Bonacci G, Koenitzer JR, Woodcock SR, Freeman BA, Schopfer FJ. Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine. J Lipid Res 54: 1998–2009, 2013. doi: 10.1194/jlr.M037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandoval YH, Atef ME, Levesque LO, Li Y, Anand-Srivastava MB. Endothelin-1 signaling in vascular physiology and pathophysiology. Curr Vasc Pharmacol 12: 202–214, 2014. doi: 10.2174/1570161112666140226122054. [DOI] [PubMed] [Google Scholar]
- 51.Schopfer FJ, Vitturi DA, Jorkasky DK, Freeman BA. Nitro-fatty acids: New drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide 79: 31–37, 2018. doi: 10.1016/j.niox.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis 14: 121, 2015. doi: 10.1186/s12944-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassègue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 95: 773–779, 2004. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 54.Sukkar MB, Harris J. Potential impact of oxidative stress induced growth inhibitor 1 (OSGIN1) on airway epithelial cell autophagy in chronic obstructive pulmonary disease (COPD). J Thorac Dis 9: 4825–4827, 2017. doi: 10.21037/jtd.2017.10.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turell L, Vitturi DA, Coitiño EL, Lebrato L, Möller MN, Sagasti C, Salvatore SR, Woodcock SR, Alvarez B, Schopfer FJ. The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid. J Biol Chem 292: 1145–1159, 2017. doi: 10.1074/jbc.M116.756288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 32, Suppl 2: S314–S321, 2009. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villacorta L, Chang L, Salvatore SR, Ichikawa T, Zhang J, Petrovic-Djergovic D, Jia L, Carlsen H, Schopfer FJ, Freeman BA, Chen YE. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc Res 98: 116–124, 2013. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villacorta L, Gao Z, Schopfer FJ, Freeman BA, Chen YE. Nitro-fatty acids in cardiovascular regulation and diseases: characteristics and molecular mechanisms. Front Biosci 21: 873–889, 2016. doi: 10.2741/4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villacorta L, Minarrieta L, Salvatore SR, Khoo NK, Rom O, Gao Z, Berman RC, Jobbagy S, Li L, Woodcock SR, Chen YE, Freeman BA, Ferreira AM, Schopfer FJ, Vitturi DA. In situ generation, metabolism and immunomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox Biol 15: 522–531, 2018. doi: 10.1016/j.redox.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol 293: H770–H776, 2007. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang G, Zhou H, Strulovici-Barel Y, Al-Hijji M, Ou X, Salit J, Walters MS, Staudt MR, Kaner RJ, Crystal RG. Role of OSGIN1 in mediating smoking-induced autophagy in the human airway epithelium. Autophagy 13: 1205–1220, 2017. doi: 10.1080/15548627.2017.1301327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Khalil RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol 81: 241–330, 2018. doi: 10.1016/bs.apha.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wierzbicki AS, Chowienczyk PJ, Cockcroft JR, Brett SE, Watts GF, Jenkins BS, Ritter JM. Cardiovascular risk factors and endothelial dysfunction. Clin Sci (Lond) 107: 609–615, 2004. doi: 10.1042/CS20040078. [DOI] [PubMed] [Google Scholar]
- 64.Wong BW, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J 36: 2187–2203, 2017. doi: 10.15252/embj.201696150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodcock SR, Salvatore SR, Bonacci G, Schopfer FJ, Freeman BA. Biomimetic nitration of conjugated linoleic acid: formation and characterization of naturally occurring conjugated nitrodienes. J Org Chem 79: 25–33, 2014. doi: 10.1021/jo4021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright MM, Kim J, Hock TD, Leitinger N, Freeman BA, Agarwal A. Human haem oxygenase-1 induction by nitro-linoleic acid is mediated by cAMP, AP-1 and E-box response element interactions. Biochem J 422: 353–361, 2009. doi: 10.1042/BJ20090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright MM, Schopfer FJ, Baker PR, Vidyasagar V, Powell P, Chumley P, Iles KE, Freeman BA, Agarwal A. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci USA 103: 4299–4304, 2006. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng Y, Liu J. Role of glypican-1 in endothelial NOS activation under various steady shear stress magnitudes. Exp Cell Res 348: 184–189, 2016. doi: 10.1016/j.yexcr.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res 107: 540–548, 2010. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z, Coomans C, David G. Membrane heparan sulfate proteoglycan-supported FGF2-FGFR1 signaling: evidence in support of the “cooperative end structures” model. J Biol Chem 276: 41921–41929, 2001. doi: 10.1074/jbc.M106608200. [DOI] [PubMed] [Google Scholar]