Abstract
A reflex arising from contracting hindlimb muscle is responsible in part for the increases in arterial pressure and heart rate evoked by exercise. The afferent arm of this reflex comprises group III and IV afferents. δ-Opioid receptors are expressed predominately on the spinal endings of group III afferents, whereas μ-opioid receptors are expressed predominately on the spinal endings of group IV afferents. Using stimuli that activated group III afferents, namely static contraction, calcaneal tendon stretch, and lactic acid injection into the superficial epigastric artery, we tested the hypothesis that, in rats with either patent or ligated femoral arteries, activation of pre- and postsynaptic δ-opioid receptors in the dorsal horn attenuated pressor reflex responses to these stimuli. In rats with patent arteries or ligated femoral arteries, [d-Pen2,5]enkephalin (DPDPE), a δ-opioid agonist injected intrathecally (10 μg in 10 μl), significantly attenuated the pressor responses to contraction, stretch, and lactic acid (all P < 0.05). Naltrindole, a δ-opioid receptor antagonist, prevented the attenuation. In contrast, DPDPE did not attenuate the pressor response to capsaicin injection into the superficial epigastric artery in either group of rats (both P > 0.05). Intrathecal injection of saline (10 μl), the vehicle for DPDPE, had no effect on the pressor responses in either group of rats. We conclude that activation of spinal δ-opioid receptors attenuates reflexes evoked by group III afferents in both freely perfused and ligated rats.
Keywords: autonomic nervous system, capsaicin, lactic acid, static contraction, sympathetic nervous system, tendon stretch
INTRODUCTION
The exercise pressor reflex is evoked by contraction of skeletal muscles and results in increases in arterial pressure, heart rate, and ventilation (4, 19). These cardiovascular and ventilatory effects function to increase both blood flow and oxygen to the contracting muscles (1, 26). The afferent arm of the exercise pressor reflex arising from muscle contraction comprises thinly myelinated group III afferents and unmyelinated group IV afferents (5, 19). In contrast, group I and II muscle afferents have been shown to play no role in evoking the exercise pressor reflex (5, 14, 40).
The discharge properties of group III muscle afferents differ from those of group IV muscle afferents. Group III afferents, which are also called Aδ-fibers, respond to nonnoxious probing of their receptive fields and to nonnoxious tendon stretch. In contrast, group IV afferents, which are also called C-fibers, frequently do not respond to either nonnoxious probing of their receptive fields or to vigorous tendon stretch (16). Nevertheless, both group III and IV afferents respond vigorously to injection of lactic acid in their arterial supply (30, 32, 35). In contrast, only a small percentage of group III afferents respond to injection of capsaicin in their arterial supply, whereas a large percentage of group IV afferents respond to injection of this algesic chemical stimulus (9, 15). Overall, group III afferents are believed to be more responsive to mechanical stimuli than are group IV afferents, whereas group IV afferents are believed to be more responsive to chemical stimuli than are group III afferents (16).
The spinal endings of group III and IV afferents contain μ- and δ-opioid receptors that, when stimulated, function to decrease neurotransmitter release, an effect that, in turn, has been shown to reduce the exercise pressor reflex (8, 13). Previously, we showed that stimulation of spinal μ-opioid receptors by intrathecal injection of [d-Ala2,N-MePhe4,Gly-ol]enkephalin attenuated the exercise pressor reflex in decerebrate unanesthetized cats and rats when the arterial supply to their contracting hindlimb muscles was patent (8, 13). The effect of stimulation of δ-opioid receptors by intrathecal injection on the exercise pressor reflex in decerebrate rats whose arterial supply to the contracting hindlimb muscles is either patent or ligated is unknown. Scherrer et al. (31) provided evidence that δ-opioid receptors are found predominately on the spinal endings of thinly myelinated afferents. This finding prompted us to investigate the possibility that attenuating the input of group III mechanoreceptors by intrathecal injection of a δ-opioid receptor agonist may provide some information about the role played by these thinly myelinated afferents in evoking the exercise pressor reflex in rats whose arterial supply to contracting muscles is either patent or ligated.
METHODS
The Institutional Animal Care and Use Committee of the Pennsylvania State College of Medicine reviewed and approved all procedures. Adult male Sprague-Dawley rats (n = 55; weight range: 300–450 grams) were used in these experiments. The rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12-h light-dark cycle and had free access to a standard diet and tap water. In 30 rats, the femoral artery was ligated just proximal to the inguinal ligament 72 h before the experiment was initiated; in the remaining rats, the femoral artery remained patent. To perform the ligation procedure, we anesthetized the rats with isoflurane (2–3%) in oxygen. Using sterile procedure, we ligated the femoral artery with a 6–0 silk suture, after which we allowed the rats to recover in their cages for 72 h before starting the experiment. Ligating the femoral artery near the inguinal ligament has been shown to have little effect on resting hindlimb blood flow; ligation, however, has been shown to reduce blood flow capacity to ~10–20% of normal (28, 43). In the experiments to be described, we will refer to rats whose femoral arteries were ligated as “ligated.” Likewise, we will refer to rats whose femoral arteries were not ligated as “freely perfused.”
Surgical procedures.
On the day of the experiment, we anesthetized the rats with isofluorane (2–3%) in oxygen. We subsequently cannulated the trachea, one jugular vein, and both carotid arteries. The lungs were then ventilated with the anesthetic gas mixture. Arterial blood pressure was measured through a catheter placed in one carotid artery, and drugs and fluids were administered through the catheter in the jugular vein. Arterial blood gases and pH were measured with a blood gas analyzer (ABL80). Arterial pH and Pco2 were maintained within normal limits by adjusting ventilation and injecting a sodium bicarbonate solution (8.5%), respectively. Body temperature was maintained between 36.5 and 38°C by using an isothermal heating pad and lamp.
We performed a laminectomy to expose the L2–L6 spinal segments. We then incised the dura at the L3 or L4, and a saline-filled PE-10 catheter was inserted with its tip pointed rostrally. The catheter was secured at L2 with Kwik-Sil (World Precision Instruments). The left sciatic nerve was then surgically exposed and isolated, and the common peroneal nerve was denervated. The left calcaneal bone was severed and attached to a force transducer (FT10; Grass Instruments).
Next, we placed the rats in a Kopf customized spinal frame and stereotaxic instrument. A rostral lumbar vertebrae and the pelvis were clamped to hold the rat securely in place. Dexamethasone (0.2 mg) was then injected intravenously to minimize brain-stem edema. We then performed a precollicular decerebration. All central neural tissue rostral to the section, which was made <1 mm anterior to the superior colliculus, was removed. Bleeding was controlled, and the cranial cavity was packed with cotton gauze. The isofluorane anesthesia was terminated, and the lungs were subsequently ventilated with room air. We initiated the experimental protocol only after the lungs were ventilated with room air for 60 min. Experiments were performed in decerebrated rats instead of in anesthetized rats because the exercise pressor reflex is often not manifested in the latter (33).
Experimental protocols.
We examined the effect of intrathecal injection of [d-Pen2,5]enkephalin (DPDPE;10 µg in 10 µl), a selective δ-opioid agonist, on the pressor reflex responses to static contraction of the hindlimb muscles, to stretch of the calcaneal tendon, to intra-arterial injection of lactic acid (12 mM; 200 µl), and to intra-arterial injection of capsaicin (0.2 µg; 200 µl). In control experiments, we substituted the vehicle, namely 10 µl of saline, for DPDPE. In all experiments, the rat was tilted 25–30 degrees with its nose up; this was done to minimize spread of the injectate to the medulla. All intra-arterial injections were made in a catheter (PE-8) placed in the superficial epigastric artery. In freely perfused rats, a snare (2–0 silk suture) was placed around both iliac artery and vein and then the snare was tightened before every intra-arterial injection. All intrathecal injections were made in the catheter secured under the dura at L3 or L4. We statically contracted the hindlimb muscles by electrically stimulating the tibial nerve (40 Hz; 0.01-ms pulse duration; 1.5–2 times motor threshold) for 30 s. We stretched the calcaneal tendon and its attached muscles by turning a rack and pinion that was attached to the force transducer for 30 s. The interval between the intrathecal injection of DPDPE and the next stimulus (i.e., contraction, stretch, lactic acid, or capsaicin) was 15–20 min. In a few experiments, we injected intrathecally DPDPE (10 μg) with naltrindole (100 μg), δ-opioid receptor antagonist; the purpose of these combined intrathecal injections was to show that the attenuating effects of DPDPE on the reflexes evoked by contraction, tendon stretch, and lactic acid were specific to δ-opioid receptors.
Control experiments.
We injected Evans blue dye (10 µl) in the intrathecal catheter and visualized the extent of spread after completing all data collection. We looked for evidence of spread of the dye to the medulla. In all experiments in which we contracted the hindlimb muscles, we injected pancuronium bromide intravenously (0.5 mg/kg) and stimulated the tibial nerve with the same frequencies, currents, and pulse durations as those used before we injected this paralyzing agent. After pancuronium injection, we measured arterial pressure and heart rate while stimulating the tibial nerve. This control experiment informed us whether the pressor response to electrically induced static contraction was caused by contraction or by electrically driving the axons of group III and IV afferents. Additionally, at the end of all intra-arterial injection experiments, we injected Evans blue dye intra-arterially to determine that both lactic acid and capsaicin were delivered to the triceps surae muscle. After completing all of the protocols, we killed the rats by ventilating their lungs with 4% isofluorane followed by intravenous injection of a saturated potassium chloride solution. Subsequently, the chest was opened to ensure death.
Data analysis.
In all experiments, mean arterial blood pressure (MAP, in mmHg), heart rate (HR, in beats/min), and muscle tension were displayed continuously in real time with a Spike 2 data acquisition system (Cambridge Electronic Design). Data were recorded and stored on a computer hard drive (Dell) for future off-line analysis. Baseline MAP and HR values were determined from the 30-s baseline period that preceded contraction. The pressor (increases in MAP) and cardioaccelerator (increases in HR) responses to static contraction, calcaneal tendon stretch, and both lactic acid and capsaicin injections were calculated as the difference between the peak MAP and HR values obtained during contraction and the baseline values. The tension-time index (TTI, in kg*s) for static contraction was calculated by subtracting the area under the tension trace for the 30-s baseline period from the area under the tension trace for the 30-s contraction period (contraction − baseline). All data are expressed as means ± SE. Statistical comparisons were performed with paired t-tests. The criteria for statistical significance was P < 0.05.
RESULTS
Effect of 72 h ligation on pressor response.
The pressor responses to static contraction of the hindlimb muscles were significantly greater in ligated rats (P = 0.0008) than were the pressor responses to contraction in freely perfused rats. Likewise, the pressor responses to lactic acid injection were significantly greater in ligated rats (P = 0.001) than were the pressor responses to lactic acid injection in freely perfused rats. However, the pressor responses to tendon stretch and to capsaicin injection were not significantly different between ligated and freely perfused rats (Fig. 1).
Fig. 1.
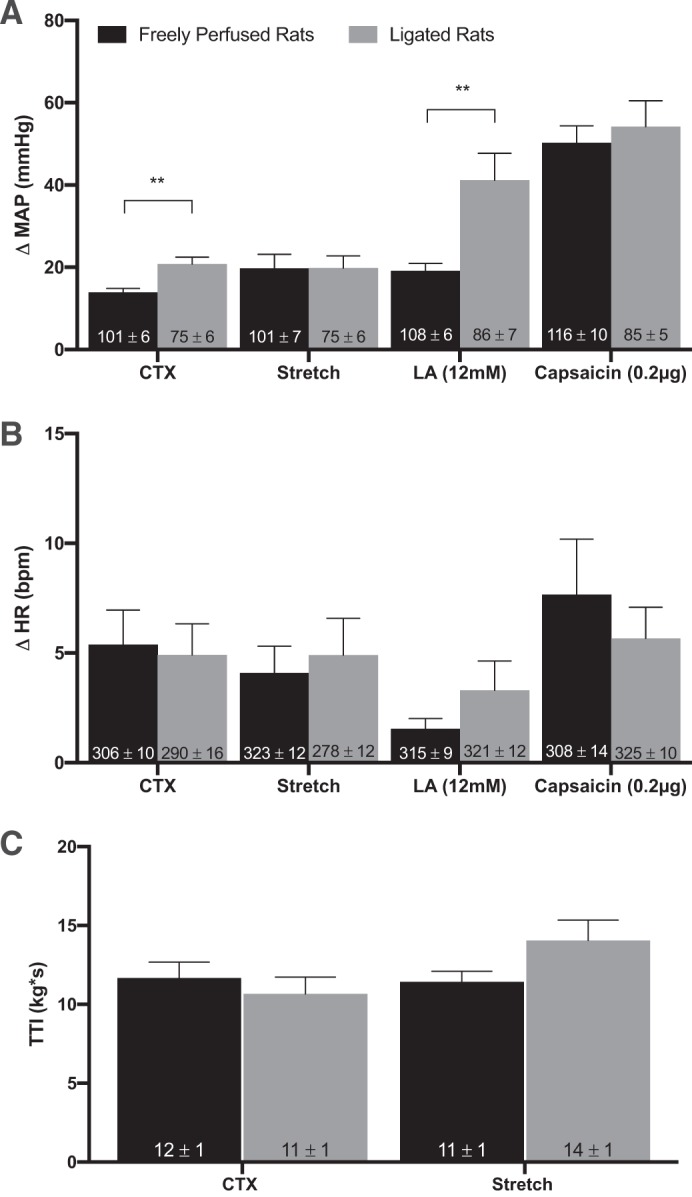
Effects of 72 h ligation on pressor responses. Peak change in pressor responses (A) and cardioaccelerator responses (B) to static contraction [n = 15 freely perfused (FP) and 10 ligated (Lig)], tendon stretch (n = 16 FP; n = 10 Lig), intra-arterial injection of lactic acid (n = 14 FP; n = 10 Lig), and capsaicin (n = 7 FP; n = 11 Lig) in FP and Lig rats. 72-h-Ligated rats had significantly greater pressor response to static contraction and lactic acid injection, whereas 72 h femoral ligation had no effect on the peak pressor response to stretch or capsaicin injection. B: peak cardioaccelerator response between FP and Lig rats was not significantly different. C: tension-time indexes (TTIs) between FP and Lig rats were not significantly different. Δ, Change; MAP, mean arterial pressure; HR, heart rate; bpm, beats/min; CTX, contraction; LA, lactic acid. Bars represent means, and vertical brackets represent SEs. **P < 0.005. Nos. within the vertical bars represent baseline values expressed as means ± SE.
Effect of intrathecal DPDPE injection on pressor responses to contraction, stretch, lactic acid, and capsaicin.
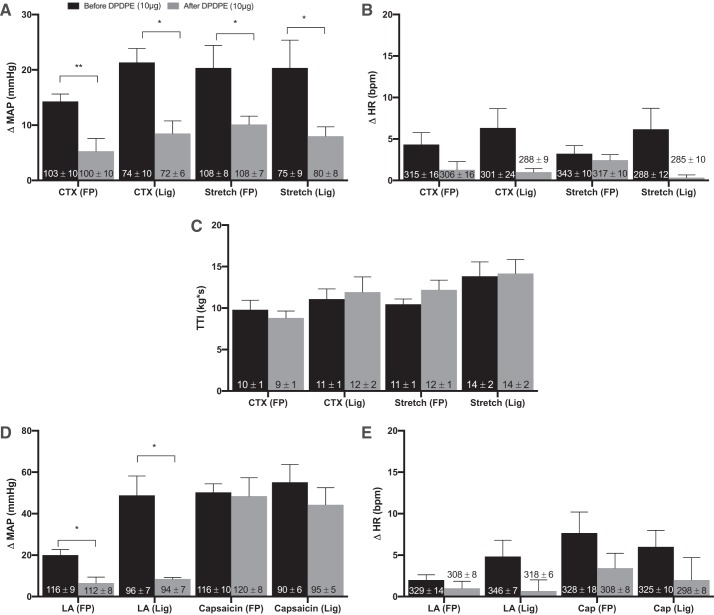
In both freely perfused (n = 7) and ligated (n = 6) rats, intrathecal injection of DPDPE (10 μg) significantly attenuated the pressor response to static contraction. On average, DPDPE decreased the pressor response from 14.3 ± 1.3 to 5.3 ± 2.3 mmHg in freely perfused rats (P = 0.0027); likewise, DPDPE decreased the pressor response from 21.3 ± 5 to 8.5 ± 2.3 mmHg in ligated rats (P = 0.0157). TTI did not differ significantly between the freely perfused and the ligated rats either before or after DPDPE injection (Fig. 2). DPDPE decreased the pressor response to contraction in each of the seven freely perfused rats tested; likewise, DPDPE decreased the pressor response to contraction in each of the six ligated rats tested.
Fig. 2.
Effects of [d-Pen2,5]enkephalin (DPDPE) on pressor responses to contraction, stretch, lactic acid, and capsaicin. Peak change in pressor (A and D) and cardioaccelerator (B and E) responses to static contraction [n = 7 freely perfused (FP) and 6 ligated (Lig)], tendon stretch (n = 9 FP; n = 6 Lig), arterial injection of lactic acid (12 mM; n = 7 FP; n = 6 Lig), and arterial injection of capsaicin (0.2 μg; n = 7 FP; n = 8 Lig) in both FP and Lig rats before and after it DPDPE (10 μg) injection. DPDPE significantly decreased the peak pressor responses to static contraction, tendon stretch, and lactic acid injection in both FP and Lig rats. DPDPE had no effect on the peak pressor and cardioaccelerator responses to capsaicin. DPDPE also had no effect on baseline mean arterial blood pressure or heart rate. C: tension-time indexes (TTIs) after it DPDPE injection were not significantly different from the TTIs before DPDPE. Δ, Change; MAP, mean arterial pressure; HR, heart rate; bpm, beats/min; CTX, contraction; LA, lactic acid. Bars represent means, and vertical brackets represent SEs. Nos. within the vertical bars represent baseline values expressed as means ± SE. *P < 0.05 and **P < 0.005.
DPDPE intrathecal injection also significantly reduced the pressor response to tendon stretch in both freely perfused (n = 9) and ligated (n = 6) rats. On average, DPDPE decreased the pressor response from 20.3 ± 4.1 to 10.1 ± 1.5 mmHg in freely perfused rats (P = 0.0337). DPDPE also decreased the pressor response from 21.3 ± 2.5 to 8 ± 1.7 mmHg in ligated rats (P = 0.0356). TTI did not differ significantly between freely perfused and ligated rats as well as before and after DPDPE injection (Fig. 2). Of the nine freely perfused rats tested, DPDPE decreased the pressor response to stretch in eight and increased the pressor response in the remaining one rat. DPDPE decreased the pressor response to stretch in each of the six ligated rats tested.
We also measured the pressor responses to lactic acid (12 mM, 0.2 ml) and capsaicin (0.2 μg, 0.2 ml) injected in the arterial supply of the hindlimb, both before and after intrathecal injection of DPDPE (10 μg). DPDPE significantly attenuated the pressor response to lactic acid injection in seven freely perfused rats (20 ± 2.7 to 6.6 ± 2.8 mmHg; P = 0.0137) and in six ligated rats (48.8 ± 9.3 to 8.5 ± 0.7 mmHg; P = 0.0091) (Fig. 3). DPDPE decreased the pressor responses to lactic acid in six of the freely perfused rats tested and had no effect on the pressor response in the remaining one. DPDPE decreased the pressor responses to lactic acid in each of the six ligated rats tested. DPDPE, however, did not have any effect on the pressor responses to capsaicin injections (Fig. 2). In each of the rats contributing data to this report, intrathecal injection of Evans blue dye (10 μl) reached the cervical spinal cord but did not reach the medulla.
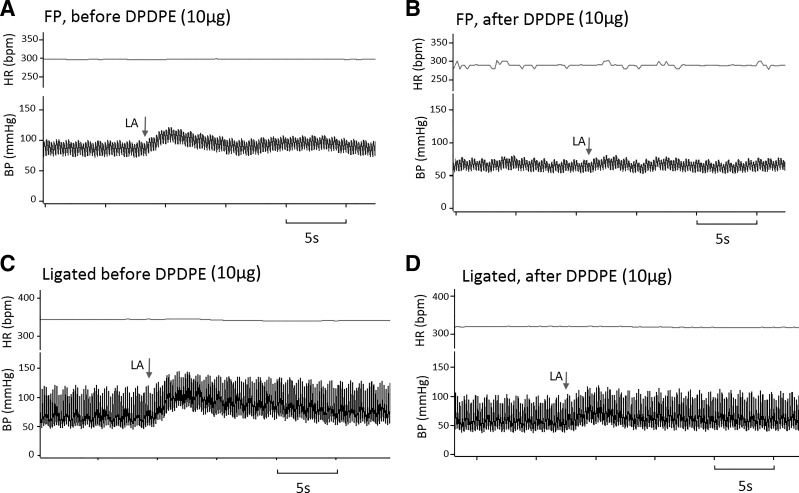
Fig. 3.
Schematic showing the pressor and cardioaccelerator responses to arterial injection of lactic acid in both freely perfused (FP) and ligated rats. Blood pressure responses to arterial injection of lactic acid (12 mM) in FP (A and B) and ligated (C and D) rats. The arrow indicates the bolus injection of lactic acid. A and B: in FP rat, the pressor response was attenuated from 20 to 0 mmHg after [d-Pen2,5]enkephalin (DPDPE, 10 μg). C and D: in ligated rat, the pressor response was attenuated from 31 to 10 mmHg. HR, heart rate; BP, blood pressure; bpm, beats/min; LA, lactic acid.
Control experiments.
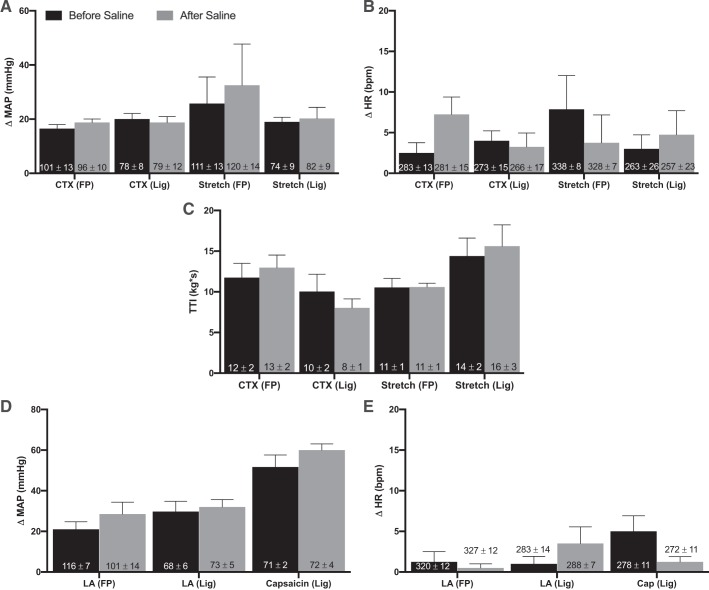
We then tested the pressor response to intrathecal injection of saline in both freely perfused and ligated rats. The pressor response to contraction did not differ either before or after intrathecal saline injections in both freely perfused and ligated rats (n = 5 and 4, respectively). Additionally, intrathecal injection of saline had no effect on tendon stretch in either freely perfused or ligated rats (n = 4). Intrathecal saline injection had no effect on the TTI evoked by static contraction and tendon stretch (Fig. 4). Intrathecal injection of saline also did not affect the pressor response to lactic acid injection in either freely perfused or ligated rats (n = 4). Last, intrathecal injection of saline had no effect on the pressor response to capsaicin injection in ligated rats.
Fig. 4.
Saline control. Peak changes in pressor responses (A and D) and cardioaccelerator response (B and E) to static contraction [n = 4 freely perfused (FP) and 4 ligated (Lig)], tendon stretch (n = 4 FP; n = 4 Lig), arterial injection of lactic acid (12 mM; n = 4 FP; n = 4 Lig), and capsaicin (0.2 μg; n = 3 Lig) in both FP and Lig rats before and after it saline (10 μl) injection. A, B, D, and E: saline had no effect on the peak pressor responses and cardioaccelerator responses to static contraction, tendon stretch, lactic acid injection, and capsaicin injection in either FP or Lig rats. Saline also had no effect on baseline mean arterial blood pressure or heart rate. C: tension-time indexes (TTIs) after it saline injection were not significantly different from TTIs before [d-Pen2,5]enkephalin (DPDPE). Bars represent means, and vertical brackets represent SEs. Intrathecal injection of DPDPE had no effect on the pressor response to capsaicin in FP rats. Therefore, there was no need to perform a vehicle control experiment with saline. Δ, Change; MAP, mean arterial pressure; HR, heart rate; bpm, beats/min. Nos. within the vertical bars represent baseline values expressed as means ± SE.
Effect of naltrindole.
In three freely perfused rats, the pressor responses to static contraction, tendon stretch, and lactic acid injection in the superficial epigastric artery were not attenuated when naltrindole (100 μg) was simultaneously injected with DPDPE (10 μg). The TTIs evoked by static contraction and tendon stretch were not affected by simultaneous intrathecal injection of naltrindole and DPDPE (Fig. 5).
Fig. 5.
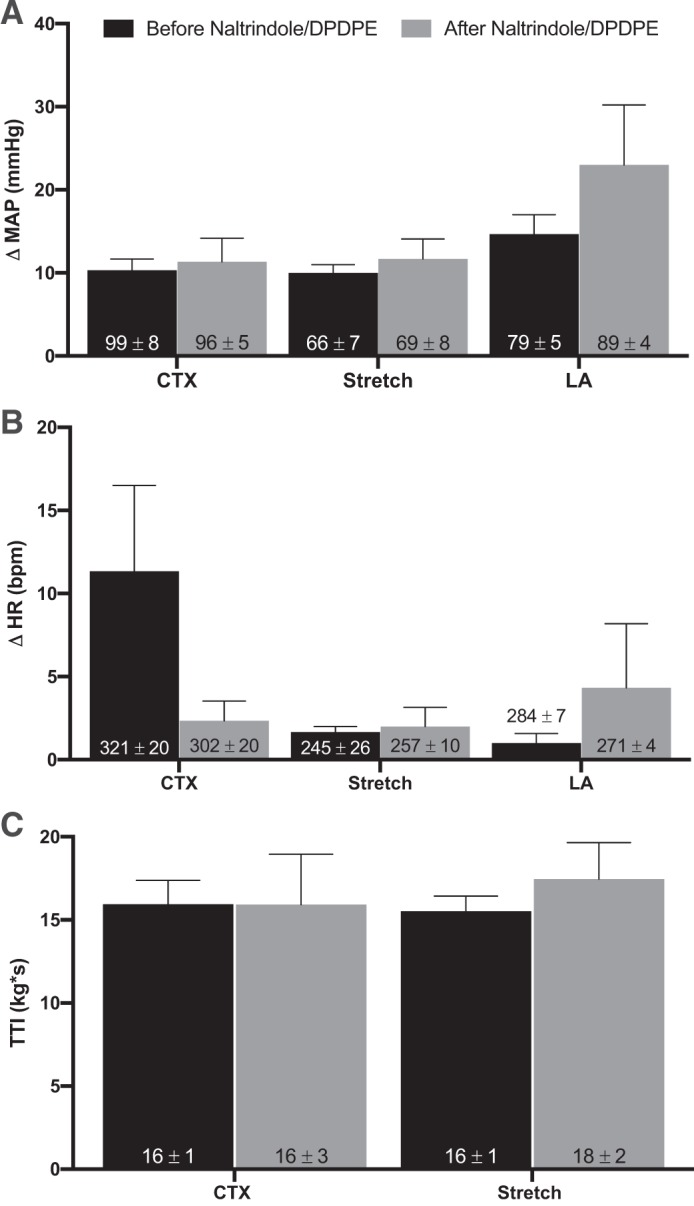
Effects of combined it injection of naltrindole and [d-Pen2,5]enkephalin (DPDPE) on pressor responses to contraction, stretch, and lactic acid. Peak changes in pressor responses (A) and cardioaccelerator responses (B) to static contraction (n = 3), tendon stretch (n = 3), and arterial injection of lactic acid (12 mM; n = 3) in freely perfused (FP) rats before and after combined it injection of naltrindole (100 μg) and DPDPE (10 μg). Note that naltrindole prevented the attenuating effect of DPDPE on the peak pressor and cardioaccelerator responses to static contraction, tendon stretch, and lactic acid in FP rats. Naltrindole/DPDPE injection had no effect on baseline mean arterial blood pressure or heart rate. C: tension-time indexes (TTIs) after it naltrindole/DPDP injection were not significantly different from TTIs before DPDPE. Δ, Change; MAP, mean arterial pressure; HR, heart rate; bpm, beats/min; CTX, contraction; LA, lactic acid. Bars represent means, and vertical brackets represent SEs. Nos. within the vertical bars represent baseline values expressed as means ± SE.
DISCUSSION
The overall goal of our experiments was to reveal the role played by group III afferents in evoking the exercise pressor reflex. To accomplish this task, we used DPDPE, which is a δ-opioid receptor agonist, to attenuate the spinal input of these thinly myelinated muscle afferents, which when stimulated evoked reflex pressor responses. The first stimulus that we used was static muscle contraction, which is both a mechanical and metabolic stimulus to group III and IV afferents (16, 17). We found that stimulation of δ-opioid receptors with DPDPE attenuated by more than half the pressor reflex responses to static contraction in both rats with patent femoral arteries and in rats with ligated femoral arteries. The second stimulus used was calcaneal tendon stretch, which is a pure mechanical stimulus to many group III afferents but only a few group IV afferents (9, 34). Similarly, we found that DPDPE attenuated by half the reflex pressor responses to stretch in both groups of rats. The third stimulus was lactic acid injection, which is an endogenously produced metabolite that activates both group III and IV afferents (30, 32, 35). Intrathecal injection of DPDPE attenuated by more than half the pressor reflex response to lactic acid injection in rats with patent femoral arteries and in rats with ligated femoral arteries. The fourth stimulus was capsaicin injection, which is a potent stimulus to group IV afferents but a weak stimulus to group III afferents (9, 15, 16). Intrathecal injection of DPDPE had no effect on the pressor reflex responses to capsaicin injection in either rats with patent or ligated femoral arteries.
In the present experiments, we found that the magnitude of the pressor response to capsaicin injected in the superficial epigastric artery was the same in both freely perfused and ligated rats. This finding at first glance contrasts with that reported previously by our laboratory in which the pressor response to capsaicin injected in the abdominal aorta of ligated rats (38) was greater than that in freely perfused rats. Consequently, the concentration of capsaicin reaching the nerve endings in the hindlimb muscles was most likely higher in the present report than that in the previous one (38). We speculate that the high concentration of capsaicin reaching the group IV afferent endings in the muscle in the present report might have evoked a maximal effect on the discharge of group IV afferents innervating the freely perfused muscles, thereby preventing a larger response to this algesic agent in the group IV afferents innervating the muscles of the ligated rats.
In healthy humans, group III mechanoreceptors play a significant role in evoking the exercise pressor reflex (2, 12). Moreover, activation of these thinly myelinated muscle afferents by exercise was found to require the production of a cyclooxygenase product of arachidonic acid (21). Activation of group III mechanoreceptors in healthy humans by passive stretch of skeletal muscle (7), by passive cycling (24, 25), and by compression (42) has also been shown to evoke pressor responses. In addition, the role played by group III mechanoreceptors in disease states such as heart failure appears to be exaggerated when compared with that evoked by these myelinated muscle afferents in health (22)
The classical view of the exercise pressor reflex is that it functions to correct a mismatch between blood/oxygen supply and demand in contracting muscles. The reflex accomplishes this correction by increasing both arterial pressure and cardiac output, both of which in conjunction with sympatholysis increase blood flow to exercising muscles (29, 36). Group IV muscle afferents, which are stimulated by a metabolic by-product of contraction, are believed to transmit this “error signal” to the spinal cord and brainstem. The concentration of this by-product is believed to be directly proportional to the level of mismatch between the muscle’s blood and oxygen supply and its metabolic demand. The evidence that such an exercise-induced mechanism, often called the muscle metaboreflex, exerts a potent effect on arterial pressure, cardiac output, and blood flow to contracting muscles is extensive and convincing (1, 26).
An additional view of the exercise pressor reflex is that it has a mechanical component as well as a metabolic component. The mechanical component has received far less attention than has the metabolic component. This is the case even though the existence of group III afferents that were responsive to tendon stretch, nonnoxious probing of their receptive fields, and contraction has been known for many years (16, 20, 27). Our laboratory, in particular, has provided evidence in both cats and rats that the contraction-induced stimulation of group III mechanoreceptors played a significant role in evoking the exercise pressor reflex. For example, we showed in anesthetized cats that static contraction markedly increased renal sympathetic discharge, the onset latency of which was far too short to be caused by the production of muscle metabolites (39). In decerebrated cats, we showed that gadolinium, a molecule that is thought to physically block the mechanogated channel, markedly decreased the exercise pressor reflex (10), a finding subsequently replicated in decerebrated rats (23). We also showed that gadolinium markedly attenuated the responses of group III mechanoreceptors, but not group IV metaboreceptors, to dynamic exercise (11). Last, we showed that GSMTx4, which is believed to block mechanogated Piezo 2 channels, greatly decreased the mechanical component of the exercise pressor reflex in decerebrate rats (6).
Recently, our laboratory has reported that the exercise pressor reflex was exaggerated in rats whose femoral artery was ligated 72 h before the start of the experiment (37). We used this preparation because it simulates the blood flow patterns to a hindlimb with peripheral artery disease. For example, at rest, femoral artery ligation has minimal effect on blood flow to the hindlimb, presumably because of its perfusion by collateral vessels. During exercise, however, ligation decreased blood flow reserve capacity to 10–20% of normal (28, 43). We found that ligation of the femoral artery increased the amount of protein comprising the δ-opioid receptor in dorsal root ganglia innervating the hindlimb (18). This increase in the δ-opioid receptor in the dorsal root ganglia in rats after ligating a femoral artery for 72 h prompted us to perform our experiments in both rats with patent femoral arteries and in rats with ligated femoral arteries. We found that femoral ligation resulted in exaggerated pressor responses to contraction and lactic acid injection and that intrathecal injection of DPDPE reduced the pressor responses to these stimuli to levels similar to those evoked in rats with freely perfused femoral arteries.
The limitations of our findings and conclusions need to be recognized and discussed. First, although δ-opioid receptors are found on myelinated afferents, these receptors are also found on nonpeptidergic unmyelinated afferents innervating the hindlimb (31). Nonpeptidergic afferents, which possess P2X3 receptors, when stimulated, do not release either substance P or calcitonin gene-related peptide. Consequently, some of the pressor responses to contraction and lactic acid injection that were attenuated by DPDPE in our experiments might have been attributable to a loss of nonpeptidergic group IV afferent input. This limitation is less likely to apply to the attenuation of the pressor responses to tendon stretch in our experiments because stretch is a weak stimulus, at best, to nonpeptidergic group IV afferents. A second limitation concerns the fact that δ- and μ-opioid receptors share ~60% amino acid sequence identity (41). As a result, the possibility exists that DPDPE injected intrathecally might have exerted its attenuating effect on the reflex pressor responses evoked in our experiments by stimulating μ- and δ-opioid receptors. We addressed this limitation by showing in selected experiments that naltrindole, a selective antagonist to δ-opioid receptors, prevented the attenuation of these reflex pressor responses induced by DPDPE. A third limitation is that our method of injecting DPDPE intrathecally may have stimulated both pre- and postsynaptic δ-opioid receptors in the spinal cord.
In our experiments, intrathecal injection of DPDPE had no effect on the pressor responses to capsaicin, which was injected in the arterial supply of the hindlimb. Capsaicin stimulates transient receptor potential vanilloid 1 receptors, which are found on peptidergic unmyelinated afferents but not on nonpeptidergic afferents (3). Consequently, our finding that DPDPE had no effect on the pressor responses to capsaicin is consistent with the immunocytochemical finding that peptidergic unmyelinated (i.e., group IV) afferents do not contain δ-opioid receptors. Instead, these afferents contain µ-opioid receptors (31). Moreover, substantial evidence exists showing the complete segregation of δ-opioid receptors, which are found on myelinated and unmyelinated nonpeptidergic afferents from µ-opioid receptors, which are found on unmyelinated peptidergic afferents (31, 41).
Perspectives and Significance
Our experiments have shown that activation of spinal δ-opioid receptors with DPDPE attenuated the pressor reflex responses to static contraction of the hindlimb muscles, to calcaneal tendon stretch, and to intra-arterial injection of lactic acid. Each of these three stimuli is well known to increase the discharge of group III muscle afferents, whose spinal endings in the dorsal horn contain δ-opioid receptors. In our experiments, activation of spinal δ-opioid receptors had no effect on the pressor reflex response to intra-arterial injection of capsaicin, a transient receptor potential vanilloid 1 agonist to group IV afferents possessing µ-opioid receptors. We interpret our findings as consistent with the hypothesis that group III muscle afferents, which often respond to nonnoxious mechanical stimulation, play a significant role in evoking the exercise pressor reflex in both muscles whose arterial supply is patent and in muscles whose arterial supply is comprised, such as that seen in peripheral artery disease.
GRANTS
This work was supported by National Institutes of Health Grants R01-AR-059397 and P01-HL-134609.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S.K. and M.P.K. conceived and designed research; J.S.K. performed experiments; J.S.K. analyzed data; J.S.K. and M.P.K. interpreted results of experiments; J.S.K. prepared figures; J.S.K. and M.P.K. drafted manuscript; J.S.K. and M.P.K. edited and revised manuscript; J.S.K. and M.P.K. approved final version of manuscript.
REFERENCES
- 1.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batman BA, Hardy JC, Leuenberger UA, Smith MB, Yang QX, Sinoway LI. Sympathetic nerve activity during prolonged rhythmic forearm exercise. J Appl Physiol (1985) 76: 1077–1081, 1994. doi: 10.1152/jappl.1994.76.3.1077. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 4.Coote JH, Hilton SM, Pérez-González JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol 208: 261–278, 1970. doi: 10.1113/jphysiol.1970.sp009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copp SW, Kim JS, Ruiz-Velasco V, Kaufman MP. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol 594: 641–655, 2016. doi: 10.1113/JP271714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drew RC, Blaha CA, Herr MD, Cui R, Sinoway LI. Muscle mechanoreflex activation via passive calf stretch causes renal vasoconstriction in healthy humans. Am J Physiol Regul Integr Comp Physiol 312: R956–R964, 2017. doi: 10.1152/ajpregu.00322.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estrada JA, Kaufman MP. µ-Opioid receptors inhibit the exercise pressor reflex by closing N-type calcium channels but not by opening GIRK channels in rats. Am J Physiol Regul Integr Comp Physiol 314: R693–R699, 2018. doi: 10.1152/ajpregu.00380.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harms J, Stone AJ, Kaufman MP. Peripheral µ-opioid receptors attenuate the responses of group III and IV afferents to contraction in rats with simulated peripheral artery disease. J Neurophysiol 119: 2052–2058, 2018. doi: 10.1152/jn.00034.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol 280: H2153–H2161, 2001. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- 11.Hayes SG, McCord JL, Koba S, Kaufman MP. Gadolinium inhibits group III but not group IV muscle afferent responses to dynamic exercise. J Physiol 587: 873–882, 2009. doi: 10.1113/jphysiol.2008.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. J Appl Physiol (1985) 86: 767–772, 1999. doi: 10.1152/jappl.1999.86.2.767. [DOI] [PubMed] [Google Scholar]
- 13.Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol (1985) 68: 2466–2472, 1990. doi: 10.1152/jappl.1990.68.6.2466. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson HJ, Matthews PB. The ineffectiveness of excitation of the primary endings of the muscle spindle by vibration as a respiratory stimulant in the decerebrate cat. J Physiol 194: 555–563, 1968. doi: 10.1113/jphysiol.1968.sp008424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res 50: 133–139, 1982. doi: 10.1161/01.RES.50.1.133. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- 18.Leal AK, Yamauchi K, Kim J, Ruiz-Velasco V, Kaufman MP. Peripheral δ-opioid receptors attenuate the exercise pressor reflex. Am J Physiol Heart Circ Physiol 305: H1246–H1255, 2013. doi: 10.1152/ajpheart.00116.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol 342: 383–397, 1983. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol 287: H1944–H1949, 2004. doi: 10.1152/ajpheart.00329.2004. [DOI] [PubMed] [Google Scholar]
- 22.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Cyclooxygenase products sensitize muscle mechanoreceptors in humans with heart failure. Am J Physiol Heart Circ Physiol 294: H1956–H1962, 2008. doi: 10.1152/ajpheart.01304.2007. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 300: H968–H977, 2011. doi: 10.1152/ajpheart.01145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nóbrega AC, Williamson JW, Friedman DB, Araújo CG, Mitchell JH. Cardiovascular responses to active and passive cycling movements. Med Sci Sports Exerc 26: 709–714, 1994. doi: 10.1249/00005768-199406000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Nóbrega ACL, Araújo CGS. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc 25: 37–41, 1993. doi: 10.1249/00005768-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 26.O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- 27.Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol 152: 250–270, 1960. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 29.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. doi: 10.1161/01.RES.11.3.370. [DOI] [PubMed] [Google Scholar]
- 30.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985) 64: 2306–2313, 1988. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- 31.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137: 1148–1159, 2009. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol 69: 1053–1059, 1993. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- 33.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. doi: 10.1113/jphysiol.2001.012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol (1985) 65: 1539–1547, 1988. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- 35.Thimm F, Tibes U. Effect of K+, osmolality, lactic acid, orthophosphate and epinephrine on muscular receptors with group I, III, and IV afferents [proceedings] J Physiol 284: 182P–183P, 1978. [PubMed] [Google Scholar]
- 36.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchimochi H, McCord JL, Kaufman MP. Peripheral mu-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 299: H557–H565, 2010. doi: 10.1152/ajpheart.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol 589: 6173–6189, 2011. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res 64: 592–599, 1989. doi: 10.1161/01.RES.64.3.592. [DOI] [PubMed] [Google Scholar]
- 40.Waldrop TG, Rybicki KJ, Kaufman MP. Chemical activation of group I and II muscle afferents has no cardiorespiratory effects. J Appl Physiol 56: 1223–1228, 1984. doi: 10.1152/jappl.1984.56.5.1223. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Tawfik VL, Corder G, Low SA, François A, Basbaum AI, Scherrer G. functional divergence of delta and mu opioid receptor organization in CNS pain circuits. Neuron 98: 90–108.e5, 2018. doi: 10.1016/j.neuron.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williamson JW, Mitchell JH, Olesen HL, Raven PB, Secher NH. Reflex increase in blood pressure induced by leg compression in man. J Physiol 475: 351–357, 1994. doi: 10.1113/jphysiol.1994.sp020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000. doi: 10.1152/ajpheart.2000.278.6.H1966. [DOI] [PubMed] [Google Scholar]