Abstract
Nitric oxide (NO) is a potent vasodilator, which improves perfusion and oxygen delivery during tissue hypoxia in terrestrial animals. The vertebrate dive response involves vasoconstriction in select tissues, which persists despite profound hypoxia. Using tissues collected from Weddell seals at necropsy, we investigated whether vasoconstriction is aided by downregulation of local hypoxia signaling mechanisms. We focused on NO–soluble guanylyl cyclase (GC)-cGMP signaling, a well-known vasodilatory transduction pathway. Seals have a lower GC protein abundance, activity, and capacity to respond to NO stimulation than do terrestrial mammals. In seal lung homogenates, GC produced less cGMP (20.1 ± 3.7 pmol·mg protein−1·min−1) than the lungs of dogs (−80 ± 144 pmol·mg protein−1·min−1 less than seals), sheep (−472 ± 96), rats (−664 ± 104) or mice (−1,160 ± 104, P < 0.0001). Amino acid sequences of the GC enzyme α-subunits differed between seals and terrestrial mammals, potentially affecting their structure and function. Vasoconstriction in diving Weddell seals is not consistent across tissues; perfusion is maintained in the brain and heart but decreased in other organs such as the kidney. A NO donor increased median GC activity 49.5-fold in the seal brain but only 27.4-fold in the kidney, consistent with the priority of cerebral perfusion during diving. Nos3 expression was high in the seal brain, which could improve NO production and vasodilatory potential. Conversely, Pde5a expression was high in the seal renal artery, which may increase cGMP breakdown and vasoconstriction in the kidney. Taken together, the results of this study suggest that alterations in the NO-cGMP pathway facilitate the diving response.
Keywords: dive response, NO-cGMP signaling, pinniped, soluble guanylate cyclase
INTRODUCTION
Deep-diving, air-breathing vertebrates rely on a well-developed dive response to support extended periods of submergence. Cardiovascular control is central to the dive response, characterized by bradycardia and peripheral vasoconstriction, with redistribution of circulation to preserve O2 delivery to the brain and heart during diving (8, 51). The cardiovascular elements of the dive response have a strong component of neural regulation (48, 60); parasympathetic stimulation via the vagus nerve produces bradycardia, and sympathetic activation drives peripheral vasoconstriction. Currently, a role for local vasoregulation of perfusion during diving is unclear.
Hypoxia-tolerant diving animals are ideal species to explore the cellular and molecular mechanisms essential to matching O2 supply with demand. In nondiving vertebrates, hypoxic vasodilation is a fast-acting vascular response, which fine-tunes local perfusion in response to falling O2 levels (34). A locally regulated vasodilatory reaction that increases perfusion to hypoxic tissues would be in direct conflict with the peripheral vasoconstriction required for the dive response (29, 62). Here, we investigate molecular mechanisms that could limit localized hypoxic vasodilation, thereby allowing the centrally mediated diving reflex to control vasomotor tone.
Despite the presumed dominant role for central control of the cardiovascular dive response, specifically the maintenance of peripheral vasoconstriction by sympathetic tone, evidence from the deep-diving Weddell seal (Leptonychotes weddellii) points to local control of blood flow during diving, at least to some extent. While blood flow is reduced six- to sevenfold in the uterus of the nonpregnant Weddell seal (62), blood flow to the uterus of the pregnant seal is preserved during diving (38). If vasoconstriction were mediated exclusively by sympathetic tone and peripheral vasomotor fibers, a similar reduction in uterine blood flow would be expected regardless of pregnancy. Moreover, perfusion to many organs is profoundly reduced during forced submersions, yet the extent of this hypoperfusion differs among tissues suspected to be of varied importance underwater (62).
The gasotransmitter nitric oxide (NO) and the downstream second messenger cGMP are important mediators of the vasodilatory response to hypoxia in terrestrial mammals (45, 47, 53). The NO-cGMP signal transduction pathway regulates blood flow in a wide variety of species, including humans (16, 28, 42). Soluble guanylyl cyclase (GC) is a heterodimeric enzyme that catalyzes the production of cGMP upon activation by NO. The amplitude and duration of a cGMP signal are controlled by the activity of cGMP-generating enzymes such as GC (18) and through catabolism of cGMP by phosphodiesterases (PDEs) such as PDE5A. In terrestrial mammals, NO is present in exhaled breath (22, 31). In contrast, NO could not be detected in the early or late exhaled gas of Weddell seals following a dive (detectable limit was 0.5 parts per billion) (12). Even though a large proportion of exhaled NO is believed to be generated by the epithelium of the paranasal sinuses, it is unlikely that this lack of exhaled NO is due solely to the absence of open sinus air cavities in the Weddell seal; NO was low but measurable in the exhaled gas of a shallow-diving dolphin (Tursiops truncatus) (61) and the terrestrial Hamadryas baboon (Papio hamadryas) (37), species which lack paranasal sinuses.
In addition to the possibility of reduced NO production and/or NO bioavailability during diving, several studies point to additional alterations in gasotransmitter signaling in marine mammals (10, 11, 46, 56). For example, NO-cGMP signaling mediates the acetylcholine-dependent relaxation of vascular smooth muscle cells in both the large and small arteries of terrestrial mammals, thereby counteracting vasoconstriction induced by neural control and local mediators (16). However, in ringed seals (Pusa hispida), acetylcholine causes vasorelaxation in large-conduit coronary arteries but not in coronary resistance arteries (10). While the absence of an acetylcholine-induced vasodilatory response in specific vascular beds is not unique to seals (14), this phenomenon may be particularly advantageous to divers; silencing potent, NO-mediated local vasodilation in resistance arteries would facilitate the maintenance of peripheral vasoconstriction despite local tissue hypoxia.
To better understand the control of peripheral vasoconstriction during diving, we investigated the NO-cGMP pathway in a model deep diver, the Antarctic Weddell seal. Weddell seals breed in a relatively accessible polar location, making it possible to obtain cold-preserved tissues following natural mortality. In this study, we tested the following hypotheses related to mechanisms by which NO-cGMP signaling could be altered in seals: 1) NO-cGMP signaling is blunted in seals compared with terrestrial mammals, through altered expression and/or activity of enzymes in the NO-GC-cGMP pathway; 2) sequence differences in genes encoding proteins involved in NO-cGMP signaling could explain the differences in the activity of this signaling pathway between seals and other mammals; and 3) tissue-specific differences in the regulation and activity of the NO-cGMP signaling pathway facilitate distinct patterns of blood perfusion observed in different tissues of diving seals.
METHODS
Sample collection.
Samples of lung, cerebral cortex, cerebellum, kidney, heart, longissimus dorsi muscle, and arteries were collected at necropsy from freshly dead Weddell seals found near McMurdo Station, Antarctica, during the 2015 and 2016 breeding seasons (n = 12 preweaning pups, n = 6 adults). All animals died of natural causes (pups generally perished following abandonment). In all cases, necropsies were performed before freezing began in any sampled tissues, which were removed, rinsed in cold PBS, and blotted before either snap-freezing in liquid nitrogen or transfer to RNA stabilization solution (RNA Later, Ambien). Seal platelets were isolated by centrifugation from venous blood samples collected from individual healthy adults (n = 9) and weaned pups (n = 8) during a brief handling event. We also collected longissimus dorsi muscle biopsies from n = 9 adult seals that were sedated and then released, following previously described protocols (26). For species comparisons, lung samples from mouse (Mus musculus, n = 5), rat (Rattus norvegicus, n = 5), dog (Canis familiaris, n = 2), and sheep (Ovis aries, n = 8) were likewise collected at necropsy following unrelated terminal experiments. We also collected cerebral cortex, cerebellum, kidney, and artery samples from sheep. All sample collection was approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Weddell seal samples were collected under scientific permit authorizations from the National Marine Fisheries Service (no. 19439) and the Antarctic Conservation Act (no. 2016-005).
Transcript sequencing and homology assessment.
The NO-cGMP pathway genes examined in this study were as follows: endothelial NO synthase (Nos3), GC subunit isoforms α1, α2, and β1 (Gucy1a1, Gucy1a2, Gucy1b1), and cGMP-specific phosphodiesterase-5a (Pde5a). The currently available NCBI genome annotation for the Weddell seal (LepWed1.0 assembly, Annotation Release 100) is not supported by experimental RNA evidence. Therefore, to confirm that these genes are expressed in Weddell seals and to design gene expression probes, we conducted RNA-seq on samples from seven tissues (skeletal muscle, left ventricle, lung, liver, cerebral cortex, testes, and placenta). RNA-seq libraries were constructed via the Illumina Tru-Seq Stranded mRNA Library prep kit and sequenced on an Illumina Hi-Seq 2000 (paired-end reads 2×101) to a depth of 50 million reads per library. Sequence data are available in the NCBI Sequence Read Archive (BioProject PRJNA474945). The resulting reads were assembled using Trinity v.2.4.0 (21), and the seal transcript sequences with the closest alignment to the dog sequence were extracted using BLAST+ v.2.2.30 (5). The Gucy1a2 transcript was shorter in Weddell seal than in the dog and was missing a region known to be GC rich in many species. In a follow up to determine the extent of the Weddell seal transcript, we selected oligonucleotide primers, based on the dog sequence, that spanned the missing region of the seal transcript, but we were unable to amplify any additional sequence with PCR.
To evaluate evolutionary pressure on the five target genes, we identified synonymous and nonsynonymous substitutions (dN/dS ratios) in Weddell seal sequences compared with select placental mammals (human, mouse, rat, dog, sheep, and cow (Bos taurus), whose sequences were retrieved from NCBI. A phylogenetic tree for these species of interest was constructed using TimeTree (24, 25, 35), with branch lengths identifying molecular time estimates (25). Pairwise dN/dS ratios (ω) across the entire protein sequence (seal vs. each terrestrial species) were calculated using SNAP (v.2.1.1, https://www.hiv.lanl.gov/) (33). Nucleotide sequences from Weddell seals were converted to amino acid sequences to investigate conserved binding domains, which were identified by Homologene (www.ncbi.nlm.nih.gov/homologene).
GC activity assay and measurement of cGMP.
We measured cGMP production to detect differences in GC activity between tissues and among species. All enzyme activities were normalized to the total protein content of the raw tissue homogenate. Arteriolar density of sampled tissues was not assessed. Frozen tissues were homogenized on ice (Omni Tissue Homogenizer TH, Omni International) in protein extraction buffer (in mM: 1 EDTA, 1 DTT, and 2 PMSF in 50 mM Tris·HCl). After a 20-min centrifugation (10,500 g at 4°C) period, the soluble fraction of the homogenate was drawn off, and protein concentration was determined using a BCA assay (no. 22660, Pierce). Aliquots of homogenate were stored at −80°C until analysis. GC enzyme activity was measured over 10 min at 37°C for 50 µl of sample protein (1 µg/µl) in 100 µl of reaction cocktail per assay condition (1 mM IBMX, 15 mM creatine phosphate, 0.4 M creatine phosphokinase, 2 mM GTP, 8 mM MgCl2, and 2 mM l-NAME in 100 mM Tris·HCl). This assay was repeated in the presence of 50 µl of either water, to determine baseline activity, or a NO donor (100 µM DETA-NONOate), to determine NO-inducible activity. The amount of reaction product, cGMP, was measured using a competitive-binding ELISA following the manufacturer’s protocol (Cayman Cyclic GMP EIA Kit, no. 581021).
Western blotting.
The amount of GC enzyme was measured in a subset of homogenates by Western blot. We measured the amount of GUCY1B1 (GCβ) in tissue because the β-subunit is a necessary component of functional GC and because amino acid sequence, and therefore the antibody epitope, is conserved between species. We normalized the amount of immunoreactive GUCY1B1 to total protein as a loading control. β-Actin immunoblotting was also used to confirm equal loading of protein in each lane. Tissues were homogenized in RIPA buffer (BP-411, Boston BioProducts) with protease and phosphatase inhibitors (HALT, 1862209 and 1861277, ThermoFisher). Samples were sonicated (3 × 10 s) and then centrifuged as above to remove cellular debris. Protein concentrations in the supernatant were determined by BCA assay. Samples were prepared for Western blotting with a 10-min incubation at 65°C with 1× reducing agent (B0009, Invitrogen Bolt) and LDS Sample Buffer (B0007). Proteins were separated by electrophoresis in 4–12% Bis-Tris gels (Invitrogen Bolt) and then transferred to PVDF membranes (Immobilon-FL, Millipore) using a semidry transfer cell. We quantified total protein on the membranes with REVERT Total Protein Stain (Li-Cor). Membranes were blocked for 1 h (Odyssey Blocking Buffer) and then incubated overnight at 4°C in fresh blocking buffer with 0.2% Tween 20 and the primary antibodies of interest: rabbit anti-GUCY1B1 antiserum (RRID:AB_259906; cat. no. G4405, Sigma-Aldrich), diluted 1:1,000, and mouse monoclonal anti-β-actin antibody (RRID:AB 626632; cat. no. sc-47778, Santa Cruz Biotechnology) diluted 1:1,000. Membranes were subsequently washed and incubated in fresh blocking buffer with 0.2% Tween, 0.02% SDS, and 1:10,000 2° antibodies (goat anti-rabbit IgG or goat anti-mouse IgG, each conjugated with IRdye 800CW, Li-Cor). An Odyssey CLx imaging system (Li-Cor) was used to detect bound antibody, and results were analyzed with ImageJ (v.1.50i, NIH) to quantify total protein and antibody-specific signals.
Measurement of expression of genes in the NO-cGMP pathway.
Species-specific oligonucleotide primers and qPCR were used to measure NO-cGMP pathway gene expression. Primers for genes of interest in the NO-cGMP pathway (Nos3, Gucy1a1, Gucy1a2, Gucy1b1, and Pde5a) are listed in Table 1. Total RNA was extracted from tissues in TRIzol, quantified using NanoDrop Lite (Thermo Scientific), and used to produce cDNA by use of a high-capacity cDNA reverse transcriptase kit (4368813, Applied Biosciences). The identities of PCR products in each species were confirmed by Sanger sequencing. We determined transcript expression using SYBR Green Real-time PCR (Applied Biosystems, 7500 Fast Real Time PCR System) with 400 nM primer sets. Expression data in each sample were normalized by ΔCT to the expression of 18S RNA amplified with human Taqman primers (Hs03003631_g1, ThermoFisher). We also quantified Nos3 and Pde5a transcripts in seals and sheep against standard curves derived from plasmid DNA containing the species-specific amplicon.
Table 1.
Primer sequences for NO-cGMP pathway genes in sheep and Weddell seal
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Weddell seal (Leptonychotes weddellii) | ||
| Nos3 (set 1) | GAAGCACCTGGAGAATGAGC | GCATTGGCCACTTCCTTAAA |
| Nos3 (set 2) | AACATGCTGCTGGAAATCGG | TCTTTCCACAGCGATGAGGT |
| Gucy1a1 | ATGCGAATTGGACTGCACTC | CGTTGTTGGGCTGACATTGA |
| Gucy1a2 | GGAAACAATAGGCGATGCGT | CCCGAGTGAATGCCTATCCT |
| Gucy1b1 | TGACACACTGACTGACTCCC | GCCATCCACTTGAACCTGAC |
| Pde5a | CCTGGCCTATTCAACAACGG | CGTGGGTCAGAGCCTCATAT |
| Sheep (Ovis aries) | ||
| Nos3 | GGAGAGGCTGCATGACATTG | GATGGTTGCTTTCACTCGCT |
| Gucy1a1 | CCCAAAATCAGCCAGACGTT | AGTCTTCCAGTCTGTCCACG |
| Gucy1a2 | TGGGCTGAAGAAGAGGATGG | TCAAACTTTCTGGCCTGCAC |
| Gucy1b1 | TATTCCGTGCTCCCTCCATC | AGAGGTCGTTGAGGAGGTTG |
| Pde5a | GGGGATCCAGAACACACAGA | GAGGTCAGTGAACATCCGGA |
GUCY1a1, -1a2, -1b1, guanylyl cyclase enzyme subunits; NO-cGMP, nitric oxide-cGMP; NOS, NO synthase; PDE5a, phosphodiesterase-5a.
Analysis and species selection.
To evaluate NO-cGMP signal transduction in the Weddell seal, we compared tissues of seals to those of other mammals. Despite being the closest carnivore relative in our species set, we were able to obtain only limited high-quality samples from dogs (2 lung samples). To support statistical comparisons of GC activity, protein content and mRNA expression between species, we compared Weddell seals against the next closest related mammal in our data set, domestic sheep. Through ongoing studies at our facility, we had access to complete necropsies from n = 8 sheep. We analyzed GC activity (stimulated and unstimulated) across species, or compared seal pups and adults, using a two-way ANOVA (comparing species or age, level of stimulation, and their interaction) and Sidak’s pairwise post hoc tests. We also calculated NO responsiveness as the fold increase in enzyme activity upon stimulation compared with baseline activity for the same samples. These results were analyzed with t-tests to compare species/age, or with one-way ANOVA when there were more than two groups. Western blot quantifications and linearized qPCR data were compared using t-tests or an ANOVA between species or age. Where there were no significant developmental differences between seal pups and adults, their results were pooled for analysis of GC activity, GUCY1B1 protein content, and mRNA expression of target genes. Data are presented as means ± SD, and fold differences for species comparisons are presented with 95% confidence intervals (CIs). Statistical significance was set at α = 0.05. All analyses were performed using Prism 7 GraphPad software.
RESULTS
Gene sequences and selective pressure.
To investigate whether critical components of the NO-cGMP signaling pathway are present and expressed in seals, we sequenced the transcripts encoding Nos3, Gucy1a1, Gucy1a2, Gucy1b1, and Pde5a. Full-length sequences of all target genes were retrieved from an RNA-seq-based Trinity assembly, except for Gucy1a2 (Fig. 1). The recovered Gucy1a2 transcript is missing part of exon 1, which varies in size in other species. Compared with the canine, the Weddell seal Gucy1a2 sequence is missing the first 91 amino acids of exon 1. This region is GC rich in other species (79% GC content in canine), suggesting that the seal Gucy1a2 transcript is shorter than expected due to sequencing difficulties. The existing transcript has 97.5% amino acid similarity to the dog and is in frame, with no indication that the seal protein has an alternative start site.
Fig. 1.
A: phylogenetic tree for relevant mammals, developed in TimeTree. Scale bar represents molecular time estimate. B–F: amino acid (AA) sequences from Weddell seal, aligned against dog. Conserved domains from dog are in boldface. AAs denoted in blue differ in seals compared with all other mammal sequences (dog, human, cow, sheep, rat, mouse) collected from NCBI Homologene but indicate a substitution with a physically similar AA. AAs denoted in red are also unique to seal but represent a significant substitution with an AA of altered charge or polarity compared with other mammals.
Substitution rates were low in the seal transcript from each of the target NO-cGMP pathway genes. dN/dS ratios were ≤0.1 for all pairwise comparisons between the seal and terrestrial placental mammals (human, mouse, rat, dog, sheep, and cow; Fig. 1A), suggesting evolutionary pressure to maintain the ancestral sequence across the placental mammal lineage. The GC1α (Gucy1a1) had the highest dN/dS ratio in the data set (ω = 0.075 ± 0.023), whereas Gucy1b1 had the lowest dN/dS ratio of all the genes examined (ω = 0.007 ± 0.001). The difference in dN/dS ratios between Gucy1a1 and Gucy1b1 is particularly interesting, considering that both α- and β-subunits are necessary to form the NO-binding heme pocket of the functional GC heterodimer. The β-subunit is very highly conserved, suggesting that any seal-specific differences in GC function arising from amino acid differences likely occur in the GC α-subunit.
Despite generally strong conservation in the gene sequences of interest among species, unique amino acid sequence modifications in seal proteins may have important functional effects. The majority of seal-specific sequence dissimilarities occurred in the GC α-subunits, with both Gucy1a1 and Gucy1a2 carrying several substitutions that are predicted to affect protein charge and/or hydrophobicity. Each Weddell seal GC α-subunit also lacks one cysteine relative to the proteins of terrestrial species, which could affect the secondary structure of the protein (Gucy1a1 Y-176, Gucy1a2 G-291; Fig. 1). The majority of sequence differences occurred in the heme-binding or heme-binding-associated domain of the GCα protein isoforms. Seal-specific amino acid modifications also appeared in the heme-binding oxygenase domain of Nos3 (Fig. 1B). Key differences of Weddell seal sequences in the NO-binding domains of these three proteins of interest suggest that there may be species-specific differences in NO signal transduction.
GC activity differs among species.
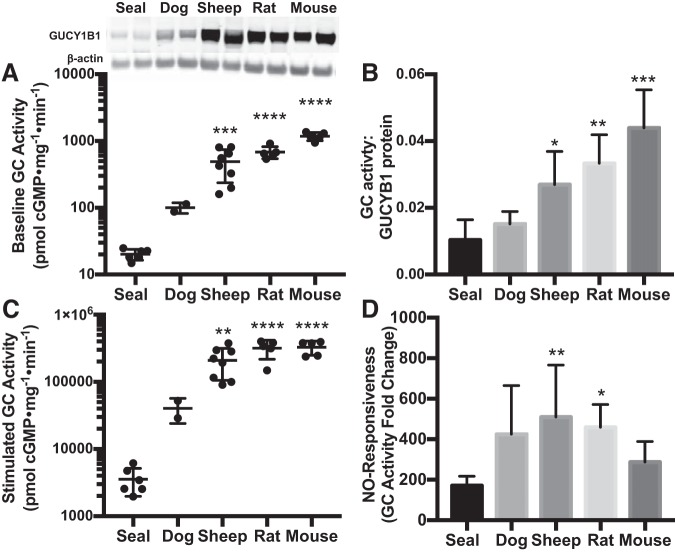
A key mechanism by which NO-cGMP signaling could be attenuated is via reduced GC enzyme activity. We initially focused on the lung, a tissue classically used to characterize GC function. Under baseline conditions (with no enzyme stimulation), GC activity was lowest in Weddell seal lung homogenates (20.1 ± 3.7 pmol cGMP·mg protein−1·min−1, species P < 0.0001) and highest in mice (1,179.1 ± 166.3 pmol·mg−1·min−1) compared with all other species examined (adult animals only; Fig. 2A). The level of GC activity in the lungs of these species is consistent with lung GCβ protein levels, which were also lowest in seals and highest in mice (Fig. 2A; post hoc P = 0.0004). When GC activity was normalized per unit GCβ protein, GC activity remained lowest in seals (Fig. 2B; species P < 0.0001), suggesting that low GC abundance does not completely explain the low GC activity in seal lung homogenates. NO-stimulated GC activity also differed among species (Fig. 2C). The NO responsiveness of GC (NO-stimulated activity normalized to baseline activity) in lung was highest in sheep and lowest in seals (510 ± 256 vs. 171 ± 46, P = 0.006; Fig. 2D). Taken together, these results identify at least three mechanisms by which NO-cGMP signaling could be limited in seals: GC is less abundant in Weddell seals compared with terrestrial mammals; the enzyme present in adult seal lung homogenates is less active at baseline; and the GC that is present in seal lung is less responsive to NO.
Fig. 2.
Guanylyl cyclase (GC, GUCY) activity in adult lung is lower in seals than in terrestrial mammals. A: baseline GC activity in lung is lower in seal (n = 6) than in dog (n = 2), sheep (n = 8), rat (n = 5), and mouse (n = 5). Inset: protein content of the GC β-subunit (GUCY1B1, 70-kDa band) was also lowest in seal lung. B: after baseline GC activity to GC protein level was normalized, enzyme activity per unit protein remains higher in lungs of mice, rats, and sheep than in Weddell seals. C: GC activity stimulated with an in vitro nitric oxide (NO) donor is also lowest in seals. D: stimulated GC activity relative to baseline indicates the capacity for NO to stimulate GC (NO responsiveness), which is also lower in seal lungs than in sheep or rats. Error bars represent means ± SD. Asterisks denote significant differences from adult seals by Sidak post hoc pairwise comparisons following an overall species difference detected by ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Changes in GC activity with maturation in seals.
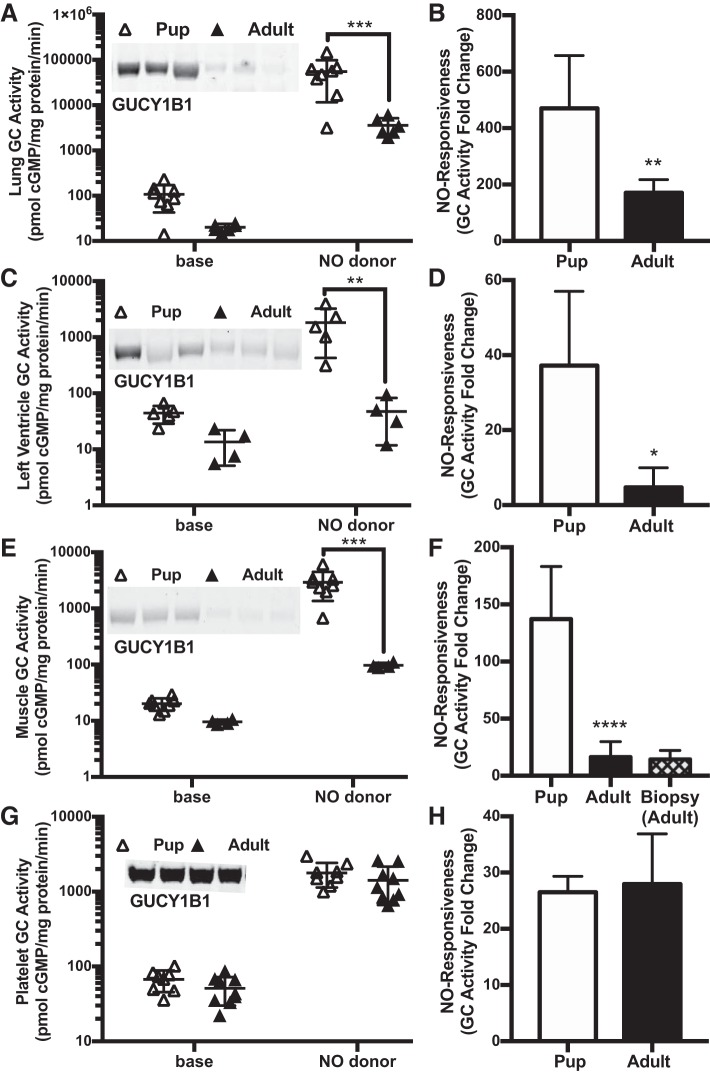
GC abundance and NO-stimulated activity were lower in adult seals than in pups in some but not all tissues. We detected an age-dependent decrease in GC activity in the seal lung (age effect P = 0.01), heart (P = 0.04), and swimming muscle (P = 0.005; Fig. 3, A, C, and E), consistent with an age-dependent decrease in GCβ protein abundance in each of these tissues. Because of a significant interaction term between age and experimental condition in all three ANOVA models, only NO-stimulated GC activity demonstrated post hoc significance between age groups (Fig. 3, A, C, and E). GC responsiveness to NO was also more than twofold lower in adult seal lung, heart, and muscle than in pups (Fig. 3, B, D, and F). In contrast, GC level, activity and NO responsiveness in platelets did not differ between pups and adults (Fig. 3, G and H) nor were there differences in other tissues examined (cerebral cortex, cerebellum, kidney, arteries; see below, next section). Taken together, these results suggest that age-related downregulation of GC protein levels, activity, and NO responsiveness during development occurs in select tissues only.
Fig. 3.
Guanylyl cyclase (GC, GUCY) protein content and nitric oxide (NO)-stimulated activity decrease with age in Weddell seal lung (A: n = 8 pups, n = 6 adults), heart (C: n = 5 pups, n = 4 adults) and swimming muscle (E: n = 8 pups, n = 4 adults). No age differences were noted in brain, kidney, arteries, and platelets (G: n = 8 pups, n = 9 adults). GC activity is presented at baseline (base) and after stimulation with a NO donor. A representative immunoblot of GUCY1B1 (70 kDa) of pups and adults is provided for each tissue type as insets. NO responsiveness of GC is also lower in lung (B), heart (D), and swimming muscle (F) from adults than from pups but is similar in adult and pup platelets (H). Error bars represent means ± SD. Asterisks denote significant Sidak post hoc differences for NO-stimulated GC activity and t-tests for NO responsiveness (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
GC activity in brain versus kidney tissues.
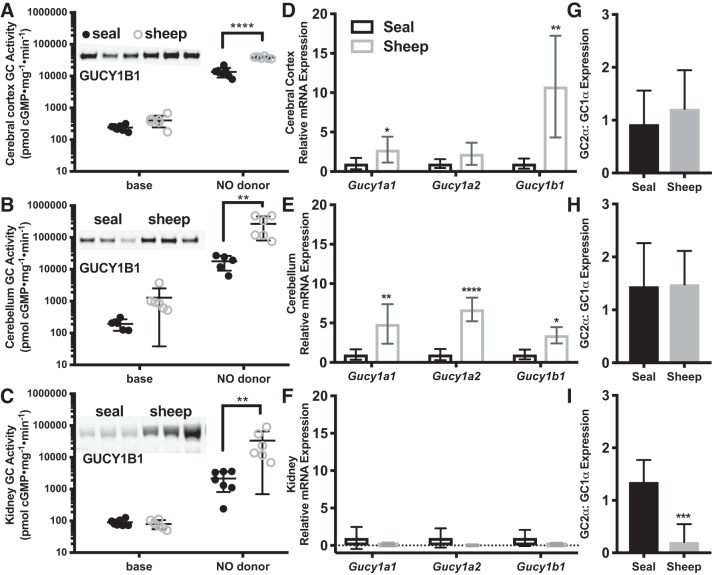
In the diving seal, blood flow is markedly decreased to the kidneys but not to the brain (6, 62). If NO-cGMP signaling plays a role in tissue-specific vasoregulation during diving, then differences in blood perfusion between the brain and the kidney could arise from differences in GC activity in the microvasculature between the two organs and/or differences in their conduit arteries [the site of adrenergic innervation in seals (59)]. To consider the possibility that differences in the activity of the NO-cGMP signal transduction pathway within these two organs contribute to the differences in blood flow observed during submergence, we measured GC activity in brain and kidney of seal and sheep (Fig. 4, A–C). GC activity was higher in all sheep tissues (species P < 0.0001 in cerebral cortex, P = 0.02 in cerebellum, P = 0.03 in kidney), consistent with greater GCβ protein content (Fig. 4, A–C). Upon NO stimulation, species-specific differences in GC activity increased (all 2-way ANOVA models had a significant interaction term), with stimulated GC activity 3-fold higher in sheep cerebral cortex and 15-fold higher in sheep cerebellum and kidney than in the corresponding seal tissue. Although in general GC activity was lower in seal than in sheep tissues, the GC enzyme in seals does respond to NO and therefore could play an important role in regulating perfusion to the seal brain.
Fig. 4.
Nitric oxide (NO)-stimulated guanylyl cyclase (GC) activity is higher in sheep brain (A: cerebral cortex, B: cerebellum) and kidney (C) than in Weddell seal (sheep: n = 6 each tissue; seal: n = 7 cerebral cortex and kidney, n = 5 cerebellum). Insets: Western blots of representative seal and sheep are presented for each tissue (A–C, 70-kDa band). mRNA expression of GC enzyme subunits (Gucy1a1, Gucy1b3, each normalized to 18S) were higher in brains of sheep than in seal (D: cerebral cortex, E: cerebellum) but were similar in kidney (F) between species (sheep: n = 4 brain, n = 5 kidney; seal: n = 6 brain, n = 5 kidney). After internal normalization to 18S within each sample, qPCR data are presented relative to seal values for each tissue. The ratio Gucy1a2/Gucy1a1 mRNA did not differ between species in the brain (G: cerebral cortex, H: cerebellum) but was higher in seal kidney compared with sheep (I). Because GC activity and gene expression were similar in seal adults and pups, data from adults and pups were combined for these analyses. Error bars represent means ± SD. Asterisks denote significant species differences with Sidak post hoc tests for NO-stimulated GC activity and relative mRNA expression and with t-tests for relative expression of A2:A1 (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Although GCβ protein levels in all three tissues were higher in sheep than in seals, mRNA expression of GC subunits was higher in the sheep cerebral cortex and cerebellum, but not the kidney (vs. the same tissues in seals; Fig. 4, D–F). Gucy1a1, Gucy1a2, and Gucy1b1 mRNA expression did not differ between sheep and seal kidney tissues (Fig. 4F) despite higher GUCY1B1 protein abundance in the sheep kidney (95% CI: +0.5–4.4-fold GUCY1B1 compared with seal kidney, P = 0.03). In addition to the lack of correlation between GCβ protein and mRNA levels, the relative expression of GC α-subunits differed between species in the kidney (Fig. 4I). Whereas GCα2/GCα1 ratios were similar between sheep and seal brain (Fig. 4, G and H), the ratio was higher in seal than in sheep kidney (P = 0.0004; Fig. 4I). This indicates that the composition of the GC heterodimer differs between species in the kidney and points to differences in stability or translation of the GC α-subunit isoforms.
GC activity in vascular tissue.
To further investigate a role for NO-cGMP signaling in differential perfusion between the seal brain and kidney, we compared GC activity in various arteries of the Weddell seal and sheep. In the seal, GC activity did not differ among carotid artery, renal artery, and thoracic aorta under either baseline or NO-stimulated conditions (Fig. 5, A and B). Both GCβ abundance and GC activities were higher in sheep carotid artery and thoracic aorta than in the same arteries in seals (Fig. 5, A and B). In the renal artery, both GCβ protein level (P = 0.96) and baseline GC activity (P = 0.54) were similar between the species (Fig. 5A); only NO-stimulated GC activity was higher in sheep (P < 0.0001; Fig. 5B). As a result, calculated NO responsiveness was significantly higher in the sheep than in the seal renal artery (177× mean activity increase with NO stimulation in seal, 333× in sheep, P = 0.02; Fig. 5C). NO responsiveness was similar between seal and sheep carotid artery (130× vs 156×, P = 0.96) and was even more pronounced in seal than in sheep in the thoracic aorta (263× vs 103×, P = 0.01; Fig. 5C). Overall, we detected the greatest degree of tissue specificity in the NO responsiveness metric, which suggests a mechanism for species differences in NO-cGMP signaling and responses to hypoxia.
Fig. 5.
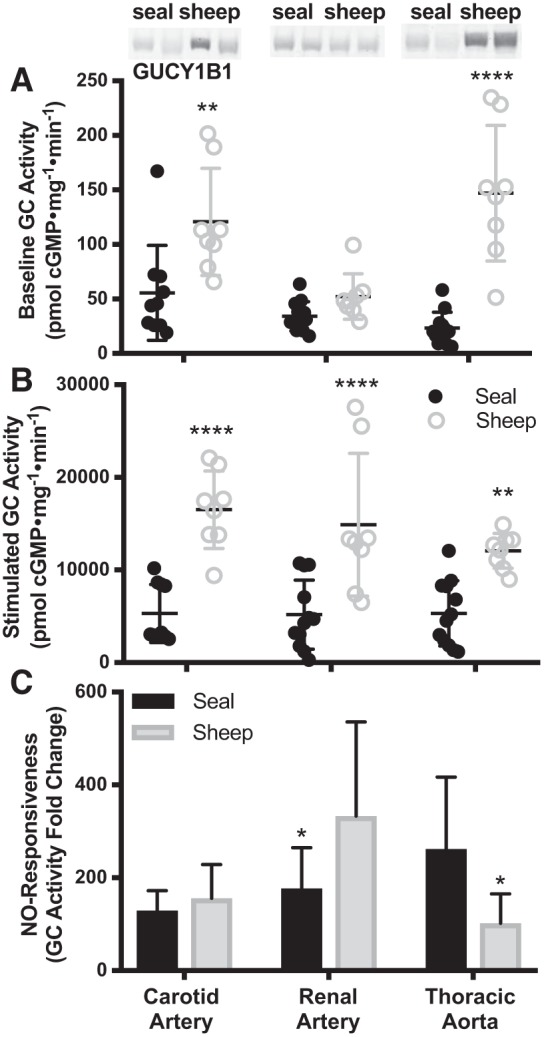
Guanylyl cyclase (GC, GUCY) activity at baseline (A), after nitric oxide (NO) stimulation (B), and NO responsiveness (NO-stimulated GC activity normalized to baseline GC activity in the same sample; C), were compared across arteries and between seals and sheep with two-way ANOVAs. GC β-subunit protein abundance is included for representative samples as an inset (70-kDa band; reordered lanes taken from a single, otherwise unmanipulated gel). Based on pairwise post hoc comparisons, GC activity was higher in sheep than in the same tissues of seals with the exception of baseline GC activity in renal arteries, which did not differ between species. NO responsiveness is lower in seal renal artery vs. sheep and in sheep thoracic aorta vs. seal. Sheep: n = 8 all arteries; seal: carotid n = 10, renal and thoracic n = 12). Error bars are means ± SD. Asterisks denote significant Sidak post hoc differences between species (*P < 0.05, **P < 0.01, ****P < 0.0001).
NO-cGMP signaling in cerebral versus renal circulation.
On the basis of observed differences between seals and sheep in GC abundance, activity, and subunit composition in kidney and brain tissues as well as their conduit arteries, we examined whether the intrinsic capacity for NO-induced vasodilation aligned with how cerebral (cerebral cortex and carotid artery) and renal (kidney and renal artery) circulations respond to hypoxia in the two species. NO responsiveness was plotted as a heat map to visualize species-specific patterns (Fig. 6A). In sheep, NO responsiveness was more pronounced in the renal circulation than in the cerebral circulation. Conversely, NO responsiveness was significantly lower in seal than sheep kidney (Figs. 4C and 6A). Because seals have limited hypoxic vasodilation potential in the kidney but not in the brain, these data are consistent with a potential role for the NO-cGMP signal transduction pathway in facilitating the redistribution of blood flow observed during diving.
Fig. 6.
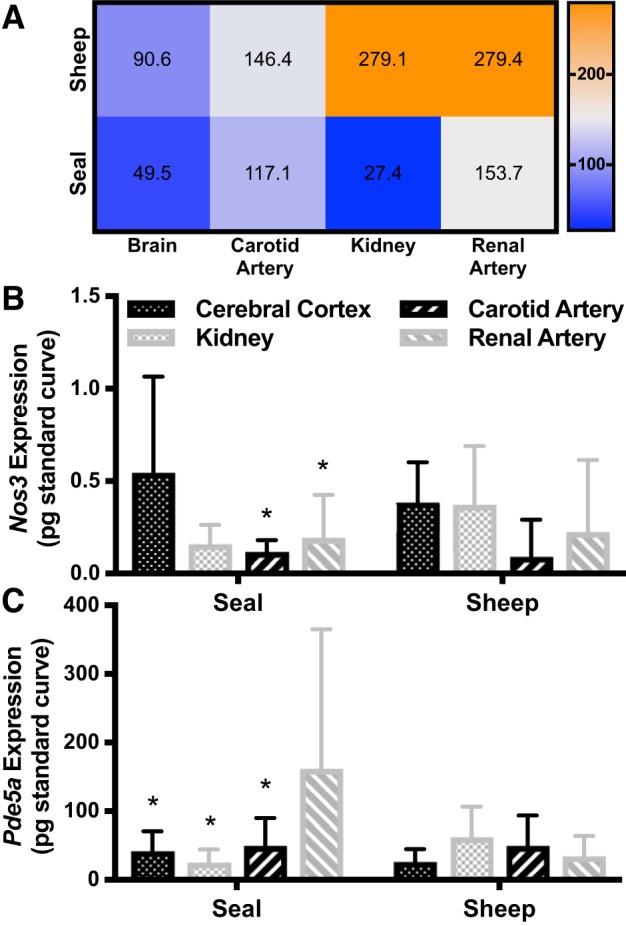
A: heat map of median nitric oxide (NO) responsiveness to compare species-specific differences among tissues. NO responsiveness is highest in sheep kidney and renal artery and lowest in seal kidney, possibly limiting vasodilation potential. NO responsiveness is calculated as NO-stimulated guanylyl cyclase (GC) activity normalized to baseline GC activity in the same sample; therefore, only samples that could be resolved within the standard curve in both assay conditions are included (sheep: brain n = 6, carotid n = 8, kidney n = 4, renal n = 8; seal: brain n = 7, carotid n = 9, kidney n = 7, renal n = 11). B: Nos3 (NO synthase-3) expression was higher in seal brain (cerebral cortex) than in kidney and renal artery. C: phosphodiesterase-5a (Pde5a) expression was higher in seal renal artery than in other seal tissues. There were no differences between sheep tissues in expression of either mRNA. Error bars represent means ± SD. Asterisks (*P < 0.05) denote significant Sidak post hoc differences between seal tissues from cerebral cortex (Nos3 comparison) and from renal artery (Pde5 comparison).
Weddell seals exhibited tissue-specific expression patterns of upstream and downstream components of the NO-cGMP pathway that are consistent with a higher capacity for NO-induced vasodilation in the brain compared with the kidney. Nos3 and Pde5a mRNA levels were similar in the renal and cerebral tissues and arteries of sheep (Fig. 6, B and C). Nos3 expression in seals was, however, higher in the cerebral cortex than in renal tissues (P = 0.01; Fig. 6B), which would increase cerebral NO availability and might promote NO-induced vasodilation in the brain relative to the kidney. Pde5a was also differentially expressed in seal tissues, and the levels were highest in the renal artery, suggesting an enhanced capacity to decrease cGMP bioavailability in the renal circulation, thereby limiting vasodilation in the seal (P = 0.04; Fig. 6C).
DISCUSSION
Bradycardia is a key cardiovascular component of the vertebrate dive response (8, 51). This acute decline in heart rate occurs in concert with pronounced peripheral vasoconstriction, which serves to maintain central arterial pressure (29, 62). Peripheral vasoconstriction is mediated by the sympathetic nervous system, although the roles of local vasodilators, such as NO, and vasoconstrictors, such as endothelin or thromboxane, have not yet been investigated. This study aimed to explore the molecular mechanisms that underlie maintenance of peripheral vasoconstriction in hypoxia by characterizing the NO-cGMP signaling pathway in tissues and vasculature of the Weddell seal. The data support our hypothesis that NO-cGMP signaling is downregulated in Weddell seals to avoid competing signals and potential dysregulation of vascular tone in hypoxia. NO-cGMP signal transduction could be limited in seal tissues and conduit arteries as a result of low GC abundance and activity. Species differences in GC activity are supported by key amino acid sequence dissimilarities, unique to Weddell seals among other nonmarine mammals examined, that may affect enzyme structure, stability, and function. Despite an overall reduction in GC activity, abundance, and NO responsiveness in seals, we observed regional differences in GC, Nos3, and Pde5a that could facilitate cerebral perfusion during diving through increased bioavailability of NO.
NO-cGMP signal transduction in Weddell seals is limited via GC.
The lower activity of GC in seals than in terrestrial mammals appears to be primarily driven by low enzyme abundance. In the vasculature of mice, GC is likely expressed in excess of levels needed to effectively translate NO signals into cGMP (39). Low GC abundance observed in seals could attenuate cGMP signaling during hypoperfusion and hypoxia, when NO may accumulate. In addition to low enzyme abundance, basal activity and the ability of NO to activate GC enzyme activity are lower in seals than in other mammals. Species differences in GC activity may arise from seal-specific differences detected in protein sequences. Although the β-subunit sequence (Gucy1b1) is highly conserved among mammals, α-subunit isoforms of Weddell seals (Gucy1a1 and Gucy1a2) each contain five to six substitutions, not present in any terrestrial mammal examined, that are predicted to impact the charge or hydrophobicity of residues in the critical NO-binding domain. Moreover, the seal Gucy1a1 (Y-176) and Gucy1a2 (G-291) isoforms each lack a cysteine, potentially affecting GC α-subunit structure. The fact that seal-specific amino acid substitutions are largely constrained to the NO binding suggests a functional effect related specifically to NO signal transduction. However, a crystal structure for the complete GC heterodimer, required to validate any functional effects of sequence differences on the activity and NO-binding of Weddell seal GC, is not yet available.
GC activity in pups and adults.
GC abundance and function were reduced with age in three seal tissues: lung, muscle, and heart. The timing of these changes coincides with the onset of physiological maturation that supports the adult Weddell seal’s diving capability. These results suggest the possibility that vasoregulation and hypoxia responses are altered developmentally in the lung, muscle, and heart. The lungs of adult deep divers undergo collapse and reexpansion (13, 32), with reduced transpulmonary gas exchange, to prevent decompression sickness from nitrogen absorption. Pups are not affected by lung collapse or decompression sickness, as their diving depths at weaning are relatively shallow (4). It is possible that decreased GC activity in adult lungs protects the lungs during deep diving by facilitating pulmonary ventilation-perfusion mismatch, which has been suggested to further limit nitrogen uptake during submergence (19). Of all other tissues examined, GC activity changed with age only in skeletal and heart muscle. Seal muscles develop to rely heavily on lipid-based aerobic metabolism, but they also improve their anaerobic capability (3, 17, 30, 49), suggesting that adult tissues become better able to survive hypoperfusion. For example, seal swimming muscle and heart both develop high internal oxygen stores with maturation (3, 36, 44). Reduced perfusion to seal muscles, possibly related to decreased abundance and activity of GC, may even be necessary to allow adult seals to capitalize on their large tissue oxygen stores bound to myoglobin during diving (2, 7). It is possible that GC activity does not decrease in the adult cerebral cortex because of the need to maintain cerebral perfusion during diving.
Regional differences in NO-cGMP signaling.
Despite lower levels of GC protein and activity in the Weddell seal than in terrestrial mammals, seal GC still responds to NO and could therefore contribute to the control of tissue perfusion under hypoxia. Seals experience global hypoxemia, with arterial oxygen saturations falling as low as 28% in freely diving Weddell seals (50). Yet, perfusion differs among vascular beds (62) and with dive duration (6); these differences could be partly explained by small regional changes in vasoregulation.
Several aspects of these data are consistent with observations from Weddell seals that the brain remains perfused, whereas the kidneys experience reduced perfusion under water (62), particularly during extended dives (6). Expression of genes in the NO-cGMP pathway is relatively consistent across sheep tissues; in contrast, expression levels of these genes in seals differ between the renal and cerebral tissues, possibly modulating regional bioavailability of NO and cGMP. Nos3 expression was relatively high in the seal brain, which could result in higher local levels of NO and downstream GC activation. Pde5a expression was highest in the seal renal artery, implying a site of increased cGMP breakdown. Decreased cGMP bioavailability may be expected in tissues that remain vasoconstricted during diving (59). Expression and activity of phosphodiesterases, including PDE5, have not been extensively studied in diving mammals in general or Weddell seals in particular. However, PDE5 is a therapeutic target in a wide variety of indications (41), illustrating its relevance to vascular homeostasis.
Species-specific differences in GC activation by NO were inconsistent between the brain and kidney. Weddell seal kidneys were less responsive to NO than sheep kidneys, whereas in the brain, species-specific differences in NO responsiveness were less apparent. Two possibilities for these observations emerge. First, it is conceivable that GC isoforms are differentially expressed in sheep and seal kidneys. In terrestrial mammals, GC exists as two heterodimers: GCα1β1 and GCα2β1 (1, 27, 40). GCα2β1 is highly expressed in the brain (40), whereas GCα1β1 is the predominant isoform in many other cell types and tissues, including the vasculature and the kidney. Contrary to expectations, in seal kidneys, GCα2β1 appears to be the primary isoform. Although GC isoforms are assumed to have similar enzymatic properties, subcellular localization and in vitro activity levels within tissue homogenates may differ. Furthermore, although GC mRNA was similar between seal and sheep kidneys, GC protein abundance was lower in seals, pointing to differences in stability or translation of the GC subunit isoforms. Whether differences in GC activity between species are solely driven by differential GC expression and activity in vascular cell types or also by differences in nonvascular cells [e.g., fibroblasts and podocytes in the kidney (54) or neurons in the brain (43)], remains to be determined.
Second, it is possible that differences in baseline levels of enzyme oxidation exist between seal tissues. Oxidation of ferrous heme results in heme dissociation from GC, preserving baseline enzyme activity but eliminating its ability to bind and be stimulated by NO (55). Repeated cycles of hypoxia and ischemia followed by reoxygenation and reperfusion on surfacing increase the exposure of diving mammals to oxidative stress (58, 63). Therefore, redox signaling and the oxidation status of tissues may differ between divers and non-divers or between different tissues of divers based on degree of perfusion, with potential impacts to the function of proteins such as GC.
Interestingly, thoracic aorta was the only tissue tested in which the NO responsiveness of GC activity was more pronounced in seals than in sheep. The thoracic aorta is a large-diameter, conduit vessel and typically not involved in regulating aortic flow. However, altered NO-cGMP signaling in the large arteries could mediate compliance of the vessel wall to accommodate altered blood volume distribution during diving. A large, bulbous aortic arch is an anatomic specialization of marine mammals (9, 52). Corresponding biochemical specializations in the nearby downstream thoracic aorta could potentiate the windkessel function of the aortic arch, and even protect against stretch-activation of baroreceptors during diving.
Perspectives and Significance
The molecular framework supporting the dive response, particularly the control by mediators of peripheral vasoconstriction in the face of hypoxia, is underexplored (20). This study characterizes components of the NO-cGMP pathway in the elite diving Weddell seal, and provides mechanistic insights into molecular features that support the dive response. In addition to an overall reduction in GC activity, abundance, and NO responsiveness in seals that may facilitate peripheral vasoconstriction during diving, we also observed regional differences in the NO-cGMP pathway that are consistent with improved perfusion potential to the seal brain compared with kidney. Limitations of this approach are the focus of investigation to one vasoregulatory pathway in one species of marine mammal. Future studies that extend these results to other species of marine mammals and integrate the functions of other vasodilators (such as hydrogen sulfide (46) and prostacyclin), other modulators of GC activity (such as carbon monoxide), as well as vasoconstrictors, will be important next steps in developing a wholistic picture of hypoxemic regulation of perfusion in divers. While the present study identified reduced vasodilatory signaling responses to NO in tissues of Weddell seal compared with tissues from terrestrial mammals, it will also be important to determine whether NO bioavailability is reduced in diving seals. It is clear that seal tissues tolerate hypoxic challenge better than other species (15, 23), requiring innate tissue-level protective mechanisms to control metabolism or prevent hypoxic damage. Reduced NO availability during ischemia could be one such mechanism by complementing the enhanced antioxidant defenses of seals (57), for example by limiting generation of harmful byproducts such as peroxynitrite.
GRANTS
This study was supported by a grant from the National Science Foundation (no. 1443554) to A. G. Hindle, D. P. Costa, E. S. Buys, and W. M. Zapol. Funding from the National Human Genome Research Institute (U54 HG-003067-08, awarded to the Broad Institute, is also gratefully acknowledged.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.G.H., K.L.-T., D.P.C., W.M.Z., and E.S.B. conceived and designed research; A.G.H., K.N.A., A.J.B., L.A.H., J.T.-M., S.A.S., and E.S.B. performed experiments; A.G.H., K.N.A., A.J.B., J.T.-M., J.J., K.L.-T., and E.S.B. analyzed data; A.G.H., K.N.A., A.J.B., J.T.-M., J.J., E.K., K.L.-T., W.M.Z., and E.S.B. interpreted results of experiments; A.G.H. and A.J.B. prepared figures; A.G.H. drafted manuscript; A.G.H., K.N.A., A.J.B., L.A.H., K.L.-T., D.P.C., D.B.B., W.M.Z., and E.S.B. edited and revised manuscript; A.G.H., K.N.A., A.J.B., L.A.H., J.T.-M., S.A.S., J.J., E.K., K.L.-T., D.P.C., D.B.B., W.M.Z., and E.S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the Antarctic Support Contractor personnel at McMurdo Station. We thank the Broad Institute Genomics Platform for generating the RNA-seq data.
Present addresses: A. G. Hindle, School of Life Sciences, Univ. of Nevada Las Vegas, 4505 S. Maryland Pkwy, Las Vegas, NV 89154; K. N. Allen, Dept. of Integrative Biology, Univ. of California Berkeley, Valley Life Sciences Bldg. 5043, Berkeley, CA 94720; S. A. Schulberg, Scripps Inst. of Oceanography, Univ. of California San Diego, 9500 Gilman Dr., La Jolla, CA 92093.
REFERENCES
- 1.Bamberger AM, Koglin M, Kempfert J, Löning T, Scholz H, Behrends S. Expression and tissue localization of soluble guanylyl cyclase in the human placenta using novel antibodies directed against the α 2 subunit. J Clin Endocrinol Metab 86: 909–912, 2001. doi: 10.1210/jcem.86.2.7409. [DOI] [PubMed] [Google Scholar]
- 2.Blix AS. Adaptations to deep and prolonged diving in phocid seals. J Exp Biol 221: jeb182972, 2018. doi: 10.1242/jeb.182972. [DOI] [PubMed] [Google Scholar]
- 3.Burns JM, Skomp N, Bishop N, Lestyk K, Hammill M. Development of aerobic and anaerobic metabolism in cardiac and skeletal muscles from harp and hooded seals. J Exp Biol 213: 740–748, 2010. doi: 10.1242/jeb.037929. [DOI] [PubMed] [Google Scholar]
- 4.Burns JM. The development of diving behavior in juvenile Weddell seals: pushing physiological limits in order to survive. Can J Zool 77: 737–747, 1999. doi: 10.1139/z99-022. [DOI] [Google Scholar]
- 5.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics 10: 421, 2009. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis RW, Castellini MA, Kooyman GL, Maue R. Renal glomerular filtration rate and hepatic blood flow during voluntary diving in Weddell seals. Am J Physiol Regul Integr Comp Physiol 245: R743–R748, 1983. doi: 10.1152/ajpregu.1983.245.5.R743. [DOI] [PubMed] [Google Scholar]
- 7.Davis RW, Kanatous SB. Convective oxygen transport and tissue oxygen consumption in Weddell seals during aerobic dives. J Exp Biol 202: 1091–1113, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Davis RW, Williams TM. The marine mammal dive response is exercise modulated to maximize aerobic dive duration. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 198: 583–591, 2012. doi: 10.1007/s00359-012-0731-4. [DOI] [PubMed] [Google Scholar]
- 9.Drabek CM. Some anatomical aspects of the cardiovascular system of Antarctic seals and their possible functional significance in diving. J Morphol 145: 85–105, 1975. doi: 10.1002/jmor.1051450106. [DOI] [PubMed] [Google Scholar]
- 10.Elsner R, de la Lande IS. Heterogeneous cholinergic reactions of ringed seal coronary arteries. Comp Biochem Physiol A Mol Integr Physiol 119: 1019–1025, 1998. doi: 10.1016/S1095-6433(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 11.Fago A, Parraga DG, Petersen EE, Kristensen N, Giouri L, Jensen FB. A comparison of blood nitric oxide metabolites and hemoglobin functional properties among diving mammals. Comp Biochem Physiol A Mol Integr Physiol 205: 35–40, 2017. doi: 10.1016/j.cbpa.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Falke KJ, Busch T, Hoffmann O, Liggins GC, Liggins J, Mohnhaupt R, Roberts JD Jr, Stanek K, Zapol WM. Breathing pattern, CO2 elimination and the absence of exhaled NO in freely diving Weddell seals. Respir Physiol Neurobiol 162: 85–92, 2008. doi: 10.1016/j.resp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Falke KJ, Hill RD, Qvist J, Schneider RC, Guppy M, Liggins GC, Hochachka PW, Elliott RE, Zapol WM. Seal lungs collapse during free diving: evidence from arterial nitrogen tensions. Science 229: 556–558, 1985. doi: 10.1126/science.4023700. [DOI] [PubMed] [Google Scholar]
- 14.Feigl EO. Neural control of coronary blood flow. J Vasc Res 35: 85–92, 1998. doi: 10.1159/000025569. [DOI] [PubMed] [Google Scholar]
- 15.Folkow LP, Ramirez JM, Ludvigsen S, Ramirez N, Blix AS. Remarkable neuronal hypoxia tolerance in the deep-diving adult hooded seal (Cystophora cristata). Neurosci Lett 446: 147–150, 2008. doi: 10.1016/j.neulet.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. doi: 10.1096/fasebj.3.9.2545495. [DOI] [PubMed] [Google Scholar]
- 17.Fuson AL, Cowan DF, Kanatous SB, Polasek LK, Davis RW. Adaptations to diving hypoxia in the heart, kidneys and splanchnic organs of harbor seals (Phoca vitulina). J Exp Biol 206: 4139–4154, 2003. doi: 10.1242/jeb.00654. [DOI] [PubMed] [Google Scholar]
- 18.Garbers DL, Radany EW. Characteristics of the soluble and particulate forms of guanylate cyclase. Adv Cyclic Nucleotide Res 14: 241–254, 1981. [PubMed] [Google Scholar]
- 19.Garcia Párraga D, Moore M, Fahlman A. Pulmonary ventilation-perfusion mismatch: a novel hypothesis for how diving vertebrates may avoid the bends. Proc Biol Sci 285: 20180482, 2018. doi: 10.1098/rspb.2018.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooden BA, Elsner R. What diving animals might tell us about blood flow regulation. Perspect Biol Med 28: 465–474, 1985. doi: 10.1353/pbm.1985.0009. [DOI] [PubMed] [Google Scholar]
- 21.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29: 644–652, 2011. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 181: 852–857, 1991. doi: 10.1016/0006-291X(91)91268-H. [DOI] [PubMed] [Google Scholar]
- 23.Halasz NA, Elsner R, Garvie RS, Grotke GT. Renal recovery from ischemia: a comparative study of harbor seal and dog kidneys. Am J Physiol 227: 1331–1335, 1974. doi: 10.1152/ajplegacy.1974.227.6.1331. [DOI] [PubMed] [Google Scholar]
- 24.Hedges SB, Dudley J, Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics 22: 2971–2972, 2006. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- 25.Hedges SB, Marin J, Suleski M, Paymer M, Kumar S. Tree of life reveals clock-like speciation and diversification. Mol Biol Evol 32: 835–845, 2015. doi: 10.1093/molbev/msv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hindle AG, Horning M, Mellish JA, Lawler JM. Diving into old age: muscular senescence in a large-bodied, long-lived mammal, the Weddell seal (Leptonychotes weddellii). J Exp Biol 212: 790–796, 2009. doi: 10.1242/jeb.025387. [DOI] [PubMed] [Google Scholar]
- 27.Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol Sci 18: 484–491, 1997. doi: 10.1016/S0165-6147(97)90687-8. [DOI] [PubMed] [Google Scholar]
- 28.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irving L, Scholander P, Grinnell S. The regulation of arterial blood pressure in the seal during diving. Am J Physiol 135: 557–566, 1942. doi: 10.1152/ajplegacy.1942.135.3.557. [DOI] [Google Scholar]
- 30.Kanatous SB, Hawke TJ, Trumble SJ, Pearson LE, Watson RR, Garry DJ, Williams TM, Davis RW. The ontogeny of aerobic and diving capacity in the skeletal muscles of Weddell seals. J Exp Biol 211: 2559–2565, 2008. doi: 10.1242/jeb.018119. [DOI] [PubMed] [Google Scholar]
- 31.Khedin U, Röken BO, Nyman G, Frostell C, Gustafsson LE. Endogenous nitric oxide in the airways of different animal species. Acta Anaesthesiol Scand 41: 1133–1141, 1997. doi: 10.1111/j.1399-6576.1997.tb04855.x. [DOI] [PubMed] [Google Scholar]
- 32.Kooyman GL, Hammond DD, Schroeder JP. Bronchograms and tracheograms of seals under pressure. Science 169: 82–84, 1970. doi: 10.1126/science.169.3940.82. [DOI] [PubMed] [Google Scholar]
- 33.Korber B. HIV signature and sequence variation analysis. In: Computational Analysis of HIV Molecular Sequences, edited by Rodrigo AG, Learn GH. Dordrecht, The Netherlands: Kluwer Academic Publishers, 2000, p. 55–72. [Google Scholar]
- 34.Kulandavelu S, Balkan W, Hare JM. Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc Natl Acad Sci USA 112: 6254–6255, 2015. doi: 10.1073/pnas.1506523112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Stecher G, Suleski M, Hedges SB. TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol 34: 1812–1819, 2017. doi: 10.1093/molbev/msx116. [DOI] [PubMed] [Google Scholar]
- 36.Lestyk KC, Folkow LP, Blix AS, Hammill MO, Burns JM. Development of myoglobin concentration and acid buffering capacity in harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals from birth to maturity. J Comp Physiol B 179: 985–996, 2009. doi: 10.1007/s00360-009-0378-9. [DOI] [PubMed] [Google Scholar]
- 37.Lewandowski K, Busch T, Lohbrunner H, Rensing S, Keske U, Gerlach H, Falke KJ. Low nitric oxide concentrations in exhaled gas and nasal airways of mammals without paranasal sinuses. J Appl Physiol (1985) 85: 405–410, 1998. doi: 10.1152/jappl.1998.85.2.405. [DOI] [PubMed] [Google Scholar]
- 38.Liggins GC, Qvist J, Hochachka PW, Murphy BJ, Creasy RK, Schneider RC, Snider MT, Zapol WM. Fetal cardiovascular and metabolic responses to simulated diving in the Weddell seal. J Appl Physiol 49: 424–430, 1980. doi: 10.1152/jappl.1980.49.3.424. [DOI] [PubMed] [Google Scholar]
- 39.Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest 116: 1731–1737, 2006. doi: 10.1172/JCI27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mergia E, Russwurm M, Zoidl G, Koesling D. Major occurrence of the new alpha2beta1 isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal 15: 189–195, 2003. doi: 10.1016/S0898-6568(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 41.Mostafa T. Non-sexual implications of phosphodiesterase type 5 inhibitors. Sex Med Rev 5: 170–199, 2017. doi: 10.1016/j.sxmr.2016.02.004. [DOI] [Google Scholar]
- 42.Murad F, Waldman S, Molina C, Bennett B, Leitman D. Regulation and role of guanylate cyclase-cyclic GMP in vascular relaxation. Prog Clin Biol Res 249: 65–76, 1987. [PubMed] [Google Scholar]
- 43.Nagasaka Y, Wepler M, Thoonen R, Sips PY, Allen K, Graw JA, Yao V, Burns SM, Muenster S, Brouckaert P, Miller K, Solt K, Buys ES, Ichinose F, Zapol WM. Sensitivity to Sevoflurane anesthesia is decreased in mice with a congenital deletion of Guanylyl Cyclase-1 alpha. BMC Anesthesiol 17: 76, 2017. doi: 10.1186/s12871-017-0368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noren SR, Iverson SJ, Boness DJ. Development of the blood and muscle oxygen stores in gray seals (Halichoerus grypus): implications for juvenile diving capacity and the necessity of a terrestrial postweaning fast. Physiol Biochem Zool 78: 482–490, 2005. doi: 10.1086/430228. [DOI] [PubMed] [Google Scholar]
- 45.Nuñez C, Victor VM, Martí M, D’Ocon P. Role of endothelial nitric oxide in pulmonary and systemic arteries during hypoxia. Nitric Oxide 37: 17–27, 2014. doi: 10.1016/j.niox.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Olson KR, Whitfield NL, Bearden SE, St Leger J, Nilson E, Gao Y, Madden JA. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol 298: R51–R60, 2010. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park KH, Rubin LE, Gross SS, Levi R. Nitric oxide is a mediator of hypoxic coronary vasodilatation. Relation to adenosine and cyclooxygenase-derived metabolites. Circ Res 71: 992–1001, 1992. doi: 10.1161/01.RES.71.4.992. [DOI] [PubMed] [Google Scholar]
- 48.Ponganis PJ, McDonald BI, Tift MS, Williams CL. Heart rate regulation in diving sea lions: the vagus nerve rules. J Exp Biol 220: 1372–1381, 2017. doi: 10.1242/jeb.146779. [DOI] [PubMed] [Google Scholar]
- 49.Prewitt JS, Freistroffer DV, Schreer JF, Hammill MO, Burns JM. Postnatal development of muscle biochemistry in nursing harbor seal (Phoca vitulina) pups: limitations to diving behavior? J Comp Physiol B 180: 757–766, 2010. doi: 10.1007/s00360-010-0448-z. [DOI] [PubMed] [Google Scholar]
- 50.Qvist J, Hill RD, Schneider RC, Falke KJ, Liggins GC, Guppy M, Elliot RL, Hochachka PW, Zapol WM. Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. J Appl Physiol (1985) 61: 1560–1569, 1986. doi: 10.1152/jappl.1986.61.4.1560. [DOI] [PubMed] [Google Scholar]
- 51.Scholander PF. Experimental investigations on the respiratory function in diving mammals and birds. Hvalradets Skrifter 22: 1–131, 1940. [Google Scholar]
- 52.Shadwick RE, Gosline JM. Arterial Windkessels in marine mammals. Symp Soc Exp Biol 49: 243–252, 1995. [PubMed] [Google Scholar]
- 53.Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol 495: 553–560, 1996. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theilig F, Bostanjoglo M, Pavenstädt H, Grupp C, Holland G, Slosarek I, Gressner AM, Russwurm M, Koesling D, Bachmann S. Cellular distribution and function of soluble guanylyl cyclase in rat kidney and liver. J Am Soc Nephrol 12: 2209–2220, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Thoonen R, Cauwels A, Decaluwe K, Geschka S, Tainsh RE, Delanghe J, Hochepied T, De Cauwer L, Rogge E, Voet S, Sips P, Karas RH, Bloch KD, Vuylsteke M, Stasch J-P, Van de Voorde J, Buys ES, Brouckaert P. Cardiovascular and pharmacological implications of haem-deficient NO-unresponsive soluble guanylate cyclase knock-in mice. Nat Commun 6: 8482, 2015. doi: 10.1038/ncomms9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tift MS, Ponganis PJ, Crocker DE. Elevated carboxyhemoglobin in a marine mammal, the northern elephant seal. J Exp Biol 217: 1752–1757, 2014. doi: 10.1242/jeb.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vázquez-Medina JP, Zenteno-Savín T, Elsner R, Ortiz RM. Coping with physiological oxidative stress: a review of antioxidant strategies in seals. J Comp Physiol B 182: 741–750, 2012. doi: 10.1007/s00360-012-0652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vázquez-Medina JP, Zenteno-Savín T, Tift MS, Forman HJ, Crocker DE, Ortiz RM. Apnea stimulates the adaptive response to oxidative stress in elephant seal pups. J Exp Biol 214: 4193–4200, 2011. doi: 10.1242/jeb.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White FN, Ikeda M, Elsner RW. Adrenergic innervation of large arteries in the seal. Comp Gen Pharmacol 4: 271–276, 1973. doi: 10.1016/0010-4035(73)90008-6. [DOI] [PubMed] [Google Scholar]
- 60.Williams TM, Fuiman LA, Kendall T, Berry P, Richter B, Noren SR, Thometz N, Shattock MJ, Farrell E, Stamper AM, Davis RW. Exercise at depth alters bradycardia and incidence of cardiac anomalies in deep-diving marine mammals. Nat Commun 6: 6055, 2015. doi: 10.1038/ncomms7055. [DOI] [PubMed] [Google Scholar]
- 61.Yeates LC, Carlin KP, Baird M, Venn‐Watson S, Ridgway S. Nitric oxide in the breath of bottlenose dolphins: effects of breath hold duration, feeding, and lung disease. Mar Mamm Sci 30: 272–281, 2014. doi: 10.1111/mms.12037. [DOI] [Google Scholar]
- 62.Zapol WM, Liggins GC, Schneider RC, Qvist J, Snider MT, Creasy RK, Hochachka PW. Regional blood flow during simulated diving in the conscious Weddell seal. J Appl Physiol 47: 968–973, 1979. doi: 10.1152/jappl.1979.47.5.968. [DOI] [PubMed] [Google Scholar]
- 63.Zenteno-Savín T, Clayton-Hernández E, Elsner R. Diving seals: are they a model for coping with oxidative stress? Comp Biochem Physiol C Toxicol Pharmacol 133: 527–536, 2002. doi: 10.1016/S1532-0456(02)00075-3. [DOI] [PubMed] [Google Scholar]